Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Anthocyanin-Rich Juice and the Anthocyanin-Depleted Placebo
2.2. Study Design and Study Subjects
2.3. Collection of Plasma and Urine Samples
2.4. Isolation of Plasma Anthocyanins and Their Metebaolites by Solid Phase Extraction
2.5. Cell Culture
2.6. Cell Migration of Colon Cancer Cells In Vitro
2.7. Assessment of Cell Viability by Flow Cytometry
2.8. Oxidative Biomarkers in Plasma and Urine Samples
2.9. Statistical Analyses
3. Results
3.1. Composition of the Anthocyanin-Rich Juice and the Anthocyanin-Depleted Placebo
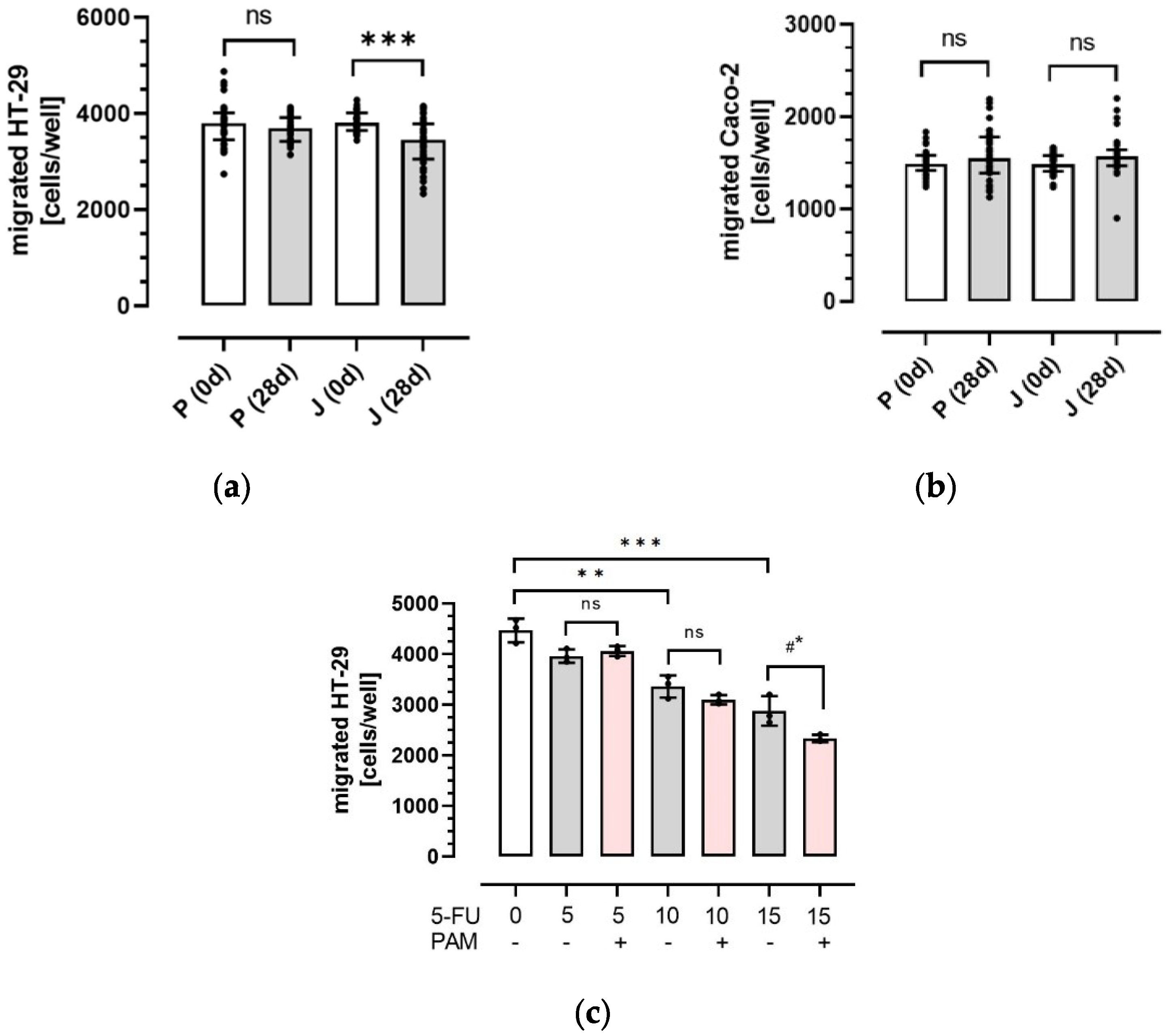
3.2. Influence of Plasma Anthocyanins and Their Metabolites on HT-29 and Caco-2 Colon Cancer Cell Migration
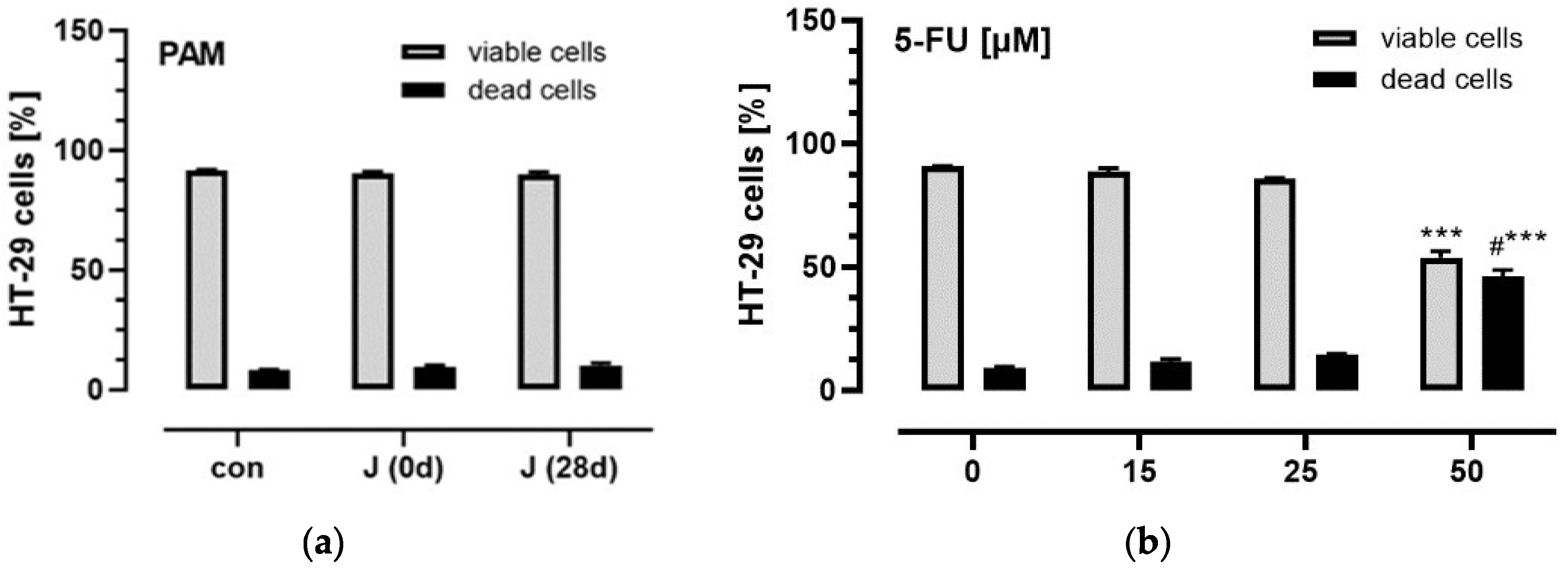
3.3. Influence of Plasma Anthocyanins and Their Metabolites on HT-29 and Caco-2 Cell Viability
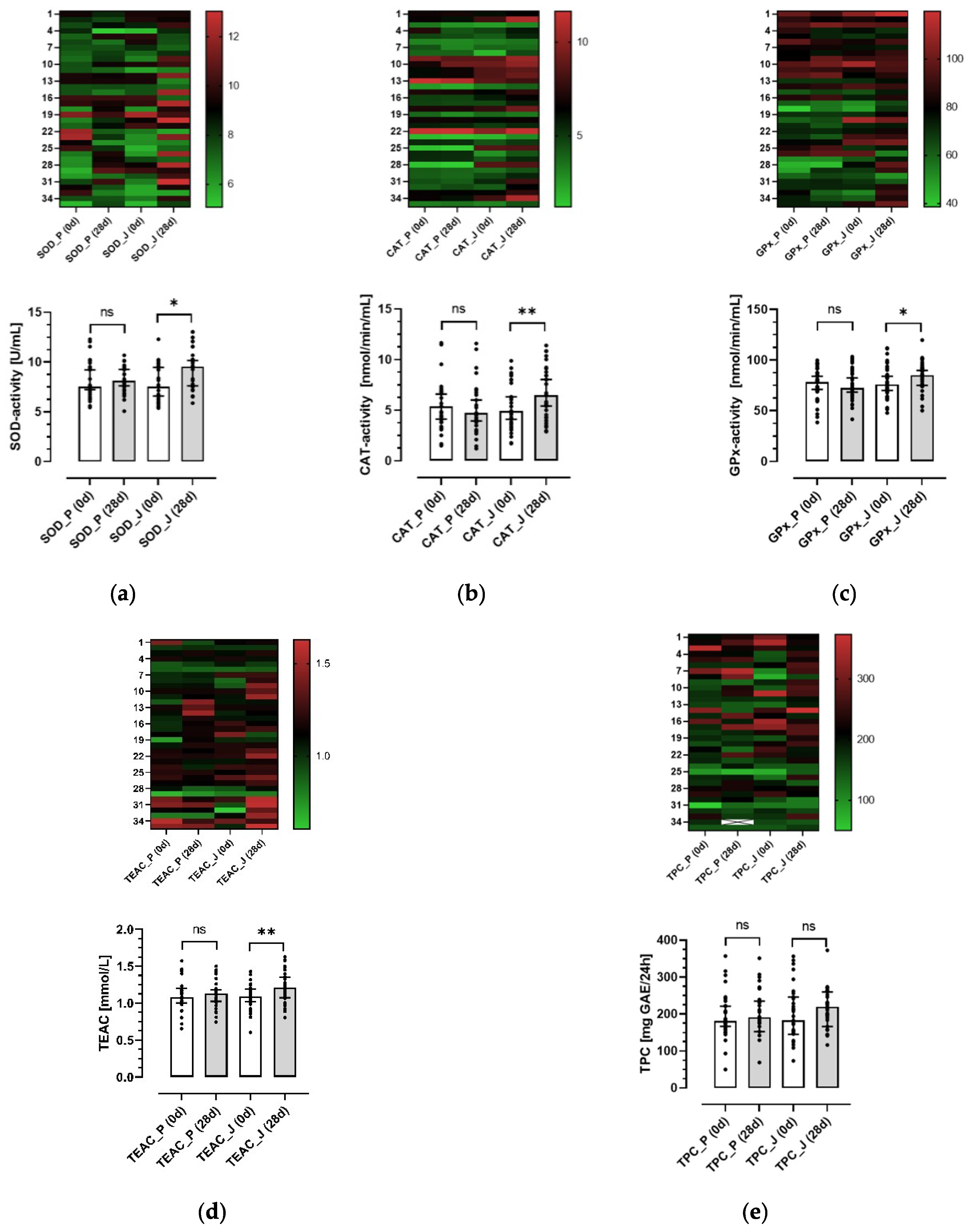
3.4. Effects of the Anthocyanin-Rich Juice and the Anthocyanin-Depleted Placebo on Antioxidative Biomarkers in Plasma and Urine
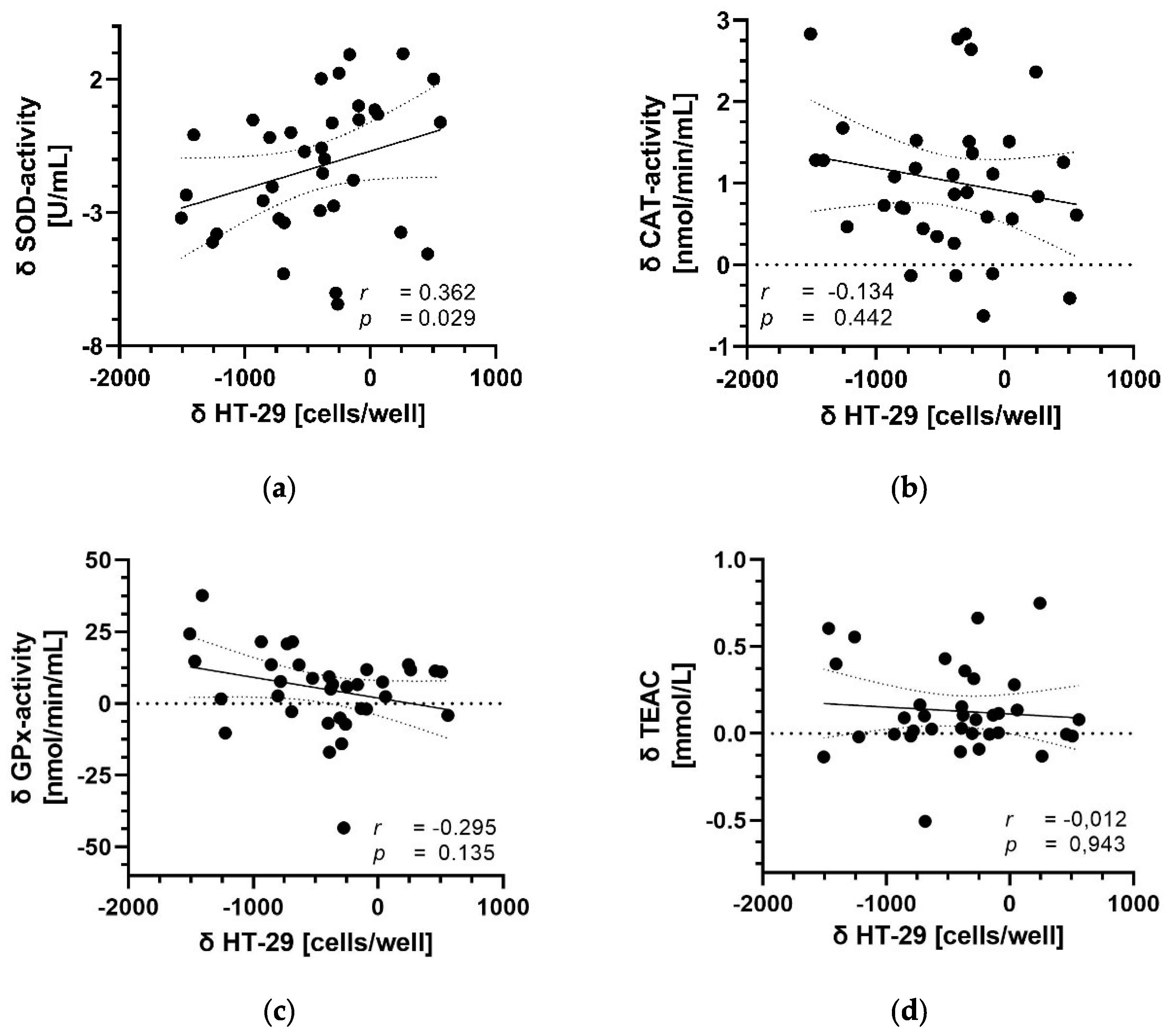
3.5. Correlation between Parameters of Antioxidant Capacity and Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Circulating Interleukin-6 Level, Dietary Antioxidant Capacity, and Risk of Colorectal Cancer. Antioxidants 2019, 8, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, S.J.D. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, I.; Brugge, J.S. The enemy of my enemy is my friend. Nature 2015, 527, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A Mitochondrial Switch Promotes Tumor Metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef] [Green Version]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.G.; Wikman, H.; Pantel, K.; Haigis, M.C.; De Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Allesina, S.; Alonso, D.; Pascual, M. A General Model for Food Web Structure. Science 2008, 320, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordheim, M.; Måge, F.; Andersen, Ø.M. Anthocyanins in Berries of Ribes Including Gooseberry Cultivars with a High Content of Acylated Pigments. J. Agric. Food Chem. 2007, 55, 5529–5535. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. Int. Rev. J. 2015, 6, 620–622. [Google Scholar] [CrossRef] [Green Version]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Chen, X.; Chen, T. Anthocyanins in Colorectal Cancer Prevention Review. Antioxidants 2021, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, D.-Y.; Yang, L.-Q.; Zhao, W.-Z.; Cai, L.-Y.; Shi, H.-P. Anthocyanin Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. J. Am. Coll. Nutr. 2018, 38, 470–477. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging Capacity of Berry Crops on Superoxide Radicals, Hydrogen Peroxide, Hydroxyl Radicals, and Singlet Oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant Activities and Antitumor Screening of Extracts from Cranberry Fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.A.M.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019, 53, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Not, C.; Guino, E.; Lujan-Barroso, L.; Garcia, R.M.; Biondo, S.; Salazar, R.; Moreno, V. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case–control study (the Bellvitge Colorectal Cancer Study). Cancer Causes Control 2012, 24, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Negri, E.; Talamini, R.; Bosetti, C.; Parpinel, M.; Gnagnarella, P.; Franceschi, S.; Dal Maso, L.; Montella, M.; Giacosa, A.; et al. Flavonoids and Colorectal Cancer in Italy. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1555–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Kim, H.G.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909. [Google Scholar] [CrossRef]
- Lee, W.S.; Shin, D.Y.; Lu, J.N.; Kim, G.-Y.; Jung, J.M.; Kang, H.S.; Choi, Y.H. Lee Anti-invasive activities of anthocyanins through modulation of tight junctions and suppression of matrix metalloproteinase activities in HCT-116 human colon carcinoma cells. Oncol. Rep. 2011, 25, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, J.; Mao, L.; Li, Q.; Guo, M.; Mu, T.; Zhang, Q.; Bi, X. Up-regulation of miR-24-1-5p is involved in the chemoprevention of colorectal cancer by black raspberry anthocyanins. Br. J. Nutr. 2018, 122, 518–526. [Google Scholar] [CrossRef]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 53, S92–S101. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Chandra, P.; Rathore, A.S.; Kay, K.L.; Everhart, J.L.; Curtis, P.; Burton-Freeman, B.; Cassidy, A. Contribution of Berry Polyphenols to the Human Metabolome. Molecules 2019, 24, 4220. [Google Scholar] [CrossRef] [Green Version]
- Kuntz, S.; Kunz, C.; Rudloff, S. Inhibition of pancreatic cancer cell migration by plasma anthocyanins isolated from healthy volunteers receiving an anthocyanin-rich berry juice. Eur. J. Nutr. 2017, 56, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Sutkowy, P.; Czuczejko, J.; Małkowski, B.; Szewczyk-Golec, K.; Łopatto, R.; Maruszak, M.; Woźniak, A. Redox State and Lysosomal Activity in Women with Ovarian Cancer with Tumor Recurrence and Multiorgan Metastasis. Molecules 2021, 26, 4039. [Google Scholar] [CrossRef] [PubMed]
- Kundaktepe, B.P.; Sozer, V.; Durmus, S.; Kocael, P.C.; Kundaktepe, F.O.; Papila, C.; Gelisgen, R.; Uzun, H. The evaluation of oxidative stress parameters in breast and colon cancer. Medicine 2021, 100, e25104. [Google Scholar] [CrossRef]
- Von Kleist, S.; Chany, E.; Burtin, P.; King, M.; Fogh, J. Immunohistology of the Antigenic Pattern of a Continuous Cell Line from a Human Colon Tumor. JNCI J. Natl. Cancer Inst. 1975, 55, 555–560. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- De Both, N.J.; Vermey, M.; Dinjens, W.N.; Bosman, F.T. A comparative evaluation of various invasion assays testing colon carcinoma cell lines. Br. J. Cancer 1999, 81, 934–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuntz, S.; Rudloff, S.; Kunz, C. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. Br. J. Nutr. 2008, 99, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie In Vitro and In Vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [Green Version]
- Spormann, T.M.; Albert, F.W.; Rath, T.; Dietrich, H.; Will, F.; Stockis, J.-P.; Eisenbrand, G.; Janzowski, C. Anthocyanin/Polyphenolic–Rich Fruit Juice Reduces Oxidative Cell Damage in an Intervention Study with Patients on Hemodialysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3372–3380. [Google Scholar] [CrossRef] [Green Version]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar] [CrossRef]
- Gagnon, M.; Zihler Berner, A.; Chervet, N.; Chassard, C.; Lacroix, C. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods 2013, 94, 274–279. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Porchet, N.; Aubert, J.P.; Swallow, D.; Gum, J.R.; Kim, Y.S.; Real, F.X.; Zweibaum, A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993, 106, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, J.; Billaut-Laden, I.; Happillon, M.; Lo-Guidice, J.-M.; Maunoury, V.; Imbenotte, M.; Broly, F. Gene Expression Profiling of Systems Involved in the Metabolism and the Disposition of Xenobiotics: Comparison between Human Intestinal Biopsy Samples and Colon Cell Lines. Drug Metab. Dispos. 2012, 40, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martinez-Gonzalez, M.A.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef]
- Msc, M.R.; Cherubini, A.; Zamora-Ros, R.; Urpi-Sarda, M.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C. Low Levels of a Urinary Biomarker of Dietary Polyphenol Are Associated with Substantial Cognitive Decline over a 3-Year Period in Older Adults: The Invecchiare in Chianti Study. J. Am. Geriatr. Soc. 2015, 63, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of Antioxidant Potency of Commonly Consumed Polyphenol-Rich Beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Düsman, E.; Almeida, I.; Pinto, E.; Lucchetta, L.; Vicentini, V. Influence of processing and storage of integral grape juice (Vitis labrusca L.) on its physical and chemical characteristics, cytotoxicity, and mutagenicity in vitro. Genet. Mol. Res. 2017, 16, gmr16029670. [Google Scholar] [CrossRef]
- Fröhling, B.; Patz, C.-D.; Dietrich, H.; Will, F. Anthocyanins, total phenolics and antioxidant capacities of commercial red grape juices, black curant and sour cherry nectars. Fruit Processing 2012, 3, 100–104. [Google Scholar]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2019, 206, 107447. [Google Scholar] [CrossRef]
- Wang, N.; Yang, L.; Dai, J.; Wu, Y.; Zhang, R.; Jia, X.; Liu, C. 5-FU inhibits migration and invasion of CRC cells through PI3K/AKT pathway regulated by MARCH1. Cell Biol. Int. 2020, 45, 368–381. [Google Scholar] [CrossRef]
- Sak, K. Chemotherapy and Dietary Phytochemical Agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [Green Version]
- Guyot, F.; Faivre, J.; Manfredi, S.; Meny, B.; Bonithon-Kopp, C.; Bouvier, A.M. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann. Oncol. 2005, 16, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Serala, K.; Steenkamp, P.; Mampuru, L.; Prince, S.; Poopedi, K.; Mbazima, V. In vitro antimetastatic activity of Momordica balsamina crude acetone extract in HT -29 human colon cancer cells. Environ. Toxicol. 2021, 36, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknaes, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Gao, Y.; Zhang, Q.; Chang, A.K.; Yang, Z.; Bi, X. Black raspberry anthocyanins increased the antiproliferative effects of 5-Fluorouracil and Celecoxib in colorectal cancer cells and mouse model. J. Funct. Foods 2021, 87, 104801. [Google Scholar] [CrossRef]
- Chavda, V.; Chaurasia, B.; Garg, K.; Deora, H.; Umana, G.E.; Palmisciano, P.; Scalia, G.; Lu, B. Molecular mechanisms of oxidative stress in stroke and cancer. Brain Disord. 2021, 5, 100029. [Google Scholar] [CrossRef]
- Paramanantham, A.; Kim, M.J.; Jung, E.J.; Nagappan, A.; Yun, J.W.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Lee, W.S. Pretreatment of Anthocyanin from the Fruit of Vitis coignetiae Pulliat Acts as a Potent Inhibitor of TNF-α Effect by Inhibiting NF-κB-Regulated Genes in Human Breast Cancer Cells. Molecules 2020, 25, 2396. [Google Scholar] [CrossRef]
- Ho, M.-L.; Chen, P.-N.; Chu, S.-C.; Kuo, D.-Y.; Kuo, W.-H.; Chen, J.-Y.; Hsieh, Y.-S. Peonidin 3-Glucoside Inhibits Lung Cancer Metastasis by Downregulation of Proteinases Activities and MAPK Pathway. Nutr. Cancer 2010, 62, 505–516. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Jayarathne, S.; Stull, A.J.; Park, O.; Kim, J.H.; Thompson, L.; Moustaid-Moussa, N. Protective Effects of Anthocyanins in Obesity-Associated Inflammation and Changes in Gut Microbiome. Mol. Nutr. Food Res. 2019, 63, e1900149. [Google Scholar] [CrossRef]
- Gu, J.; Thomas-Ahner, J.; Riedl, K.; Bailey, M.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary Black Raspberries Impact the Colonic Microbiome and Phytochemical Metabolites in Mice. Mol. Nutr. Food Res. 2019, 63, e1800636. [Google Scholar] [CrossRef]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Liu, X.; Tian, Y.; Xie, C.; Li, Q.; Cui, H.; Sun, C. Flavonoids, Flavonoid Subclasses, and Esophageal Cancer Risk: A Meta-Analysis of Epidemiologic Studies. Nutrients 2016, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Arteche, R.; Muñiz, P.; Cavia-Saiz, M.; García-Girón, C.; Garcia-Gonzalez, M.; Llorente-Ayala, B.; Corral, M.J.C.-D. Cancer chemotherapy reduces plasma total polyphenols and total antioxidants capacity in colorectal cancer patients. Mol. Biol. Rep. 2012, 39, 9355–9360. [Google Scholar] [CrossRef] [PubMed]
- Pahlke, G.; Ahlberg, K.; Oertel, A.; Janson-Schaffer, T.; Grabher, S.; Mock, H.-P.; Matros, A.; Marko, D. Antioxidant Effects of Elderberry Anthocyanins in Human Colon Carcinoma Cells: A Study on Structure–Activity Relationships. Mol. Nutr. Food Res. 2021, 65, 2100229. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. J. Cereb. Blood Flow Metab. 2016, 174, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Kropat, C.; Mueller, D.; Boettler, U.; Zimmermann, K.; Heiss, E.H.; Dirsch, V.; Rogoll, D.; Melcher, R.; Richling, E.; Marko, D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol. Nutr. Food Res. 2013, 57, 545–550. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the biological activity of anthocyanins and polyphenols in blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef]

| Anthocyanin-Rich Juice | Anthocyanin-Depleted Placebo | |||
|---|---|---|---|---|
| Anthocyanins | (mg/L) | (%) | mg/L | (%) |
| peonidin-3,5-O-diglucoside | 346 ± 12.5 | 36.8 | 1.7 ± 0.02 | 26.9 |
| malvidin-3,5-O-diglucoside | 138 ± 8.4 | 14.7 | 0.88 ± 0.06 | 14.0 |
| peonidin-3-O-glucoside | 83.5 ± 6.4 | 8.9 | 0.37 ± 0.01 | 5.9 |
| malvidin-3-O-glucoside | 63.4 ± 3.8 | 6.7 | 0.30 ± 0.01 | 4.7 |
| delphinidin-3-O-glucoside | 61.5 ± 2.9 | 6.5 | 0.80 ± 0.03 | 12.7 |
| delphinidin-3-O-galactoside | 53.6 ± 0.6 | 5.7 | 0.75 ± 0.01 | 11.9 |
| delphinidin-3-O-arabinoside | 53.4 ± 1.6 | 5.7 | 0.56 ± 0.02 | 7.4 |
| petunidin-3-O-glucoside | 43.7 ± 1.9 | 4.6 | 0.37 ± 0.02 | 5.8 |
| cyanidin-3-O-arabinoside | 27.2 ± 1.7 | 2.9 | 0.11 ± 0.02 | 1.7 |
| cyanidin-3,5-O-diglucoside | 18.2 ± 1.6 | 1.9 | 0.29 ± 0.00 | 4.6 |
| malvidin-3-(6”-O-coumaryl)-5-O-diglucoside | 17.1 ± 0.4 | 1.8 | n.d. | n.d. |
| petunidin-3-O-galactoside | 13.2 ± 0.3 | 1.4 | 0.11 ± 0.01 | 1.8 |
| petunidin-3-O-arabinoside | 8.8 ± 0.0 | 0.9 | 0.05 ± 0.01 | 0.8 |
| malvidin-3-O-arabinoside | 5.3 ± 0.0 | 0.6 | 0.02 ± 0.00 | 0.3 |
| peonidin-3-O-galactoside | 4.3 ± 0.0 | 0.5 | 0.01 ± 0.00 | 0.1 |
| delphinidin-3,5-O-diglucoside | 3.4 ± 0.0 | 0.4 | 0.09 ± 0.01 | 1.4 |
| Sum | 942 ± 10 | 100 | 6.3 ± 0.5 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behrendt, I.; Röder, I.; Will, F.; Mostafa, H.; Gonzalez-Dominguez, R.; Meroño, T.; Andres-Lacueva, C.; Fasshauer, M.; Rudloff, S.; Kuntz, S. Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study. Antioxidants 2022, 11, 1341. https://doi.org/10.3390/antiox11071341
Behrendt I, Röder I, Will F, Mostafa H, Gonzalez-Dominguez R, Meroño T, Andres-Lacueva C, Fasshauer M, Rudloff S, Kuntz S. Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study. Antioxidants. 2022; 11(7):1341. https://doi.org/10.3390/antiox11071341
Chicago/Turabian StyleBehrendt, Inken, Isabella Röder, Frank Will, Hamza Mostafa, Raúl Gonzalez-Dominguez, Tomás Meroño, Cristina Andres-Lacueva, Mathias Fasshauer, Silvia Rudloff, and Sabine Kuntz. 2022. "Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study" Antioxidants 11, no. 7: 1341. https://doi.org/10.3390/antiox11071341
APA StyleBehrendt, I., Röder, I., Will, F., Mostafa, H., Gonzalez-Dominguez, R., Meroño, T., Andres-Lacueva, C., Fasshauer, M., Rudloff, S., & Kuntz, S. (2022). Influence of Plasma-Isolated Anthocyanins and Their Metabolites on Cancer Cell Migration (HT-29 and Caco-2) In Vitro: Results of the ATTACH Study. Antioxidants, 11(7), 1341. https://doi.org/10.3390/antiox11071341








