Phytochemical Screening, Antioxidant, and Enzyme Inhibitory Properties of Three Prangos Species (P. heyniae, P. meliocarpoides var. meliocarpoides, and P. uechtritzii) Depicted by Comprehensive LC-MS and Multivariate Data Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Total Phenolic and Flavonoid Contents
2.3. LC-DAD-MSn
2.4. Antioxidant Assays
2.5. Enzyme Inhibitory Assays
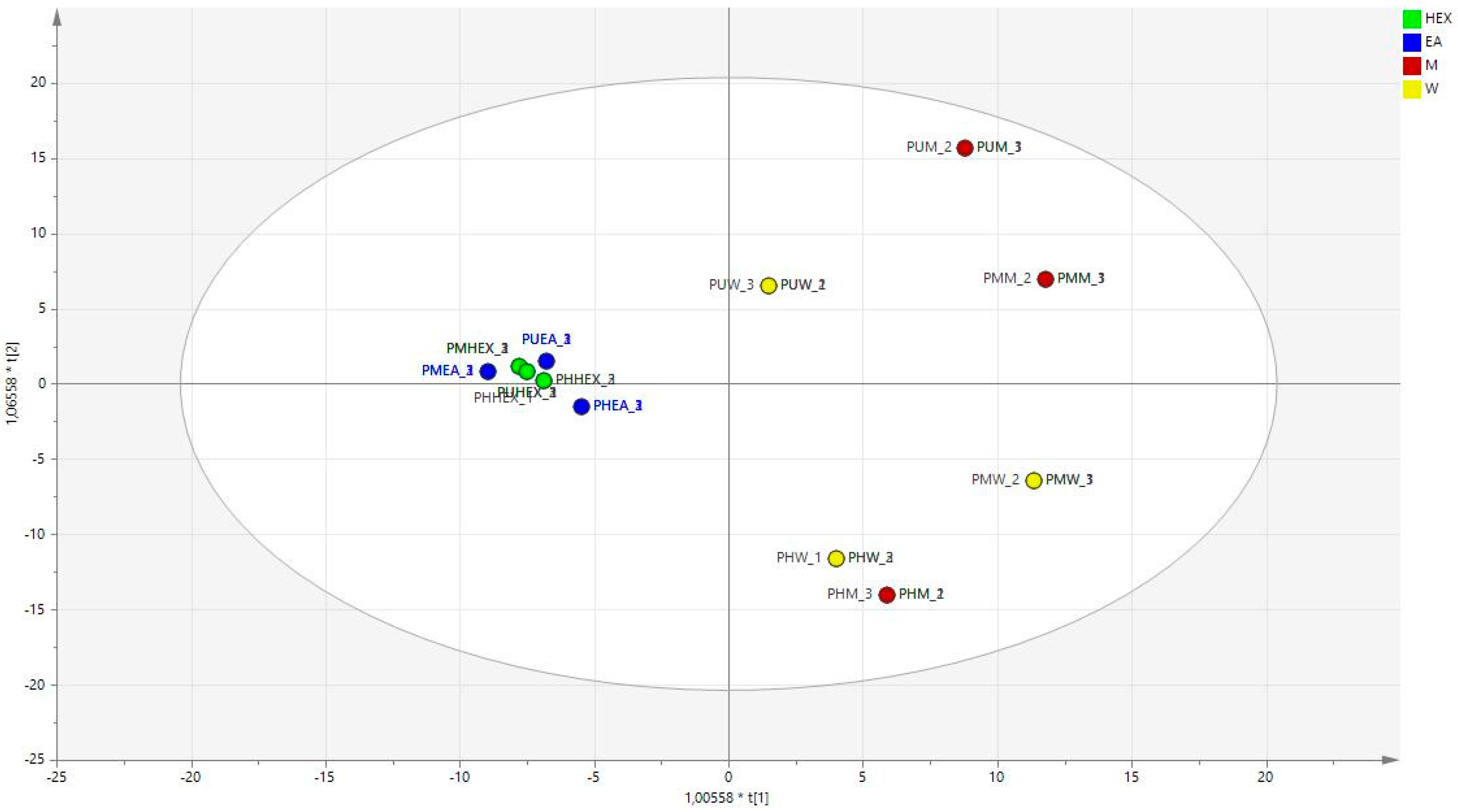
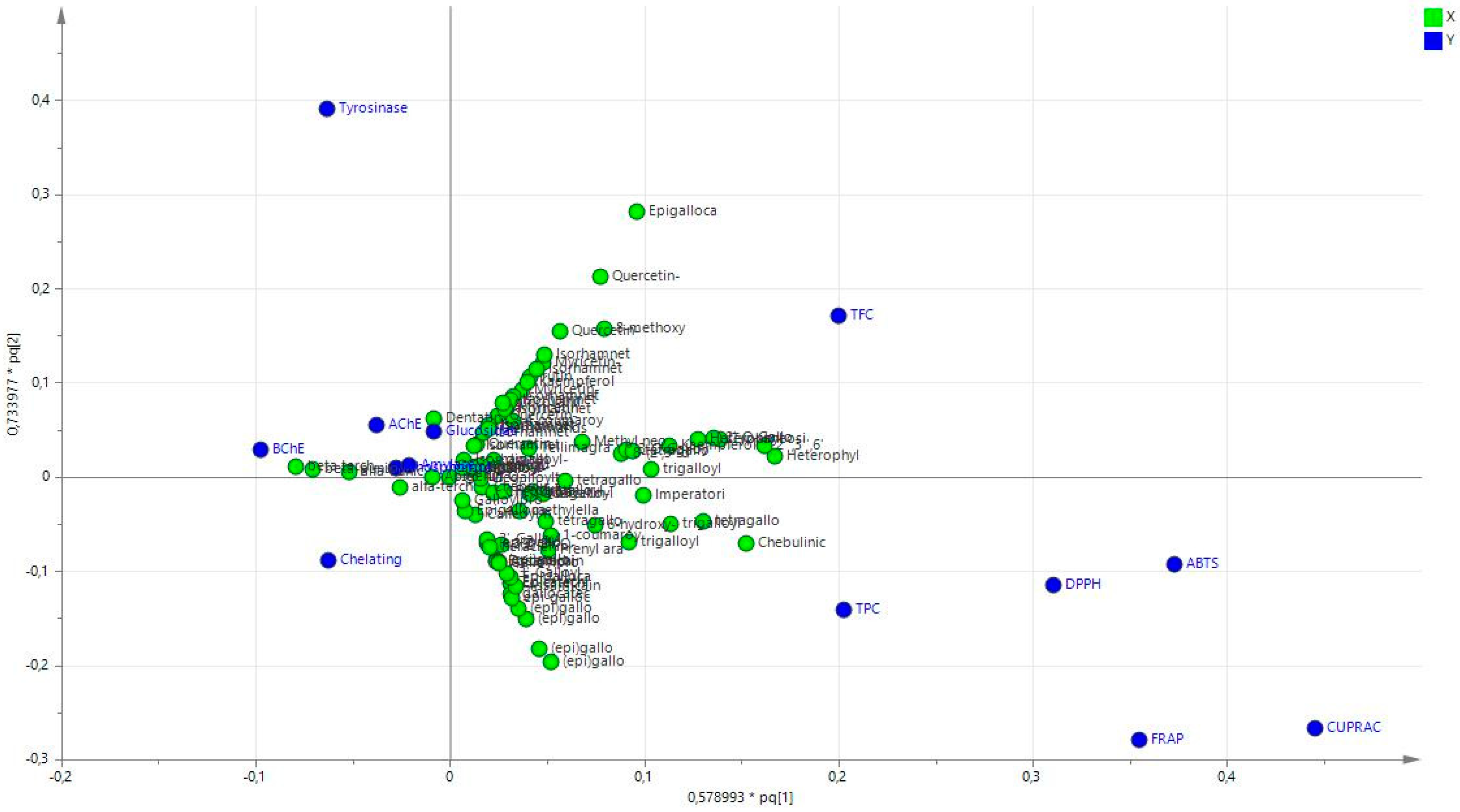
2.6. Statistical Analysis
3. Results and Discussion
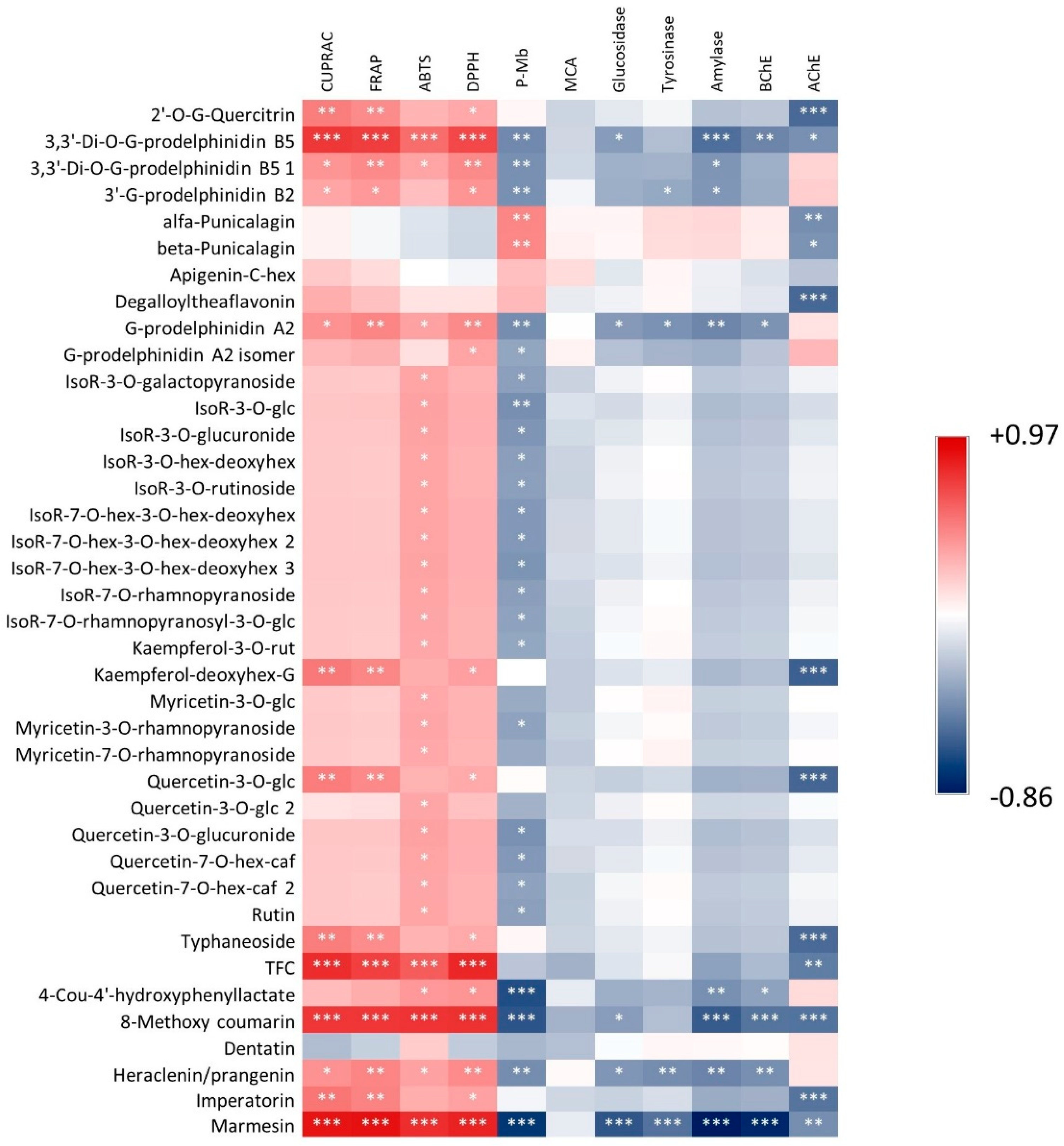
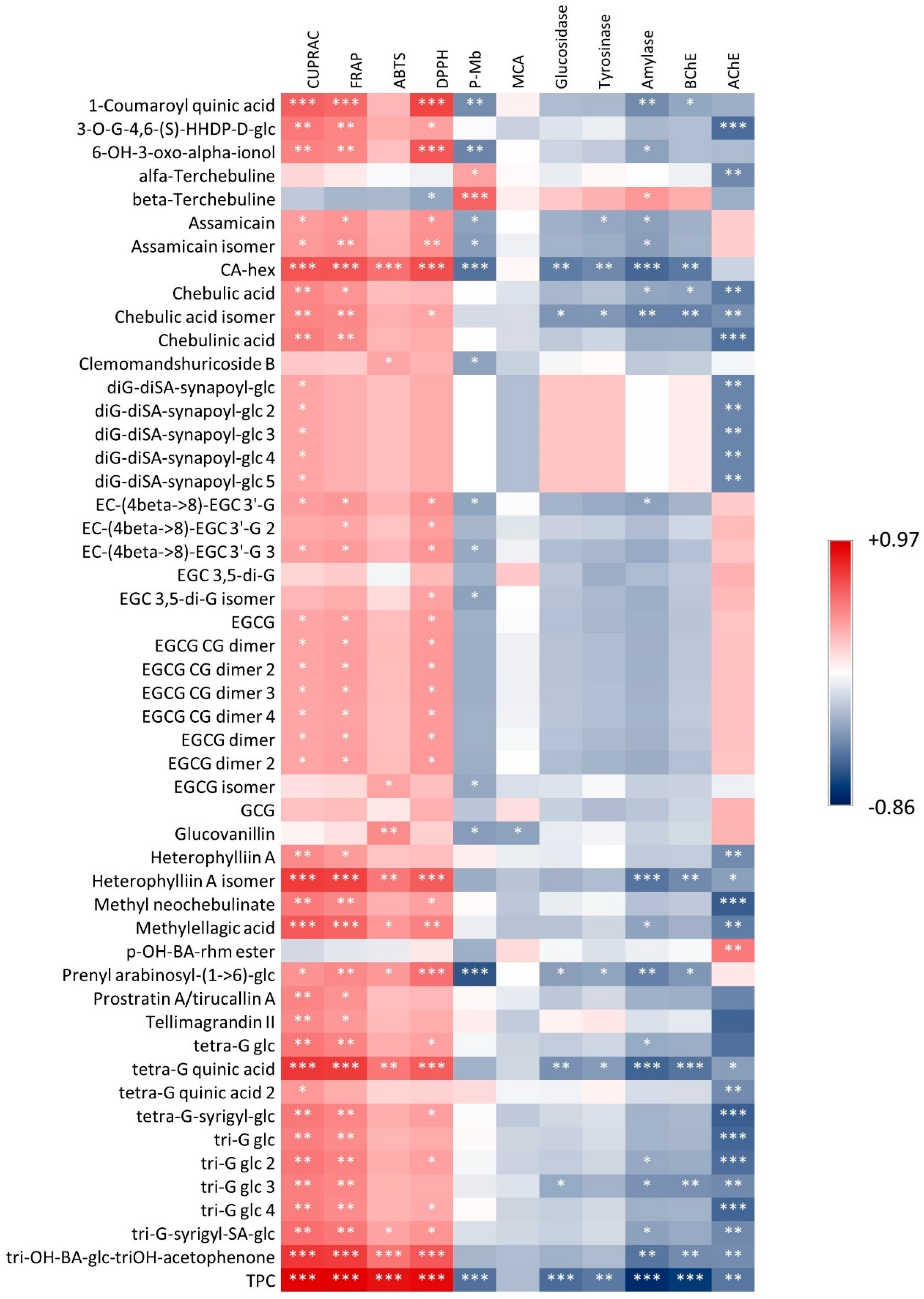
3.1. Characterization of Bioactive Secondary Metabolites
3.2. Antioxidant Effects
3.3. Enzyme Inhibitory Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, B.; Zhang, Y. Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives. Drug Discov. Today 2022, 27, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.; Pellati, F.; Pinzi, L.; Gamberini, M.C. Repositioning natural products in drug discovery. Molecules 2020, 25, 1154. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nature Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar]
- Rammohan, A.; Bhaskar, B.V.; Camilo, A.; Gunasekar, D.; Gu, W.; Zyryanov, G.V. In Silico, In Vitro antioxidant and density functional theory based structure activity relationship studies of plant polyphenolics as prominent natural antioxidants. Arab. J. Chem. 2020, 13, 3690–3701. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; Dall Acqua, S. Nutraceuticals, a New Challenge for Medicinal Chemistry. Curr. Med. Chem. 2016, 23, 3198–3223. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Kiss, T.; Tóth, B.; Csupor, D. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020, 19, 1449–1470. [Google Scholar] [CrossRef]
- Başer, K.; Kırımer, N. Essential oils of Anatolian Apiaceae—A profile. Nat. Volatiles Essent. Oils 2014, 1, 1–50. [Google Scholar]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. The nonvolatile and volatile metabolites of Prangos ferulacea and their biological properties. Planta Med. 2019, 85, 815–824. [Google Scholar] [CrossRef]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. Composition and biological activities of the essential oil from a Sicilian accession of Prangos ferulacea (L.) Lindl. Nat. Prod. Res. 2021, 35, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Delnavazi, M.R.; Soleimani, M.; Hadjiakhoondi, A.; Yass, N. Isolation of phenolic derivatives and essential oil analysis of Prangos ferulacea (L.) Lindl. Aerial Parts. Iran. J. Pharm. Res. IJPR 2017, 16, 207–215. [Google Scholar] [PubMed]
- Yousefi, K.; Hamedeyazdan, S.; Hodaei, D.; Lotfipour, F.; Baradaran, B.; Orangi, M.; Fathiazad, F. An In Vitro ethnopharmacological study on Prangos ferulacea: A wound healing agent. Bioimpacts 2017, 7, 75–82. [Google Scholar] [CrossRef][Green Version]
- Shokoohinia, Y.; Sajjadi, S.E.; Gholamzadeh, S.; Fattahi, A.; Behbahani, M. Antiviral and cytotoxic evaluation of coumarins from Prangos ferulacea. Pharm. Biol. 2014, 52, 1543–1549. [Google Scholar] [CrossRef]
- Azadpour Motlagh, A.M.S.S.; Dolatian, M.P.; Mojab, F.P.; Nasiri, M.P.; Ezatpour, B.P.; Sahranavard, Y.M.; Shakiba, H.B.; Rahimy, B.M.; Ghanati, K. The effect of Prangos ferulacea vaginal cream on accelerating the aecovery of bacterial vaginosis: A randomized controlled clinical trial. Int. J. Community Based Nurs. Midwifery 2018, 6, 100–110. [Google Scholar]
- Numonov, S.; Bobakulov, K.; Numonova, M.; Sharopov, F.; Setzer, W.N.; Khalilov, Q.; Begmatov, N.; Habasi, M.; Aisa, H.A. New coumarin from the roots of Prangos pabularia. Nat. Prod. Res. 2018, 32, 2325–2332. [Google Scholar] [CrossRef]
- Dissanayake, A.A.; Ameen, B.A.H.; Nair, M.G. Lipid peroxidation and cyclooxygenase enzyme inhibitory compounds from Prangos haussknechtii. J. Nat. Prod. 2017, 80, 2472–2477. [Google Scholar] [CrossRef]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial activities of pyrenylated coumarins from the roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef]
- Ahmed, J.; Güvenç, A.; Küçükboyaci, N.; Baldemir, A.; Coşkun, M. Total phenolic contents and antioxidant activities of Prangos Lindl.(Umbelliferae) species growing in Konya province (Turkey). Turk. J. Biol. 2011, 35, 353–360. [Google Scholar] [CrossRef]
- Albayrak, G.; Demir, S.; Kose, F.A.; Baykan, S. New coumarin glycosides from endemic Prangos heyniae H. Duman & M.F. Watson. Nat. Prod. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Karahisar, E.; Köse, Y.B.; İşcan, G.; Kurkcuoglu, M.; Tugay, O. Chemical composition and anticandidal activity of essential oils obtained from different parts of Prangos heyniae H. Duman & MF Watson. Rec. Nat. Prod. 2022, 16, 74–83. [Google Scholar]
- Özek, G.; Bedir, E.; Tabanca, N.; Ali, A.; Khan, I.A.; Duran, A.; Başer, K.H.; Özek, T. Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H. Duman & MF Watson essential oil and mosquitocidal activity of the essential oils. Open Chem. 2018, 16, 453–467. [Google Scholar]
- Oke Altuntas, F.; Aslim, B.; Duman, H. The anti-lipid peroxidative, metal chelating, and radical scavenging properties of the fruit extracts from endemic Prangos meliocarpoides Boiss var. meliocarpoides. Gazi Univ. J. Sci. 2016, 29, 537–542. [Google Scholar]
- Uzel, A.; Dirmenci, T.; Celik, A.; Arabaci, T. Composition and antimicrobial activity of Prangos platychlaena and P-uechtritzii. Chem. Nat. Compd. 2006, 42, 169–171. [Google Scholar] [CrossRef]
- Ozcan, M.; Bagci, Y.; Akgul, A.; Dural, H.; Novak, J. Chemical composition of the essential oil of Prangos uechtritzii Boiss. et Hausskn. fruits from Turkey. J. Essent. Oil Res. 2000, 12, 183–185. [Google Scholar] [CrossRef]
- Ozcan, M. Antifungal effects of Micromeria myrtifolia Boiss. & Hohen. in Boiss. and Prangos uechtritzii Boiss. Hawsskn decoctions. Acta Aliment. 1999, 28, 355–360. [Google Scholar]
- Sevin, G.; Alan, E.; Demir, S.; Albayrak, G.; Demiroz, T.; Yetik-Anacak, G.; Baykan, S. Comparative evaluation of relaxant effects of three Prangos species on mouse corpus cavernosum: Chemical characterization and the relaxant mechanisms of action of P. pabularia and (+)-oxypeucedanin. J. Ethnopharmacol. 2022, 284, 114823. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Yıldıztugay, E.; Jugreet, S.; Khan, S.U.; Dall’Acqua, S.; Mollica, A.; Bouyahya, A.; Montesano, D. Chemical composition, biological activities and in silico analysis of essential oils of three endemic Prangos species from Turkey. Molecules 2022, 27, 1676. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Trad. Complement. Alt. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Etienne, O.K.; Mahomoodally, M.F.; Lobine, D.; Zengin, G. Evaluation of antioxidant and enzyme inhibition properties of Croton hirtus L’Hér. extracts obtained with different solvents. Molecules 2021, 26, 1902. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In Vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar]
- Deen, F.; Visvanathan, R.; Liyanage, R. Extraction Methods: Conventional and non-conventional. In Bioactive Compounds from Plant Origin; CRC Press: Boca Raton, FL, USA, 2019; p. 24. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Peña Malacara, C.F.; Petricevich, V.L. Characterization of chemical compounds with antioxidant and cytotoxic activities in Bougainvillea x buttiana Holttum and Standl, (var. rose) extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef]
- Abdul Razak, M.; Yong, P.; Shah, Z.; Abdullah, L.; Yee, S.; Yaw, I. The effects of varying solvent polarity on extraction yield of Orthosiphon stamineus leaves. J. Appl. Sci. 2012, 12, 1207–1210. [Google Scholar] [CrossRef]
- Lee, R.J.; Lee, V.S.; Tzen, J.T.; Lee, M.R. Study of the release of gallic acid from (–)-epigallocatechin gallate in old oolong tea by mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 851–858. [Google Scholar]
- De Lima Paula, P.; de Oliveira Lemos, A.S.; Campos, L.M.; Ferreira, T.G.; de Souza, T.F.; Queiroz, L.S.; Guedes, M.C.M.R.; Martins, M.M.; Goulart Filho, L.R.; Macedo, G.C. Pharmacological investigation of antioxidant and anti-inflammatory activities of leaves and branches extracts from Plinia cauliflora (Jaboticaba). J. Ethnopharmacol. 2021, 280, 114463. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Lin, C.-C.; Lin, T.-C. Antiviral properties of prodelphinidin B-2 3′-O-gallate from green tea leaf. Antivir. Chem. Chemother. 2002, 13, 223–229. [Google Scholar] [CrossRef]
- Li, M.; Shen, Y.; Ling, T.; Ho, C.-T.; Li, D.; Guo, H.; Xie, Z. Analysis of differentiated chemical components between Zijuan purple tea and Yunkang green tea by UHPLC-Orbitrap-MS/MS Combined with Chemometrics. Foods 2021, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Juang, L.J.; Sheu, S.J.; Lin, T.C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2004, 27, 718–724. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.W.; Yang, H.; Sung, S.H. Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their α-glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.; Gomes, E.T.; Wolfender, J.-L.; Marston, A.; Hostettmann, K. Application of high performance liquid chromatography coupled with ultraviolet spectroscopy and electrospray mass spectrometry to the characterisation of ellagitannins from Terminalia macroptera roots. Pharm. Res. 2000, 17, 1396–1401. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.-i.; Nishioka, I. Tannins and Related Compounds. LXXVII.: Novel Chalcan-flavan Dimers, Assamicains A, B and C, and a New Flavan-3-ol and Proanthocyanidins from the fresh leaves of Camella sinensis L. var. assamica kitamura. Chem. Pharm. Bull. 1989, 37, 77–85. [Google Scholar] [CrossRef]
- Wyrepkowski, C.C.; Gomes da Costa, D.L.M.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Dos Santos, L.C. Characterization and quantification of the compounds of the ethanolic extract from Caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Comm. 2016, 11, 1934578X1601100227. [Google Scholar]
- Ben Said, R.; Arafa, I.H.; Usam, A.M.; Abdullah Sulaiman, A.-A.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Barry, K.; Davies, N.; Mohammed, C. Identification of hydrolysable tannins in the reaction zone of Eucalyptus nitens wood by high performance liquid chromatography–electrospray ionisation mass spectrometry. Phytochem. Anal. 2001, 12, 120–127. [Google Scholar] [CrossRef]
- Farooq, S.; Dangroo, N.A.; Priya, D.; Banday, J.A.; Sangwan, P.L.; Qurishi, M.A.; Koul, S.; Saxena, A.K. Isolation, cytotoxicity evaluation and HPLC-quantification of the chemical constituents from Prangos pabularia. PLoS ONE 2014, 9, e108713. [Google Scholar]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv.“Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic composition influences the health-promoting potential of bee-pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-H.; Xu, H.-Y.; Wang, J.-Y.; Duan, S.; Wang, Y.-C.; Ma, C.-M. In Vivo hepatoprotective activity and the underlying mechanism of chebulinic acid from Terminalia chebula fruit. Phytomedicine 2021, 83, 153479. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Sharma, S.; Pareek, A.; Dwivedi, J.; Yadav, S.; Sharma, S. Phytochemistry, traditional uses and cancer chemopreventive activity of Amla (Phyllanthus emblica): The Sustainer. J. Appl. Pharm. Sci. 2012, 2, 176–183. [Google Scholar]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Lazarova, I.; Zengin, G.; Sinan, K.I.; Aneva, I.; Uysal, S.; Picot-Allain, M.C.N.; Aktumsek, A.; Bouyahya, A.; Mahomoodally, M.F. Metabolomics profiling and biological properties of root extracts from two Asphodelus species: A. albus and A. aestivus. Food Res. Int. 2020, 134, 109277. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Zengin, G.; Sinan, K.I.; Polat, R.; Canlı, D.; Picot-Allain, M.C.N.; Mahomoodally, M.F. Impact of different extraction solvents and techniques on the biological activities of Cirsium yildizianum (Asteraceae: Cynareae). Ind. Crops Prod. 2020, 144, 112033. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Rocchetti, G.; Senizza, B.; Montesano, D.; Zengin, G.; Uysal, A.; Jeewon, R.; Lucini, L.; Mahomoodally, M.F. Untargeted metabolomic profiling, multivariate analysis and biological evaluation of the true mangrove (Rhizophora mucronata Lam.). Antioxidants 2019, 8, 489. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.R.; Fawzi Mahomoodally, M. Assessment of the pharmacological properties and phytochemical profile of Bruguiera gymnorhiza (l.) lam using in vitro studies, in silico docking, and multivariate analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Sinan, K.I.; Ferrarese, I.; Sut, S.; Bene, K.; Mahomoodally, M.F.; Sadeer, N.B.; Ak, G.; Zengin, G. Chromatographic separation of Breynia retusa (Dennst.) Alston bark, fruit and leaf constituents from bioactive extracts. Molecules 2020, 25, 5537. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Llorent-Martínez, E.J.; Bene, K.; Mahomoodally, M.F.; Mollica, A.; Sinan, K.I.; Stefanucci, A.; Ruiz-Riaguas, A.; Fernández-de Córdova, M.L.; Zengin, G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019, 174, 19–33. [Google Scholar] [CrossRef]
- Darvesh, S. Butyrylcholinesterase as a diagnostic and therapeutic target for Alzheimer’s disease. Curr. Alzheimer. Res. 2016, 13, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- DeBay, D.R.; Darvesh, S. Chapter 17-Butyrylcholinesterase as a biomarker in Alzheimer’s disease. In Diagnosis and Management in Dementia; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 263–280. [Google Scholar]
- Abbas-Mohammadi, M.; Moridi Farimani, M.; Salehi, P.; Nejad Ebrahimi, S.; Sonboli, A.; Kelso, C.; Skropeta, D. Acetylcholinesterase-inhibitory activity of Iranian plants: Combined HPLC/bioassay-guided fractionation, molecular networking and docking strategies for the dereplication of active compounds. J. Pharm. Biomed. Anal. 2018, 158, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Maggi, F.; Dinparast, L. Chemical composition of essential oil, antioxidant, antidiabetic, anti-obesity, and neuroprotective properties of Prangos gaubae. Nat. Prod. Comm. 2017, 12, 1934578X1701201233. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Jekő, J.; Cziáky, Z.; et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crops Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Menichini, F.; Bonesi, M.; Piccolo, V.; Statti, G.A.; de Cindio, B.; Houghton, P.J.; Menichini, F. In Vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. J. Ethnopharmacol. 2008, 119, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci.Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]

| Species | Solvents | TPC (mg GAE/g) | TFC (mg RE/g) | PBD (mmol TE/g) |
|---|---|---|---|---|
| P. heyniae | Hexane | 17.55 ± 0.16 g | 3.43 ± 0.14 j | 1.93 ± 0.02 b |
| EA | 21.85 ± 0.36 f | 12.93 ± 0.35 f | 2.30 ± 0.10 a | |
| MeOH | 32.13 ± 0.76 c | 28.75 ± 0.36 b | 1.51 ± 0.11 de | |
| Water | 38.77 ± 0.01 b | 16.19 ± 0.24 d | 1.39 ± 0.03 ef | |
| P. meliocarpoides var. meliocarpoides | Hexane | 22.63 ± 0.16 f | 8.19 ± 0.23 h | 2.52 ± 0.06 a |
| EA | 26.15 ± 1.40 e | 19.40 ± 0.60 c | 2.49 ± 0.05 a | |
| MeOH | 40.03 ± 0.68 b | 44.66 ± 0.68 a | 1.82 ± 0.11 bc | |
| Water | 44.28 ± 0.27 a | 11.00 ± 0.18 g | 1.46 ± 0.02 def | |
| P. uechtritzii | Hexane | 18.70 ± 0.08 g | 1.72 ± 0.07 k | 1.79 ± 0.04 bc |
| EA | 25.45 ± 0.12 e | 5.72 ± 0.05 i | 2.35 ± 0.17 a | |
| MeOH | 31.20 ± 0.16 c | 28.22 ± 0.54 b | 1.67 ± 0.13 cd | |
| Water | 29.62 ± 0.07 d | 14.75 ± 0.30 e | 1.21 ± 0.03 f |
| M-H | Molecular Formula | Fragments | Name and References | P. heyniae- Hexane | P. heyniae- EA | P. heyniae- MeOH | P. heyniae- Water | P. meliocarpoides- Hexane | P. meliocarpoides-EA | P. meliocarpoides-MeOH | P. meliocarpoides-Water | P. uechtritzii-Hexane | P. uechtritzii-EA | P. uechtritzii-MeOH | P. uechtritzii-Water |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condensed Tannins | |||||||||||||||
| 609.088050 | C29H21O15 | 457 305 249 | Epigallocatechin 3,5-di-gallate [38,39] | 0.15 ± 0.01 | 0.08 ± 0.01 | 0.47 ± 0.02 | 0.89 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd |
| 609.088050 | C29H21O15 | 457 305 249 | Epigallocatechin di-gallate isomer [38,39] | 0.05 ± 0.01 | 0.03 ± 0.01 | 7.93 ± 0.05 | 3.21 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd |
| M + H | |||||||||||||||
| 763,151 | C37H31O18 | 611 595 458 443 425 317 305 287 | Galloylprodelphinidin isomer 1 [40] | nd | nd | 2.33 ± 0.05 | 1.85 ± 0.04 | nd | nd | nd | nd | nd | nd | nd | nd |
| 761,1398 | C37H28O18 | 611 595 458 443 425 317 305 287 | Galloylprodelphinidin isomer 2 [40] | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.31 ± 0.03 | 0.25 ± 0.02 | nd | nd | nd | nd | nd | nd | nd | nd |
| 763,1505 | C37H30O18 | 611 595 458 443 425 317 305 287 | Galloylprodelphinidin isomer 3 [40] | 0.01 ± 0.01 | nd | 4.66 ± 0.05 | 5.79 ± 0.07 | nd | nd | nd | nd | nd | nd | nd | nd |
| 761,1398 | C37H28O18 | 611 595 458 443 425 317 305 287 | Galloylprodelphinidin isomer 4 [40] | nd | nd | 3.22 ± 0.05 | 5.47 ± 0.09 | nd | nd | nd | nd | nd | nd | nd | nd |
| 915,162 | C44H34O22 | 611 595 458 443 425 317 305 287 | di-O-galloylprodelphinidin dimer isomer 1 [40] | 0.01 ± 0.01 | nd | 3.11 ± 0.05 | 2.81 ± 0.06 | 0.34 ± 0.02 | 0.23 ± 0.02 | 0.48 ± 0.01 | 0.11 ± 0.01 | nd | nd | 0.22 ± 0.03 | 0.24 ± 0.02 |
| 915,1615 | C44H34O22 | 611 595 458 443 425 317 305 287 | di-O-galloylprodelphinidin dimer isomer 2 [40] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 459,0925 | C23H20O10 | 303,0579 | Epigallocatechin-gallate * [41] | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.09 ± 0.08 | 53.52 ± 0.22 | 31.25 ± 0.44 |
| 345,0614 | C17H13O8 | 315 | Methylellagic acid * [42,43] | nd | nd | 1.62 ± 0.05 | 1.27 ± 0.05 | 0.93 ± 0.04 | 1.86 ± 0.04 | 2.43 ± 0.04 | 2.04 ± 0.02 | nd | nd | nd | nd |
| 357.0461 | C14H12O11 | Chebulic acid [44] | nd | nd | nd | nd | 0.02 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.01 | 0.88 ± 0.01 | nd | nd | nd | nd | |
| 357.046 | C14H12O11 | Chebulic acid isomer 1 [44] | nd | nd | nd | nd | nd | 0.02 ± 0.01 | 0.01 ± 0.01 | 1.71 ± 0.03 | nd | nd | nd | nd | |
| 761,136 | C37H30O18 | 593 423 | (epi)gallocatechin-gallocatechin-gallate dimer [39,45] | nd | 0.56 ± 0.02 | 4.81 ± 0.05 | 2.63 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd |
| 761,136 | C37H30O18 | 593 423 | (epi)gallocatechin-gallocatechin-gallate dimer [39,45] | nd | 0.35 ± 0.01 | 3.00 ± 0.05 | 1.63 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd |
| 761,136 | C37H30O18 | 593 423 | (epi)gallocatechin-gallocatechin-gallate dimer [39,45] | nd | 3.12 ± 0.06 | 23.54 ± 0.25 | 12.4 ± 0.11 | nd | nd | nd | nd | nd | nd | nd | nd |
| 761,136 | C37H30O18 | 593 423 | (epi)gallocatechin-gallocatechin-gallate dimer [39,45] | nd | 2.22 ± 0.05 | 13.64 ± 0.11 | 7.52 ± 0.11 | nd | nd | nd | nd | nd | nd | nd | nd |
| 745,141 | C37H30O17 | Epicatechin-(4beta- > 8)-epigallocatechin 3′-gallate [39,45] | nd | 0.59 ± 0.06 | 7.31 ± 0.07 | 4.48 ± 0.10 | nd | nd | nd | nd | nd | nd | nd | nd | |
| 913,1468 | C44H33O22 | 761 423 | (epi)gallocatechin-gallate dimer [39,45] | nd | 3.46 ± 0.11 | 18.02 ± 0.11 | 12.39 ± 0.22 | nd | nd | nd | nd | nd | nd | nd | nd |
| 913,1468 | C44H33O22 | 761 423 | (epi)gallocatechin-gallate dimer [39,45] | nd | 1.85 ± 0.09 | 9.71 ± 0.11 | 8.46 ± 0.10 | nd | nd | nd | nd | nd | nd | nd | nd |
| 745,141 | C37H30O18 | Epicatechin-(4beta- > 8)-epigallocatechin 3′-gallate [39,45] | nd | 0.40 ± 0.05 | 5.79 ± 0.08 | 6.57 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd | |
| 745,141 | C37H30O19 | Epicatechin-(4beta- > 8)-epigallocatechin 3′-gallate [39,45] | nd | 0.86 ± 0.05 | 5.89 ± 0.09 | 1.96 ± 0.02 | nd | nd | nd | nd | nd | nd | nd | nd | |
| 915,162 | C44H36O22 | 457 | Assamicain [46] | nd | 0.17 ± 0.05 | 3.52 ± 0.22 | 4.58 ± 0.04 | nd | nd | nd | nd | nd | nd | nd | nd |
| 457,0771 | C22H17O11 | 331 305 169 | gallocatechin gallate* [46] | 0.01 ± 0.01 | 1.61 ± 0.05 | 7.23 ± 0.09 | 7.32 ± 0.05 | nd | nd | nd | nd | nd | nd | nd | nd |
| 457,0771 | C22H17O11 | 331 305 169 | epi-gallocatechin gallate* | nd | 1.77 ± 0.05 | 8.61 ± 0.11 | 6.61 ± 0.09 | nd | nd | nd | nd | nd | nd | nd | nd |
| 915,162 | C44H36O22 | 457 | Assamicain B [46] | nd | 0.05 ± 0.01 | 7.24 ± 0.09 | 5.73 ± 0.07 | nd | nd | nd | nd | nd | nd | nd | nd |
| TOTAL | 0.24 | 17.12 | 141.95 | 103.77 | 1.29 | 2.15 | 2.97 | 4.73 | nd | 5.09 | 53.74 | 31.49 | |||
| M-H | Hydrolisable tannins | ||||||||||||||
| 801,1135 | C35H29O23 | tetragalloylquinic acid [39,47,48,49] | nd | nd | 0.99 ± 0.05 | 1.51 ± 0.09 | 0.26 ± 0.03 | 0.21 ± 0.01 | 1.33 ± 0.06 | 3.02 ± 0.06 | nd | nd | nd | nd | |
| 618,0935 | C27H23O17 | 3-O-Galloyl-4,6-(S)-HHDP-d-glucose [39,47,48,49] | nd | nd | nd | nd | 0.19 ± 0.02 | 0.16 ± 0.03 | 13.35 ± 0.36 | 6.11 ± 0.06 | nd | nd | nd | nd | |
| 787,0994 | C34H27O22 | 617 321 | 1,3-Digalloyl-4,6-HHDP-glucose/Heterophyliin A [39,47,48,49] | nd | nd | nd | nd | 1.28 ± 0.04 | 0.01 ± 0.01 | 26.06 ± 0.35 | 12.48 ± 0.12 | nd | nd | nd | nd |
| 787,1152 | C35H30O21 | 2″,3″,6″-Tris-O-(3,4,5-trihydroxybenzoyl)-3′-Glucosyl-2′,4′,6′-trihydroxyacetophenone [39,47,48,49] | nd | nd | 1.71 ± 0.06 | 1.16 ± 0.06 | 0.22 ± 0.03 | 0.15 ± 0.03 | 37.97 ± 0.99 | 18.84 ± 0.17 | nd | nd | nd | nd | |
| 787,0994 | C34H27O22 | 617 321 | Heterophylliin A isomer | nd | nd | 1.86 ± 0.05 | 1.16 ± 0.09 | 0.47 ± 0.05 | nd | 36.29 ± 0.85 | 22.62 ± 0.23 | nd | nd | nd | nd |
| 801,155 | C35H29O22 | tetragalloylquinic acid [39,47,48,49] | nd | nd | nd | nd | 8.58 ± 0.06 | 0.36 ± 0.03 | 18.63 ± 0.88 | 12.13 ± 0.21 | nd | nd | nd | nd | |
| 1237,71 | C55H34O34 | Prostratin A or Tirucallin A | nd | nd | nd | nd | 0.11 ± 0.02 | 0.04 ± 0.02 | 0.32 ± 0.01 | 2.11 ± 0.06 | nd | nd | nd | nd | |
| 1083.0581 | C48H27O30 | alfa-Punicalagin * [48] | nd | nd | nd | nd | 4.26 ± 0.14 | 8.82 ± 0.11 | 0.11 ± 0.01 | 0.65 ± 0.05 | nd | nd | nd | nd | |
| 1083.0581 | C48H27O30 | beta-punicalagin * [48] | nd | nd | nd | nd | 9.12 ± 0.16 | 14.64 ± 0.23 | 0.13 ± 0.01 | 1.13 ± 0.02 | nd | nd | nd | nd | |
| 1083.0581 | C48H27O300 | 601 | Punicalagin derivative [48] | nd | nd | nd | nd | 4.72 ± 0.22 | 8.40 ± 0.22 | 0.32 ± 0.01 | 4.93 ± 0.09 | nd | nd | nd | nd |
| 1083.0581 | C48H28O30 | 601 | Terchebuline [39,45,48] | nd | nd | nd | nd | 8.71 ± 0.03 | 17.53 ± 0.85 | nd | nd | nd | nd | nd | nd |
| 637.1052 | C27H24O18 | 483 465 313 | trigalloyl glucose [39,47,48,49] | nd | nd | nd | nd | 0.11 ± 0.03 | 0.23 ± 0.09 | 1.32 ± 0.02 | 3.67 ± 0.04 | nd | nd | nd | nd |
| 637.1048 | C27H24O18 | 483 465 313 | trigalloyl glucose [39,47,48,49] | nd | nd | nd | nd | 0.04 ± 0.01 | 0.07 ± 0.03 | 1.43 ± 0.04 | 4.48 ± 0.04 | nd | nd | nd | nd |
| 637.1048 | C27H24O18 | 483 465 313 | trigalloyl glucose [39,47,48,49] | nd | nd | nd | nd | 0.08 ± 0.01 | 0.13 ± 0.02 | 0.37 ± 0.02 | 27.63 ± 0.34 | nd | nd | nd | nd |
| 971.1733 | C43H38O26 | 817 635 465 | tetragalloyl-syrigylglucose [39,47,48,49] | nd | nd | nd | nd | 0.01 ± 0.01 | 0.07 ± 0.03 | 3.43 ± 0.08 | 4.37 ± 0.06 | nd | nd | nd | nd |
| 637.1045 | C27H24O18 | trigalloyl glucose [39,47,48,49] | nd | nd | nd | nd | 0.46 ± 0.04 | 1.35 ± 0.05 | 7.63 ± 0.09 | 28.29 ± 0.33 | nd | nd | nd | nd | |
| 939.1110 | C41H30O26 | Tellimagrandin II [47,48,49,50] | nd | nd | nd | nd | 0.05 ± 0.02 | 0.04 ± 0.01 | 4.77 ± 0.08 | 0.19 ± 0.01 | nd | nd | nd | nd | |
| 989.1483 | C42H36O28 | Methyl neochebulinate [44] | nd | nd | nd | nd | 0.04 ± 0.03 | 0.11 ± 0.03 | 10.02 ± 0.33 | 2.07 ± 0.06 | nd | nd | nd | nd | |
| 787,0997 | C34H27O22 | tetragalloyl glucose [39,47,48,49] | nd | nd | nd | nd | 0.48 ± 0.05 | 0.66 ± 0.03 | 11.15 ± 0.34 | 32.53 ± 0.22 | nd | nd | nd | nd | |
| 955 | C41H32O27 | Chebulinic acid [44,47,48] | nd | nd | nd | nd | 2.02 ± 0.07 | 2.55 ± 0.09 | 13.5 ± 0.08 | 53.83 ± 0.76 | nd | nd | nd | nd | |
| 1001.2199 | C45H45O26 | 909 617 465 | digalloyl-dishikimoyl-synapoylglucose [39,45,47,49] | nd | nd | nd | nd | nd | nd | 0.55 ± 0.01 | nd | nd | nd | nd | nd |
| 1001.2199 | C45H45O26 | 910 617 465 | digalloyl-dishikimoyl-synapoylglucose [39,45,47,49] | nd | nd | nd | nd | nd | nd | 1.54 ± 0.05 | nd | nd | nd | nd | nd |
| 1001.2199 | C45H45O26 | 911 617 465 | digalloyl-dishikimoyl-synapoylglucose [39,45,47,49] | nd | nd | nd | nd | nd | nd | 0.74 ± 0.01 | nd | nd | nd | nd | nd |
| 1001.2199 | C45H45O26 | 912 617 465 | digalloyl-dishikimoyl-synapoylglucose [39,45,47,49] | nd | nd | nd | nd | nd | nd | 0.37 ± 0.01 | nd | nd | nd | nd | nd |
| 987.1887 | C43H40O26 | 799 771 617 465 313 | trigalloyl-syrigyl-syìhykimil-glucose [39,45,47,49] | nd | nd | nd | nd | 0.11 ± 0.02 | nd | 12.29 ± 0.08 | 10.84 ± 0.07 | nd | nd | nd | nd |
| 1001.2199 | C45H45O26 | 913 617 465 | digalloyl-dishikimoyl-synapoylglucose [39,45,47,49] | nd | nd | nd | nd | nd | nd | 0.42 ± 0.03 | nd | nd | nd | nd | nd |
| TOTAL | nd | nd | 4.56 | 3.82 | 41.29 | 55.52 | 204.04 ± 1.52 | 251.9 ± 0.99 | nd | nd | nd | nd | |||
| M + H | Coumarins | ||||||||||||||
| 177,0552 | C10H9O3 | 133 | 8-methoxy coumarin * [8,51] | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.86 ± 0.05 | 0.84 ± 0.04 | 0.19 ± 0.02 | 0.23 ± 0.03 | 5.83 ± 0.11 | 1.04 ± 0.02 | 0.05 ± 0.01 | 0.09 ± 0.01 | 14.39 ± 0.11 | 16.12 ± 0.33 |
| 327,0896 | C18H15O6 | 4-coumaroyl-4′-hydroxyphenyllactate | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.51 ± 0.03 | 0.87 ± 0.05 | nd | nd | nd | nd | 0.02 ± 0.01 | 0.01 ± 0.01 | 3.88 ± 0.07 | 2.38 ± 0.06 | |
| 287,1016 | C16H15O5 | 269 245 201 | heraclenin/prangenin * [8,51] | nd | nd | 2.07 ± 0.11 | 4.14 ± 0.09 | nd | nd | nd | nd | nd | nd | nd | nd |
| 247,097 | C14H14O4 | 188 146 | 8-(2′,3′-dihydroxyisopentyl)-7-hydroxycoumarin /marmesin* [8,51] | nd | nd | 0.74 ± 0.05 | 3.25 ± 0.09 | 0.52 ± 0.03 | nd | 2.41 ± 0.04 | 7.06 ± 0.04 | nd | nd | 4.71 ± 0.06 | 5.87 ± 0.11 |
| 271,091 | C16H13O4 | 203 175 | Imperatorin* [8,51] | nd | nd | nd | nd | 0.25 ± 0.03 | 0.21 ± 0.03 | 8.28 ± 0.11 | 14.72 ± 0.33 | nd | nd | nd | nd |
| 327,166 | C20H23O4 | Dentatin * [8,51] | nd | nd | nd | nd | nd | nd | nd | nd | 4.05 ± 0.03 | 1.54 ± 0.03 | 2.61 ± 0.05 | 3.68 ± 0.11 | |
| TOTAL | 0.07 | 0.04 | 4.18 | 9.11 | 0.94 | 0.43 | 16.51 | 22.82 | 4.12 | 1.64 | 25.59 | 28.05 | |||
| M-H | Flavonoid derivatives | ||||||||||||||
| 785,162 | C36H32O20 | Degalloyltheaflavonin | nd | nd | nd | nd | 2.04 ± 0.03 | 2.65 ± 0.11 | 2.17 ± 0.09 | 3.16 ± 0.22 | nd | nd | nd | nd | |
| 771,2372 | C34H42O20 | 625,1824 479,0928 317,0729 | Typhaneoside | nd | nd | nd | nd | 1.37 ± 0.05 | 1.05 ± 0.08 | 30.82 ± 0.55 | 15.9 ± 0.78 | nd | nd | nd | nd |
| 433,1171 | C21H21O10 | Apigenin-C-hexoside | nd | nd | nd | nd | 1.26 ± 0.09 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.28 ± 0.02 | nd | nd | nd | nd | |
| 601,1193 | C28H25O15 | 431 329 | 2′-O-Galloylquercitrin | nd | nd | nd | nd | 1.25 ± 0.02 | 1 | 29.98 ± 0.66 | 14.72 ± 0.22 | nd | nd | nd | nd |
| 585,1269 | C28H25O14 | 431 415 285 | Kaempferol-deoxyhexoside-gallate [39,45,49] | nd | nd | nd | nd | 0.12 ± 0.01 | 0.32 ± 0.03 | 19.61 ± 0.44 | 9.87 ± 0.08 | nd | nd | nd | nd |
| 769.2205 | C34H42O20 | 605 314 | Isorhamnetin-7-O-hexoside-3-O-hexosidedeoxyhexoside [39,45,49] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.24 ± 0.02 | 0.11 ± 0.01 |
| 625.1198 | C30H26O15 | 448 301 | Quercetin-7-O-hexoside-caffeoyl [39,45,49] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 3.08 ± 0.09 | 1.4 ± 0.02 |
| 625.1196 | C30H26O15 | 449 301 | Quercetin-7-O-hexoside-caffeoyl isomer [39,45,49] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.94 ± 0.06 | 0.25 ± 0.01 |
| 479.0815 | C21H20O13 | 316 271 179 | Myricetin-3-O-glucopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.52 ± 0.09 | 0.71 ± 0.02 |
| 769.2204 | C34H42O20 | 605 314 | Isorhamnetin-7-O-hexoside-3-O-hexosidedeoxyhexoside [39,45,49] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.54 ± 0.11 | 0.71 ± 0.03 |
| 769.2200 | C34H42O20 | 605 314 | Isorhamnetin-7-O-hexoside-3-O-hexosidedeoxyhexoside [39,45,49] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.78 ± 0.08 | 0.43 ± 0.01 |
| 609.1464 | C27H30O16 | 301 271 179 | Rutin * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 8.55 ± 0.11 | 2.75 ± 0.11 |
| 463.0884 | C21H20O12 | 316 271 | Myricetin-3-O-rhamnopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 11.7 ± 0.22 | 3.07 ± 0.11 |
| 463.0887 | C21H20O12 | 316 271 | Myricetin-7-O-rhamnopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 7.37 ± 0.13 | 1.11 ± 0.22 |
| 623.1627 | C28H32O16 | 461 314 315 299 | Isorhamnetin-7-O-rhamnopyranosyl-3-O-glucopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.51 ± 0.22 | 1.86 ± 0.22 |
| 463.0969 | C21H20O12 | 301 | Quercetin-3-O-galactopyranoside * | nd | nd | nd | nd | 0.06 ± 0.03 | 0.16 ± 0.03 | 1.05 ± 0.02 | 3.69 ± 0.05 | nd | nd | nd | nd |
| 477.0684 | C21H18O13 | 301 271 | Quercetin-3-O-glucuronide * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 16.33 ± 0.99 | 10.17 ± 0.22 |
| 623.1638 | C28H32O16 | 315 300 | Isorhamnetin-3-O-hexosyl-deoxyhexoside [39,45,49] | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.18 ± 0.11 | 0.73 ± 0.05 |
| 623.1622 | C28H32O16 | 315 300 | Isorhamnetin-3-O-rutinoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 5.05 ± 0.09 | 1.61 ± 0.07 |
| 477.1054 | C22H22O12 | 314 300 271 255 | Isorhamnetin-3-O-glucopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.02 ± 0.05 | 1.4 ± 0.07 |
| 463.0854 | C21H20O12 | 301 271 255 179 151 | Quercetin-3-O-glucopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 34.3 ± 0.51 | 10.57 ± 0.33 |
| 477.1043 | C22H22O12 | 314 300 271 255 | Isorhamnetin-3-O-galactopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 10.03 ± 0.11 | 3.11 ± 0.05 |
| 491.0840 | C22H20O13 | 315 300 271 255 | Isorhamnetin-3-O-glucuronide * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 11.93 ± 0.22 | 6.18 ± 0.07 |
| 431.0978 | C21H20O10 | 285 | Kaempferol-3-O-rhamnoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 8.18 ± 0.08 | 1.71 ± 0.06 |
| 461.1083 | C22H21O11 | 314 300 | Isorhamnetin-7-O-rhamnopyranoside * | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 4.12 ± 0.11 | 1.06 ± 0.05 |
| TOTAL | nd | nd | nd | nd | 6.10 ± 0.05 | 5.19 ± 0.09 | 83.65 ± 0.99 | 47.62 ± 0.08 | nd | 2.68 ± 0.03 | 138.03 ± 1.25 | 48.92 ± 0.66 | |||
| M + H | Hydroxycinnamic derivatives | nd | |||||||||||||
| 339,11 | C16H19O8 | 191 | 1-coumaroyl quinic acid [39,45,49] | 1.83 ± 0.01 | 5.48 ± 0.07 | 8.52 ± 0.05 | 6.32 ± 0.07 | 0.13 ± 0.01 | 1.44 ± 0.07 | 3.06 ± 0.04 | 0.75 ± 0.05 | nd | nd | 4.11 ± 0.04 | 3.91 ± 0.04 |
| M-H | nd | nd | |||||||||||||
| 341,0873 | C15H17O9 | 179 | Caffeoyl hexose [39,45,49] | nd | 0.16 ± 0.05 | 0.76 ± 0.06 | 1.31 ± 0.09 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | nd | nd | 0.08 ± 0.01 | 0.23 ± 0.02 |
| 501,1619 | C22H29O13 | 417 399 285 152 | Clemomandshuricoside B | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 2.09 ± 0.06 | 0.55 ± 0.02 |
| TOTAL | 1.83 | 5.64 | 9.26 ± 0.10 | 7.61 ± 0.09 | 0.14 ± 0.05 | 1.46 ± 0.06 | 3.07 ± 0.05 | 0.77 ± 0.03 | nd | nd | 6.27 ± 0.07 | 4.69 ± 0.02 | |||
| M + H | Other compounds | nd | nd | ||||||||||||
| 225,1399 | C13H20O3 | 147 119 103 79 88 | 6-hydroxy-3-oxo-alpha-ionol | 3.72 ± 0.01 | 3.12 ± 0.05 | 10.2 ± 0.20 | 10.8 ± 0.11 | nd | 0.89 ± 0.03 | 5.05 ± 0.11 | 0.08 ± 0.01 | nd | nd | 6.52 ± 0.05 | 6.57 ± 0.02 |
| 381,1658 | C16H29O10 | 364 219 200 | Prenyl arabinosyl-(1- > 6)-glucoside | 0.11 ± 0.01 | 0.27 ± 0.05 | 7.36 ± 0.11 | 7.92 ± 0.10 | nd | nd | nd | nd | nd | nd | 3.25 ± 0.05 | 3.61 ± 0.04 |
| 787,1152 | C35H31O31 | 2″,3″,6″-Tris-O-(3,4,5-trihydroxybenzoyl)-3′-Glucosyl-2′,4′,6′-trihydroxyacetophenone | nd | nd | 1.71 ± 0.06 | 1.16 ± 0.05 | 0.21 ± 0.05 | 0.15 ± 0.03 | 37.97 ± 0.54 | 18.84 ± 0.22 | nd | nd | nd | nd | |
| 285,0974 | C13H17O7 | p-hydroxy-benzoic acid rhamnosyl ester | 4.92 ± 0.02 | 3.83 ± 0.06 | 3.52 ± 0.09 | 3.65 ± 0.09 | nd | nd | nd | nd | 0.57 ± 0.03 | 2.54 ± 0.05 | 5.46 ± 0.11 | 2.91 ± 0.03 | |
| 315.1088 | C14H18O8 | 167 | glucovanillin | nd | nd | 2.98 ± 0.09 | 2.27 ± 0.11 | nd | nd | nd | nd | 2.21 ± 0.09 | 6.78 ± 0.09 | 10.27 ± 0.22 | 3.67 ± 0.05 |
| TOTAL | 8.73 | 7.22 | 25.77 | 25.8 | 0.2 | 1.04 | 43.02 | 18.92 | 2.78 | 9.32 | 25.5 | 16.75 |
| Species | Solvents | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|
| P. heyniae | Hexane | 5.42 ± 0.37 g | na | 35.97 ± 1.80 h | 20.27 ± 0.55 i | 27.34 ± 0.34 a |
| EA | 10.15 ± 0.47 e | 10.44 ± 0.91 g | 59.74 ± 0.63 ef | 31.60 ± 0.62 gh | 26.18 ± 0.16 ab | |
| MeOH | 46.65 ± 0.34 c | 60.98 ± 0.04 d | 108.43 ± 1.52 c | 70.21 ± 3.22 d | 15.53 ± 0.90 e | |
| Water | 50.66 ± 0.42 b | 90.74 ± 1.35 a | 129.89 ± 3.24 b | 93.14 ± 1.75 b | 27.79 ± 0.32 a | |
| P. meliocarpoides var. meliocarpoides | Hexane | 4.13 ± 0.33 g | 8.90 ± 0.95 g | 55.98 ± 0.84 f | 28.61 ± 1.00 h | 26.38 ± 0.15 ab |
| EA | 9.70 ± 0.09 e | 17.72 ± 0.08 f | 62.26 ± 1.02 e | 34.22 ± 0.55 g | 19.91 ± 0.22 cd | |
| MeOH | 52.27 ± 0.28 a | 77.88 ± 1.07 b | 133.19 ± 1.09 b | 80.79 ± 1.03 c | 18.55 ± 0.61 d | |
| Water | 52.01 ± 0.52 ab | 92.84 ± 0.44 a | 154.04 ± 2.10 a | 104.34 ± 1.07 a | 21.17 ± 0.54 c | |
| P. uechtritzii | Hexane | 2.18 ± 0.62 h | 17.00 ± 0.74 f | 35.91 ± 1.32 h | 21.79 ± 0.85 i | 18.50 ± 1.92 d |
| EA | 8.11 ± 0.35 f | 26.29 ± 0.44 e | 45.62 ± 2.18 g | 27.99 ± 1.69 h | 20.75 ± 0.25 c | |
| MeOH | 34.55 ± 0.63 d | 75.03 ± 0.43 c | 96.69 ± 2.56 d | 56.31 ± 0.36 f | 17.88 ± 0.83 d | |
| Water | 34.52 ± 0.96 d | 74.74 ± 0.58 c | 95.43 ± 0.80 d | 65.86 ± 0.82 e | 24.57 ± 0.40 b |
| Species | Solvents | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| P. heyniae | Hexane | 2.39 ± 0.06 a | 7.83 ± 0.18 a | 56.07 ± 1.46 ef | 0.36 ± 0.01 c | 0.67 ± 0.04 bc |
| EA | 1.58 ± 0.38 cd | 7.64 ± 0.15 ab | 54.21 ± 1.32 f | 0.41 ± 0.01 b | 0.62 ± 0.04 cd | |
| MeOH | 2.36 ± 0.18 a | 4.28 ± 0.16 c | 65.20 ± 0.89 c | 0.17 ± 0.01 e | 0.46 ± 0.08 e | |
| Water | 0.35 ± 0.08 e | na | 17.34 ± 0.38 i | 0.06 ± 0.01 g | na | |
| P. meliocarpoides var. meliocarpoides | Hexane | 1.16 ± 0.27 d | 7.97 ± 0.06 a | 81.15 ± 0.19 a | 0.46 ± 0.01 a | 0.61 ± 0.02 cd |
| EA | na | 7.32 ± 0.80 ab | 59.92 ± 0.96 d | 0.40 ± 0.02 b | 0.56 ± 0.01 d | |
| MeOH | na | 3.34 ± 0.46 d | 70.57 ± 0.59 b | 0.21 ± 0.01 d | 0.74 ± 0.01 ab | |
| Water | 0.19 ± 0.01 e | na | 21.23 ± 1.33 h | 0.05 ± 0.01 g | na | |
| P. uechtritzii | Hexane | 2.34 ± 0.12 ab | 7.63 ± 0.39 ab | 58.77 ± 1.76 de | 0.39 ± 0.01 b | 0.64 ± 0.01 cd |
| EA | 2.13 ± 0.18 ab | 6.91 ± 0.17 b | 61.03 ± 1.10 d | 0.40 ± 0.01 b | 0.59 ± 0.01 cd | |
| MeOH | 1.76 ± 0.14 bc | 1.58 ± 0.12 e | 68.03 ± 0.39 bc | 0.20 ± 0.01 d | 0.78 ± 0.01 a | |
| Water | na | 0.34 ± 0.04 f | 27.54 ± 1.03 g | 0.09 ± 0.01 f | na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Acqua, S.; Sut, S.; Zengin, G.; Peron, G.; Elbasan, F.; Yildiztugay, E.; Bibi Sadeer, N.; Mahomoodally, M.F. Phytochemical Screening, Antioxidant, and Enzyme Inhibitory Properties of Three Prangos Species (P. heyniae, P. meliocarpoides var. meliocarpoides, and P. uechtritzii) Depicted by Comprehensive LC-MS and Multivariate Data Analysis. Antioxidants 2022, 11, 1712. https://doi.org/10.3390/antiox11091712
Dall’Acqua S, Sut S, Zengin G, Peron G, Elbasan F, Yildiztugay E, Bibi Sadeer N, Mahomoodally MF. Phytochemical Screening, Antioxidant, and Enzyme Inhibitory Properties of Three Prangos Species (P. heyniae, P. meliocarpoides var. meliocarpoides, and P. uechtritzii) Depicted by Comprehensive LC-MS and Multivariate Data Analysis. Antioxidants. 2022; 11(9):1712. https://doi.org/10.3390/antiox11091712
Chicago/Turabian StyleDall’Acqua, Stefano, Stefania Sut, Gokhan Zengin, Gregorio Peron, Fevzi Elbasan, Evren Yildiztugay, Nabeelah Bibi Sadeer, and Mohamad Fawzi Mahomoodally. 2022. "Phytochemical Screening, Antioxidant, and Enzyme Inhibitory Properties of Three Prangos Species (P. heyniae, P. meliocarpoides var. meliocarpoides, and P. uechtritzii) Depicted by Comprehensive LC-MS and Multivariate Data Analysis" Antioxidants 11, no. 9: 1712. https://doi.org/10.3390/antiox11091712
APA StyleDall’Acqua, S., Sut, S., Zengin, G., Peron, G., Elbasan, F., Yildiztugay, E., Bibi Sadeer, N., & Mahomoodally, M. F. (2022). Phytochemical Screening, Antioxidant, and Enzyme Inhibitory Properties of Three Prangos Species (P. heyniae, P. meliocarpoides var. meliocarpoides, and P. uechtritzii) Depicted by Comprehensive LC-MS and Multivariate Data Analysis. Antioxidants, 11(9), 1712. https://doi.org/10.3390/antiox11091712










