Adenophora Stricta Root Extract Protects Lung Injury from Exposure to Particulate Matter 2.5 in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of A. stricta Root Extract (AsE)
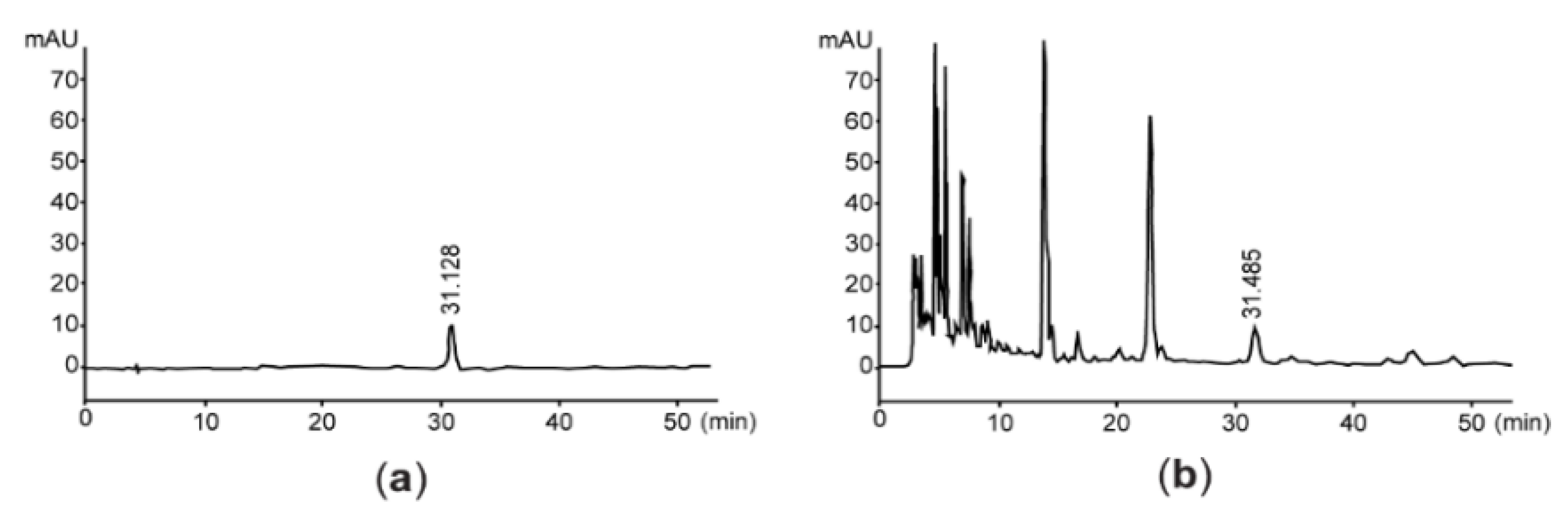
2.2. Quantification of Vanillic Acid 4-β-D-Glucopyranoside and Total Flavonoids in AsE
2.3. Animal Model and Drug Administration
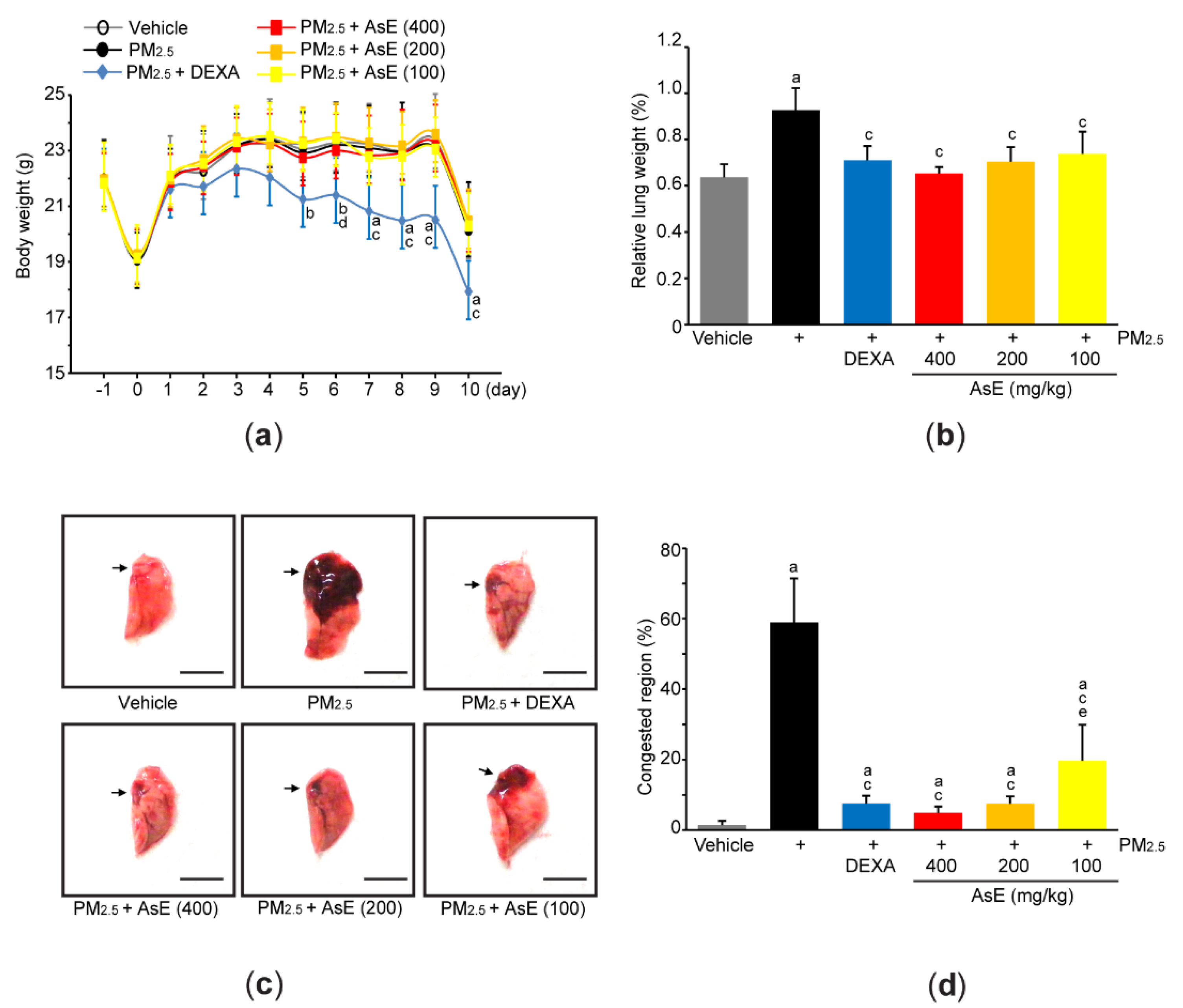
2.4. Measurement of Body and Lung Weight
2.5. Lung Sampling and Gross Inspection
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Cytological Analysis
2.9. Measurement of Reactive Oxygen Species (ROS)
2.10. Measurement of Lipid Peroxidation, Reduced Glutathione Levels, Catalase and Superoxide Dismutase Activity
2.11. Statistical Analysis
3. Results
3.1. AsE Decreases Pulmonary Congestion Induced by PM2.5
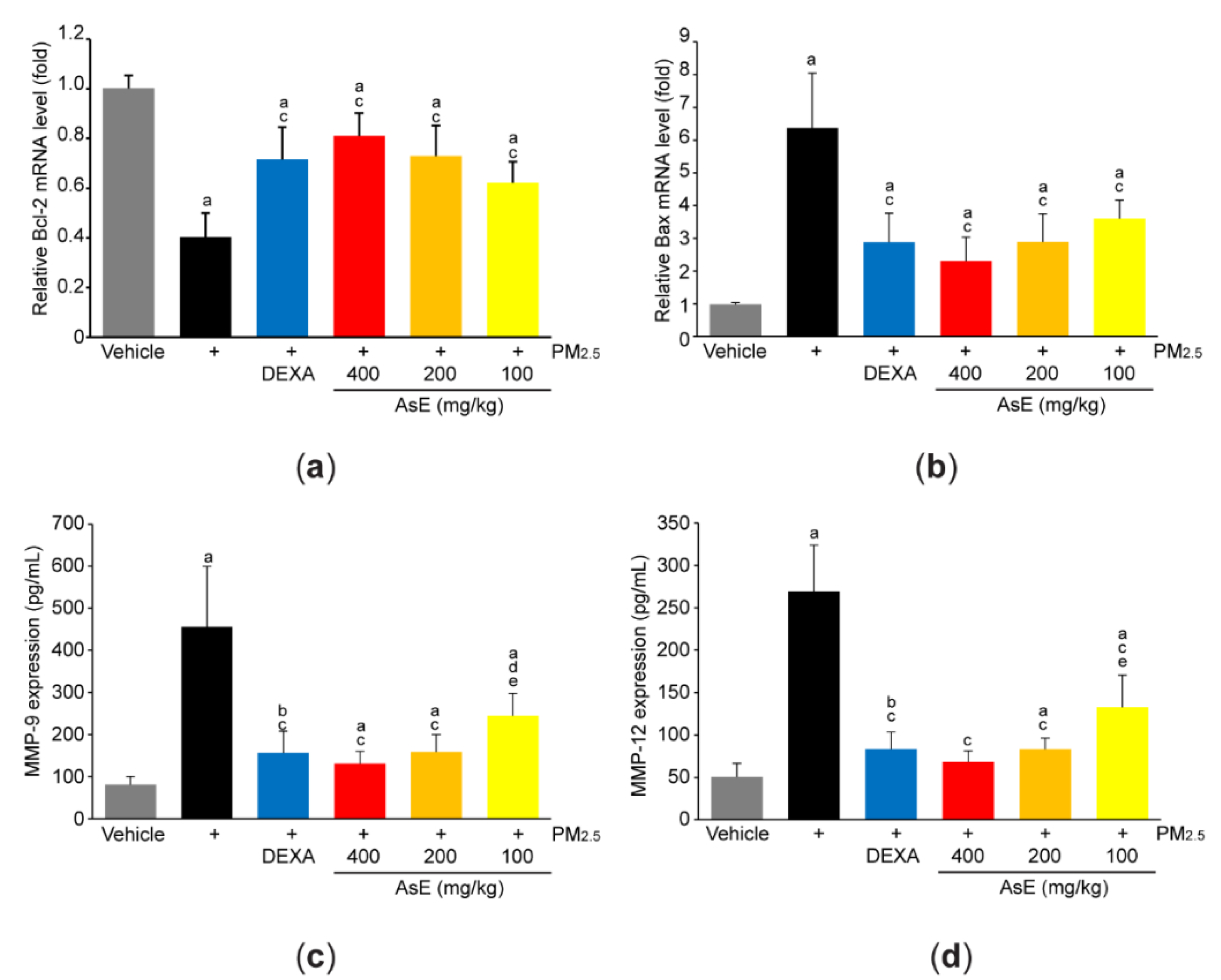
3.2. AsE Protects Lung Tissue from PM2.5-Mediated Injuries
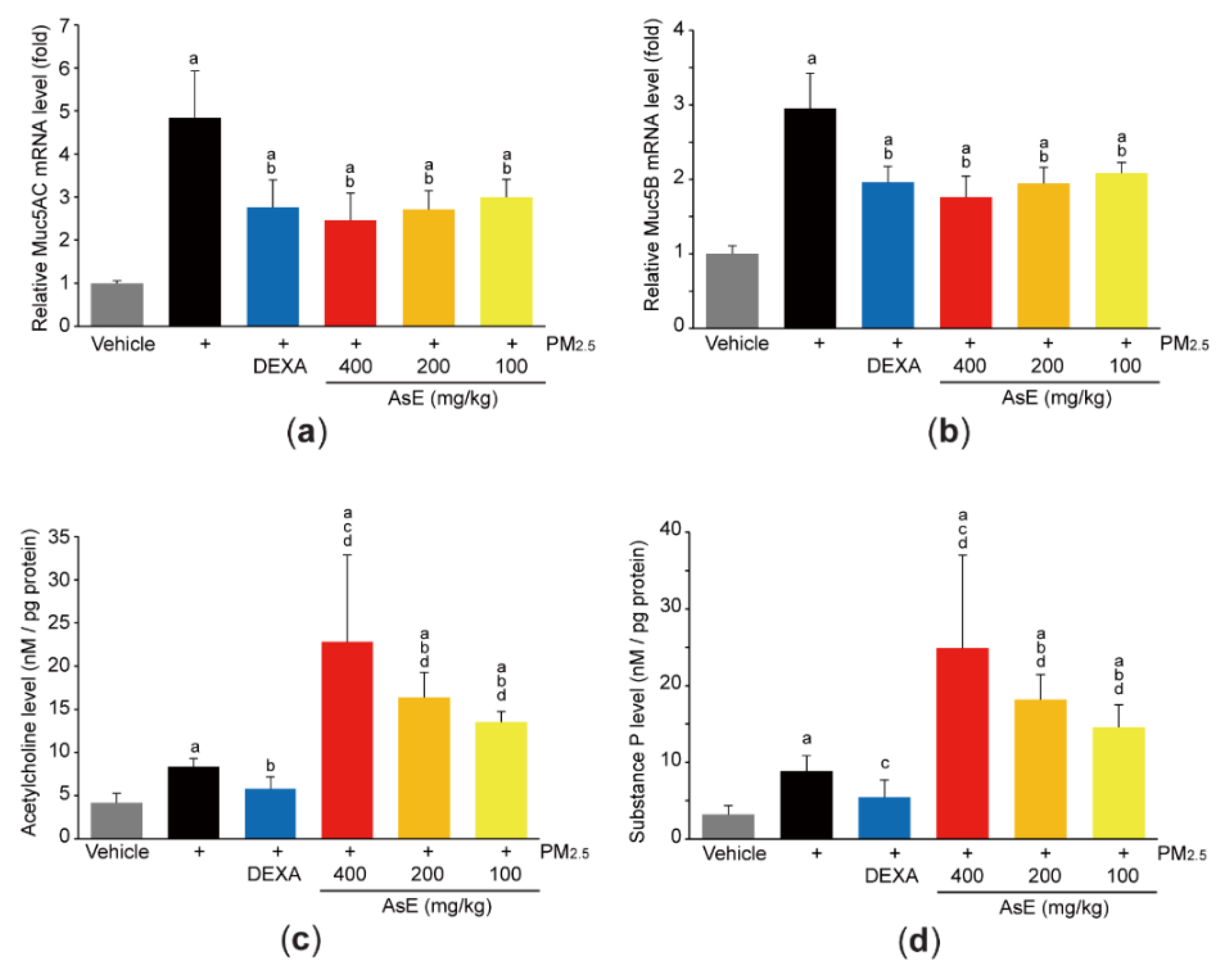
3.3. AsE Inhibits Mucus Stasis
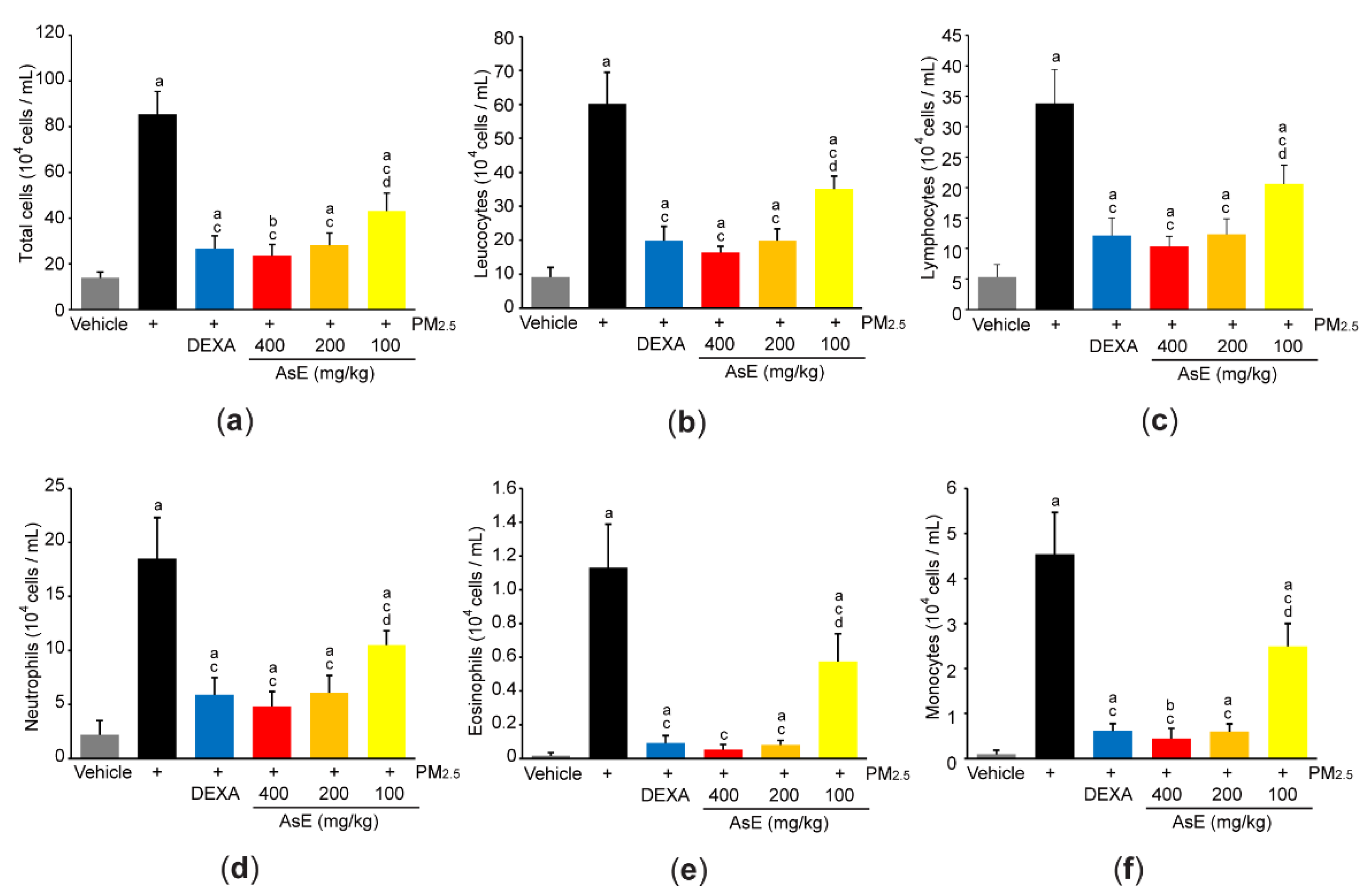
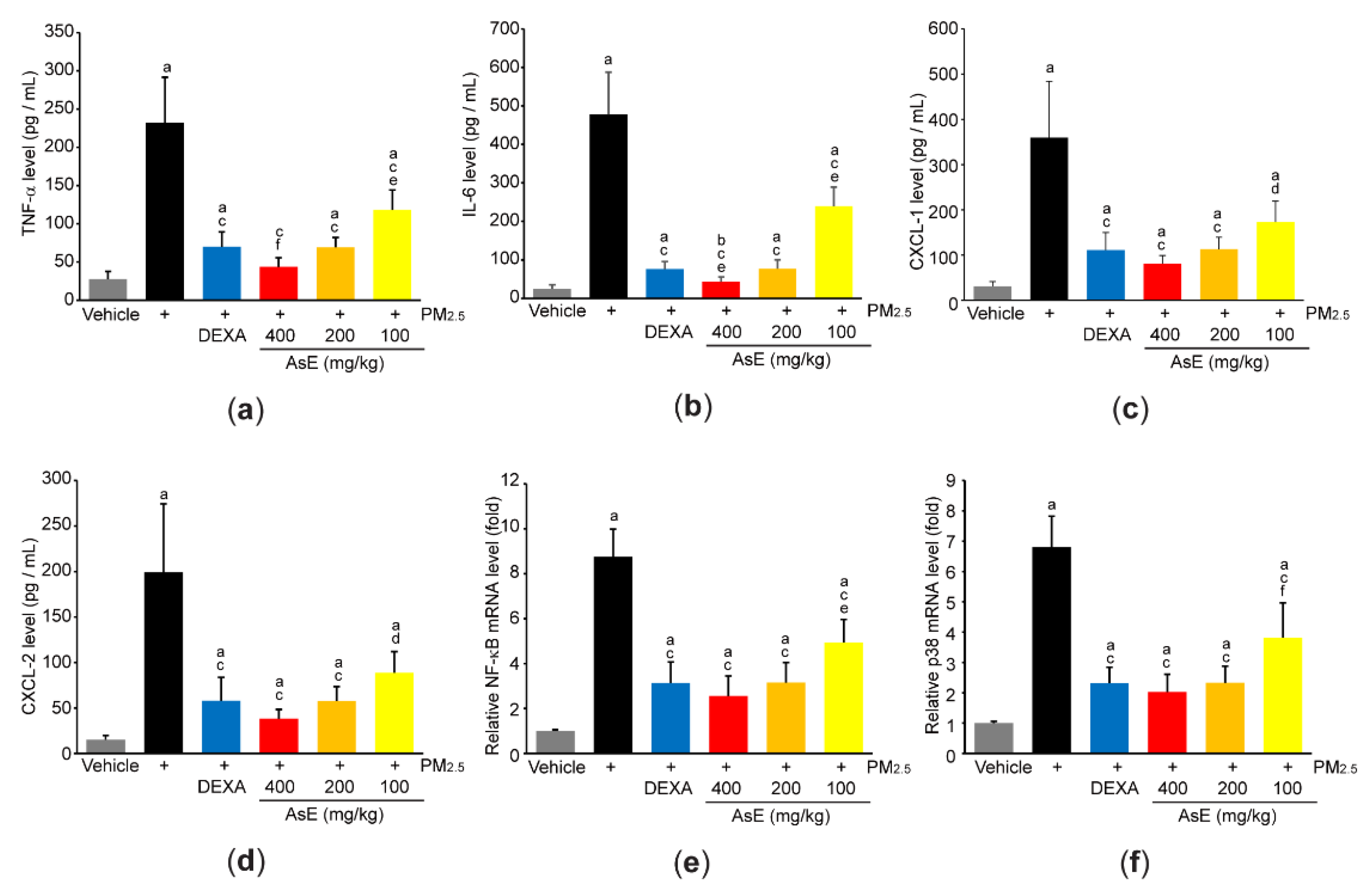
3.4. AsE Reduces PM2.5-Mediated Inflammation in the Lungs
3.5. AsE Diminishes Oxidative Stress in the Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Li, T.; Yan, Y.; Xian, H.; Al-Aly, Z. The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet. Health 2018, 2, e301–e312. [Google Scholar] [CrossRef]
- Bu, X.; Xie, Z.; Liu, J.; Wei, L.; Wang, X.; Chen, M.; Ren, H. Global PM2.5-attributable health burden from 1990 to 2017: Estimates from the Global Burden of disease study 2017. Environ. Res. 2021, 197, 111123. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Jiang, C.; Chen, J.; Feng, X.; Chen, W.; Zhang, J.; Dong, H.; Zhang, W. A traditional herbal formula, Deng-Shi-Qing-Mai-Tang, regulates TLR4/NF-κB signaling pathway to reduce inflammatory response in PM2.5-induced lung injury. Phytomedicine 2021, 91, 153665. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory Effects of Black Ginseng on Particulate Matter-Induced Pulmonary Injury. Am. J. Chin. Med. 2019, 47, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; An, X.; Zhang, L.; Li, X.; Wang, L.; Zhu, G. Continuous exposure of PM2.5 exacerbates ovalbumin-induced asthma in mouse lung via a JAK-STAT6 signaling pathway. Adv. Clin. Exp. Med. 2020, 29, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, H.; Yamazaki, S.; Tamura, K.; Hwang, Y.H.; Ono, R.; Amimoto, Y.; Askew, D.J.; Odajima, H. Association between daily ambient air pollution and respiratory symptoms in children with asthma and healthy children in western Japan. J. Asthma 2018, 55, 712–719. [Google Scholar] [CrossRef]
- Pei, C.; Wang, F.; Huang, D.; Shi, S.; Wang, X.; Wang, Y.; Li, S.; Wu, Y.; Wang, Z. Astragaloside IV Protects from PM2.5-Induced Lung Injury by Regulating Autophagy via Inhibition of PI3K/Akt/mTOR Signaling in vivo and in vitro. J. Inflamm. Res. 2021, 14, 4707–4721. [Google Scholar] [CrossRef]
- Mao, M.; Li, J.; Bi, A.; Jia, H.; Li, Q.; Liu, Y.; Jiang, X.; Huang, D.; Xia, S. Thymoquinone ameliorates the PM2.5-induced lung injury in rats. Exp. Lung Res. 2020, 46, 297–307. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J. Toxicol. Environ. Health A 2019, 82, 338–350. [Google Scholar] [CrossRef] [PubMed]
- The Korean Herbal Pharmacopoeia. Available online: http://www.law.go.kr/admRulLsInfoP.do?admRulSeq=2000000021929 (accessed on 10 May 2022).
- Park, J.H. The Encyclopedia of Natural Product Drugs, 1st ed.; Shinilbooks: Seoul, Korea, 2018; pp. 356–358. [Google Scholar]
- World Health Organization. WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region, 1st ed.; WHO: Geneva, Switzerland, 2007; pp. 223–263. [Google Scholar]
- Kim, H.C. Herbal Pharmacology, 1st ed.; Jipmoondang: Paju, Korea, 2001; pp. 474–475. [Google Scholar]
- Roh, S.S.; Kim, S.H.; Lee, Y.C.; Seo, Y.B. Effects of radix adenophorae and cyclosporine A on an OVA-induced murine model of asthma by suppressing to T cells activity, eosinophilia, and bronchial hyperresponsiveness. Mediat. Inflamm. 2008, 2008, 781425. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Jung, C.J.; Ku, S.M.; Jung, D.H.; Ku, S.K.; Choi, J.S. Antitussive, expectorant, and anti-inflammatory effects of Adenophorae Radix powder in ICR mice. J. Ethnopharmacol. 2019, 239, 111915. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, E.H.; Lee, T.J.; Kim, S.W.; Kim, B.H. Anti-obesity effect and action mechanism of Adenophora triphylla root ethanol extract in C57BL/6 obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2013, 77, 544–550. [Google Scholar] [CrossRef]
- Kang, M.; Ha, I.J.; Chun, J.; Kang, S.S.; Kim, Y.S. Separation of two cytotoxic saponins from the roots of Adenophora triphylla var. japonica by high-speed counter-current chromatography. Phytochem. Anal. 2013, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Lee, C.M.; Choi, H.; Lee, J.H.; Oh, J.S.; Kwak, J.H.; Zee, O.P. Evaluation of oriental medicinal herbs for estrogenic and antiproliferative activities. Phytother. Res. 2006, 20, 1017–1019. [Google Scholar] [CrossRef]
- Cho, I.J.; Shin, J.H.; Choi, B.R.; Park, H.R.; Park, J.E.; Hong, S.H.; Kwon, Y.S.; Oh, W.S.; Ku, S.K. Lemon Balm and Corn Silk Mixture Alleviates Metabolic Disorders Caused by a High-Fat Diet. Antioxidants 2022, 11, 730. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Fan, X.; Zhu, Z.; Zhu, Y. Effect of San’ao decoction on aggravated asthma mice model induced by PM2.5 and TRPA1/TRPV1 expressions. J. Ethnopharmacol. 2019, 236, 82–90. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Heidari, R.; Taheri, V.; Rahimi, H.R.; Yeganeh, B.S.; Niknahad, H.; Najibi, A. Sulfasalazine-induced renal injury in rats and the protective role of thiol-reductants. Ren. Fail. 2016, 38, 137–141. [Google Scholar] [CrossRef]
- Gupta, R.; Dubey, D.K.; Kannan, G.M.; Flora, S.J.S. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol. Int. 2007, 31, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Rho, T.; Yoon, K.D. Phytochemical Study of Adenophora stricta Roots. Kor. J. Pharmacogn. 2019, 50, 73–80. [Google Scholar]
- Wang, L.; Jiao, X.F.; Wu, C.; Li, X.Q.; Sun, H.X.; Shen, X.Y.; Zhang, K.Z.; Zhao, C.; Liu, L.; Wang, M.; et al. Trimetazidine attenuates dexamethasone-induced muscle atrophy via inhibiting NLRP3/GSDMD pathway-mediated pyroptosis. Cell Death Discov. 2021, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Fappi, A.; Godoy, T.S.; Maximino, J.R.; Rizzato, V.R.; Neves, J.D.C.; Chadi, G.; Zanoteli, E. The effects of omega-3 fatty acid supplementation on dexamethasone-induced muscle atrophy. Biomed. Res. Int. 2014, 2014, 961438. [Google Scholar] [CrossRef]
- Skjøt-Arkil, H.; Clausen, R.E.; Nguyen, Q.H.; Wang, Y.; Zheng, Q.; Martinez, F.J.; Hogaboam, C.M.; Han, M.; Klickstein, L.B.; Larsen, M.R.; et al. Measurement of MMP-9 and -12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm. Med. 2012, 12, 34. [Google Scholar] [CrossRef]
- Ridley, C.; Thornton, D.J. Mucins: The frontline defence of the lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef]
- Atanasova, K.R.; Reznikov, L.R. Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis. Respir. Res. 2018, 19, 149. [Google Scholar] [CrossRef]
- Buels, K.S.; Fryer, A.D. Muscarinic receptor antagonists: Effects on pulmonary function. In Muscarinic Receptors. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 208, pp. 317–341. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.S.; Rhee, M.H.; Sung, G.H.; Yoo, B.C.; Cho, J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 352371. [Google Scholar] [CrossRef]
- Tu, P.F.; Xu, G.J.; Yang, X.W.; Masao, H.; Tsuneo, N. A triterpene from the roots of Adenophora stricta subsp. sessilifolia. Jpn. J. Pharmacogn. 1990, 44, 98–100. [Google Scholar]
- Park, S.M.; Jung, E.H.; Kim, J.K.; Jegal, K.H.; Park, C.A.; Cho, I.J.; Kim, S.C. 20S-Protopanaxadiol, an aglycosylated ginsenoside metabolite, induces hepatic stellate cell apoptosis through liver kinase B1-AMP-activated protein kinase activation. J. Ginseng Res. 2017, 41, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Somade, O.T.; Adeyi, O.E.; Ajayi, B.O.; Asunde, O.O.; Iloh, P.D.; Adesanya, A.A.; Babalola, O.I.; Folorunsho, O.T.; Olakunle, D.A.; Lawal, O.F. Syringic and ascorbic acids prevent NDMA-induced pulmonary fibrogenesis, inflammation, apoptosis, and oxidative stress through the regulation of PI3K-Akt/PKB-mTOR-PTEN signaling pathway. Metab. Open 2022, 14, 100179. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Fang, L.; Hu, H.; Yang, Y.; Feng, X.; Sun, D. Vanillic acid mitigates the ovalbumin (OVA)-induced asthma in rat model through prevention of airway inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Su, Z.; Wang, Q.; Hou, W.; Li, J.; Zhang, L.; Zhang, J. Vanillin protects lipopolysaccharide-induced acute lung injury by inhibiting ERK1/2, p38 and NF-κB pathway. Futur. Med. Chem. 2019, 11, 2081–2094. [Google Scholar] [CrossRef]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef]
- Ballard, S.T.; Spadafora, D. Fluid secretion by submucosal glands of the tracheobronchial airways. Respir. Physiol. Neurobiol. 2007, 159, 271–277. [Google Scholar] [CrossRef][Green Version]
- Malerba, M.; Ragnoli, B. Ambroxol in the 21st century: Pharmacological and clinical update. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1119–1129. [Google Scholar] [CrossRef]
- Hu, J.R.; Jung, C.J.; Ku, S.M.; Jung, D.H.; Bashir, K.M.I.; Ku, S.K.; Choi, J.S. Anti-inflammatory, expectorant, and antitussive properties of Kyeongok-go in ICR mice. Pharm. Biol. 2021, 59, 321–334. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Phillai, S. Cellular and Molecular Immunology, 7th ed.; Saunders: Philadelphia, PA, USA, 2012; pp. 41–88. [Google Scholar]
- Wong, C.K.; Wang, C.B.; Ip, W.K.; Tian, Y.P.; Lam, C.W. Role of p38 MAPK and NF-kB for chemokine release in coculture of human eosinophils and bronchial epithelial cells. Clin. Exp. Immunol. 2005, 139, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Audousset, C.; McGovern, T.; Martin, J.G. Role of Nrf2 in Disease: Novel Molecular Mechanisms and Therapeutic Approaches—Pulmonary Disease/Asthma. Front. Physiol. 2021, 12, 727806. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Cho, H.Y.; Reddy, S.P.; Yamamoto, M.; Kleeberger, S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004, 18, 1258–1260. [Google Scholar] [CrossRef]
- Pan, H.; Wang, H.; Zhu, L.; Mao, L.; Qiao, L.; Su, X. Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem. Res. 2011, 36, 2434–2441. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef]
- Kim, J.E.; You, D.J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.I. Suppression of NF-kappaB signaling by KEAP1 regulation of IKKbeta activity through autophagic degradation and inhibition of phosphorylation. Cell. Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Seldon, M.P.; Silva, G.; Pejanovic, N.; Larsen, R.; Gregoire, I.P.; Filipe, J.; Anrather, J.; Soares, M.P. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-kappaB RelA phosphorylation at serine 276. J. Immunol. 2007, 179, 7840–7851. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, D.J.; Hwang, O. KMS99220 Exerts Anti-Inflammatory Effects, Activates the Nrf2 Signaling and Interferes with IKK, JNK and p38 MAPK via HO-1. Mol. Cells 2019, 42, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Zakkar, M.; Van der Heiden, K.; Luong, L.A.; Chaudhury, H.; Cuhlmann, S.; Hamdulay, S.S.; Krams, R.; Edirisinghe, I.; Rahman, I.; Carlsen, H.; et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1851–1857. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Sense Primer | Antisense Primer | RefSeq No. | Amplicon Size (bp) |
|---|---|---|---|---|
| β-actin | 5′-GCTGAGAGGGAAATCG TGCGT-3′ | 5′-GAAGCATTTGCGGTGCACGATG-3′ | NM_007393.5 | 516 |
| Bcl-2 | 5′-TGAGAGCAACCGAACGCCCG-3′ | 5′-CCGTGGCAAAGCGTCCCCTC-3′ | NM_009741.5 | 230 |
| Bax | 5′-GGGTGGCAGCTGACATGTTT-3′ | 5′-GCCTTGAGCACCAGTTTGCT-3′ | NM_007527.3 | 91 |
| Muc5AC | 5′-CACCATCTCTACAACCCAAACT-3′ | 5′-TGAGGTCCAGGTCTTTGTGTCT-3′ | NM_010844.3 | 517 |
| Muc5B | 5′-GCCCTCACTGCCTCTGCTCCAC-3′ | 5′-TTTTACAGTGCCAGGGTTTATT-3′ | NM_028801.2 | 387 |
| NF-κB | 5′-CGTTGTTTCCTGGTACAGACC-3′ | 5′-CCATTTCTTCTTGGTCAAGGG-3′ | NM_009045.5 | 307 |
| p38 | 5′-CGTTGTTTCCTGGTACAGACC-3′ | 5′-CCATTTCTTCTTGGTCAAGGG-3′ | NM_011951.3 | 414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-M.; Jung, C.-J.; Lee, D.-G.; Choi, B.-R.; Ku, T.-H.; La, I.-J.; Cho, I.-J.; Ku, S.-K. Adenophora Stricta Root Extract Protects Lung Injury from Exposure to Particulate Matter 2.5 in Mice. Antioxidants 2022, 11, 1376. https://doi.org/10.3390/antiox11071376
Park S-M, Jung C-J, Lee D-G, Choi B-R, Ku T-H, La I-J, Cho I-J, Ku S-K. Adenophora Stricta Root Extract Protects Lung Injury from Exposure to Particulate Matter 2.5 in Mice. Antioxidants. 2022; 11(7):1376. https://doi.org/10.3390/antiox11071376
Chicago/Turabian StylePark, Seok-Man, Cheol-Jong Jung, Dae-Geon Lee, Beom-Rak Choi, Tae-Hun Ku, Im-Joung La, Il-Je Cho, and Sae-Kwang Ku. 2022. "Adenophora Stricta Root Extract Protects Lung Injury from Exposure to Particulate Matter 2.5 in Mice" Antioxidants 11, no. 7: 1376. https://doi.org/10.3390/antiox11071376
APA StylePark, S.-M., Jung, C.-J., Lee, D.-G., Choi, B.-R., Ku, T.-H., La, I.-J., Cho, I.-J., & Ku, S.-K. (2022). Adenophora Stricta Root Extract Protects Lung Injury from Exposure to Particulate Matter 2.5 in Mice. Antioxidants, 11(7), 1376. https://doi.org/10.3390/antiox11071376






