The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Determination of the Shallot Sugar Profile
2.3. Determination of the Shallot Flavonoid Profile
2.4. Total Phenolic Content and Total Antioxidant Capacity
2.5. Pyruvic Acid Content
2.6. Determination of the Shallot Mineral Composition
2.7. Statistical Analysis
3. Results
3.1. Dry Matter, Sugar Profile and Pyruvic Acid Content in Shallot Accessions
3.2. Phenolic Compound Content and Antioxidant Activity
3.3. Mineral Content
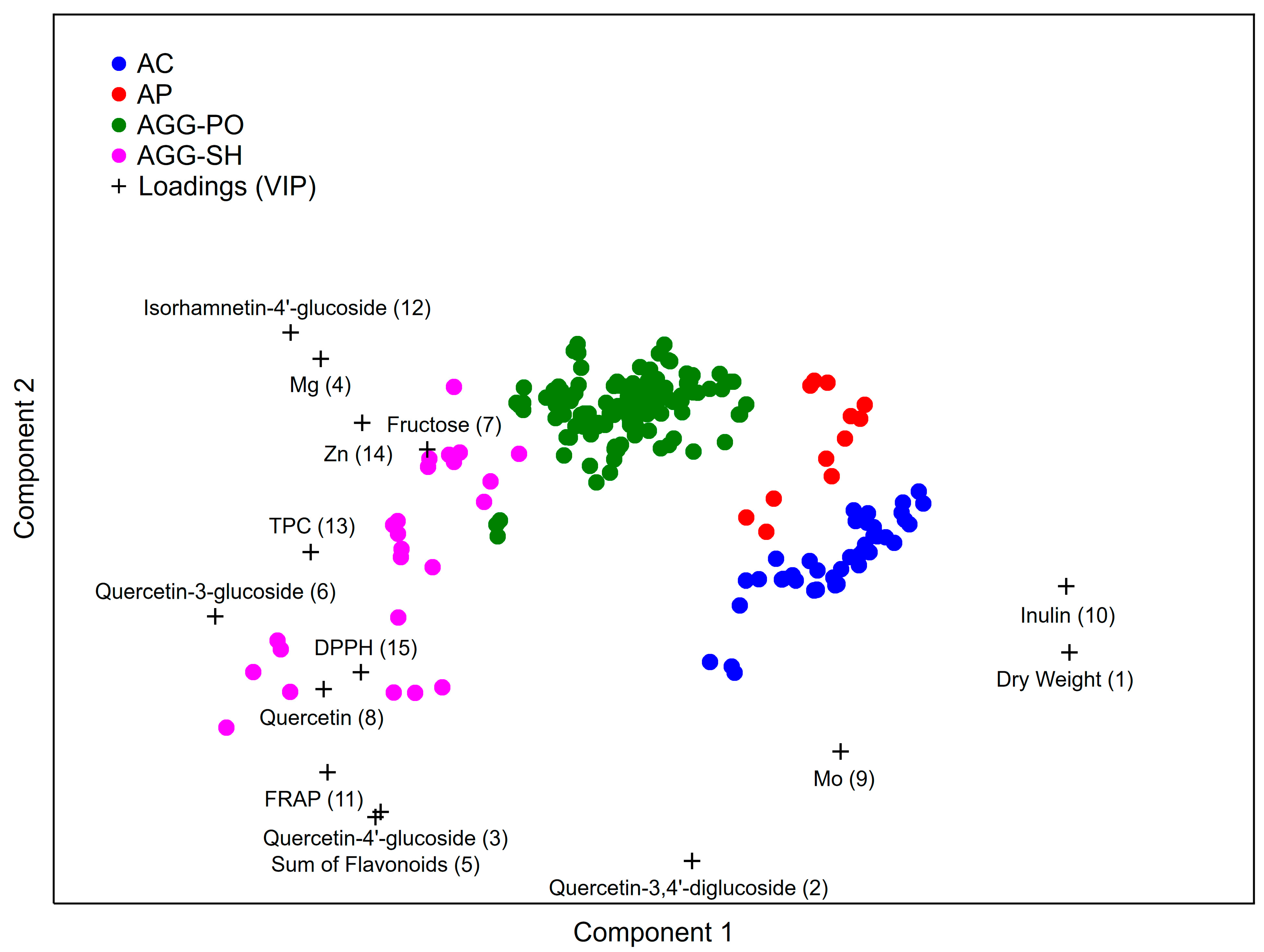
3.4. Discrimination of the Shallot Species and Subtypes Based on a PLS-DA Model
4. Discussion
4.1. Dry Matter, Sugar Profile and Pyruvic Acid Content in the Shallot Accessions
4.2. Antioxidant Activity and Phenolic Compounds Content
4.3. Mineral Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, D.; Checa, A.; de Ancos, B.; Wheelock, C.E.; Sánchez-Moreno, C. New Insights into the effects of onion consumption on lipid mediators using a diet-induced model of hypercholesterolemia. Redox Biol. 2017, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Abouzed, T.K.; del Mar Contreras, M.; Sadek, K.M.; Shukry, M.; Abdelhady, D.H.; Gouda, W.M.; Abdo, W.; Nasr, N.E.; Mekky, R.H.; Segura-Carretero, A.; et al. Red onion scales ameliorated streptozotocin-induced diabetes and diabetic nephropathy in wistar rats in relation to their metabolite fingerprint. Diabetes Res. Clin. Pract. 2018, 140, 253–264. [Google Scholar] [CrossRef]
- Yu, S.; Li, H.; Cui, T.; Cui, M.; Piao, C.; Wang, S.; Ju, M.; Liu, X.; Zhou, G.; Xu, H.; et al. Onion (Allium cepa L.) peel extract effects on 3T3-L1 adipocytes and high-fat diet-induced obese mice. Food Biosci. 2021, 41, 101019. [Google Scholar] [CrossRef]
- Loredana, L.; Adiletta, G.; Nazzaro, F.; Florinda, F.; Marisa, D.M.; Donatella, A. Biochemical, antioxidant properties and antimicrobial activity of different onion varieties in the mediterranean area. J. Food Meas. Charact. 2019, 13, 1232–1241. [Google Scholar] [CrossRef]
- Law, Y.Y.; Chiu, H.F.; Lee, H.H.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-AGED and post-menopausal healthy subjects. Food Funct. 2016, 7, 902–912. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium Cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031. [Google Scholar] [CrossRef]
- Judprasong, K.; Tanjor, S.; Puwastien, P.; Sungpuag, P. Investigation of thai plants for potential sources of inulin-type fructans. J. Food Compos. Anal. 2011, 24, 642–649. [Google Scholar] [CrossRef]
- Moongngarm, A.; Trachoo, N.; Sirigungwan, N. Low molecular weight carbohydrates, prebiotic content, and prebiotic activity of selected food plants in thailand. Adv. J. Food Sci. Technol. 2011, 3, 269–274. [Google Scholar]
- Franco-Robles, E.; López, M.G. Implication of fructans in health: Immunomodulatory and antioxidant mechanisms. Sci. World J. 2015, 2015, 289267. [Google Scholar] [CrossRef] [PubMed]
- Coxam, V. Current data with inulin-type fructans and calcium, targeting bone health in adults. J. Nutr. 2007, 137, 2527S–2533S. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef]
- Gargari, B.P.; Dehghan, P.; Aliasgharzadeh, A.; Jafar-Abadi, M.A. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab. J. 2013, 37, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitch, H.D.; Kamenetsky, R. Shallot (Allium cepa, Aggregatum Group). In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2002; pp. 409–430. [Google Scholar]
- Puizina, J. Shallots in croatia-genetics, morphology and nomenclature. Acta Bot. Croat. 2013, 72, 387–398. [Google Scholar] [CrossRef][Green Version]
- Perković, J.; Major, N.; Ban, D.; Cvitan, D.; Ban, S.G. Shallot species and subtypes discrimination based on morphology descriptors. Plants 2020, 10, 60. [Google Scholar] [CrossRef]
- Fritsch, R.M.; Friesen, N. Evolution, domestication and taxonomy. In Allium Crop Science: Recent Advances; CABI: Wallingford, UK, 2002; pp. 5–30. [Google Scholar]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant Sci. 2007, 24, 227–247. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a Matter of Time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Major, N.; Goreta Ban, S.; Urlić, B.; Ban, D.; Dumičić, G.; Perković, J. Morphological and biochemical diversity of shallot landraces preserved along the croatian coast. Front. Plant Sci. 2018, 9, 1749. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucoside profile of southern italian red onion (Allium cepa L.). J. Agric. Food Chem. 2005, 53, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Garthwaite, P.H. An interpretation of partial least squares. J. Am. Stat. Assoc. 1994, 89, 122. [Google Scholar] [CrossRef]
- Helland, I. Partial least squares regression. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Chichester, UK, 2014. [Google Scholar]
- Pérez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of selenium biofortification and beneficial microorganism inoculation on yield, quality and antioxidant properties of shallot bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef]
- Woldetsadik, K.; Gertsson, U.; Ascard, J. Shallot yield, quality and storability as affected by irrigation and nitrogen. J. Hortic. Sci. Biotechnol. 2003, 78, 549–553. [Google Scholar] [CrossRef]
- Pudzianowska, M.; Gajewski, M.; Przybył, J.; Buraczyńska, A.; Gaczkowska, O.; Matuszczak, M.; Dziechciarska, M. Influence of storage conditions on flavonoids content and antioxidant activity of selected shallot (Allium Cepa Var. Ascalonicum Backer) hybrid cultivars. J. Fruit Ornam. Plant Res. 2012, 77, 101–111. [Google Scholar] [CrossRef]
- Sinclair, P.J.; Blakeney, A.B.; Barlow, E.W.R. Relationships between bulb dry matter content, soluble solids concentration and non-structural carbohydrate composition in the ONION (Allium cepa). J Sci Food Agric. 1995, 69, 203–209. [Google Scholar] [CrossRef]
- Hansen, S.L. Content and composition of dry matter in onion (Allium cepa L.) as influenced by developmental stage at time of harvest and long-term storage. Acta Agric. Scand. Sect. B-Soil Plant Sci. 1999, 49, 103–109. [Google Scholar] [CrossRef]
- Yanti, I.G.; Azhar, M.; Faridah, A.; Oktavia, B. Simple determination of inulin polymerization degree average from dahlia tuber using spectrophotometer. Int. J. Res. Rev. 2019, 6, 399–404. [Google Scholar]
- Kolida, S.; Tuohy, K.; Gibson, G.R. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 2002, 87, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef]
- Jaime, L.; Martín-Cabrejas, M.A.; Mollá, E.; López-Andréu, F.J.; Esteban, R.M. Effect of storage on fructan and fructooligosaccharide of onion (Allium cepa L.). J. Agric. Food Chem. 2001, 49, 982–988. [Google Scholar] [CrossRef]
- Kleniewska, P.; Hoffmann, A.; Pniewska, E.; Pawliczak, R. The influence of probiotic lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxid. Med. Cell. Longev. 2016, 2016, 1340903. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Khalili, M.; Mirtaheri, E.; Gargari, B.P.; Tavakoli, F.; Farhangi, M.A.; Babaei, H.; Dehghan, P. A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: A randomized controlled clinical trial. Adv. Pharm. Bull. 2015, 5, 507. [Google Scholar] [CrossRef]
- Dhumal, K.; Datir, S.; Pandey, R. Assessment of bulb pungency level in different indian cultivars of onion (Allium cepa L.). Food Chem. 2007, 100, 1328–1330. [Google Scholar] [CrossRef]
- Pöhnl, T.; Schweiggert, R.M.; Carle, R. Impact of cultivation method and cultivar selection on soluble carbohydrates and pungent principles in onions (Allium cepa L.). J. Agric. Food Chem. 2018, 66, 12827–12835. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; Lázaro, A. Physicochemical and textural properties of a spanish traditional garlic (Allium Sativum L.) variety: Characterizing distinctive properties of “fino de chinchón” garlic. Eur. Food Res. Technol. 2021, 247, 2399–2408. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Singh, N.P.; Hegade, P.M.; Sorty, A.M. Growth, bulb yield, water productivity and quality of onion (Allium cepa L.) as affected by deficit irrigation regimes and exogenous application of plant bio–regulators. Agric. Water Manag. 2018, 199, 1–10. [Google Scholar] [CrossRef]
- Soininen, T.H.; Jukarainen, N.; Auriola, S.O.K.; Julkunen-Tiitto, R.; Karjalainen, R.; Vepsäläinen, J.J. Quantitative metabolite profiling of edible onion species by NMR and HPLC-MS. Food Chem. 2014, 165, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, D.; WÓJcik-Stopczyska, B. Influence of plant covering on some organic compound content and pungency of shallot grown for bunching harvest. Veg. Crop. Res. Bull. 2007, 66, 25–30. [Google Scholar] [CrossRef]
- Schwimmer, S.; Weston, W.J. Onion flavor and odor, enzymatic development of pyruvic acid in onion as a measure of pungency. J. Agric. Food Chem. 1961, 9, 301–304. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol glucosides in allium species: A comparative study by means of HPLC–DAD–ESI-MS–MS. Food Chem. 2008, 107, 1668–1673. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Cikeš-Čulić, V.; Puizina, J. Chemical composition and biological activity of allium cepa L. and allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef]
- Zhao, T.T.; Yang, T.L.; Gong, L.; Wu, P. Isorhamnetin protects against hypoxia/reoxygenation-induced injure by attenuating apoptosis and oxidative stress in H9c2 cardiomyocytes. Gene 2018, 666, 92–99. [Google Scholar] [CrossRef]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.W.; Ohkohchi, N.; Isoda, H. Isorhamnetin alleviates steatosis and fibrosis in mice with nonalcoholic steatohepatitis. Sci. Rep. 2019, 9, 16210. [Google Scholar] [CrossRef]
- Thong, N.M.; Vo, Q.V.; Huyen, T.L.; Van Bay, M.; Tuan, D.; Nam, P.C. Theoretical study for exploring the diglycoside substituent effect on the antioxidative capability of isorhamnetin extracted from anoectochilus roxburghii. ACS Omega 2019, 4, 14996–15003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Plumb, G.W.; Uda, Y.; Price, K.R.; Rhodes, M.J. Dietary quercetin glycosides: Antioxidant activity and induction of the anticarcinogenic phase II marker enzyme quinone reductase in Hepalclc7 cells. Carcinogenesis 1996, 17, 2385–2387. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from propolis: A theoretical study. Sci. Rep. 2017, 7, 7543. [Google Scholar] [CrossRef]
- Victoria Ibiiiez, M.; Rinch, F.; Amaro, M.; Martinez, B. Intrinsic variability of mineral composition of chickpea (Cicer arietinum, L.). Food Chem. 1998, 63, 5. [Google Scholar]
- USDA. USDA National Nutrient Database for Standard Reference; USDA: Washington, DC, USA, 2018. [Google Scholar]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics, and elemental composition of garlic and onion under selenium supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Balen, G.P.; Van Den Berg, D.J.; Bast, A.; Van Der Vijgh, W.J.F. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem. Pharmacol. 1998, 56, 935–943. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Strigunova, E.N.; Kostyuk, T.V.; Afanas’ev, I.B. Experimental evidence that flavonoid metal complexes may act as mimics of superoxide dismutase. Arch. Biochem. Biophys. 2004, 428, 204–208. [Google Scholar] [CrossRef]
- Porfírio, D.A.; Ferreira, R.D.Q.; Malagutti, A.R.; Valle, E.M.A. Electrochemical study of the increased antioxidant capacity of flavonoids through complexation with Iron(II) ions. Electrochim. Acta 2014, 141, 33–38. [Google Scholar] [CrossRef]
| Dry Weight | Pyruvic Acid | Inulin | Sucrose | Glucose | Fructose | |
|---|---|---|---|---|---|---|
| % | mmol/kg DW 1 | g/100 g DW | ||||
| Harvest year | ||||||
| 2018 | 19.9 ± 0.5 | 18.4 ± 0.7 | 31.7 ± 0.5 | 1.56 ± 0.07 | 0.98 ± 0.06 | 2.79 ± 0.11 |
| 2019 | 16.6 ± 0.6 | 24.8 ± 0.9 | 35.1 ± 1.4 | 4.36 ± 0.16 | 1.91 ± 0.17 | 1.57 ± 0.09 |
| p-value | *** | *** | *** | *** | *** | *** |
| Species | ||||||
| A. × cornutum | 25.4 ± 0.4 a 2 | 22.1 ± 2 ab | 47.5 ± 1.1 a | 1.64 ± 0.18 c | 0.59 ± 0.06 b | 1.2 ± 0.07 b |
| A. cepa Aggregatum potato onion type | 16 ± 0.2 b | 20.4 ± 0.5 c | 29.6 ± 0.5 b | 3.48 ± 0.17 a | 1.73 ± 0.13 a | 2.43 ± 0.09 a |
| A. cepa Aggregatum shallot type | 14.1 ± 0.8 c | 24 ± 2 ab | 22.5 ± 1.1 c | 3.02 ± 0.29 ab | 1.87 ± 0.3 a | 3.00 ± 0.36 a |
| A. × proliferum | 25.1 ± 0.3 a | 27.4 ± 3.5 a | 44.8 ± 3.0 a | 2.07 ± 0.29 bc | 0.64 ± 0.14 b | 1.37 ± 0.17 b |
| p-value | *** | ** | *** | *** | *** | *** |
| Harvest year × species | ||||||
| A. × cornutum | ||||||
| 2018 | 27.3 ± 0.3 a | 16.6 ± 0.5 c | 40.7 ± 0.6 b | 0.51 ± 0.04 f | 0.38 ± 0.06 d | 1.42 ± 0.08 cd |
| 2019 | 23.6 ± 0.2 c | 27.6 ± 3.6 ab | 54.3 ± 1.8 a | 2.77 ± 0.12 c | 0.79 ± 0.24 cd | 0.99 ± 0.2 d |
| A. cepa Aggregatum potato onion type | ||||||
| 2018 | 17.5 ± 0.2 d | 18.1 ± 0.8 c | 29.3 ± 0.3 cd | 1.9 ± 0.07 de | 1.27 ± 0.06 c | 2.99 ± 0.1 b |
| 2019 | 14.5 ± 0.2 e | 22.7 ± 0.4 b | 29.9 ± 0.8 cd | 5.05 ± 0.13 a | 2.18 ± 0.06 ab | 1.87 ± 0.18 c |
| A. cepa Aggregatum shallot type | ||||||
| 2018 | 16.6 ± 0.1 d | 14.8 ± 0.5 c | 26.4 ± 0.6 d | 1.89 ± 0.03 cde | 0.61 ± 0.09 cd | 4.63 ± 0.11 a |
| 2019 | 11.6 ± 0.3 f | 33.1 ± 0.8 a | 18.7 ± 0.5 e | 4.16 ± 0.05 ab | 3.13 ± 0.14 a | 1.37 ± 0.11 cd |
| A. × proliferum | ||||||
| 2018 | 25.6 ± 0.2 ab | 35.5 ± 5.3 a | 35.2 ± 1 bc | 1.12 ± 0.19 f | 0.82 ± 0.23 bcd | 1.81 ± 0.12 cd |
| 2019 | 24.5 ± 0.6 bc | 19.2 ± 0.2 bc | 54.5 ± 1.4 a | 3.02 ± 0.32 bcd | 0.47 ± 0.31 cd | 0.92 ± 0.17 cd |
| p-value | *** | *** | *** | ** | *** | *** |
| Accessions | ||||||
| A. × cornutum | ||||||
| IPT021 | 25.2 ± 0.7 a | 39.5 ± 12.2 a | 46 ± 4.1 a–d | 1.69 ± 0.47 b | 0.43 ± 0.07 d | 0.8 ± 0.16 c |
| IPT022 | 24.9 ± 0.6 a | 19.5 ± 2.1 b | 47.3 ± 4.2 a–c | 1.74 ± 0.4 b | 0.81 ± 0.1 d | 1.09 ± 0.16 bc |
| IPT211 | 26.4 ± 1.2 a | 16.3 ± 0.5 b | 51.1 ± 3 a | 1.54 ± 0.48 b | 0.25 ± 0.03 d | 0.82 ± 0.23 c |
| IPT212 | 26 ± 1.5 a | 18.1 ± 0.5 b | 46.7 ± 2 a–c | 1.76 ± 0.5 b | 0.53 ± 0.2 d | 1.04 ± 0.12 bc |
| IPT213 | 25.8 ± 1.4 a | 19.6 ± 0.1 b | 46.9 ± 3.4 a–c | 1.59 ± 0.54 b | 0.74 ± 0.23 d | 1.37 ± 0.02 bc |
| IPT214 | 25.4 ± 1.6 a | 21.9 ± 2.1 ab | 45.6 ± 1.7 a–d | 1.66 ± 0.6 b | 0.63 ± 0.16 d | 1.59 ± 0.07 a–c |
| IPT215 | 24.5 ± 0.8 a | 19.7 ± 0.7 b | 48.7 ± 3.1 ab | 1.49 ± 0.53 b | 0.71 ± 0.18 d | 1.71 ± 0.15 a–c |
| A. cepa Aggregatum potato onion type | ||||||
| IPT176 | 15.5 ± 1.3 bc | 27.8 ± 5.3 ab | 30.1 ± 2.6 e–i | 5.04 ± 0.99 ab | 1.41 ± 0.12 b–d | 2.3 ± 0.04 a–c |
| IPT208 | 15.2 ± 0.1 bc | 26.1 ± 3.6 ab | 39.2 ± 3.5 a–f | 4.6 ± 0.39 ab | 1.02 ± 0.04 b–d | 2.89 ± 0.27 a–c |
| IPT216 | 17 ± 0.9 b | 19.3 ± 0.5 b | 34 ± 1.4 d–h | 4.04 ± 1.11 ab | 0.63 ± 0.16 d | 1.75 ± 0.43 a–c |
| IPT217 | 18.2 ± 1.9 b | 20.7 ± 0.3 b | 35.1 ± 1.9 c–h | 4.31 ± 1.28 ab | 1.25 ± 0.18 b–d | 2.7 ± 0.08 a–c |
| IPT218 | 17 ± 0.5 b | 17 ± 1.7 b | 37.5 ± 3.2 b–f | 3.95 ± 0.91 ab | 0.86 ± 0.1 cd | 2.41 ± 0.11 a–c |
| IPT225 | 16.8 ± 1.3 b | 19.6 ± 1.2 b | 32.7 ± 0.7 e–i | 5.68 ± 1.58 a | 0.91 ± 0.07 cd | 1.8 ± 0.17 a–c |
| IPT226 | 16.4 ± 0.6 bc | 20.6 ± 0.4 b | 31 ± 1.2 e–i | 3.73 ± 0.93 ab | 1.29 ± 0.12 b–d | 2.39 ± 0.35 a–c |
| IPT228 | 14.8 ± 1.7 bc | 21.5 ± 2.5 b | 26.4 ± 1 g–j | 3.64 ± 0.67 ab | 2.61 ± 0.75 a–d | 2.45 ± 0.09 a–c |
| IPT229 | 16.6 ± 0.9 bc | 19.8 ± 1 b | 30 ± 0.7 f–i | 2.55 ± 0.36 ab | 2.05 ± 0.23 b–d | 3.06 ± 1.05 a–c |
| IPT230 | 14.4 ± 1.1 bc | 22.1 ± 2 ab | 20.9 ± 1.9 ij | 4.29 ± 0.95 ab | 4.46 ± 0.9 a | 2.46 ± 0.29 a–c |
| IPT231 | 15.7 ± 1.1 bc | 20.9 ± 1.6 b | 27.1 ± 0.4 f–j | 3.08 ± 0.63 ab | 1.71 ± 0.19 b–d | 2.57 ± 0.47 a–c |
| IPT232 | 16 ± 0.8 bc | 19.1 ± 1 b | 29 ± 0.2 f–j | 2.96 ± 0.48 ab | 1.67 ± 0.25 b–d | 3.74 ± 0.71 a |
| IPT233 | 16.8 ± 0.7 b | 18.3 ± 1.9 b | 28.4 ± 2.1 f–j | 2.8 ± 0.41 ab | 2.19 ± 0.95 a–d | 1.81 ± 0.2 a–c |
| IPT234 | 16.5 ± 0.8 bc | 22.2 ± 2.5 ab | 27.7 ± 1.9 f–j | 2.84 ± 0.57 ab | 1.11 ± 0.18 b–d | 2.57 ± 0.43 a–c |
| IPT235 | 14.9 ± 0.9 bc | 16.9 ± 1.6 b | 30.1 ± 0.1 e–i | 2.92 ± 0.39 ab | 1.7 ± 0.23 b–d | 2.5 ± 0.25 a–c |
| IPT236 | 15.8 ± 1.2 bc | 18.4 ± 1.7 b | 29.5 ± 0.8 f–j | 3 ± 0.56 ab | 1.8 ± 0.18 b–d | 2.5 ± 0.59 a–c |
| IPT237 | 15 ± 0.7 bc | 19 ± 2.7 b | 26.6 ± 0.5 g–j | 2.75 ± 0.52 ab | 0.93 ± 0.04 b–d | 2.34 ± 0.52 a–c |
| IPT238 | 16 ± 1 bc | 20.6 ± 3.5 b | 26.9 ± 0.9 f–j | 2.72 ± 0.63 ab | 1.02 ± 0.12 b–d | 2.24 ± 0.4 a–c |
| IPT242 | 16.3 ± 0.8 bc | 22.8 ± 2.8 ab | 23.7 ± 1.6 g–j | 2.94 ± 0.6 ab | 3.28 ± 1.24 ab | 2.17 ± 0.18 a–c |
| IPT243 | 16.6 ± 1 bc | 16.4 ± 1.6 b | 27.6 ± 1.1 f–j | 2.25 ± 0.38 ab | 3.21 ± 0.8 a–c | 2.29 ± 0.3 a–c |
| IPT244 | 15.3 ± 1 bc | 19.8 ± 2.1 b | 28.5 ± 0.8 f–j | 2.89 ± 0.53 ab | 1.15 ± 0.09 b–d | 2.06 ± 0.39 a–c |
| A. Cepa Aggregatum shallot type | ||||||
| IPT239 | 15.1 ± 1.2 bc | 22.7 ± 4.3 ab | 25.6 ± 1.3 g–j | 3.46 ± 0.62 ab | 1.12 ± 0.37 b–d | 2.86 ± 0.64 a–c |
| IPT240 | 15.2 ± 1.2 bc | 22.4 ± 3.8 ab | 25.8 ± 1.6 g–j | 3.48 ± 0.53 ab | 1.66 ± 0.56 b–d | 3.08 ± 0.41 a–c |
| IPT241 | 12.1 ± 2.3 c | 26.5 ± 4.8 ab | 17.1 ± 2.5 j | 3.34 ± 0.65 ab | 2.24 ± 0.61 a–d | 2.83 ± 0.94 a–c |
| IPT245 | 14 ± 1.2 bc | 24.2 ± 3.4 ab | 21.6 ± 1.7 h–j | 1.82 ± 0.27 b | 2.44 ± 0.81 a–d | 3.23 ± 0.95 ab |
| A. × proliferum | ||||||
| IPT023 | 25.1 ± 0.1 a | 21.8 ± 1 ab | 42.4 ± 5 a–e | 2.26 ± 0.39 ab | 1.01 ± 0.18 b–d | 1.67 ± 0.27 a–c |
| IPT210 | 25 ± 0.6 a | 33 ± 6.4 ab | 47.2 ± 3.7 a–c | 1.87 ± 0.46 b | 0.27 ± 0.01 d | 1.06 ± 0.14 bc |
| p-value | *** | ** | *** | *** | *** | ** |
| Quercetin-7,4′-diglucoside | Quercetin-3,4′-diglucoside | Isorhamnetin-3,4′-diglucoside | Quercetin-3-glucoside | Quercetin-4′-glucoside | Isorhamnetin-4′-glucoside | Quercetin | Sum of Flavonoids | DPPH | FRAP | TPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/100 g DW 1 | TEQ µmol/g DW | Fe2+ EQ µmol/g DW | GAEQ mg/g DW | ||||||||
| Harvest year | |||||||||||
| 2018 | 1.14 ± 0.05 | 86.8 ± 3.3 | 1.96 ± 0.11 | 8.07 ± 0.46 | 156.6 ± 5.3 | 25.7 ± 1.6 | 8.14 ± 0.69 | 288 ± 9 | 9.93 ± 0.26 | 21.8 ± 0.6 | 4.42 ± 0.10 |
| 2019 | 1.96 ± 0.04 | 87.5 ± 2.4 | 3.09 ± 0.12 | 8.53 ± 0.43 | 142.1 ± 4.2 | 30.2 ± 1.4 | 12.3 ± 0.95 | 286 ± 7 | 6.03 ± 0.22 | 19.7 ± 0.5 | 6.09 ± 0.13 |
| p-value | *** | n.s. | *** | n.s. | ** | ** | *** | n.s. | *** | *** | *** |
| Species | |||||||||||
| A. × cornutum | 1.68 ± 0.11 b 2 | 118.4 ± 2.9 a | 1.34 ± 0.08 b | 4.43 ± 0.3 c | 163 ± 4.7 b | 10.4 ± 0.7 c | 8.12 ± 0.84 b | 307 ± 9 b | 6.83 ± 0.31 b | 20.7 ± 0.6 b | 4.63 ± 0.16 c |
| A. cepa Aggregatum Potato onion type | 1.37 ± 0.05 c | 71.8 ± 1.2 c | 2.95 ± 0.11 a | 8.01 ± 0.19 b | 135.3 ± 2.2 c | 33.3 ± 0.9 b | 8.12 ± 0.21 b | 261 ± 4 c | 7.62 ± 0.21 b | 19.5 ± 0.3 b | 5.17 ± 0.1 b |
| A. cepa Aggregatum Shallot type | 1.89 ± 0.1 ab | 110 ± 7.6 ab | 2.78 ± 0.28 a | 17.38 ± 1.13 a | 236.7 ± 11.6 a | 41 ± 3.9 a | 26.63 ± 3.26 a | 436 ± 20 a | 12.95 ± 0.78 a | 30.6 ± 1.3 a | 7.36 ± 0.32 a |
| A. × proliferum | 2.31 ± 0.2 a | 93.6 ± 11.6 b | 1.76 ± 0.14 b | 6.72 ± 0.69 b | 73.8 ± 7.1 d | 6.8 ± 0.8 c | 6.73 ± 0.36 b | 192 ± 20 d | 5.79 ± 0.47 b | 13.8 ± 0.4 c | 4.03 ± 0.22 c |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Harvest year × species | |||||||||||
| A. × cornutum | |||||||||||
| 2018 | 1.08 ± 0.04 c | 108.2 ± 4.3 b | 0.93 ± 0.08 d | 4.59 ± 0.32 d | 147.9 ± 5.7 c | 7.6 ± 0.6 c | 5.7 ± 0.19 c | 276 ± 11 c | 8.44 ± 0.23 | 20.1 ± 0.7 cd | 3.82 ± 0.1 |
| 2019 | 2.29 ± 0.35 a | 128.6 ± 19 a | 1.75 ± 0.22 c | 4.26 ± 1.11 d | 178.2 ± 12.3 b | 13.3 ± 1.4 c | 10.54 ± 0.51 c | 339 ± 35 b | 7.17 ± 0.36 | 21.4 ± 0.8 c | 3.37 ± 0.17 |
| A. cepa Aggregatum potato onion type | |||||||||||
| 2018 | 0.93 ± 0.04 c | 68.1 ± 2.1 d | 2.12 ± 0.11 c | 7.27 ± 0.23 c | 145.7 ± 3.5 c | 29.9 ± 1.3 b | 6.69 ± 0.19 c | 261 ± 7 c | 9.68 ± 0.17 | 21 ± 0.4 c | 4.32 ± 0.07 |
| 2019 | 1.81 ± 0.17 b | 75.5 ± 9.5 cd | 3.78 ± 0.4 a | 8.75 ± 1.49 b | 124.9 ± 16 d | 36.6 ± 5.8 a | 9.56 ± 4.33 c | 261 ± 24 c | 15.19 ± 0.92 | 18.1 ± 1.5 de | 6.47 ± 0.44 |
| A. cepa Aggregatum shallot type | |||||||||||
| 2018 | 1.82 ± 0.11 b | 134.8 ± 2.5 a | 3.15 ± 0.07 ab | 18.6 ± 0.52 a | 266.4 ± 6 a | 44.3 ± 1 a | 21.06 ± 1.52 b | 490 ± 10 a | 5.21 ± 0.27 | 33 ± 1.1 a | 5.44 ± 0.18 |
| 2019 | 1.96 ± 0.22 ab | 85.1 ± 8.8 c | 2.41 ± 0.16 bc | 16.17 ± 0.71 a | 207.1 ± 6.6 b | 37.7 ± 0.7 ab | 32.21 ± 0.34 a | 383 ± 17 b | 4.41 ± 0.25 | 28.2 ± 0.2 b | 4.69 ± 0.06 |
| A. × proliferum | |||||||||||
| 2018 | 2.16 ± 0.04 ab | 112.8 ± 1.1 ab | 1.55 ± 0.1 cd | 7.63 ± 0.26 bcd | 82.1 ± 2.1 e | 6.7 ± 1.1 c | 6 ± 0.27 c | 219 ± 4 cd | 5.56 ± 0.13 | 13.9 ± 0.3 e | 6.02 ± 0.13 |
| 2019 | 2.46 ± 0.12 a | 74.3 ± 6.2 cd | 1.97 ± 0.39 bcd | 5.81 ± 1.69 bcd | 65.4 ± 12.3 e | 6.9 ± 5.2 c | 7.46 ± 4.47 c | 164 ± 25 d | 10.71 ± 0.88 | 13.7 ± 1.9 e | 8.24 ± 0.29 |
| p-value | *** | *** | *** | ** | *** | * | * | *** | n.s. | ** | *** |
| Accessions | |||||||||||
| A. × cornutum | |||||||||||
| IPT021 | 1.69 ± 0.16 ab | 127.5 ± 0.8 a–c | 1.22 ± 0.18 f–h | 4.81 ± 0.21 i–l | 157 ± 1.7 d–g | 7.8 ± 0.7 ef | 6.56 ± 0.34 d | 307 ± 2 c–g | 7.12 ± 0.82 c–e | 18.2 ± 0.6 d–g | 3.98 ± 0.24 e |
| IPT022 | 1.77 ± 0.25 ab | 130 ± 3.8 a–c | 1.6 ± 0.09 d–h | 4.1 ± 0.8 kl | 171.4 ± 7.7 c–f | 10.6 ± 0.9 ef | 6.08 ± 0.19 d | 326 ± 13 c–g | 7.07 ± 1.38 c–e | 19.7 ± 1.2 d–g | 4.26 ± 0.23 de |
| IPT211 | 1.62 ± 0.28 ab | 107.7 ± 2.1 a–g | 1.12 ± 0.09 h | 2.5 ± 0.35 l | 144.6 ± 3 d–h | 7.4 ± 0.7 ef | 5.16 ± 0.19 d | 270 ± 4 d–g | 6.32 ± 0.91 de | 18.8 ± 1.5 d–g | 4.44 ± 0.2 c–e |
| IPT212 | 1.44 ± 0.44 b | 112.2 ± 11.1 a–e | 1.44 ± 0.33 d–h | 5.63 ± 1.69 g–l | 165.2 ± 23.4 c–g | 12.5 ± 3.1 ef | 15.34 ± 4.95 cd | 314 ± 45 c–g | 7.41 ± 0.27 c–e | 21.8 ± 3.2 d–f | 4.94 ± 0.73 c–e |
| IPT213 | 1.61 ± 0.32 ab | 108.7 ± 9.7 a–g | 1.18 ± 0.26 gh | 4.22 ± 0.44 j–l | 153.2 ± 14.9 d–g | 8.9 ± 1.5 ef | 8.63 ± 1.38 d | 286 ± 28 d–g | 5.85 ± 0.77 de | 21.1 ± 0.5 d–f | 4.79 ± 0.49 c–e |
| IPT214 | 1.87 ± 0.36 ab | 109.8 ± 8.6 a–f | 1.45 ± 0.32 d–h | 4.81 ± 0.16 i–l | 174.4 ± 16.7 c–f | 12.6 ± 3.1 ef | 7.49 ± 0.7 d | 312 ± 30 c–g | 6.75 ± 0.58 c–e | 23.6 ± 2 c–e | 5.07 ± 0.56 c–e |
| IPT215 | 1.78 ± 0.29 ab | 133 ± 6 ab | 1.37 ± 0.14 d–h | 4.92 ± 0.51 h–l | 175.3 ± 1.5 c–f | 13.2 ± 1.1 ef | 7.57 ± 0.35 d | 337 ± 8 c–f | 7.28 ± 0.77 c–e | 21.9 ± 0.9 d–f | 4.91 ± 0.28 c–e |
| A. cepa Aggregatum potato onion type | |||||||||||
| IPT176 | 1.35 ± 0.25 b | 68.5 ± 5.2 g–i | 2.82 ± 0.32 a–h | 6.17 ± 0.5 g–l | 133 ± 1.8 d–h | 31.1 ± 1.5 b–d | 7.46 ± 0.49 d | 250 ± 9 f–h | 6.01 ± 1.07 de | 17.6 ± 0.3 e–g | 4.09 ± 0.14 de |
| IPT208 | 1.55 ± 0.36 ab | 72.3 ± 3 e–i | 2.38 ± 0.24 b–h | 7.56 ± 0.79 d–k | 130.1 ± 8.9 e–h | 29.6 ± 1.2 b–d | 8.07 ± 0.74 d | 251 ± 13 f–h | 7.02 ± 1.16 c–e | 17.2 ± 1.5 e–g | 4.35 ± 0.28 c–e |
| IPT216 | 1.38 ± 0.24 b | 68.6 ± 5.1 g–i | 2.31 ± 0.48 b–h | 6.81 ± 0.54 f–k | 136.7 ± 2.9 d–h | 29.7 ± 3.1 b–d | 9.5 ± 1.04 d | 255 ± 11 e–g | 7.61 ± 0.97 c–e | 19.3 ± 1.6 d–g | 5.05 ± 0.46 c–e |
| IPT217 | 1.36 ± 0.17 b | 71.9 ± 2.9 e–i | 2.06 ± 0.48 c–h | 6.11 ± 0.18 g–l | 135.1 ± 8.7 d–h | 19.7 ± 3.5 c–e | 7.43 ± 0.14 d | 244 ± 3 f–h | 7.01 ± 1.43 c–e | 19.9 ± 1.4 d–g | 5.14 ± 0.19 c–e |
| IPT218 | 1.41 ± 0.2 b | 73.3 ± 3.1 e–i | 3.32 ± 0.57 a–e | 6.71 ± 0.23 f–l | 117.1 ± 6.2 f–i | 36.2 ± 0.7 b | 6.93 ± 0.67 d | 245 ± 8 f–h | 7.14 ± 0.83 c–e | 17.7 ± 0.8 e–g | 4.33 ± 0.21 c–e |
| IPT225 | 1.21 ± 0.26 b | 71.1 ± 1.5 f–i | 3.04 ± 0.71 a–h | 6.88 ± 0.82 f–k | 105.8 ± 4.2 g–i | 31 ± 2.9 b–d | 6.63 ± 0.58 d | 226 ± 11 gf | 7.05 ± 0.63 c–e | 17.3 ± 0.6 e–g | 4.54 ± 0.55 c–e |
| IPT226 | 1.25 ± 0.24 b | 72.3 ± 3.6 e–i | 3.08 ± 0.76 a–h | 9.69 ± 0.22 d–g | 135.6 ± 8.2 d–h | 31.1 ± 4.1 b–d | 7.49 ± 0.46 d | 261 ± 5 e–g | 7.34 ± 1.37 c–e | 19.3 ± 0.5 d–g | 5.08 ± 0.43 c–e |
| IPT228 | 1.3 ± 0.15 b | 73.7 ± 5.7 e–i | 2.48 ± 0.35 b–h | 8.71 ± 0.32 d–i | 138.5 ± 9.2 d–h | 33 ± 1 bc | 7.19 ± 0.55 d | 265 ± 15 d–g | 7.74 ± 1.1 c–e | 19.8 ± 1.7 d–g | 5.22 ± 0.26 c–e |
| IPT229 | 1.33 ± 0.21 b | 65.1 ± 3.9 i | 3.23 ± 0.63 a–f | 7.63 ± 0.27 d–k | 135.5 ± 3.9 d–h | 34 ± 4.4 b | 8.73 ± 1.24 d | 256 ± 7 e–g | 7.52 ± 0.71 c–e | 19.5 ± 0.2 d–g | 4.88 ± 0.51 c–e |
| IPT230 | 1.51 ± 0.35 ab | 80.4 ± 6.8 d–i | 1.49 ± 0.05 d–h | 9.09 ± 1.25 d–h | 123.6 ± 5.1 e–h | 13.4 ± 2.6 ef | 7.94 ± 1.31 d | 237 ± 12 f–h | 6.98 ± 0.54 c–e | 19.1 ± 1.1 d–g | 6.46 ± 0.91 a–d |
| IPT231 | 1.41 ± 0.35 b | 58.5 ± 6.6 i | 2.66 ± 0.44 a–h | 7.7 ± 0.74 d–k | 140.7 ± 1.5 d–h | 34 ± 3.5 b | 7.73 ± 0.9 d | 253 ± 13 e–g | 7.21 ± 0.89 c–e | 19.6 ± 0.5 d–g | 5.2 ± 0.57 c–e |
| IPT232 | 1.44 ± 0.18 b | 65.2 ± 7.6 i | 2.86 ± 0.43 a–h | 6.32 ± 0.66 g–l | 117 ± 2.3 f–i | 29.1 ± 3.9 b–d | 6.17 ± 0.36 d | 228 ± 15 gh | 7 ± 0.47 c–e | 18.3 ± 0.4 d–g | 4.9 ± 0.33 c–e |
| IPT233 | 1.41 ± 0.23 b | 75.7 ± 5.6 e–i | 3.33 ± 0.38 a–d | 8.29 ± 1.3 d–k | 129.7 ± 8.3 e–h | 34 ± 4.6 b | 8.03 ± 1.26 d | 260 ± 22 e–g | 7 ± 0.68 c–e | 18.9 ± 0.7 d–g | 5.46 ± 0.51 c–e |
| IPT234 | 1.6 ± 0.3 ab | 72.7 ± 4.6 e–i | 2.93 ± 0.68 a–h | 11.21 ± 1.23 de | 146.7 ± 2.5 d–h | 38.9 ± 4.1 b | 9.28 ± 1.08 d | 283 ± 10 d–g | 8.15 ± 0.88 c–e | 21.1 ± 0.5 d–f | 5.62 ± 0.54 b–e |
| IPT235 | 1.34 ± 0.07 b | 73.9 ± 4.2 e–i | 3.64 ± 0.11 a–c | 6.82 ± 0.37 f–k | 126.4 ± 14.6 e–h | 36.2 ± 1.8 b | 7.42 ± 0.28 d | 256 ± 21 e–g | 7.64 ± 1.06 c–e | 18.4 ± 1.3 d–g | 5.34 ± 0.12 c–e |
| IPT236 | 1.17 ± 0.15 b | 74.9 ± 3 e–i | 3.2 ± 0.36 a–g | 7.68 ± 0.2 d–k | 150 ± 24.3 d–h | 36.5 ± 1.6 b | 7.17 ± 0.57 d | 281 ± 28 d–g | 8.85 ± 0.9 b–e | 22.5 ± 1.3 de | 5.6 ± 0.72 b–e |
| IPT237 | 1.62 ± 0.08 ab | 98.2 ± 8.9 b–i | 4.56 ± 0.19 a | 10.65 ± 0.62 d–g | 180.2 ± 15.5 c–e | 56.7 ± 1.9 a | 8.44 ± 0.49 d | 360 ± 27 b–e | 9.84 ± 1.59 a–e | 24.6 ± 2.9 b–e | 6.13 ± 0.39 a–e |
| IPT238 | 1.36 ± 0.12 b | 64 ± 3.9 i | 3.03 ± 0.34 a–h | 8.74 ± 0.56 d–i | 146.5 ± 2 d–h | 36.7 ± 1.3 b | 8.71 ± 1.11 d | 269 ± 6 d–g | 8.71 ± 0.93 b–e | 20.8 ± 0.9 d–f | 5.52 ± 0.48 b–e |
| IPT242 | 1.15 ± 0.2 b | 70.2 ± 3.9 f–i | 2.89 ± 0.24 a–h | 9.77 ± 0.32 d–g | 152.1 ± 6.2 d–h | 34.8 ± 0.9 b | 10.9 ± 1.99 cd | 282 ± 7 d–g | 8.22 ± 0.62 c–e | 21.2 ± 0.2 d–f | 5.75 ± 0.48 b–e |
| IPT243 | 1.12 ± 0.21 b | 63.1 ± 4.5 i | 2.76 ± 0.37 a–h | 7.34 ± 0.19 e–k | 122.3 ± 8.2 e–h | 31.4 ± 1.2 b–c | 8.9 ± 1.08 d | 237 ± 4 f–h | 7.48 ± 1.04 c–e | 18.3 ± 0.7 d–g | 4.75 ± 0.32 c–e |
| IPT244 | 1.51 ± 0.22 ab | 74.8 ± 3.6 e–i | 3.82 ± 0.34 a–c | 8.37 ± 0.81 d–j | 138.9 ± 4.5 d–h | 41.3 ± 1.6 b | 10.49 ± 0.35 cd | 279 ± 2 d–g | 8.53 ± 1.13 b–e | 19.5 ± 0.5 d–g | 5.26 ± 0.33 c–e |
| A. Cepa Aggregatum shallot type | |||||||||||
| IPT239 | 1.99 ± 0.1 ab | 107.5 ± 6.4 a–h | 3.76 ± 0.23 a–c | 17.8 ± 0.49 b | 248.6 ± 5.2 ab | 59.2 ± 2 a | 23.2 ± 3.32 bc | 462 ± 11 ab | 11.87 ± 1.32 a–c | 31.2 ± 0.9 ab | 6.73 ± 0.44 a–c |
| IPT240 | 1.43 ± 0.2 b | 91.8 ± 3.5 c–i | 4.25 ± 0.14 ab | 11.62 ± 0.4 cd | 191.3 ± 5.2 b–d | 56.3 ± 1.1 a | 15.77 ± 2.64 cd | 372 ± 9 b–d | 10.94 ± 0.67 a–d | 25.5 ± 1.3 b–d | 6.24 ± 0.53 a–e |
| IPT241 | 2.33 ± 0.13 ab | 143.2 ± 17.5 a | 1.3 ± 0.04 e–h | 24.49 ± 0.7 a | 287.9 ± 19 a | 18.5 ± 1.9 d–f | 36.15 ± 9.2 a | 514 ± 26 a | 15.23 ± 0.13 a | 35.7 ± 1.2 a | 7.92 ± 0.65 ab |
| IPT245 | 1.81 ± 0.21 ab | 97.3 ± 19.5 b–i | 1.82 ± 0.4 c–h | 15.62 ± 2.27 bc | 219.1 ± 32.5 bc | 29.9 ± 5 b–c | 31.41 ± 6.63 ab | 397 ± 65 bc | 13.76 ± 2.58 ab | 30.2 ± 4 a–c | 8.53 ± 0.53 a |
| A. × proliferum | |||||||||||
| IPT023 | 2.79 ± 0.22 a | 119.9 ± 17.4 a–d | 1.96 ± 0.16 c–h | 8.18 ± 1.07 d–k | 91.4 ± 9.8 hi | 8.6 ± 0.9 ef | 7.34 ± 0.3 d | 240 ± 29 f–h | 6.22 ± 0.8 de | 14.4 ± 0.6 fg | 4.16 ± 0.2 de |
| IPT210 | 1.84 ± 0.2 ab | 67.2 ± 2.5 hi | 1.57 ± 0.23 d–h | 5.26 ± 0.25 h–l | 56.1 ± 1.4 i | 5.0 ± 0.7 f | 6.12 ± 0.59 d | 143 ± 3 h | 5.36 ± 0.49 d | 13.2 ± 0.4 g | 3.91 ± 0.4 d |
| p-value | ** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Ca | K | P | S | Mg | |
|---|---|---|---|---|---|
| g/kg DW 1 | |||||
| Harvest year | |||||
| 2018 | 2.16 ± 0.06 | 10.6 ± 0.3 | 3.13 ± 0.55 | 0.91 ± 0.02 | 0.71 ± 0.01 |
| 2019 | 1.89 ± 0.06 | 15.8 ± 0.3 | 3.18 ± 1.14 | 2.18 ± 0.07 | 0.88 ± 0.01 |
| p-value | *** | *** | n.s. | *** | *** |
| Species | |||||
| A. × cornutum | 2.04 ± 0.04 bc 2 | 9.6 ± 0.5 c | 2.33 ± 0.26 c | 1.25 ± 0.07 c | 0.62 ± 0.01 c |
| A. cepa Aggregatum potato onion type | 1.87 ± 0.04 c | 13.7 ± 0.3 b | 3.24 ± 0.54 b | 1.53 ± 0.07 b | 0.81 ± 0.01 b |
| A. cepa Aggregatum shallot type | 2.6 ± 0.22 a | 16.6 ± 1 a | 4.42 ± 1.48 a | 2.23 ± 0.26 a | 0.97 ± 0.03 a |
| A. × proliferum | 2.41 ± 0.12 ab | 12.9 ± 0.8 b | 2.62 ± 0.32 c | 1.47 ± 0.07 bc | 0.86 ± 0.03 b |
| p-value | *** | *** | *** | *** | *** |
| Harvest year × species | |||||
| A. × cornutum | |||||
| 2018 | 2.16 ± 0.04 a–c | 6.7 ± 0.2 e | 2.48 ± 0.04 c | 0.84 ± 0.02 e | 0.54 ± 0.01 |
| 2019 | 1.92 ± 0.07 cd | 12.6 ± 0.2 d | 2.18 ± 0.05 c | 1.66 ± 0.05 c | 0.69 ± 0.01 |
| A. cepa Aggregatum potato onion type | |||||
| 2018 | 2.08 ± 0.06 bc | 11.5 ± 0.2 d | 3.25 ± 0.06 b | 0.86 ± 0.02 e | 0.73 ± 0.01 |
| 2019 | 1.67 ± 0.05 d | 16 ± 0.3 b | 3.22 ± 0.08 b | 2.19 ± 0.06 b | 0.89 ± 0.01 |
| A. cepa Aggregatum shallot type | |||||
| 2018 | 2.57 ± 0.36 ab | 12.6 ± 0.5 cd | 3.7 ± 0.17 b | 1.05 ± 0.09 de | 0.85 ± 0.02 |
| 2019 | 2.64 ± 0.28 a | 20.7 ± 1 a | 5.15 ± 0.5 a | 3.41 ± 0.12 a | 1.09 ± 0.04 |
| A. × proliferum | |||||
| 2018 | 2.22 ± 0.18 a–d | 10.3 ± 0.4 d | 2.92 ± 0.01 bc | 1.42 ± 0.14 cd | 0.76 ± 0.03 |
| 2019 | 2.6 ± 0.13 a–c | 15.5 ± 0.3 bc | 2.32 ± 0.03 c | 1.51 ± 0.01 cd | 0.96 ± 0.02 |
| p-value | * | *** | *** | *** | n.s. |
| Accessions | |||||
| A. × cornutum | |||||
| IPT021 | 1.94 ± 0.01 d–j | 9.7 ± 1.3 de | 2.18 ± 0.04 jk | 1.42 ± 0.25 | 0.63 ± 0.03 f–k |
| IPT022 | 2.09 ± 0.01 d–i | 10.7 ± 1 c–e | 2.39 ± 0.16 h–k | 1.22 ± 0.21 | 0.67 ± 0.01 e–k |
| IPT211 | 2.28 ± 0.05 c–g | 8.8 ± 1.4 d–e | 2.04 ± 0.09 k | 1.06 ± 0.12 | 0.59 ± 0.03 jk |
| IPT212 | 2.09 ± 0.07 d–i | 10.5 ± 1.8 c–e | 2.51 ± 0.03 f–k | 1.27 ± 0.15 | 0.6 ± 0.04 i–k |
| IPT213 | 2.00 ± 0.16 d–i | 8.5 ± 1.1 e | 2.53 ± 0.08 e–k | 1.24 ± 0.14 | 0.63 ± 0.04 g–k |
| IPT214 | 1.85 ± 0.08 d–j | 9.8 ± 1.5 de | 2.43 ± 0.03 g–k | 1.39 ± 0.26 | 0.62 ± 0.05 h–k |
| IPT215 | 2.03 ± 0.21 d–i | 9.4 ± 1.2 de | 2.23 ± 0.06 i–k | 1.13 ± 0.16 | 0.57 ± 0.02 k |
| A. cepa Aggregatum potato onion type | |||||
| IPT176 | 1.69 ± 0.18 e–j | 13.4 ± 0.7 a–e | 2.78 ± 0.04 d–k | 1.25 ± 0.10 | 0.84 ± 0.05 a–i |
| IPT208 | 3.09 ± 0.27 b | 12.1 ± 0.3 b–e | 2.77 ± 0.25 d–k | 1.13 ± 0.11 | 0.78 ± 0.03 b–k |
| IPT216 | 2.46 ± 0.1 b–d | 14.6 ± 1.7 a–e | 3.52 ± 0.07 c–g | 1.35 ± 0.16 | 0.89 ± 0.06 a–e |
| IPT217 | 1.57 ± 0.16 g–j | 10.7 ± 1.5 b–e | 3.11 ± 0.26 c–k | 1.31 ± 0.15 | 0.69 ± 0.06 d–k |
| IPT218 | 1.59 ± 0.06 f–j | 11 ± 0.8 b–e | 3.24 ± 0.34 c–j | 1.3 ± 0.19 | 0.66 ± 0.04 e–k |
| IPT225 | 1.43 ± 0.01 ij | 13.5 ± 1.5 a–e | 3.11 ± 0.06 c–k | 1.65 ± 0.4 | 0.72 ± 0.09 d–k |
| IPT226 | 1.58 ± 0.14 g–j | 14 ± 1.2 a–e | 3.73 ± 0.32 b–d | 1.47 ± 0.24 | 0.77 ± 0.05 b–k |
| IPT228 | 1.67 ± 0.1 e–j | 15 ± 2 a–e | 3.61 ± 0.14 c–f | 1.9 ± 0.43 | 0.88 ± 0.05 a–f |
| IPT229 | 1.81 ± 0.18 d–j | 12.4 ± 1.3 b–e | 2.89 ± 0.08 d–k | 1.47 ± 0.39 | 0.76 ± 0.01 c–k |
| IPT230 | 1.77 ± 0.06 d–j | 12.1 ± 0.4 b–e | 3.01 ± 0.01 d–k | 1.5 ± 0.41 | 0.85 ± 0.02 a–h |
| IPT231 | 2.3 ± 0.11 c–f | 15 ± 1.9 a–e | 3.33 ± 0.16 c–i | 1.49 ± 0.3 | 0.86 ± 0.04 a–h |
| IPT232 | 1.9 ± 0.12 d–j | 12.8 ± 0.8 a–e | 2.77 ± 0.03 d–k | 1.36 ± 0.21 | 0.81 ± 0.01 b–k |
| IPT233 | 1.72 ± 0.04 e–j | 13.9 ± 1.1 a–e | 3.21 ± 0.14 c–j | 1.75 ± 0.38 | 0.86 ± 0.03 a–h |
| IPT234 | 1.8 ± 0.05 d–j | 15.7 ± 0.9 a–d | 3.26 ± 0.27 c–j | 1.75 ± 0.39 | 0.84 ± 0.08 a–i |
| IPT235 | 2.01 ± 0.02 d–i | 14.1 ± 0.6 a–e | 2.77 ± 0.11 d–k | 1.43 ± 0.22 | 0.72 ± 0.01 d–k |
| IPT236 | 1.8 ± 0.21 d–j | 14.2 ± 0.7 a–e | 2.85 ± 0.03 d–k | 1.58 ± 0.33 | 0.79 ± 0.05 b–k |
| IPT237 | 1.9 ± 0.06 d–j | 16.8 ± 1.2 a–c | 3.56 ± 0.06 c–f | 1.72 ± 0.5 | 0.83 ± 0.04 b–j |
| IPT238 | 1.97 ± 0.13 d–j | 15.3 ± 0.8 a–e | 3.4 ± 0.05 c–h | 1.72 ± 0.53 | 0.88 ± 0.06 a–g |
| IPT242 | 2 ± 0.03 d–j | 15.7 ± 1.2 a–d | 3.66 ± 0.07 b–e | 2.04 ± 0.43 | 0.98 ± 0.03 a–c |
| IPT243 | 1.76 ± 0.17 d–j | 11.7 ± 0.4 b–e | 3.27 ± 0.13 c–j | 1.18 ± 0.2 | 0.74 ± 0.01 c–k |
| IPT244 | 1.53 ± 0.05 h–j | 14.4 ± 0.9 a–e | 4.14 ± 0.32 bc | 1.67 ± 0.27 | 0.87 ± 0.02 a–g |
| A. cepa Aggregatum shallot type | |||||
| IPT239 | 2.18 ± 0.07 d–i | 13.7 ± 1.3 a–e | 2.9 ± 0.08 d–k | 2.14 ± 0.7 | 0.86 ± 0.04 a–h |
| IPT240 | 2.96 ± 0.23 b–c | 15.6 ± 1.2 a–d | 4.15 ± 0.06 bc | 2.3 ± 0.52 | 0.92 ± 0.03 a–d |
| IPT241 | 3.99 ± 0.21 a | 17.7 ± 2.8 ab | 4.75 ± 0.43 b | 1.92 ± 0.38 | 1.08 ± 0.08 a |
| IPT245 | 1.29 ± 0.01 j | 19.6 ± 2 a | 5.89 ± 0.74 a | 2.57 ± 0.51 | 1.02 ± 0.07 ab |
| A. × proliferum | |||||
| IPT023 | 2.37 ± 0.23 c–e | 13.2 ± 0.9 a–e | 2.6 ± 0.15 e–k | 1.32 ± 0.1 | 0.85 ± 0.07 a–h |
| IPT210 | 2.45 ± 0.11 b–d | 12.6 ± 1.4 a–e | 2.64 ± 0.12 d–k | 1.61 ± 0.06 | 0.86 ± 0.02 a–h |
| p-value | *** | *** | *** | n.s. | *** |
| Al | B | Cu | Fe | Li | Mn | Mo | Na | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| mg/kg DW 1 | |||||||||
| Harvest year | |||||||||
| 2018 | 31.9 ± 0.8 | 7.98 ± 0.09 | 6.67 ± 0.12 | 27.6 ± 0.6 | 63.3 ± 3.8 | 10.9 ± 0.2 | 0.51 ± 0.01 | 102 ± 4 | 16.6 ± 0.4 |
| 2019 | 27.7 ± 0.7 | 6.78 ± 0.09 | 6.07 ± 0.15 | 29.6 ± 0.6 | 42 ± 0.8 | 10.5 ± 0.1 | 0.43 ± 0.01 | 147 ± 5 | 13.8 ± 0.5 |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Species | |||||||||
| A. × cornutum | 28.9 ± 0.7 | 7.86 ± 0.11 b 2 | 5.68 ± 0.19 c | 23.9 ± 0.5 c | 45.4 ± 1.1 b | 10 ± 0.2 c | 0.62 ± 0.01 a | 83 ± 5 b | 11.2 ± 0.3 c |
| A. cepa Aggregatum potato onion type | 30.2 ± 0.7 | 7 ± 0.09 c | 6.32 ± 0.1 b | 28.8 ± 0.5 b | 58.1 ± 3.2 a | 10.9 ± 0.2 b | 0.42 ± 0.01 c | 142 ± 5 a | 15.5 ± 0.3 b |
| A. cepa Aggregatum shallot type | 29.6 ± 0.9 | 8.53 ± 0.24 a | 8.18 ± 0.32 a | 35 ± 1.1 a | 47 ± 0.9 ab | 11.8 ± 0.2 a | 0.49 ± 0.02 b | 139 ± 6 a | 20.8 ± 1 a |
| A. × proliferum | 29.1 ± 3.9 | 7.33 ± 0.18 bc | 5.71 ± 0.17 bc | 30.1 ± 1.9 b | 32.6 ± 4 b | 8.9 ± 0.3 c | 0.49 ± 0.03 b | 60 ± 4 b | 15.1 ± 0.9 b |
| p-value | n.s. | *** | *** | *** | *** | *** | *** | *** | *** |
| Harvest year × species | |||||||||
| A. × cornutum | |||||||||
| 2018 | 29.2 ± 0.8 b | 8.54 ± 0.07 | 6.36 ± 0.25 c | 21.4 ± 0.5 e | 51.7 ± 1.0 | 8.8 ± 0.1 e | 0.66 ± 0.02 a | 63 ± 7 | 12.3 ± 0.4 cd |
| 2019 | 28.7 ± 1.2 b | 7.19 ± 0.05 | 5 ± 0.19 d | 26.5 ± 0.5 d | 39.1 ± 0.6 | 11.1 ± 0.2 b | 0.58 ± 0.01 ab | 102 ± 6 | 10.2 ± 0.3 d |
| A. cepa Aggregatum potato onion type | |||||||||
| 2018 | 32.1 ± 1 ab | 7.61 ± 0.12 | 6.58 ± 0.15 c | 28.1 ± 0.7 cd | 72.3 ± 5.9 | 11.8 ± 0.2 ab | 0.48 ± 0.01 c | 116 ± 5 | 17.5 ± 0.4 b |
| 2019 | 28.3 ± 1 b | 6.39 ± 0.09 | 6.06 ± 0.14 c | 29.5 ± 0.7 b–d | 43.9 ± 0.6 | 10.1 ± 0.2 cd | 0.36 ± 0.01 d | 168 ± 6 | 13.5 ± 0.4 c |
| A. cepa Aggregatum shallot type | |||||||||
| 2018 | 32.1 ± 0.9 ab | 8.99 ± 0.24 | 7.92 ± 0.29 ab | 32.9 ± 0.7 ab | 46.4 ± 0.4 | 11.2 ± 0.3 a–c | 0.48 ± 0.02 c | 118 ± 6 | 18.7 ± 0.6 b |
| 2019 | 27.2 ± 1 b | 8.08 ± 0.38 | 8.44 ± 0.57 a | 37.1 ± 1.9 a | 47.6 ± 1.7 | 12.5 ± 0.3 a | 0.51 ± 0.04 bc | 159 ± 8 | 22.9 ± 1.8 a |
| A. × proliferum | |||||||||
| 2018 | 39.9 ± 4.5 a | 7.86 ± 0.06 | 6.26 ± 0.07 b–d | 33.8 ± 2.9 a–c | 44.3 ± 1.4 | 8.8 ± 0.5 ed | 0.44 ± 0.04 cd | 56 ± 3 | 17.8 ± 0.2 b |
| 2019 | 18.2 ± 0.4 c | 6.81 ± 0.19 | 5.16 ± 0.05 cd | 26.4 ± 1.1 b–e | 20.9 ± 3.5 | 9 ± 0.3 ed | 0.54 ± 0.02 bc | 64 ± 7 | 12.3 ± 0.4 cd |
| p-value | *** | n.s. | * | ** | n.s. | *** | *** | n.s. | *** |
| Accessions | |||||||||
| A. × cornutum | |||||||||
| IPT021 | 27.3 ± 2.6 a–c | 7.56 ± 0.33 a–f | 4.85 ± 0.32 fg | 23 ± 0.2 f–h | 40.8 ± 2.1 d | 10 ± 0.9 c–g | 0.63 ± 0.03 a–d | 109 ± 4 d– | 9.9 ± 0.2 g |
| IPT022 | 32.5 ± 0.9 a–c | 7.96 ± 0.35 a–e | 5.2 ± 0.42 d–g | 27.4 ± 1.3 c–h | 43 ± 1.4 d | 10.5 ± 0.5 b–g | 0.57 ± 0.02 a–g | 106 ± 5 d–k | 10.6 ± 0.8 fg |
| IPT211 | 26.3 ± 0.9 bc | 7.76 ± 0.35 a–e | 5.06 ± 0.44 e–g | 22.8 ± 1.8 f–h | 45.4 ± 1.9 d | 10 ± 0.5 c–g | 0.55 ± 0.02 a–i | 59 ± 1 jk | 9.7 ± 0.7 g |
| IPT212 | 26.2 ± 0.2 bc | 8.09 ± 0.21 a–e | 5.97 ± 0.19 c–g | 24.3 ± 1.8 e–h | 47.8 ± 1.8 b–d | 10.4 ± 0.7 b–g | 0.69 ± 0.01 a | 68 ± 11 h–k | 11.4 ± 0.3 e–g |
| IPT213 | 29.4 ± 1.0 a–c | 7.74 ± 0.24 a–e | 5.95 ± 0.28 c–g | 24.4 ± 0.6 e–h | 47.1 ± 3.9 b–d | 10.4 ± 0.5 b–g | 0.66 ± 0.03 ab | 98 ± 27 f–k | 12.9 ± 0.5 c–g |
| IPT214 | 28.1 ± 0.3 a–c | 7.71 ± 0.22 a–e | 6 ± 0.25 c–g | 22.5 ± 1.2 gh | 47.2 ± 4.5 b–d | 9.8 ± 0.6 d–g | 0.65 ± 0.05 a–c | 66 ± 12 i–k | 12 ± 0.2 d–g |
| IPT215 | 32.7 ± 3.3 a–c | 8.23 ± 0.41 a–d | 6.75 ± 0.92 b–g | 23.3 ± 1.4 f–h | 46.5 ± 4.2 cd | 8.7 ± 0.1 e–g | 0.58 ± 0.01 a–f | 75 ± 11 g–k | 12.2 ± 1.3 d–g |
| A. cepa Aggregatum potato onion type | |||||||||
| IPT176 | 27.1 ± 1.4 bc | 6.98 ± 0.35 b–f | 6.26 ± 0.25 c–g | 25.8 ± 0.5 d–h | 43 ± 2 d | 12.7 ± 0.4 a–c | 0.46 ± 0.03 d–j | 242 ± 24 a | 13.6 ± 0.7 b–g |
| IPT208 | 30.5 ± 2 a–c | 7.23 ± 0.71 b–f | 4.82 ± 0.26 g | 30.2 ± 3.4 b–h | 44.9 ± 2.6 d | 13.4 ± 0.7 a–c | 0.47 ± 0.05 d–j | 234 ± 20 ab | 15.2 ± 2.6 b–g |
| IPT216 | 31.6 ± 0.4 a–c | 8.03 ± 0.25 a–e | 6.29 ± 0.28 c–g | 35.6 ± 0.7 a–c | 52.2 ± 3 a–d | 12.9 ± 0.6 ab | 0.39 ± 0.02 g–j | 133 ± 15 c–i | 15.7 ± 1.1 b–g |
| IPT217 | 31 ± 2.2 a–c | 7.28 ± 0.75 a–f | 6.87 ± 0.57 b–g | 27.9 ± 1.2 b–h | 101 ± 28 a–c | 11.3 ± 0.4 a–f | 0.36 ± 0.02 j | 199 ± 1 a–c | 15.3 ± 2.4 b–g |
| IPT218 | 30.4 ± 0.7 a–c | 6.34 ± 0.29 d–f | 5.9 ± 0.8 c–g | 24.6 ± 0.3 e–h | 102 ± 28 ab | 9.6 ± 0.5 d–g | 0.41 ± 0.04 f–j | 143 ± 2 c–g | 16.4 ± 3.2 b–g |
| IPT225 | 32.4 ± 0.3 a–c | 5.66 ± 0.22 f | 5.69 ± 0.26 c–g | 24.8 ± 2.8 e–h | 104 ± 29 a | 8.4 ± 0 g | 0.45 ± 0.05 d–j | 146 ± 20 c–f | 15.6 ± 0.8 b–g |
| IPT226 | 26.8 ± 0.9 bc | 6.53 ± 0.44 c–f | 7.65 ± 1.1 a–c | 25.5 ± 0.2 d–h | 104 ± 29 a | 10.2 ± 0.7 b–g | 0.38 ± 0.01 h–j | 157 ± 9 c–f | 19.5 ± 3.1 bc |
| IPT228 | 42.6 ± 9.5 a | 6.93 ± 0.16 b–f | 6.32 ± 0.17 c–g | 33.4 ± 2.8 a–e | 59.7 ± 13.4 a–d | 10.2 ± 0.7 b–g | 0.4 ± 0.02 f–j | 128 ± 5 d–j | 17 ± 0.7 b–f |
| IPT229 | 39.8 ± 3.3 ab | 6.27 ± 0.32 d–f | 5.91 ± 0.26 c–g | 30.6 ± 1.1 b–h | 43.4 ± 1.3 d | 9.8 ± 0.9 d–g | 0.4 ± 0.05 f–j | 98 ± 12 e–k | 12.9 ± 0.7 c–g |
| IPT230 | 31.1 ± 5 a–c | 7.52 ± 0.11 a–f | 6.47 ± 0.19 b–g | 34.6 ± 2.3 a–d | 45 ± 0.9 d | 12.2 ± 0.4 a–d | 0.4 ± 0.01 f–j | 173 ± 27 a–d | 15.8 ± 0.9 b–g |
| IPT231 | 24.6 ± 0.6 bc | 7.54 ± 0.39 a–f | 7.38 ± 0.22 a–d | 28.6 ± 1.5 b–h | 47.1 ± 0.3 b–d | 11 ± 0.4 a–g | 0.42 ± 0.03 f–j | 110 ± 14 d–k | 16.6 ± 0.3 b–g |
| IPT232 | 25 ± 1.1 bc | 7.09 ± 0.12 b–f | 5.62 ± 0.42 c–g | 26.4 ± 0.4 c–h | 45.7 ± 0.8 d | 12 ± 0.9 a–d | 0.47 ± 0.1 c–j | 123 ± 5 d–j | 13.2 ± 1.6 c–g |
| IPT233 | 25.9 ± 2.3 bc | 6.53 ± 0.21 c–f | 5.96 ± 0.11 c–g | 27.2 ± 1.4 c–h | 45.9 ± 1.1 cd | 10.5 ± 0.4 b–g | 0.36 ± 0.02 ij | 97 ± 12 f–k | 14.8 ± 0.6 b–g |
| IPT234 | 25 ± 0.9 bc | 6.57 ± 0.03 c–f | 6.48 ± 0.36 b–g | 29.3 ± 2.6 b–h | 46.5 ± 1 cd | 11.5 ± 0.3 a–e | 0.39 ± 0.03 g–j | 121 ± 20 d–k | 15.5 ± 0.7 b–g |
| IPT235 | 25.2 ± 1.5 bc | 7.37 ± 0.37 a–f | 5.87 ± 0.25 c–g | 24.8 ± 0.6 e–h | 48.2 ± 0.7 b–d | 9.9 ± 0.2 d–g | 0.42 ± 0.04 f–j | 113 ± 7 d–k | 14.2 ± 0.4 b–g |
| IPT236 | 24.5 ± 1.9 c | 7.09 ± 0.33 b–f | 5.31 ± 0.22 d–g | 25.9 ± 0.2 d–h | 45.8 ± 1 cd | 10.8 ± 0.1 a–g | 0.43 ± 0.02 e–j | 111 ± 8 d–k | 12.6 ± 0.8 c–g |
| IPT237 | 35.6 ± 3.5 a–c | 6.12 ± 0.14 ef | 7.12 ± 0.41 b–e | 31.7 ± 2.8 a–g | 48 ± 0.2 b–d | 10.1 ± 0.1 b–g | 0.41 ± 0.04 f–j | 127 ± 12 d–j | 15.8 ± 0.6 b–g |
| IPT238 | 38.1 ± 3.4 a–c | 6.95 ± 0.58 b–f | 7.07 ± 0.42 b–f | 34.4 ± 3.6 a–d | 46.8 ± 1.1 cd | 10.5 ± 0.3 b–g | 0.43 ± 0.04 f–j | 152 ± 22 c–f | 16 ± 0.6 b–g |
| IPT242 | 28 ± 2.1 a–c | 7.37 ± 0.65 a–f | 6.52 ± 0.19 b–g | 30.3 ± 0.3 b–h | 44.2 ± 0.9 d | 11.2 ± 0.6 a–g | 0.36 ± 0.01 j | 120 ± 11 d–k | 17.4 ± 0.5 b–f |
| IPT243 | 26.7 ± 0.3 bc | 7.9 ± 0.08 a–e | 6.23 ± 0.28 c–g | 21.8 ± 0.3 h | 51.3 ± 1.9 a–d | 10.4 ± 0.6 b–g | 0.46 ± 0.01 d–j | 129 ± 7 c–j | 13.8 ± 0.5 b–g |
| IPT244 | 32 ± 0.4 a–c | 7.72 ± 0.07 a–e | 6.98 ± 0.38 b–g | 31.6 ± 0.3 a–g | 51.6 ± 2.1 a–d | 10.8 ± 0.3 a–g | 0.49 ± 0.02 b–j | 125 ± 2 d–j | 18.7 ± 0.8 b–c |
| A. cepa Aggregatum shallot type | |||||||||
| IPT239 | 29.2 ± 3 a–c | 7.62 ± 0.63 a–f | 6.1 ± 0.22 c–g | 40.5 ± 2.3 a | 45 ± 0.5 d | 12 ± 0.8 a–d | 0.37 ± 0.01 ij | 124 ± 15 d–j | 17.8 ± 0.4 b–e |
| IPT240 | 29.2 ± 0.3 a–c | 8.86 ± 0.55 ab | 8.68 ± 0.31 ab | 36.9 ± 1.3 ab | 45.4 ± 0.2 d | 11.2 ± 0.1 a–f | 0.42 ± 0.02 f–j | 122 ± 7 d–j | 20.6 ± 0.3 ab |
| IPT241 | 30.3 ± 0.7 a–c | 9.24 ± 0.12 a | 8.57 ± 0.37 ab | 31.1 ± 0.9 b–h | 46.5 ± 1 cd | 12.8 ± 0.1 ab | 0.62 ± 0.04 a–e | 169 ± 14 b–e | 17.9 ± 0.6 b–e |
| IPT245 | 29.9 ± 1.9 a–c | 8.4 ± 0.26 a–c | 9.38 ± 0.57 a | 31.4 ± 0.9 a–g | 51 ± 2.8 a–d | 11.3 ± 0.3 a–f | 0.56 ± 0.02 a–h | 139 ± 1 c–h | 26.9 ± 2.9 a |
| A. × proliferum | |||||||||
| IPT023 | 24.5 ± 2.6 c | 7.48 ± 0.12 a–f | 5.6 ± 0.23 c–g | 28.1 ± 0.6 b–h | 30.3 ± 7.5 d | 8.7 ± 0.5 f–g | 0.52 ± 0.02 a–j | 71 ± 4 h–k | 14.8 ± 1.5 b–g |
| IPT210 | 33.6 ± 7.3 a–c | 7.19 ± 0.36 b–f | 5.82 ± 0.26 c–g | 32.1 ± 3.7 a–f | 34.8 ± 3.2 d | 9.1 ± 0.4 e–g | 0.45 ± 0.05 d–j | 50 ± 1 k | 15.4 ± 1 b–g |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, N.; Perković, J.; Palčić, I.; Bažon, I.; Horvat, I.; Ban, D.; Goreta Ban, S. The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent. Antioxidants 2022, 11, 1547. https://doi.org/10.3390/antiox11081547
Major N, Perković J, Palčić I, Bažon I, Horvat I, Ban D, Goreta Ban S. The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent. Antioxidants. 2022; 11(8):1547. https://doi.org/10.3390/antiox11081547
Chicago/Turabian StyleMajor, Nikola, Josipa Perković, Igor Palčić, Iva Bažon, Ivana Horvat, Dean Ban, and Smiljana Goreta Ban. 2022. "The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent" Antioxidants 11, no. 8: 1547. https://doi.org/10.3390/antiox11081547
APA StyleMajor, N., Perković, J., Palčić, I., Bažon, I., Horvat, I., Ban, D., & Goreta Ban, S. (2022). The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent. Antioxidants, 11(8), 1547. https://doi.org/10.3390/antiox11081547








