Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens?
Abstract
:1. Introduction
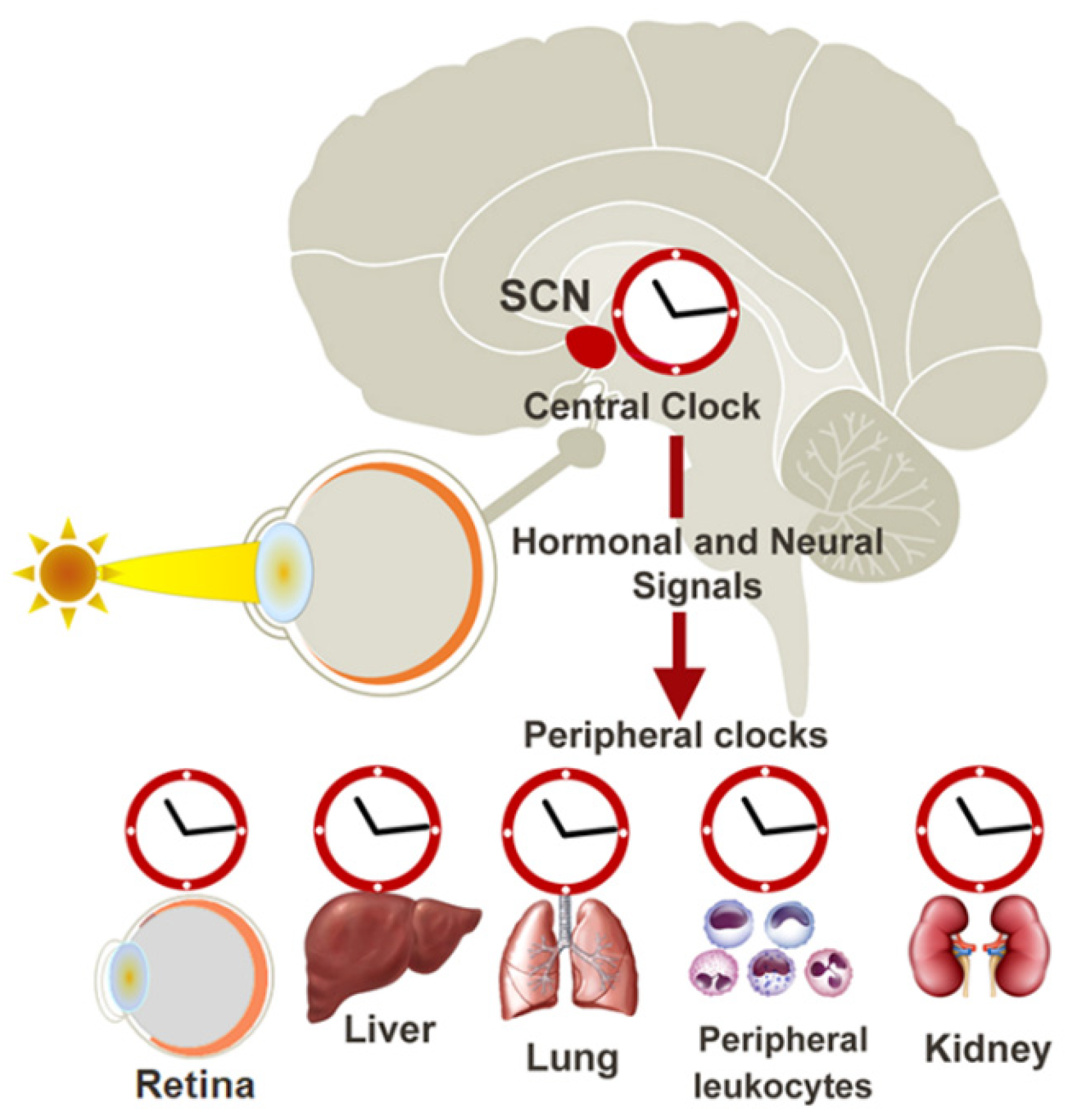
2. The Circadian Clock
3. The Molecular Clock
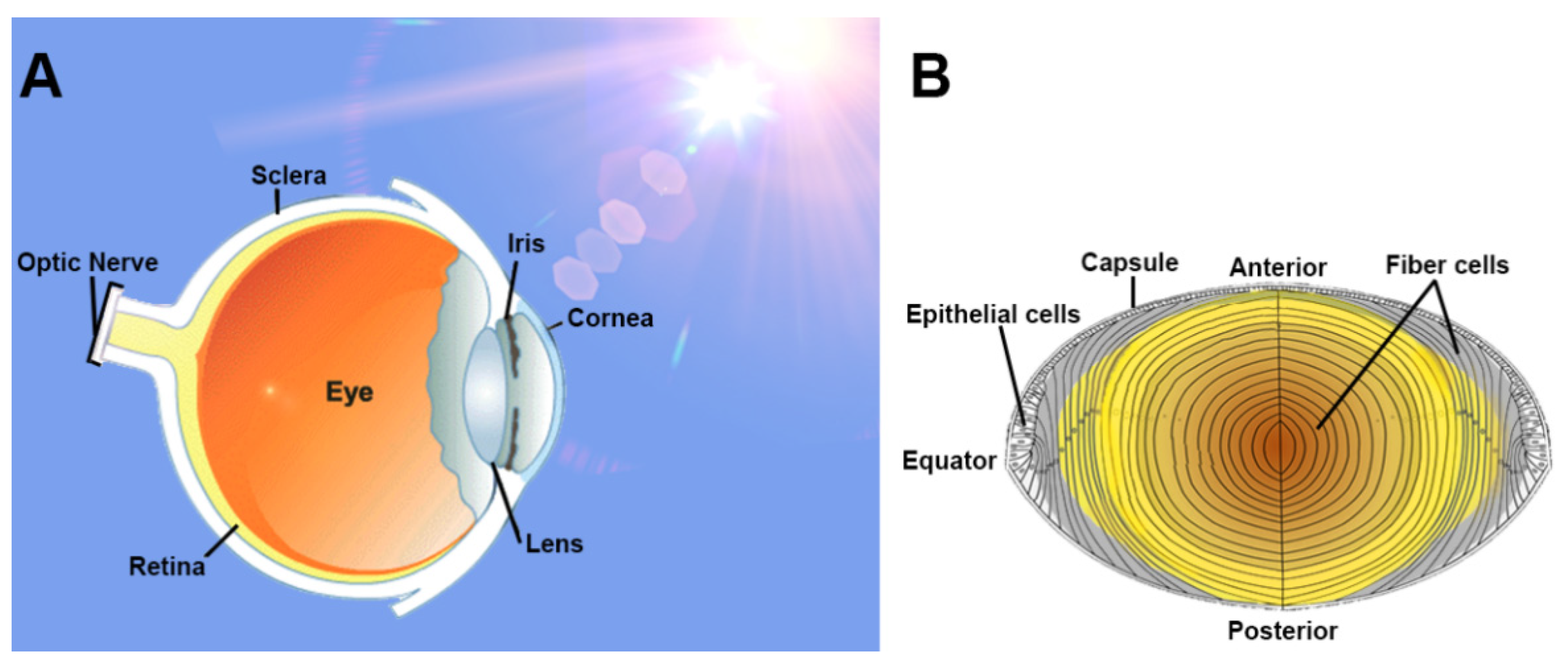
4. The Lens: Structure and Function
5. Evidence of the Molecular Clock in the Lens and Its Importance in Lens Transparency
6. The Importance of Redox Balance in the Lens
7. Evidence of Circadian Regulation of Redox Homeostasis in Non-Ocular Tissues
8. Evidence of Circadian Regulation of Redox Homeostasis in the Lens
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, A.W.; Maldonado, M.; Hidalgo, M.S.; Tan, D.-X.; Reiter, R.J. Protective effects of melatonin in experimental free radical-related ocular diseases. J. Pineal Res. 2006, 40, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Felder-Schmittbuhl, M.-P.; Buhr, E.D.; Dkhissi-Benyahya, O.; Hicks, D.; Peirson, S.N.; Ribelayga, C.P.; Sandu, C.; Spessert, R.; Tosini, G. Ocular Clocks: Adapting Mechanisms for Eye Functions and Health. Investig. Opthalmol. Vis. Sci. 2018, 59, 4856–4870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giblin, F.J. Glutathione: A Vital Lens Antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- Reddy, V.; Giblin, F.J. Metabolism and Function of Glutathione in the Lens. Ciba Found. Symp. 1984, 106, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-related nuclear cataract—Oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Michael, R.; Bron, A.J. The ageing lens and cataract: A model of normal and pathological ageing. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1278–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.-J.; Taylor, A. Nutritional antioxidants and age-related cataract and maculopathy. Exp. Eye Res. 2007, 84, 229–245. [Google Scholar] [CrossRef]
- Thrimawithana, T.R.; Rupenthal, I.D.; Räsch, S.S.; Lim, J.C.; Morton, J.D.; Bunt, C.R. Drug delivery to the lens for the management of cataracts. Adv. Drug Deliv. Rev. 2018, 126, 185–194. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative Protection by Melatonin: Multiplicity of Mechanisms from Radical Detoxification to Radical Avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Stangherlin, A.; Reddy, A.B. Regulation of Circadian Clocks by Redox Homeostasis. J. Biol. Chem. 2013, 288, 26505–26511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian Rhythm Connections to Oxidative Stress: Implications for Human Health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.; Jan, J.E.; Lyons, C.J. Light, dark, and melatonin: Emerging evidence for the importance of melatonin in ocular physiology. Eye 2006, 21, 901–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A Novel Human Opsin in the Inner Retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2016, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akashi, M.; Tsuchiya, Y.; Yoshino, T.; Nishida, E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol. Cell. Biol. 2002, 22, 1693–1703. [Google Scholar] [CrossRef] [Green Version]
- Eide, E.J.; Vielhaber, E.L.; Hinz, W.A.; Virshup, D.M. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J. Biol. Chem. 2002, 277, 17248–17254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, M.; Virshup, D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.; Zhou, W.; Santana, J.; Kyriakides, C.; Velazquez, H.; Sessa, W.C. CLOCK phosphorylation by AKT regulates its nuclear accumulation and circadian gene expression in peripheral tissues. J. Biol. Chem. 2018, 293, 9126–9136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, J.; Sahar, S.; Grimaldi, B.; Tamaru, T.; Takamatsu, K.; Nakahata, Y.; Sassone-Corsi, P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007, 450, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.O.; Izawa, J.; Nishimura, A.; Hattori, A.; Suzuki, N.; Hirayama, J. Post-translational Modifications are Required for Circadian Clock Regulation in Vertebrates. Curr. Genom. 2019, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Lange, T.; Golombek, D.; Sarkar, D.; Nakao, A.; Shibata, S.; Mazzoccoli, G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol. Int. 2013, 30, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Coto-Montes, A.; Poeggeler, B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol. Int. 2003, 20, 921–962. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, I.; Ripperger, J.A.; Baeriswyl-Aebischer, S.; Albrecht, U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010, 24, 345–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crumbley, C.; Burris, T.P. Direct Regulation of CLOCK Expression by REV-ERB. PLoS ONE 2011, 6, e17290. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Molina, L.L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Brubaker, R.F. Measurement of aqueous flow by fluorophotometry. In The Glaucomas; Ritch, R., Shields, M.B., Krupin, T., Eds.; Mosby: St. Louis, MO, USA, 1989; pp. 337–344. [Google Scholar]
- Bassnett, S.; Shi, Y.; Vrensen, G.F.J.M. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAvoy, J.W.; Chamberlain, C.G.; de Iongh, R.U.; Hales, A.M.; Lovicu, F.J. Lens development. Eye Lond. 1999, 13, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Bassnett, S.; Šikić, H. The lens growth process. Prog. Retin. Eye Res. 2017, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Michael, R.; van Marle, J.; Vrensen, G.F.; Berg, T.J.V.D. Changes in the refractive index of lens fibre membranes during maturation—Impact on lens transparency. Exp. Eye Res. 2003, 77, 93–99. [Google Scholar] [CrossRef]
- Bassnett, S.; Beebe, D.C. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev. Dyn. 1992, 194, 85–93. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, Y.V.; Samsa, W.E.; Kondratov, R.V. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging 2010, 2, 936–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant Defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Umapathy, A.; Donaldson, P.; Lim, J. Antioxidant Delivery Pathways in the Anterior Eye. BioMed Res. Int. 2013, 2013, 207250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.-S.; Lee, M.-H.; Lee, S.-H.; Bae, K. Cu/Zn superoxide dismutase is differentially regulated in period gene-mutant mice. Biochem. Biophys. Res. Commun. 2011, 409, 22–27. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Strafella, E.; Staffolani, S.; Ciarapica, V.; Copertaro, A.; Rapisarda, V.; Ledda, C.; Amati, M.; Valentino, M.; et al. Circadian Modulation of 8-Oxoguanine DNA Damage Repair. Sci. Rep. 2015, 5, 13752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, B.P.; Davies, M.H.; Schnell, R.C. Circadian variations in hepatic glutathione content, gamma-glutamylcysteine synthetase and gamma-glutamyl transferase activities in mice. Toxicol. Lett. 1987, 35, 217–223. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zhang, D.; Jin, T.; Cai, D.-J.; Wu, Q.; Lu, Y.; Liu, J.; Klaassen, C.D. Diurnal Variation of Hepatic Antioxidant Gene Expression in Mice. PLoS ONE 2012, 7, e44237. [Google Scholar] [CrossRef]
- Radha, E.; Hill, T.D.; Rao, G.H.; White, J.G. Glutathione levels in human platelets display a circadian rhythm in vitro. Thromb. Res. 1985, 40, 823–831. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Rozin, T. Diurnal variability of cysteine and glutathione content in the pancreas and liver of the mouse. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 114, 91–95. [Google Scholar] [CrossRef]
- Díaz-Muñoz, M.; Hernández-Muñoz, R.; Suárez, J.; de Sánchez, V.C. Day-night cycle of lipid peroxidation in rat cerebral cortex and their relationship to the glutathione cycle and superoxide dismutase activity. Neuroscience 1985, 16, 859–863. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, I.T.; Rezza, I.G.; Delgado, S.M.; Navigatore, L.S.; Bonomi, M.R.; Golini, R.L.; Gimenez, M.S.; Anzulovich, A. Daily oscillation of glutathione redox cycle is dampened in the nutritional vitamin A deficiency. Biol. Rhythm Res. 2012, 43, 351–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zeng, C.; Du, L.; Dong, C. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ische-mia-reperfusion. Exp. Ther. Med. 2021, 21, 190. [Google Scholar] [CrossRef]
- Ait-Hmyed Hakkari, O.; Acar, N.; Savier, E.; Spinnhirny, P.; Bennis, M.; Felder-Schmittbuhl, M.P.; Mendoza, J.; Hicks, D. Rev-Erbalpha modulates retinal visual processing and behavioral responses to light. FASEB J. 2016, 30, 3690–3701. [Google Scholar] [CrossRef] [Green Version]
- Ruan, G.X.; Allen, G.C.; Yamazaki, S.; McMahon, D.G. An autonomous circadian clock in the inner mouse retina regu-lated by dopamine and GABA. PLoS Biol. 2008, 6, e249. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Investig. Ophthalmol. Vis. Sci. 1978, 17, 105–116. [Google Scholar]
- Baba, K.; Ribelayga, C.P.; Iuvone, P.M.; Tosini, G. The Retinal Circadian Clock and Photoreceptor Viability. Adv. Exp. Med. Biol. 2018, 1074, 345–350. [Google Scholar] [CrossRef]
- Reme´, C.; Wirz-Justice, A.; Rhyner, A.; Hofmann, S. Circadian rhythm in the light response of rat retinal disk-shedding and autophagy. Brain Res. 1986, 369, 356–360. [Google Scholar] [CrossRef]
- Du Toit, R.; Vega, J.A.; Fonn, D.; Simpson, T. Diurnal Variation of Corneal Sensitivity and Thickness. Cornea 2003, 22, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, M.J.; Sande, P.H.; Bernades, J.M.; Aba, M.A.; Rosenstein, R.E. Circadian rhythm of intraocular pressure in cats. Veter- Ophthalmol. 2007, 10, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, H.; Shahab, U.; Rehman, S.; Rafi, Z.; Khan, M.Y.; Ansari, A.; Siddiqui, Z.; Ashraf, J.M.; Abdullah, S.M.S.; et al. Protein oxidation an overview of metabolism of sulphur containing amino acid cysteine. Front. Biosci. 2017, 9, 71–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Ballatori, N. Endogenous glutathione conjugates: Occurrence and biological functions. Pharmacol. Rev. 1998, 50, 335–356. [Google Scholar] [PubMed]
- Sasaki, H.; Giblin, F.J.; Winkler, B.S.; Chakrapani, B.; Leverenz, V.; Shu, C.C. A protective role for glutathi-one-dependent reduction of dehydroascorbic acid in lens epithelium. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1804–1817. [Google Scholar]
- Harding, J.J. Free and protein-bound glutathione in normal and cataractous human lenses. Biochem. J. 1970, 117, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.F.; Dickerson, J.E.; Garadi, R. The role of protein-thiol mixed disulfides in cataractogenesis. Exp. Eye Res. 1990, 50, 819–826. [Google Scholar] [CrossRef]
- Sweeney, M.H.; Truscott, R.J. An Impediment to Glutathione Diffusion in Older Normal Human Lenses: A Possible Precondition for Nuclear Cataract. Exp. Eye Res. 1998, 67, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.H.; Spector, A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc. Natl. Acad. Sci. USA 1980, 77, 1274–1277. [Google Scholar] [CrossRef] [Green Version]
- Truscott, R.; Augusteyn, R. The state of sulphydryl groups in normal and cataractous human lenses. Exp. Eye Res. 1977, 25, 139–148. [Google Scholar] [CrossRef]
- Spector, A.; Roy, D. Disulfide-linked high molecular weight protein associated with human cataract. Proc. Natl. Acad. Sci. USA 1978, 75, 3244–3248. [Google Scholar] [CrossRef] [Green Version]
- Brubaker, R.F. Flow of aqueous humor in humans [The Friedenwald Lecture]. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3145–3166. [Google Scholar]
- Abe, M.; Itoh, M.T.; Miyata, M.; Ishikawa, S.; Sumi, Y. Detection of Melatonin, Its Precursors and Related Enzyme Activities in Rabbit Lens. Exp. Eye Res. 1999, 68, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Itoh, M.T.; Miyata, M.; Shimizu, K.; Sumi, Y. Circadian rhythm of serotonin N -acetyltransferase activity in rat lens. Exp. Eye Res. 2000, 70, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Alkozi, H.A.; Wang, X.; de Lara, M.J.P.; Pintor, J. Presence of melanopsin in human crystalline lens epithelial cells and its role in melatonin synthesis. Exp. Eye Res. 2017, 154, 168–176. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Nakamura, W.; Yamazaki, S.; Kudo, T.; Cutler, T.; Colwell, C.S.; Block, G.D. Age-Related Decline in Circadian Output. J. Neurosci. 2011, 31, 10201–10205. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Penev, P.; Zhang, Y.; van Reeth, O.; Zee, P. Effects of age on the circadian system. Neurosci. Biobehav. Rev. 1995, 19, 53–58. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.C.; Suzuki-Kerr, H.; Nguyen, T.X.; Lim, C.J.J.; Poulsen, R.C. Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens? Antioxidants 2022, 11, 1516. https://doi.org/10.3390/antiox11081516
Lim JC, Suzuki-Kerr H, Nguyen TX, Lim CJJ, Poulsen RC. Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens? Antioxidants. 2022; 11(8):1516. https://doi.org/10.3390/antiox11081516
Chicago/Turabian StyleLim, Julie C., Haruna Suzuki-Kerr, Tai X. Nguyen, Christopher J. J. Lim, and Raewyn C. Poulsen. 2022. "Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens?" Antioxidants 11, no. 8: 1516. https://doi.org/10.3390/antiox11081516
APA StyleLim, J. C., Suzuki-Kerr, H., Nguyen, T. X., Lim, C. J. J., & Poulsen, R. C. (2022). Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens? Antioxidants, 11(8), 1516. https://doi.org/10.3390/antiox11081516





