Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Process—Inclusion and Exclusion Criteria
- Patients: insulin-resistant animal models or cell lines.
- Intervention: administration of OA.
- Comparison: control substances and/or another bioactive compound.
- Outcomes: signaling pathways that are altered in states of IR and inflammatory and OS biomarkers (proinflammatory cytokines, antioxidant enzymes, transcription factors, and ROS).
- Study type: experimental studies.
- Narrative and systematic reviews, letter to editor, book chapter, and other kinds of secondary studies.
- Studies that are focused on the extraction of bioactive compounds from natural plants and on the analysis of their biological activities without a more in-depth explanation of the molecular mechanisms implied.
- Studies that are focused on treating comorbidities or short-term or long-term complications of T2DM, without the approach of analyzing the effects of OA on signaling pathways involved in the development of IR.
- Administration of OA derivatives or OA combined with another bioactive compound.
- Non-English studies.
2.3. Data Extraction
2.4. Study Risk of Bias Assessment
3. Results
3.1. Study Selection
3.2. Characteristics of the Cell Experiments Selected
3.3. Characteristics of the Animal Studies Selected
3.4. Reporting Risk of Bias Assessment
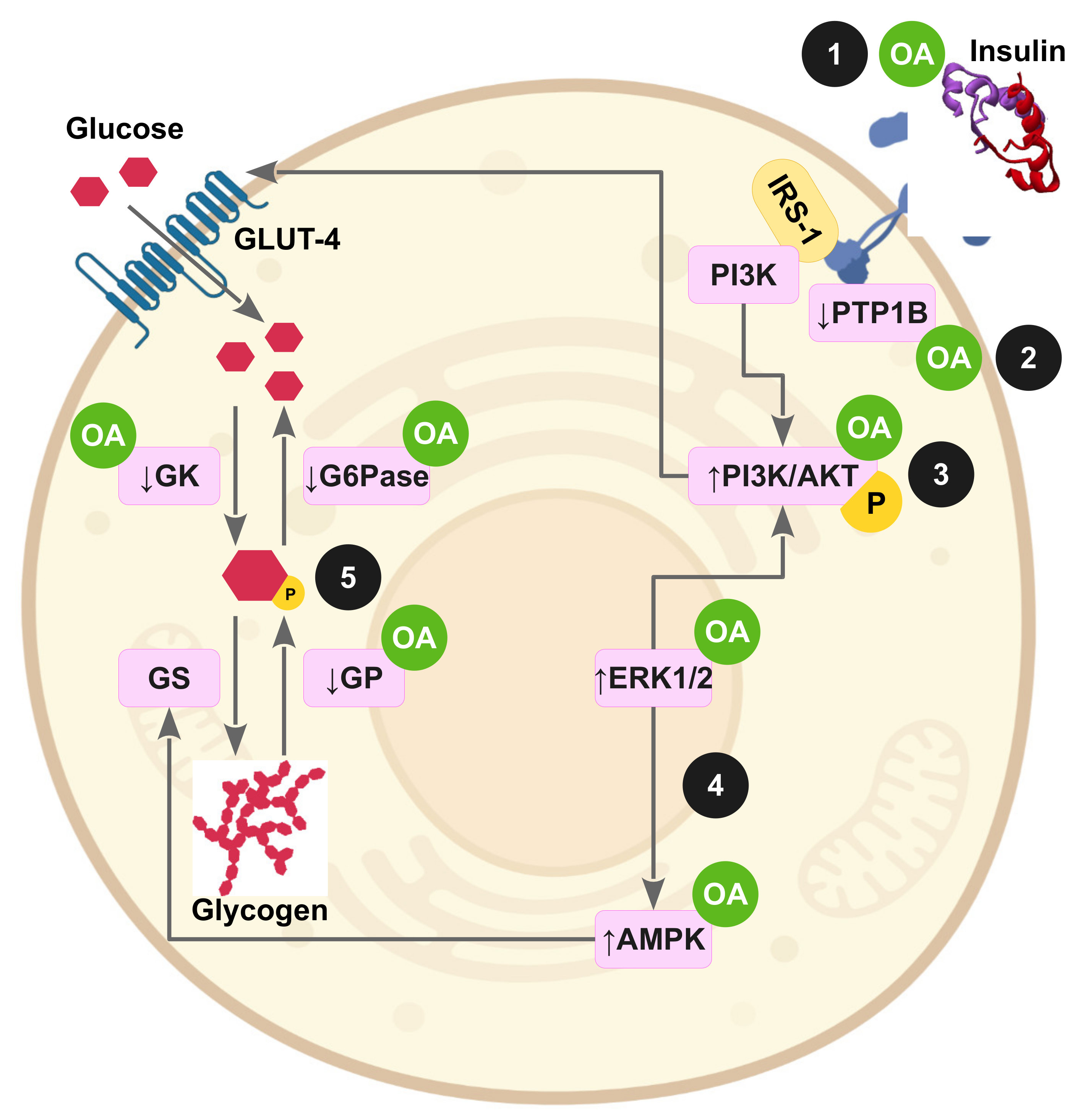
3.5. OA Effects in Insulin-Resistant Cell Lines
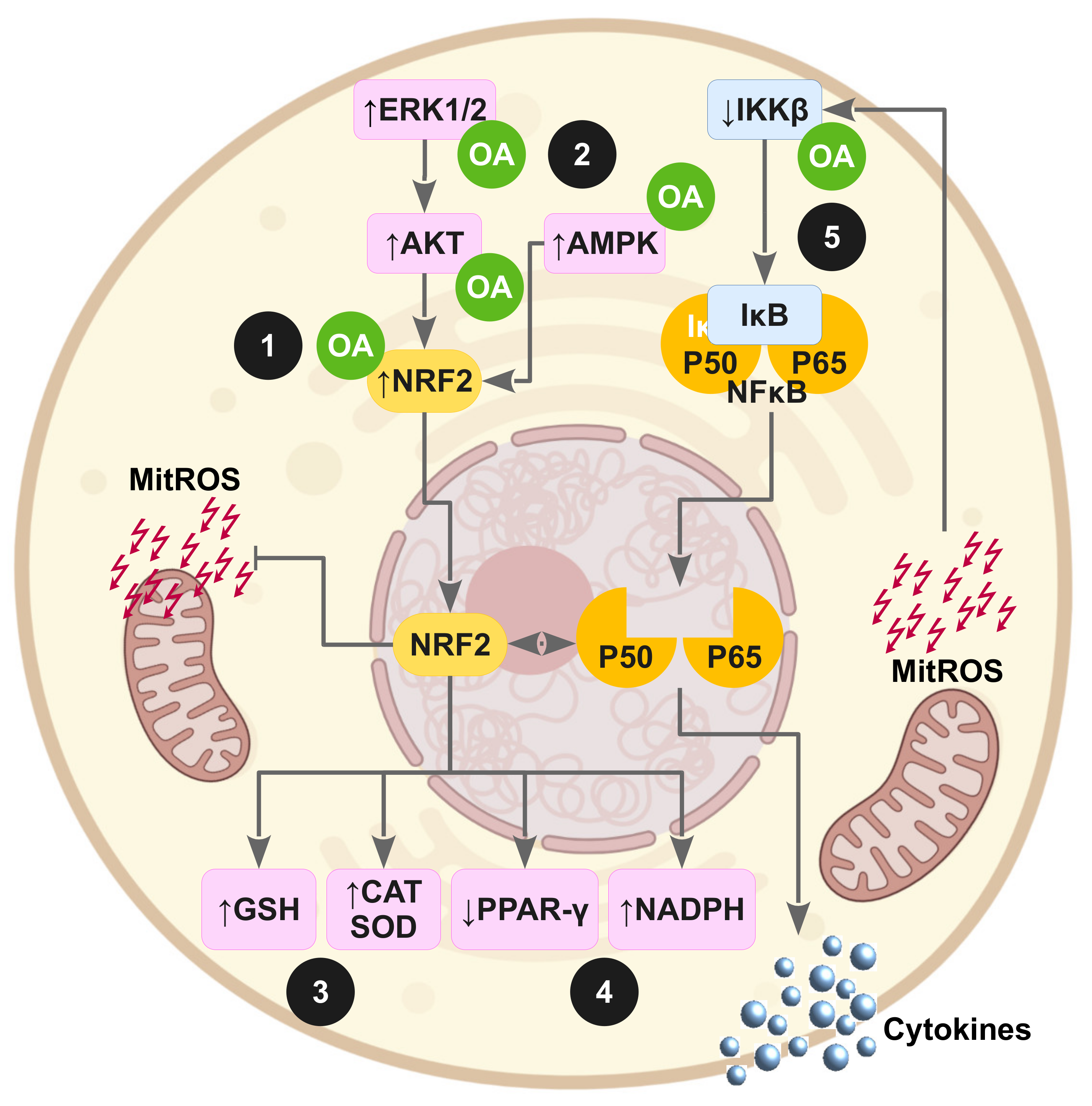
3.6. OA Effects on Impaired Signaling Pathways and on OS in Insulin-Resistant Animal Models
3.6.1. Hepatic IR
3.6.2. IR in Adipose Tissue and Skeletal Muscle
3.6.3. OA Effects on Proinflammatory Cytokines and OS Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Seventy-Fourth World Health Assembly. Reducing the Burden of Noncommunicable Diseases through Strengthening Prevention and Control of Diabetes; World Health Organization: Geneva, Switzerland, 2021; pp. 1–6. [Google Scholar]
- American Diabetes Association Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, S.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z.Q. The PI3K/AKT Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and Insulin Resistance: Pathophysiology and Treatment. Drug Discov. Today 2022, 27, 822–830. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the Underlying Defect in Insulin Action in Type 2 Diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The Role of JNk Signaling Pathway in Obesity-Driven Insulin Resistance. Diabetes Metab. Syndr. Obes. 2020, 13, 1399–1406. [Google Scholar] [CrossRef]
- Nandipati, K.C.; Subramanian, S.; Agrawal, D.K. Protein Kinases: Mechanisms and Downstream Targets in Inflammation-Mediated Obesity and Insulin Resistance. Mol. Cell. Biochem. 2017, 426, 27–45. [Google Scholar] [CrossRef]
- Ou, Y.; Zheng, Z.; Niu, B.; Su, J.; Su, H. Different MAPK Signal Transduction Pathways Play Different Roles in the Impairment of Glucose-stimulated Insulin Secretion in Response to IL-1β. Mol. Med. Rep. 2020, 22, 2973–2980. [Google Scholar] [CrossRef]
- Lima, J.E.B.F.; Moreira, N.C.S.; Sakamoto-Hojo, E.T. Mechanisms Underlying the Pathophysiology of Type 2 Diabetes: From Risk Factors to Oxidative Stress, Metabolic Dysfunction, and Hyperglycemia. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 874–875, 503437. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The Molecular Link between Oxidative Stress, Insulin Resistance, and Type 2 Diabetes: A Target for New Therapies against Cardiovascular Diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Graham, E.J.; Adler, F.R. Long-Term Models of Oxidative Stress and Mitochondrial Damage in Insulin Resistance Progression. J. Theor. Biol. 2014, 340, 238–250. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2021, 11, 79. [Google Scholar] [CrossRef]
- Morelli, N.R.; Scavuzzi, B.M.; Miglioranza, L.H.; Lozovoy, M.A.B.; Simão, A.N.C.; Dichi, I. Metabolic Syndrome Components Are Associated with Oxidative Stress in Overweight and Obese Patients. Arch. Endocrinol. Metab. 2018, 62, 309–318. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic Triterpenes: New Tools to Fight Metabolic Syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; de Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients 2020, 12, 323. [Google Scholar] [CrossRef]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of Type 2 Diabetes in Prediabetic Patients by Using Functional Olive Oil Enriched in Oleanolic Acid: The PREDIABOLE Study, a Randomized Controlled Trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef]
- Claro-Cala, C.M.; Jiménez-Altayó, F.; Zagmutt, S.; Rodriguez-Rodriguez, R. Molecular Mechanisms Underlying the Effects of Olive Oil Triterpenic Acids in Obesity and Related Diseases. Nutrients 2022, 14, 1606. [Google Scholar] [CrossRef]
- Ayeleso, T.; Matumba, M.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Sen, A. Prophylactic and Therapeutic Roles of Oleanolic Acid Its Derivatives in Several Diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, L.-J.; Fan, Y.-S.; Chen, Y.; Li, Q. Network Pharmacology-Based Analysis on the Action Mechanism of Oleanolic Acid to Alleviate Osteoporosis. ACS Omega 2021, 6, 28410–28420. [Google Scholar] [CrossRef]
- Fernández-Aparicio, Á.; Schmidt-RioValle, J.; Perona, J.S.; Correa-Rodríguez, M.; Castellano, J.M.; González-Jiménez, E. Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. J. Clin. Med. 2019, 8, 1294. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLOS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.L.; Liu, J.Z.; Liao, N.; Yu, W.H.; Zhang, X.D.; Zhang, T.; Li, W.L.; Hai, C.X. Protective Effect of Oleanolic Acid against Beta Cell Dysfunction and Mitochondrial Apoptosis: Crucial Role of ERK-NRF2 Signaling Pathway. J. Biol. Regul. Homeost. Agents 2013, 27, 55–67. [Google Scholar] [PubMed]
- Xie, Z.K.; Yu, S.Y.; He, M.; Yu, S.X.; Xiao, H.F.; Song, Y.D. Inhibitory Effect of Oleanolic Acid on Non-Enzymatic Glycation and Glycometabolism in Insulin Resistant HepG2 Cells. Acta Aliment. 2021, 50, 112–124. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Terim Kapakin, K.A.; Yenice, G.; Mohtare, B.; Bolat, I.; Hayirli, A. Effects of Oleanolic Acid on Inflammation and Metabolism in Diabetic Rats. Biotech. Histochem. 2022, 97, 269–276. [Google Scholar] [CrossRef]
- Kim, H.-S.; Han, S.-Y.; Sung, H.-Y.; Park, S.-H.; Kang, M.-K.; Han, S.-J.; Kang, Y.-H. Blockade of Visfatin Induction by Oleanolic Acid via Disturbing IL-6-TRAF6-NF-ΚB Signaling of Adipocytes. Exp. Biol. Med. 2014, 239, 284–292. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, S.; Gu, J.; Min, Z.; Wang, R. Effect of Nano-Oleanolic Acid Combined with Lipid-Lowering Ketones on Insulin Resistance in Rats with Gestational Diabetes. J. Biomed. Nanotechnol. 2022, 18, 474–480. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.L.; Wu, H.; Liu, J.Z.; Hu, J.X.; Liao, N.; Peng, J.; Cao, P.P.; Liang, X.; Hai, C.X. Antidiabetic Effect of Oleanolic Acid: A Promising Use of a Traditional Pharmacological Agent. Phyther. Res. 2011, 25, 1031–1040. [Google Scholar] [CrossRef]
- Li, M.; Han, Z.; Bei, W.; Rong, X.; Guo, J.; Hu, X. Oleanolic Acid Attenuates Insulin Resistance via NF-κ B to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 643102. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, Z.; Xu, M.-Z.; Zeng, Z.; Huang, J.; Guan, Y.-Q. Natural Plant-Derived Polygalacturonic Acid-Oleanolic Acid Assemblies as Oral-Delivered Nanomedicine for Insulin Resistance Treatment. Chem. Eng. J. 2020, 390, 124630. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Abdelkader, D.; Hassan, W.; Sun, H.; Liu, J. Combination Therapy with Oleanolic Acid and Metformin as a Synergistic Treatment for Diabetes. J. Diabetes Res. 2015, 2015, 973287. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic Acid Improves Hepatic Insulin Resistance via Antioxidant, Hypolipidemic and Anti-Inflammatory Effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Wang, Y.P.; Cantley, J.; Iseli, T.J.; Molero, J.C.; Hegarty, B.D.; Kraegen, E.W.; Ye, Y.; Ye, J.M. Oleanolic Acid Reduces Hyperglycemia beyond Treatment Period with Akt/FoxO1-Induced Suppression of Hepatic Gluconeogenesis in Type-2 Diabetic Mice. PLoS ONE 2012, 7, e42115. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Zeng, X.-Y.; Wang, H.; Li, S.; Jo, E.; Xue, C.C.L.; Tan, M.; Molero, J.C.; Ye, J.-M. Hepatic FoxO1 Acetylation Is Involved in Oleanolic Acid-Induced Memory of Glycemic Control: Novel Findings from Study 2. PLoS ONE 2014, 9, e107231. [Google Scholar] [CrossRef]
- Yunoki, K.; Sasaki, G.; Tokuji, Y.; Kinoshita, M.; Naito, A.; Aida, K.; Ohnishi, M. Effect of Dietary Wine Pomace Extract and Oleanolic Acid on Plasma Lipids in Rats Fed High-Fat Diet and Its DNA Microarray Analysis. J. Agric. Food Chem. 2008, 56, 12052–12058. [Google Scholar] [CrossRef]
- Xue, C.; Li, Y.; Lv, H.; Zhang, L.; Bi, C.; Dong, N.; Shan, A.; Wang, J. Oleanolic Acid Targets the Gut-Liver Axis to Alleviate Metabolic Disorders and Hepatic Steatosis. J. Agric. Food Chem. 2021, 69, 7884–7897. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Gu, T.; Yamahara, J.; Li, Y. Oleanolic Acid Supplement Attenuates Liquid Fructose-Induced Adipose Tissue Insulin Resistance through the Insulin Receptor Substrate-1/Phosphatidylinositol 3-Kinase/Akt Signaling Pathway in Rats. Toxicol. Appl. Pharmacol. 2014, 277, 155–163. [Google Scholar] [CrossRef]
- Li, W.; Zeng, H.; Xu, M.; Huang, C.; Tao, L.; Li, J.; Zhang, T.; Chen, H.; Xia, J.; Li, C.; et al. Oleanolic Acid Improves Obesity-Related Inflammation and Insulin Resistance by Regulating Macrophages Activation. Front. Pharmacol. 2021, 12, 697483. [Google Scholar] [CrossRef]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic Acid Attenuates PCBs-Induced Adiposity and Insulin Resistance via HNF1b-Mediated Regulation of Redox and PPARγ Signaling. Free Radic. Biol. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef]
- Matumba, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Chegou, N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. [Google Scholar] [CrossRef]
- Nyakudya, T.; Isaiah, S.; Ayeleso, A.; Ndhlala, A.; Mukwevho, E.; Erlwanger, K. Short-Term Neonatal Oral Administration of Oleanolic Acid Protects against Fructose-Induced Oxidative Stress in the Skeletal Muscles of Suckling Rats. Molecules 2019, 24, 661. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules 2019, 24, 340. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.B.; Jin, L.; Wang, Z.; Peng, J.; Liao, N.; Zhao, Y.Y.; Zhang, J.L.; Pauluhn, J.; Hai, C.X.; et al. Nano-Oleanolic Acid Alleviates Metabolic Dysfunctions in Rats with High Fat and Fructose Diet. Biomed. Pharmacother. 2018, 108, 1181–1187. [Google Scholar] [CrossRef]
- Camer, D.; Yu, Y.; Szabo, A.; Huang, X.F. The Molecular Mechanisms Underpinning the Therapeutic Properties of Oleanolic Acid, Its Isomer and Derivatives for Type 2 Diabetes and Associated Complications. Mol. Nutr. Food Res. 2014, 58, 1750–1759. [Google Scholar] [CrossRef]
- Panzhinskiy, E.; Ren, J.; Nair, S. Protein Tyrosine Phosphatase 1B and Insulin Resistance: Role of Endoplasmic Reticulum Stress/Reactive Oxygen Species/Nuclear Factor Kappa B Axis. PLoS ONE 2013, 8, e77228. [Google Scholar] [CrossRef]
- Qian, S.; Li, H.; Chen, Y.; Zhang, W.; Yang, S.; Wu, Y. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives As Inhibitors of Protein Tyrosine Phosphatase 1B. J. Nat. Prod. 2010, 73, 1743–1750. [Google Scholar] [CrossRef]
- Niu, S.-L.; Tong, Z.-F.; Zhang, Y.; Liu, T.-L.; Tian, C.-L.; Zhang, D.-X.; Liu, M.-C.; Li, B.; Tian, J.-L. Novel Protein Tyrosine Phosphatase 1B Inhibitor-Geranylated Flavonoid from Mulberry Leaves Ameliorates Insulin Resistance. J. Agric. Food Chem. 2020, 68, 8223–8231. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.-Y.; Li, J.; Hu, L.-H. Oleanolic Acid and Its Derivatives: New Inhibitor of Protein Tyrosine Phosphatase 1B with Cellular Activities. Bioorg. Med. Chem. 2008, 16, 8697–8705. [Google Scholar] [CrossRef]
- Liu, Q.-C.; Guo, T.-T.; Zhang, L.; Yu, Y.; Wang, P.; Yang, J.-F.; Li, Y.-X. Synthesis and Biological Evaluation of Oleanolic Acid Derivatives as PTP1B Inhibitors. Eur. J. Med. Chem. 2013, 63, 511–522. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, T.; Li, D.; Li, F.; Li, W. Synthesis and Evaluation of Several Oleanolic Acid Glycoconjugates as Protein Tyrosine Phosphatase 1B Inhibitors. Eur. J. Med. Chem. 2014, 79, 34–46. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, S.; Fan, P.; Li, L.; Feng, S.; Xiao, H.; Zhu, L. The Roles of Liver Inflammation and the Insulin Signaling Pathway in PM2.5 Instillation-Induced Insulin Resistance in Wistar Rats. Dis. Markers 2021, 2021, 2821673. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin Regulation of Gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake. In Blood Glucose Levels; IntechOpen: London, UK, 2020. [Google Scholar]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The Aetiology and Molecular Landscape of Insulin Resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK Expression by Macrophages Promotes Obesity-Induced Insulin Resistance and Inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Szewczuk, M.; Boguszewska, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The Role of AMPK in Metabolism and Its Influence on DNA Damage Repair. Mol. Biol. Rep. 2020, 47, 9075–9086. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK Signaling in Diabetes Mellitus, Insulin Resistance and Diabetic Complications: A Pre-Clinical and Clinical Investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The Etiology of Oxidative Stress in Insulin Resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Tan, S.M.; de Haan, J.B. Combating Oxidative Stress in Diabetic Complications with Nrf2 Activators: How Much Is Too Much? Redox Rep. 2014, 19, 107–117. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef]
- Buscemi, N.; Hartling, L.; Vandermeer, B.; Tjosvold, L.; Klassen, T.P. Single Data Extraction Generated More Errors than Double Data Extraction in Systematic Reviews. J. Clin. Epidemiol. 2006, 59, 697–703. [Google Scholar] [CrossRef]
- Horton, J.; Vandermeer, B.; Hartling, L.; Tjosvold, L.; Klassen, T.P.; Buscemi, N. Systematic Review Data Extraction: Cross-Sectional Study Showed That Experience Did Not Increase Accuracy. J. Clin. Epidemiol. 2010, 63, 289–298. [Google Scholar] [CrossRef]
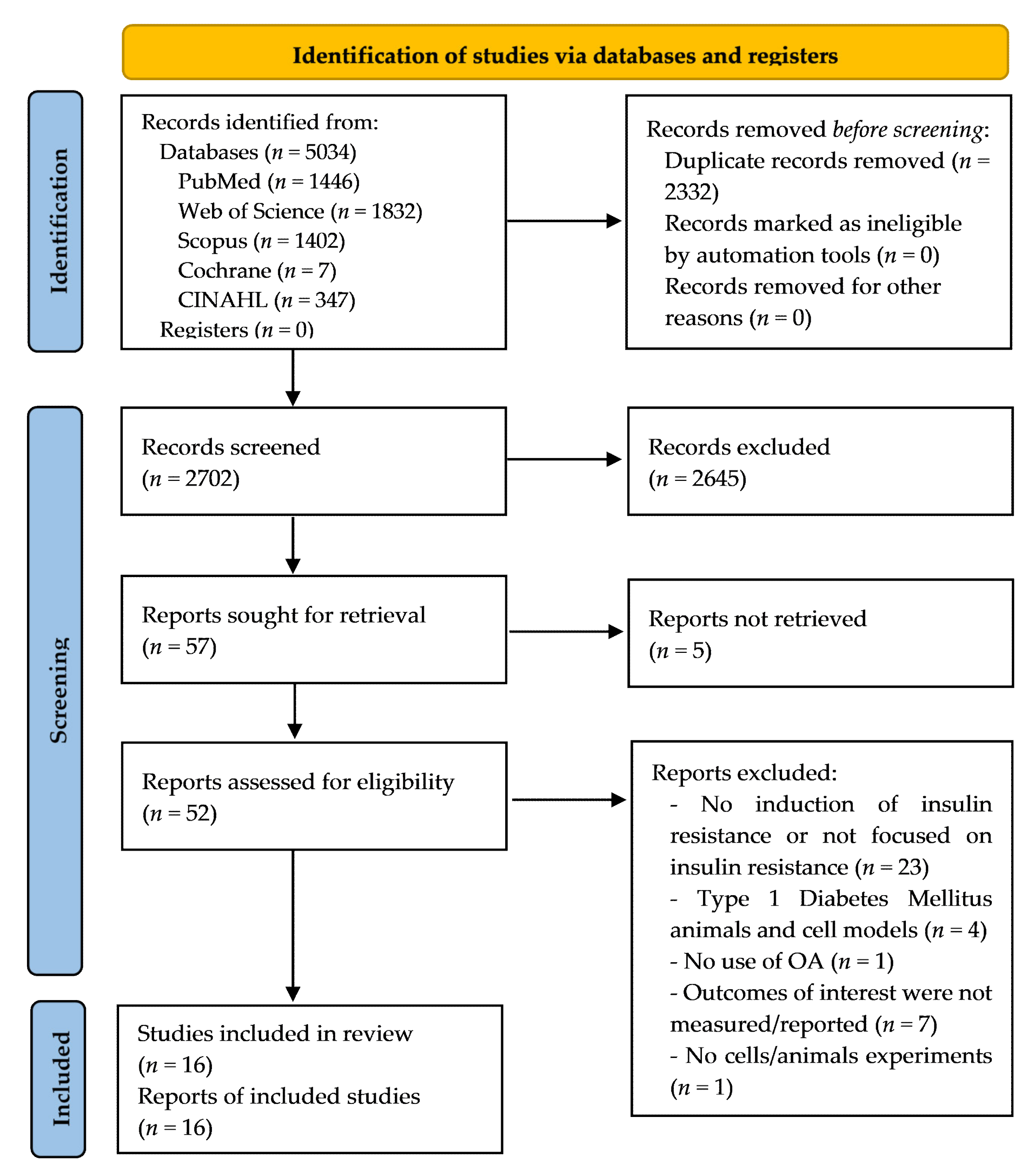

| Database | Search Field | Additional Filters |
|---|---|---|
| PubMed | All fields | Not applicable |
| Web of Science 1 | Title, Abstract, and Keywords | Document types: articles, others, and clinical trial |
| Scopus | Title, Abstract, and Keywords | Document types: article |
| Cochrane | Title, Abstract, and Keywords | Document types: article |
| CINAHL 2 | Full text | Not applicable |
| Author/Year | Subjects | Intervention and Groups | Main Findings |
|---|---|---|---|
| Wang X et al. [38] (2011) | QZG cells induced for IR with tBHP. | Intervention: Treatment with or without OA at 10 µM for 4 h, later exposition to tBHP, and finally stimulation with insulin. Groups:
| The exposition to tBHP decreased the insulin-stimulated phosphorylation of Akt and ERK, but it was inhibited by OA (p < 0.05). Non-significant (ns) actions on PGC-1α gene expression and on phosphorylation of p38 were observed. |
| Li M et al. [39] (2015) | HepG2 cells induced for IR with sodium oleate, except the CG. | Intervention: Insulin-resistant HepG2 cells treated with different doses of OA. Groups:
| ↓ Content of glucose, IL-6, and TNF-α (p < 0.05) and ↓ protein expression of NF-кB (p < 0.01); ↑ IRS and GLUT4 protein expression (p < 0.01) in all OA groups vs. non-treated insulin-resistant cells.↓ ns of TNF-α levels at OA-5 µM vs. non-treated insulin-resistant cells. |
| Zhang Y et al. [40] (2020) | Insulin-resistant HepG2 cells induced with high concentrations of insulin. | Intervention and Groups:
| ↓ PTP1B protein expression and ↑ protein expression of Akt and IRS-1 in both insulin-resistant cell models treated with OA or PGA-OA. All changes p < 0.05. |
| Author/Year | Subjects (n) | Intervention | Groups | Main Findings |
|---|---|---|---|---|
| Wang X et al. [41] (2015) | 24 male C57BL/KsJ-Lepdb (db/db) mice | Intragastric administration of vehicle (0.5% CMC-Na), OA (250 mg/kg/day), or metformin (100 mg/kg/day) for 28 days. | Groups (n = 6 per group):
| ↓ HOMA-IR; ↓ mRNA expression levels in liver (p < 0.001) of GP, PEPCK1, G-6-Pase, and GLUT2; ↓ ns of mRNA expression levels in liver of PGC-1α and ↑ ns of GS. ↑ AMPK, ACC, Akt, and PI3K and ↓ mTOR and CREB phosphorylation in livers; ↓ liver protein levels of PEPCK and of G-6-Pase. All changes reported are of OA group compared with CG. |
| Wang X et al. [42] (2013) | 24 male C57BLKS/J lar-Lepdb/db mice, and 10 wild-type mice as control | Intraperitoneal injection of OA (20 mg/kg/day) for 14 days. |
| ↓ Liver protein expression of G-6-Pase and PEPCK; and ↑ PGC-1α gene expression and AMPK phosphorylation in livers.↑ Insulin-stimulated phosphorylation of Akt in livers. ↓ Mitochondrial ROS production and GSSG, and ↑ mitochondrial GSH in liver. ↑ Protein expression of Nrf2, GCLC, SOD, and CAT in liver. ↓ IL-6, IL-1β, and TNF-α both in serum and in liver. All changes (p < 0.05) in OA-treated diabetic mice compared with non-OA-treated diabetic mice. |
| Zeng X et al. [43] (2012) | Male C57BL/6J mice | Mice fed with a normal diet or an HFD during 10 weeks, and injection of STZ into HFD-fed rats to establish a T2DM model. Later, administration of 100 mg/kg/day of OA to T2DM mice for 2 weeks. |
| phosphorylated-/total-Akt ratio similar in both diabetic mice groups; ↓ levels of p-Akt in non-treated T2DM mice vs. non-diabetic mice, while treatment with OA ↑ p-Akt levels in T2DM mice to levels of non-diabetic mice. In OA-treated T2DM mice, ↑ of phosphorylated-/total-FoxO1 ratio and ↓ in total FoxO1 protein (p < 0.05) compared to untreated T2DM mice and non-diabetic mice. All results measured in livers of mice. |
| Zhou X et al. [44] (2014) | Male C57BL/6J mice | Idem to Zeng et al. [43], but measurements of variables were undertaken after 4 weeks of OA administration (during OA treatment experiment), or after 2 weeks of OA administration followed by an OA-free HFD diet during 4 weeks (post-OA treatment). |
| In liver of diabetic mice, the following was observed: ↓ total content of FoxO1, ↑ phosphorylation of FoxO1 and acetylation of FoxO1, and ↓ gene expression of G-6-Pase during and post OA treatment. ↑ Phosphorylation of AMPK-α and ACC; ↓ of the mature form of SERBP-1c in the livers of diabetic mice during OA treatment. All changes p < 0.05 vs. untreated diabetic mice. |
| Yunoki K et al. [45] (2008) | 24 male Sprague Dawley rats | Administration of OA (50 mg/kg/day) or PEE (450 mg/kg/day) for 4 weeks |
| Downregulation of ACC, G-6-Pase, FoxO1, TNF-α, and IL-1β genes; and upregulation of genes of insulin receptor substrates and AMPK β-2 regulatory subunit in rat livers. All changes p < 0.01 vs. HFD-fed rats. |
| Xue C et al. [46] (2021) | 30 Sprague Dawley Rats | Administration of OA (25, 50, and 100 mg/kg/day) for 8 weeks. Rats were simultaneously fed with an HFD or normal diet for 12 weeks. | Groups (n = 6 per group):
| ↓ MDA and ↑ SOD, GPx, and CAT content; ↓ IL-1β, IL-6, and TNF-α overexpression; inhibition of the phosphorylation of IκB-α and p65 in liver tissues. All changes (p < 0.05), especially in rats treated with OA 50 or 100 vs. HFD-fed rats. |
| Li Y et al. [47] (2014) | 24 male Sprague-Dawley rats | Fructose induced insulin-resistant rats and oral administration of 5 or 25 mg/kg/day of OA for 10 weeks. | Groups (n = 6 per group):
| ↓ Adipo-IR; ↑ adipose mRNA expression of insulin receptor, IRS-1, PI3K, and Akt. ↑ IRS-1 protein expression, ↓ fructose-stimulated pIRS-1 protein expression, and of the ratio of pIRS-1 to total IRS-1 protein expression. ↑ Ratio of pAkt protein to Akt protein. All changes (p < 0.05) in the adipose tissue of insulin-resistant rats treated with OA 25 mg vs. non-treated insulin-resistant rats. |
| Li W et al. [48] (2021) | 21 C57BL/6J male mice | All mice fed with HFD during 12 weeks. Later, administration with distilled water, or OA 25 mg/kg or OA 50 mg/kg per day by intragastric gavage for 4 weeks. | Groups (n = 7 per group):
| ↓ HOMA-IR and Adipo-IR index; ↑ phosphorylation of Akt and ↓ gene expression of iNOS, IL-6, TNF-α, IL-1β, and Caspase 1; ↓ macrophages M1 and ↑ macrophages M2 in eWAT of mice treated with OA-25 and -50. ↓ phosphorylation of ERK and JNK in eWAT of mice treated with OA-50. All changes (p < 0.05) vs. non-OA-treated HFD-fed mice. |
| Su S et al. [49] (2018) | 40 male C57B6/J mice | Exposure to vehicle or Aroclor 1254 (100 µg/kg; PCBs) every 3 days for 10 weeks. Pretreatment with 50 mg/kg of OA for 1 h every 3 days for 10 weeks. | Groups (n = 10 per group):
| ↓ HOMA-IR, ↓ serum MDA, and ↑ serum SOD and CAT activity; ↓ mRNA expression of NOX4, GCLC, and GCLM in adipose tissue. All changes (p < 0.05) in favor of OA-pretreated mice vs. PCBs-treated mice. |
| Matumba MG et al. [50] (2019) | 40 male Sprague Dawley rat pups | OA (60 mg/kg/day) administration by orogastric gavage during the second neonatal week. Duration of the experiment was 16 weeks. |
| ↑ AMPK, GLUT4 and ↓ IL-6 and TNF-α gene expression (p < 0.001) in the skeletal muscle; and ↓ plasma concentration of IL-6 (p < 0.0001) and ns of TNF-α. All changes in OA-treated HF-fed rats vs. HF-fed rats. |
| Nyakudya T et al. [51] (2019) | 30 Sprague Dawley rat pups | Administration of OA (60 mg/kg/day b.w.) by orogastric gavage in the second postnatal week. Duration of the experiments 14 days since their birth. |
| The ↓ of GSH and CAT activity in the skeletal muscles of HF-fed rats was attenuated in OA-treated HF-fed rats (p < 0.05). Non-significant changes on GPx, SOD, and MDA. |
| Gamede M et al. [52] (2019) | 36 male Sprague-Dawley rats | Treatment with OA or OA + dietary intervention for 12 weeks after previous administration of HFHC diet during 20 weeks to induce prediabetes. | Groups (n = 6 per group): Non-prediabetic control (NC); prediabetic control (PC); metformin (MET); MET + dietary intervention; OA; OA + diet | ↓ Plasma levels of IL-6 and heart MDA concentration, and ↑ plasma level of SOD and GPx in OA-treated rats vs. prediabetic control group. All changes p < 0.05. |
| Wang S et al. [53] (2018) | 36 male Sprague-Dawley rats | 6 normal-diet-fed rats and 30 HFF-fed rats during the first four weeks. HFF-fed rats were intraperitoneally injected with tBHP during the last eight weeks of the experiments. | 6 Groups (n = 6 per group): 6 HFF-fed rats were treated with OA and another 6 with nano-OA during the last 6 weeks of the experiments. 25 mg/kg/day | ↓ Serum NO levels and ↑ serum CAT activity in OA and nano-OA groups; ↓ serum levels of MDA and ↑ serum SOD activity and ISI in nano-OA group. All changes (p < 0.05) vs. non-treated insulin-resistant rats. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Aparicio, Á.; Correa-Rodríguez, M.; Castellano, J.M.; Schmidt-RioValle, J.; Perona, J.S.; González-Jiménez, E. Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1517. https://doi.org/10.3390/antiox11081517
Fernández-Aparicio Á, Correa-Rodríguez M, Castellano JM, Schmidt-RioValle J, Perona JS, González-Jiménez E. Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants. 2022; 11(8):1517. https://doi.org/10.3390/antiox11081517
Chicago/Turabian StyleFernández-Aparicio, Ángel, María Correa-Rodríguez, Jose M. Castellano, Jacqueline Schmidt-RioValle, Javier S. Perona, and Emilio González-Jiménez. 2022. "Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review" Antioxidants 11, no. 8: 1517. https://doi.org/10.3390/antiox11081517
APA StyleFernández-Aparicio, Á., Correa-Rodríguez, M., Castellano, J. M., Schmidt-RioValle, J., Perona, J. S., & González-Jiménez, E. (2022). Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants, 11(8), 1517. https://doi.org/10.3390/antiox11081517









