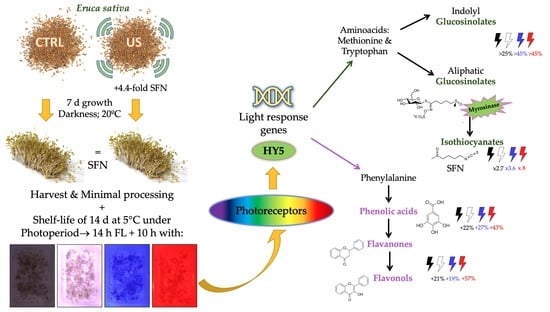
Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Ultrasound Seed Treatment
2.3. Seed Germination and Minimal Processing
2.4. Postharvest Lighting Treatments
2.5. Physiological Quality during Shelf-Life
2.6. Bioactive Compounds Determination
2.6.1. Antioxidant Compounds: Phenolic Acids and Flavonoids
2.6.2. Glucosinolates
2.6.3. Sulforaphane
2.7. Statistical Analysis
3. Results and Discussion
3.1. US Seed Treatment Effect
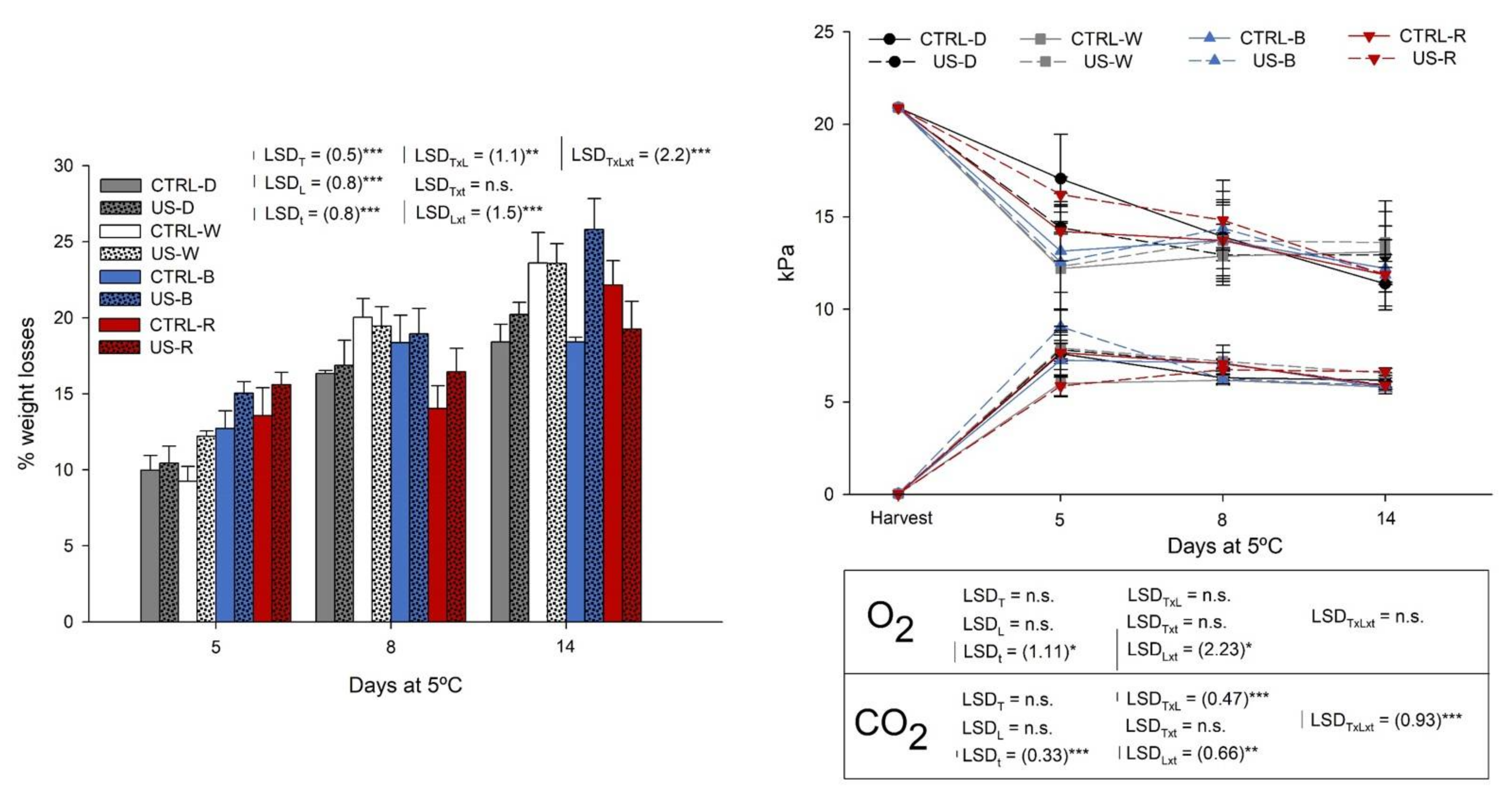
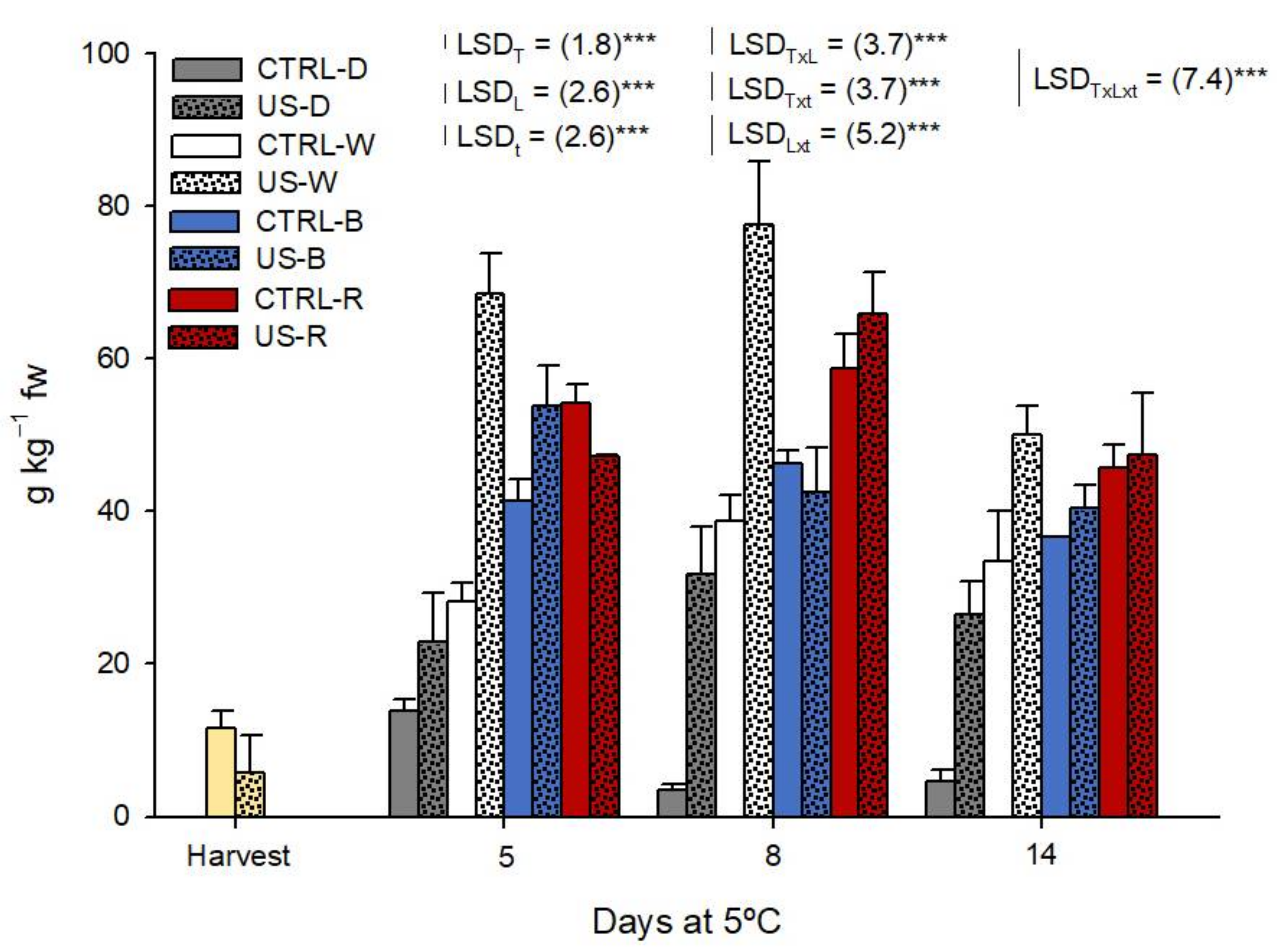
3.2. Physiological Changes during Shelf Life
3.3. Bioactive Compound Changes during Shelf Life under Illumination Treatments
3.3.1. Phenolic Compounds
3.3.2. Glucosinolates
3.3.3. Isothiocyanates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State Of Food And Agriculture, 2019: Moving Forward On Food Loss And Waste Reduction; FAO: Rome, Italy, 2019; ISBN 9789251317891. [Google Scholar]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of Ultrasound Pretreated Apple and Its Selected Physical Properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Yang, H.; Gao, J.; Yang, A.; Chen, H. The Ultrasound-Treated Soybean Seeds Improve Edibility and Nutritional Quality of Soybean Sprouts. Food Res. Int. 2015, 77, 704–710. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés-Hernández, F. Amelioration Effect of LED Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.A.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest LED Lighting: Effect of Red, Blue and Far Red on Quality of Minimally Processed Broccoli Sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Orsini, F.; Art, F.; Crepaldi, A.; Fernández, J.A.; Artés-Hernández, F. Postharvest Yellow LED Lighting Affects Phenolics and Glucosinolates Biosynthesis in Broccoli Sprouts. J. Food Compos. Anal. 2021, 103, 104101. [Google Scholar] [CrossRef]
- Pintos, F.M.; Hasperué, J.H.; Vicente, A.R.; Rodoni, L.M. Role of White Light Intensity and Photoperiod during Retail in Broccoli Shelf-Life. Postharvest Biol. Technol. 2020, 163, 111121. [Google Scholar] [CrossRef]
- Ntagkas, N.; de Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the Tomato Fruit Metabolome by LED Light. Metabolites 2020, 10, 266. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736. [Google Scholar] [CrossRef]
- Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z.; Marton, M.; Mándoki, Z.; Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z. The Role of Sprouts in Human Nutrition. A Review. Aliment. Hungarian Univ. Transylvania 2010, 3, 81–117. [Google Scholar]
- Egner, P.A.; Chen, J.G.; Zarth, A.T.; Ng, D.K.; Wang, J.B.; Kensler, K.H.; Jacobson, L.P.; Muñoz, A.; Johnson, J.L.; Groopman, J.D.; et al. Rapid and Sustainable Detoxication of Airborne Pollutants by Broccoli Sprout Beverage: Results of a Randomized Clinical Trial in China. Cancer Prev. Res. 2014, 7, 813–823. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. UV Radiation Enhanced Biosynthesis of Flavonoids and Carotenes in Bell Peppers. Postharvest Biol. Technol. Postharvest 2022, 184, 111774. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and UV-C Radiation Enhanced the Biosynthesis of Glucosinolates and Isothiocyanates in Brassicaceae Sprouts. Postharvest Biol. Technol. 2021, 181, 111650. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Li, X.; Xiao, J.; Guo, L. Enhancement of Ultrasound-Assisted Extraction of Sulforaphane from Broccoli Seeds via the Application of Microwave Pretreatment. Ultrason. Sonochem. 2022, 87, 106061. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource Use Efficiency of Indoor Lettuce (Lactuca sativa L.) Cultivation as Affected by Red: Blue Ratio Provided by LED Lighting. Nat. Sci. Reports 2019, 9, 14127. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Castillejo, N.; Gómez, P.A.; Crepaldi, A.; Fernández, J.A.; Egea-Gilabert, C.; Artés-Hernández, F.; Gianquinto, G. Spectral Composition from Led Lighting during Storage Affects Nutraceuticals and Safety Attributes of Fresh-Cut Red Chard (Beta vulgaris) and Rocket (Diplotaxis tenuifolia) Leaves. Postharvest Biol. Technol. 2021, 175, 111500. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, M.; Chitrakar, B.; Devahastin, S.; Guo, Z. Novel Combined Use of Red-White LED Illumination and Modified Atmosphere Packaging for Maintaining Storage Quality of Postharvest Pakchoi. Food Bioprocess Technol. 2022, 15, 590–605. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Adhikari, B. Extending Shelf-Life of Fresh-Cut Green Peppers Using Pressurized Argon Treatment. Postharvest Biol. Technol. 2012, 71, 13–20. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (Led) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting Broccoli Accumulate Higher Concentrations of Nutritionally Important Metabolites under Narrow-Band Light-Emitting Diode Lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Obyedul Kalam Azad, M.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of Artificial LED Light and Far Infrared Irradiation on Phenolic Compound, Isoflavones and Antioxidant Capacity in Soybean (Glycine max L.). Sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef]
- Cuellar, M.; Baroni, V.; Pfaffen, V.; Griboff, J.; Ortiz, P.; Monferrán, M.V. Uptake and Accumulation of Cr in Edible Parts of Eruca Sativa from Irrigation Water. Effects on Polyphenol Profile and Antioxidant Capacity. Heliyon 2021, 7, e06086. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium Biofortification in Radish Enhances Nutritional Quality via Accumulation of Methyl-Selenocysteine and Promotion of Transcripts and Metabolites Related to Glucosinolates, Phenolics Amino Acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating Colors: Regulation of Carotenoid Biosynthesis and Accumulation by Light. Curr. Opin. Plant Biol. 2017, 37, 49–55. [Google Scholar] [CrossRef]
- Tian, S.L.; Li, L.; Shah, S.N.M.; Gong, Z.H. The Relationship between Red Fruit Colour Formation and Key Genes of Capsanthin Biosynthesis Pathway in Capsicum Annuum. Biol. Plant. 2015, 59, 507–513. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and Visible Spectrum LED Lighting as Abiotic Elicitors of Bioactive Compounds in Sprouts, Microgreens, and Baby Leaves—A Comprehensive Review Including Their Mode of Action. Foods 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I. Signal Transduction in Responses to UV-B Radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef]
- Jenkins, G.I. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Wang, J.; Mao, S.; Wu, Q.; Yuan, Y.; Liang, M.; Wang, S.; Huang, K.; Wu, Q. Effects of LED Illumination Spectra on Glucosinolate and Sulforaphane Accumulation in Broccoli Seedlings. Food Chem. 2021, 356, 129550. [Google Scholar] [CrossRef]
- Yan, Y.; Stoddard, F.L.; Neugart, S.; Sadras, V.O.; Lindfors, A.; Morales, L.O.; Aphalo, P.J. Responses of Flavonoid Profile and Associated Gene Expression to Solar Blue and UV Radiation in Two Accessions of Vicia Faba L. from Contrasting UV Environments. Photochem. Photobiol. Sci. 2019, 18, 434–447. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Glucosinolates and Phenolic Compounds in Sprouting Canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an Anticancer Molecule: Mechanisms of Action, Synergistic Effects, Enhancement of Drug Safety, and Delivery Systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Kuno, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of Irradiation Patterns and Light Quality of Red and Blue Light-Emitting Diodes on Growth of Leaf Lettuce (Lactuca sativa L.”Greenwave”). Environ. Control Biol. 2017, 55, 129–135. [Google Scholar] [CrossRef]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary Sulforaphane-Rich Broccoli Sprouts Reduce Colonization and Attenuate Gastritis in Helicobacter Pylori-Infected Mice and Humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef]
- Lim, S.; Han, S.W.; Kim, J. Sulforaphene Identified from Radish (Raphanus sativus L.) Seeds Possesses Antimicrobial Properties against Multidrug-Resistant Bacteria and Methicillin-Resistant Staphylococcus Aureus. J. Funct. Foods 2016, 24, 131–141. [Google Scholar] [CrossRef]
- Wang, M.T.; Honn, K.V.; Nie, D. Cyclooxygenases, Prostanoids, and Tumor Progression. Cancer Metastasis Rev. 2007, 26, 525–534. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-Targeted Prevention of Cancer by Sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef]
- Liang, J.; Hänsch, G.M.; Hübner, K.; Samstag, Y. Sulforaphane as Anticancer Agent: A Double-Edged Sword? Tricky Balance between Effects on Tumor Cells and Immune Cells. Adv. Biol. Regul. 2019, 71, 79–87. [Google Scholar] [CrossRef]

| Phenolics acids (mg g−1) | ||||||
| Gallic acid | Caffeic acid | Ferulic acid | Coumaric acid | Sinapic acid | ||
| CTRL | 3.56 ± 1.19 | 1.82 ± 0.15 | 1.81 ± 0.39 | 3.07 ± 0.85 | 2.97 ± 0.39 | |
| US | 3.25 ± 1.12 | 0.45 ± 0.16 | 1.64 ± 0.12 | 2.15 ± 0.38 | 2.05 ± 0.69 | |
| Flavonoids (mg g−1) | ||||||
| Kaempferol derivative-1 | Kaempferol derivative-2 | Kaempferol derivative-3 | Rutin | Quercetin | Quercetin derivative | |
| CTRL | 2.02 ± 0.14 | 6.16 ± 0.51 | 4.15 ± 0.93 | 2.25 ± 0.43 | 11.28 ± 3.5 | 9.29 ± 2.01 |
| US | 1.88 ± 0.15 | 5.85 ± 0.19 | 3.59 ± 0.56 | 2.04 ± 0.27 | 2.31 ± 0.20 | 2.10 ± 0.22 |
| Dsf-Glucosinolates (mg g−1) | ||||||
| Glucoraphanin | Sinigrin | Glucoerucin | 4-Methoxy-Glucobrassicin | Glucobrassicin | Neoglucobrassicin | |
| CTRL | 12.10 ± 0.12 | - | 23.54 ± 1.32 | - | - | - |
| US | 13.26 ± 0.11 | - | 25.66 ± 1.05 | - | - | - |
| Sulforaphane (mg g−1) | ||||||
| CTRL | 9.33 ± 2.10 | |||||
| US | 50.15 ± 4.89 * | |||||
| Seed Treatment | Postharvest 10 h Illumination Photoperiod | Days at 5 °C | Gallic Acid | Caffeic Acid | Ferulic Acid | Coumaric Acid | Sinapic Acid | Total Phenolic Acids |
|---|---|---|---|---|---|---|---|---|
| CTRL | - | Harvest | 0.73 ± 0.16 | 0.90 ± 0.15 | 0.58 ± 0.12 | 0.65 ± 0.14 | 0.27 ± 0.02 | 3.14 ± 0.55 |
| US | 0.39 ± 0.08 | 0.46 ± 0.16 | 0.29 ± 0.09 | 0.51 ± 0.16 | 0.15 ± 0.05 | 1.80 ± 0.55 | ||
| CTRL | Darkness | 5 | 0.41 ± 0.07 | 0.33 ± 0.03 | 0.20 ± 0.01 | 0.54 ± 0.03 | 0.15 ± 0.01 | 1.63 ± 0.10 |
| 8 | 0.40 ± 0.07 | 0.30 ± 0.06 | 0.49 ± 0.09 | 0.68 ± 0.08 | 0.13 ± 0.01 | 2.00 ± 0.29 | ||
| 14 | 0.36 ± 0.02 | 0.22 ± 0.01 | 0.36 ± 0.05 | 0.73 ± 0.07 | 0.14 ± 0.01 | 1.80 ± 0.12 | ||
| White | 5 | 0.45 ± 0.07 | 0.44 ± 0.09 | 0.27 ± 0.06 | 0.65 ± 0.10 | 0.16 ± 0.01 | 1.98 ± 0.32 | |
| 8 | 0.52 ± 0.11 | 0.52 ± 0.06 | 0.59 ± 0.09 | 0.77 ± 0.06 | 0.15 ± 0.00 | 2.56 ± 0.32 | ||
| 14 | 0.34 ± 0.03 | 0.34 ± 0.05 | 0.56 ± 0.13 | 1.03 ± 0.03 | 0.18 ± 0.01 | 2.44 ± 0.19 | ||
| Blue | 5 | 0.45 ± 0.03 | 0.52 ± 0.05 | 0.32 ± 0.03 | 0.56 ± 0.06 | 0.15 ± 0.01 | 2.00 ± 0.16 | |
| 8 | 0.51 ± 0.09 | 0.56 ± 0.16 | 0.56 ± 0.14 | 0.79 ± 0.04 | 0.16 ± 0.02 | 2.58 ± 0.09 | ||
| 14 | 0.35 ± 0.05 | 0.29 ± 0.04 | 0.83 ± 0.03 | 0.97 ± 0.06 | 0.15 ± 0.01 | 2.59 ± 0.10 | ||
| Red | 5 | 0.42 ± 0.07 | 0.45 ± 0.10 | 0.33 ± 0.09 | 0.53 ± 0.07 | 0.14 ± 0.03 | 1.87 ± 0.36 | |
| 8 | 0.49 ± 0.05 | 0.59 ± 0.05 | 0.89 ± 0.18 | 0.87 ± 0.06 | 0.20 ± 0.01 | 3.04 ± 0.23 | ||
| 14 | 0.54 ± 0.08 | 0.61 ± 0.02 | 1.15 ± 0.14 | 1.09 ± 0.10 | 0.21 ± 0.03 | 3.59 ± 0.20 | ||
| US | Darkness | 5 | 0.47 ± 0.01 | 0.45 ± 0.04 | 0.29 ± 0.04 | 0.51 ± 0.05 | 0.14 ± 0.01 | 1.88 ± 0.07 |
| 8 | 0.44 ± 0.04 | 0.43 ± 0.03 | 0.59 ± 0.01 | 0.57 ± 0.05 | 0.20 ± 0.01 | 2.23 ± 0.06 | ||
| 14 | 0.43 ± 0.05 | 0.38 ± 0.04 | 0.48 ± 0.02 | 0.67 ± 0.06 | 0.16 ± 0.01 | 2.13 ± 0.14 | ||
| White | 5 | 0.48 ± 0.02 | 0.49 ± 0.07 | 0.26 ± 0.03 | 0.65 ± 0.11 | 0.15 ± 0.01 | 2.03 ± 0.23 | |
| 8 | 0.43 ± 0.05 | 0.37 ± 0.05 | 0.67 ± 0.14 | 0.82 ± 0.08 | 0.16 ± 0.02 | 2.44 ± 0.27 | ||
| 14 | 0.51 ± 0.10 | 0.59 ± 0.12 | 1.06 ± 0.12 | 0.84 ± 0.15 | 0.21 ± 0.05 | 3.21 ± 0.53 | ||
| Blue | 5 | 0.46 ± 0.03 | 0.48 ± 0.01 | 0.30 ± 0.02 | 0.59 ± 0.03 | 0.15 ± 0.01 | 1.98 ± 0.02 | |
| 8 | 0.39 ± 0.04 | 0.44 ± 0.01 | 0.56 ± 0.08 | 0.78 ± 0.09 | 0.16 ± 0.02 | 2.34 ± 0.20 | ||
| 14 | 0.56 ± 0.15 | 0.63 ± 0.18 | 1.36 ± 0.16 | 1.09 ± 0.11 | 0.23 ± 0.01 | 3.97 ± 0.30 | ||
| Red | 5 | 0.43 ± 0.02 | 0.51 ± 0.02 | 0.36 ± 0.01 | 0.57 ± 0.04 | 0.14 ± 0.01 | 2.02 ± 0.06 | |
| 8 | 0.60 ± 0.04 | 0.73 ± 0.06 | 0.53 ± 0.04 | 0.90 ± 0.04 | 0.18 ± 0.02 | 2.89 ± 0.19 | ||
| 14 | 0.48 ± 0.04 | 0.54 ± 0.07 | 0.86 ± 0.12 | 0.86 ± 0.18 | 0.18 ± 0.02 | 2.93 ± 0.13 |
| Seed Treatment | Postharvest 10 h Illumination Photoperiod | Days at 5 °C | Kaempferol Derivative-1 | Kaempferol Derivative-2 | Kaempferol Derivative-3 | Rutin | Quercetin | Quercetin Derivative | Total Flavonoids |
|---|---|---|---|---|---|---|---|---|---|
| CTRL | - | Harvest | 0.31 ± 0.03 | 0.87 ± 0.09 | 0.66 ± 0.08 | 0.39 ± 0.03 | 1.90 ± 0.20 | 0.35 ± 0.04 | 4.47 ± 0.48 |
| US | 0.20 ± 0.06 | 0.59 ± 0.09 | 0.44 ± 0.13 | 0.29 ± 0.01 | 1.25 ± 0.38 | 0.21 ± 0.07 | 2.97 ± 0.84 | ||
| CTRL | Darkness | 5 | 0.19 ± 0.00 | 0.59 ± 0.04 | 0.45 ± 0.02 | 0.24 ± 0.01 | 1.46 ± 0.11 | 0.22 ± 0.00 | 3.14 ± 0.18 |
| 8 | 0.18 ± 0.01 | 0.59 ± 0.09 | 0.41 ± 0.03 | 0.28 ± 0.04 | 1.58 ± 0.28 | 0.22 ± 0.02 | 3.26 ± 0.48 | ||
| 14 | 0.17 ± 0.01 | 0.51 ± 0.04 | 0.36 ± 0.02 | 0.21 ± 0.03 | 1.33 ± 0.07 | 0.21 ± 0.01 | 2.78 ± 0.17 | ||
| White | 5 | 0.22 ± 0.01 | 0.69 ± 0.09 | 0.54 ± 0.06 | 0.28 ± 0.02 | 1.70 ± 0.27 | 0.25 ± 0.02 | 3.67 ± 0.47 | |
| 8 | 0.20 ± 0.01 | 0.76 ± 0.03 | 0.48 ± 0.03 | 0.30 ± 0.02 | 1.98 ± 0.15 | 0.26 ± 0.01 | 3.98 ± 0.23 | ||
| 14 | 0.21 ± 0.01 | 0.64 ± 0.05 | 0.44 ± 0.04 | 0.34 ± 0.05 | 1.71 ± 0.15 | 0.25 ± 0.01 | 3.59 ± 0.29 | ||
| Blue | 5 | 0.19 ± 0.01 | 0.65 ± 0.06 | 0.52 ± 0.05 | 0.26 ± 0.02 | 1.67 ± 0.17 | 0.23 ± 0.02 | 3.53 ± 0.32 | |
| 8 | 0.21 ± 0.02 | 0.72 ± 0.05 | 0.46 ± 0.04 | 0.35 ± 0.02 | 1.96 ± 0.17 | 0.25 ± 0.02 | 3.95 ± 0.30 | ||
| 14 | 0.18 ± 0.01 | 0.56 ± 0.02 | 0.38 ± 0.01 | 0.34 ± 0.03 | 1.54 ± 0.10 | 0.23 ± 0.00 | 3.22 ± 0.16 | ||
| Red | 5 | 0.18 ± 0.03 | 0.60 ± 0.11 | 0.40 ± 0.12 | 0.26 ± 0.03 | 1.44 ± 0.13 | 0.21 ± 0.02 | 3.10 ± 0.62 | |
| 8 | 0.26 ± 0.01 | 0.92 ± 0.05 | 0.57 ± 0.02 | 0.46 ± 0.00 | 2.35 ± 0.09 | 0.35 ± 0.05 | 4.89 ± 0.16 | ||
| 14 | 0.26 ± 0.02 | 0.84 ± 0.05 | 0.57 ± 0.05 | 0.51 ± 0.07 | 2.41 ± 0.09 | 0.33 ± 0.02 | 4.91 ± 0.28 | ||
| US | Darkness | 5 | 0.20 ± 0.01 | 0.64 ± 0.04 | 0.50 ± 0.04 | 0.24 ± 0.02 | 1.59 ± 0.15 | 0.22 ± 0.01 | 3.39 ± 0.25 |
| 8 | 0.25 ± 0.01 | 0.74 ± 0.02 | 0.49 ± 0.01 | 0.34 ± 0.02 | 1.82 ± 0.08 | 0.29 ± 0.00 | 3.94 ± 0.11 | ||
| 14 | 0.19 ± 0.01 | 0.60 ± 0.01 | 0.41 ± 0.01 | 0.30 ± 0.01 | 1.68 ± 0.04 | 0.24 ± 0.01 | 3.40 ± 0.08 | ||
| White | 5 | 0.21 ± 0.02 | 0.68 ± 0.05 | 0.51 ± 0.01 | 0.28 ± 0.01 | 1.69 ± 0.14 | 0.24 ± 0.01 | 3.61 ± 0.21 | |
| 8 | 0.21 ± 0.01 | 0.72 ± 0.04 | 0.45 ± 0.05 | 0.38 ± 0.03 | 1.90 ± 0.12 | 0.26 ± 0.06 | 3.93 ± 0.25 | ||
| 14 | 0.25 ± 0.05 | 0.78 ± 0.14 | 0.48 ± 0.09 | 0.48 ± 0.08 | 2.12 ± 0.32 | 0.31 ± 0.01 | 4.43 ± 0.74 | ||
| Blue | 5 | 0.19 ± 0.00 | 0.63 ± 0.06 | 0.44 ± 0.01 | 0.26 ± 0.01 | 1.59 ± 0.12 | 0.23 ± 0.01 | 3.34 ± 0.20 | |
| 8 | 0.22 ± 0.01 | 0.70 ± 0.04 | 0.44 ± 0.02 | 0.36 ± 0.02 | 1.78 ± 0.07 | 0.26 ± 0.01 | 3.75 ± 0.18 | ||
| 14 | 0.29 ± 0.01 | 0.91 ± 0.05 | 0.60 ± 0.02 | 0.58 ± 0.01 | 2.48 ± 0.08 | 0.35 ± 0.01 | 5.20 ± 0.18 | ||
| Red | 5 | 0.20 ± 0.01 | 0.66 ± 0.03 | 0.43 ± 0.02 | 0.28 ± 0.02 | 1.63 ± 0.09 | 0.23 ± 0.01 | 3.42 ± 0.16 | |
| 8 | 0.24 ± 0.01 | 0.87 ± 0.03 | 0.54 ± 0.02 | 0.38 ± 0.01 | 2.27 ± 0.05 | 0.29 ± 0.01 | 4.58 ± 0.13 | ||
| 14 | 0.24 ± 0.02 | 0.78 ± 0.10 | 0.50 ± 0.07 | 0.52 ± 0.10 | 2.27 ± 0.22 | 0.31 ± 0.03 | 4.62 ± 0.84 |
| T | L | t | T × L | T × t | L × t | T × L × t | |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| Gallic acid | (0.03) ** | ns. | (0.05) *** | ns | (0.07) *** | ns | ns |
| Caffeic acid | (0.04) * | (0.06) *** | (0.06) *** | ns | (0.08) *** | (0.12) * | (0.16) ** |
| Ferulic acid | ns | (0.06) *** | (0.06) *** | (0.08) *** | (0.08) *** | (0.11) *** | (0.16) *** |
| Coumaric acid | (0.04) ** | (0.06) *** | (0.06) *** | ns | (0.08) * | (0.12) ** | ns |
| Sinapic acid | (0.01) *** | ns | (0.01) *** | ns | (0.02) *** | ns | ns |
| Total | (0.17) * | (0.25) *** | (0.25) *** | ns | (0.35) *** | ns | n.s |
| Flavonoids | |||||||
| Kaempferol derivative-1 | (0.01) * | (0.02) * | (0.02) *** | ns | (0.02) *** | (0.03) * | ns |
| Kaempferol derivative-2 | ns | (0.05) *** | (0.05) ** | ns | (0.08) *** | (0.11) * | ns |
| Kaempferol derivative-3 | (0.03) ** | ns | (0.04) *** | ns | (0.06) *** | (0.08) ** | ns |
| Rutin | ns | (0.02) *** | (0.02) *** | (0.03) ** | (0.03) *** | (0.04) *** | (0.06) *** |
| Quercetin | ns | (0.13) *** | (0.13) *** | ns | (0.18) *** | (0.26) *** | (0.36) * |
| Quercetin derivative | (0.01) ** | (0.02) ** | (0.02) *** | ns | (0.03) *** | (0.04) ** | ns |
| Total | (0.12) ** | (0.17) *** | (0.17) *** | (0.24) * | (0.24) *** | (0.34) *** | (0.49) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts. Antioxidants 2022, 11, 1490. https://doi.org/10.3390/antiox11081490
Martínez-Zamora L, Castillejo N, Artés-Hernández F. Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts. Antioxidants. 2022; 11(8):1490. https://doi.org/10.3390/antiox11081490
Chicago/Turabian StyleMartínez-Zamora, Lorena, Noelia Castillejo, and Francisco Artés-Hernández. 2022. "Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts" Antioxidants 11, no. 8: 1490. https://doi.org/10.3390/antiox11081490
APA StyleMartínez-Zamora, L., Castillejo, N., & Artés-Hernández, F. (2022). Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts. Antioxidants, 11(8), 1490. https://doi.org/10.3390/antiox11081490