Abstract
A cataract is a condition that causes 17 million people to experience blindness and is the most significant cause of vision loss, around 47.9%. The formation of cataracts is linked to both the production of reactive oxygen species (ROS) and the reduction of endogenous antioxidants. ROS are highly reactive molecules produced by oxygen. Examples of ROS include peroxides, super-oxides, and hydroxyl radicals. ROS are produced in cellular responses to xenobiotics and bacterial invasion and during mitochondrial oxidative metabolism. Excessive ROS can trigger oxidative stress that initiates the progression of eye lens opacities. ROS and other free radicals are highly reactive molecules because their outer orbitals have one or more unpaired electrons and can be neutralized by electron-donating compounds, such as antioxidants. Examples of natural antioxidant compounds are vitamin C, vitamin E, and beta-carotene. Numerous studies have demonstrated that plants contain numerous antioxidant compounds that can be used as cataract preventatives or inhibitors. Natural antioxidant extracts for cataract therapy may be investigated further in light of these findings, which show that consuming a sufficient amount of antioxidant-rich plants is an excellent approach to cataract prevention. Several other natural compounds also prevent cataracts by inhibiting aldose reductase and preventing apoptosis of the eye lens.
1. Introduction
A cataract is a condition where the eye’s lens clouds and can lead to progressive loss of vision. Cataracts are often associated with age, where with increasing age, the eye’s lens can turn cloudy due to the oxidative stress process, so that vision becomes blurry [1]. Based on age, cataracts are classified into senile, juvenile, and congenital cataracts [2]. Senile cataracts occur at an advanced age (age-related cataracts), juvenile cataracts are categorized when cataracts arise at a young age, and congenital cataracts are cataracts that occur at birth [3,4].
Senile cataract is one of the leading causes of visual impairment and blindness globally and is the most common form of cataract.
The oxidation process plays a vital role in lens opacities in senile cataract cases. The elderly population will increase, increasing the prevalence and incidence of senile cataract cases. Currently, the incidence of senile cataracts is 3.9% at the age of 55–64 years and increases to 92.6% at the age of 80 years and over [5,6].
The prevalence of cataracts as a cause of vision loss increases every year. The World Health Organization (WHO) claims that cataracts are the leading cause of blindness and visual impairment globally, accounting for around 47.9% of the world’s blind people. It is the cause of reversible blindness in more than 17 million (47.8%) of the 37 million blind people worldwide. Cataracts also account for 30–50% of blindness in African and Asian countries [7].
Antioxidants are one of the compounds reported to be able to inhibit the progression of cataracts. Antioxidants react with radical and non-radical species after oxidative stress to trigger defense mechanisms that protect intracellular and extracellular components [8]. Natural antioxidants are created in living cells in nutrition metabolism and immunological function to maintain an oxidation-reduction equilibrium.
Plants provide most natural antioxidants. Plants, which are plentiful in cereals, spices, and essential oils utilized in meat products for organoleptic purposes, are the most abundant source of antioxidants. Tea water extract has also been used as a source of natural antioxidants because it contains several compounds, such as catechins, tannins, and other flavonoids, with the advantage of not having a strong taste like essential oils [9]. Antioxidants and other phytochemicals are abundant in certain fruits and vegetables. Several minerals and vitamins are natural antioxidants because they act as cofactors for antioxidant enzymes. Various short, multifunctional peptides capable of neutralizing free radicals and preventing pro-oxidative metal ions have also been discovered in nature. Antioxidant peptides are produced as a result of the enzymatic breakdown of proteins [8].
Vitamin E, vitamin C, carotenoids, polyphenols, and phenolic compounds may include coumarins, cinnamic acid derivatives, flavonoids, tocopherols, and multifunctional organic acids. The flavonoid molecules flavonols, flavones, isoflavones, catechins, and chalcones are all antioxidants. There are also chlorogenic acid, caffeic acid, ferulic acid, and other cinnamic acid derivatives. The hydroxyl group (-OH) and the double bond are responsible for this property [10].
Based on the description above, in this article, we examined plants reported to have antioxidant activity and have the potential to prevent cataract progression. The most recent review compiled ethnopharmacological/ethnobotanical data on medicinal plants and plant-based natural products used for cataract treatment around the world [11], and another review [12] focused on natural chemicals with antioxidant capabilities that may be used as a large-scale interventional strategy and are also very inexpensive; now, we take a more comprehensive look. This article also includes the most recent updates of several natural products from plants are helpful in preventing cataractogenesis, a process of cataract formation. Antioxidant-containing natural products could be considered potential anticataract agents for the prevention of cataractogenesis. However, as not all natural antioxidants have anticataract properties, they were also studied in a comprehensive manner either in vitro and in vivo.
2. Methods
The approach employed is a systematic literature review (SLR) design, which is a systematic literature review by locating, analyzing, and interpreting all results on a single research subject utilizing Google Scholar, Science Direct, PubMed, and Wiley databases.
2.1. What Is a Cataract and What Are Cataract Characteristics?
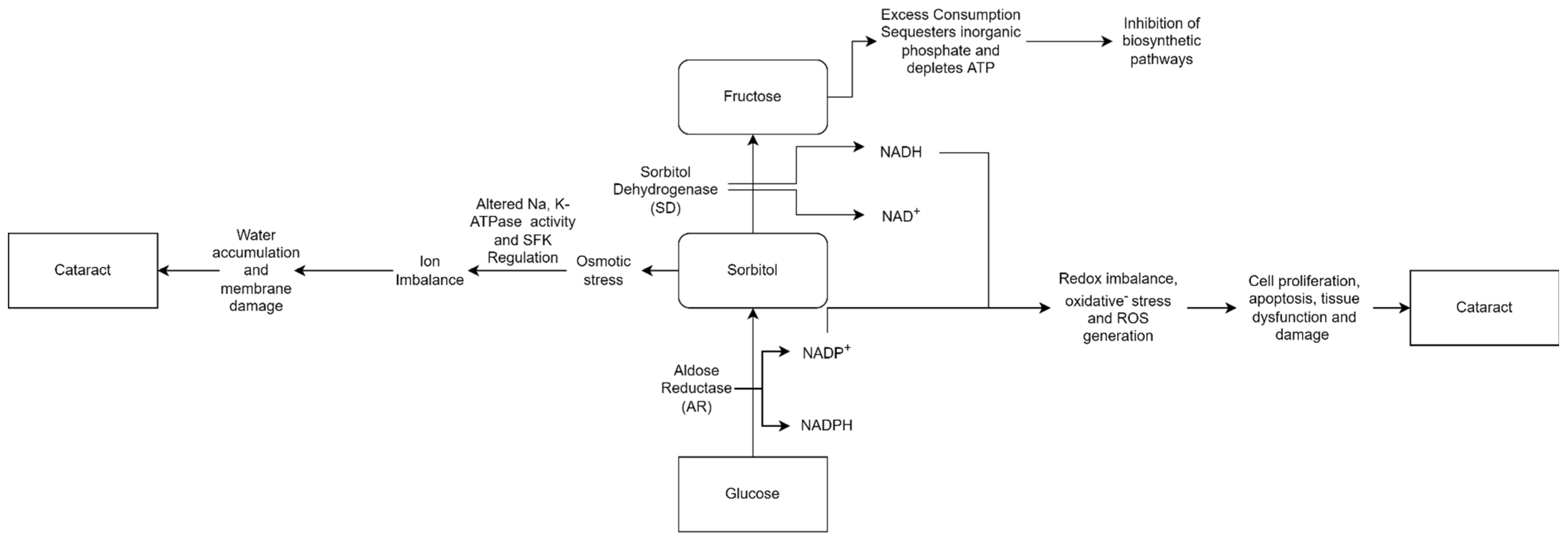
The lens is composed of transparent, flexible tissue and is located directly behind the iris and the pupil. It is the second part of your eye, after the cornea, that helps to focus light and images on your retina. Cataracts are the most common cause of blindness globally, and cataracts are a condition in which lens proteins clump together, causing the lens to become cloudy. Various factors can cause cataracts, but many cases show that free radicals are the mediators behind the pathological processes that lead to cataracts (Figure 1) [13].
Cataracts cause impaired vision function and vision loss because light cannot penetrate the lens. The capsule, lens epithelium, and lens fibers are the three main parts of the lens. Dense connective tissue forms a capsule. The entire lens body is composed of dense, concentric layers of lens fibers. The lens epithelium is a simple cuboidal epithelium that lines the anterior surface of the lens. The lens epithelium plays a crucial role in maintaining homeostasis by allowing ion permeability, nutrients, and osmolarity into the aqueous humor. The primary source of energy for lens tissue is glucose. Lens fiber osmolarity is enhanced by sodium/potassium adenosine triphosphatase and calcium adenosine triphosphatase [14,15].
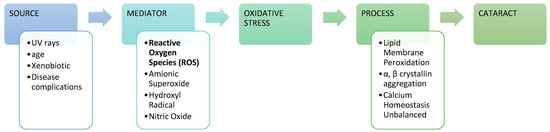
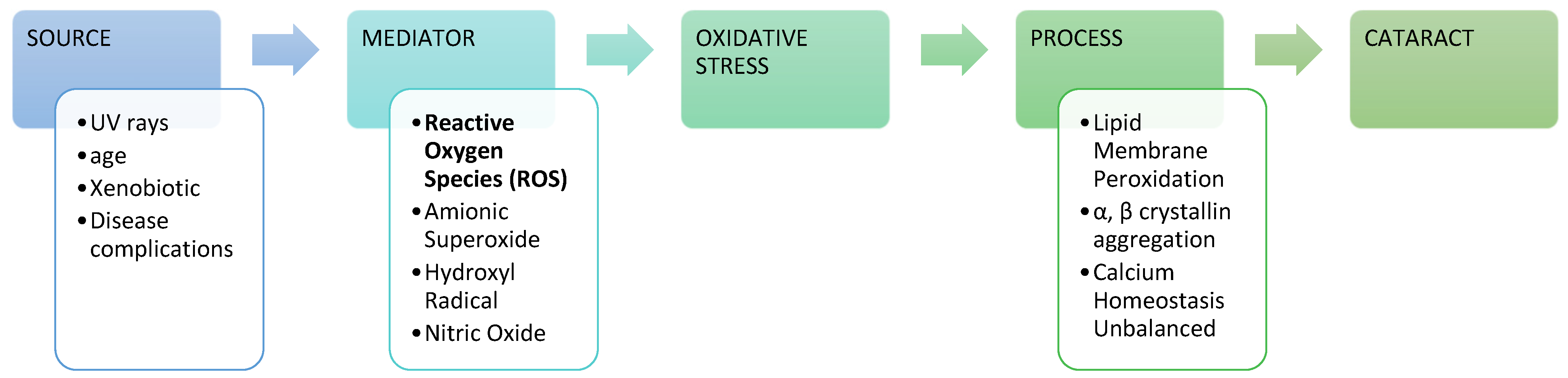
Figure 1.
Cataract progression with reactive oxygen species (ROS) mediators [16].
Figure 1.
Cataract progression with reactive oxygen species (ROS) mediators [16].
Numerous factors may produce cataracts. Pathophysiological alterations linked with disorders such as diabetes are a well-known cause of cataract development [17], and some xenobiotics have also been identified as being able to produce cataracts [18]. Cataracts can also be caused by diseases in newborns [19], injury or developmental disorder before birth or during childhood [20], smoking [21], and exposure to harmful substances such as UV rays [22] and corticosteroids [23], among many others.
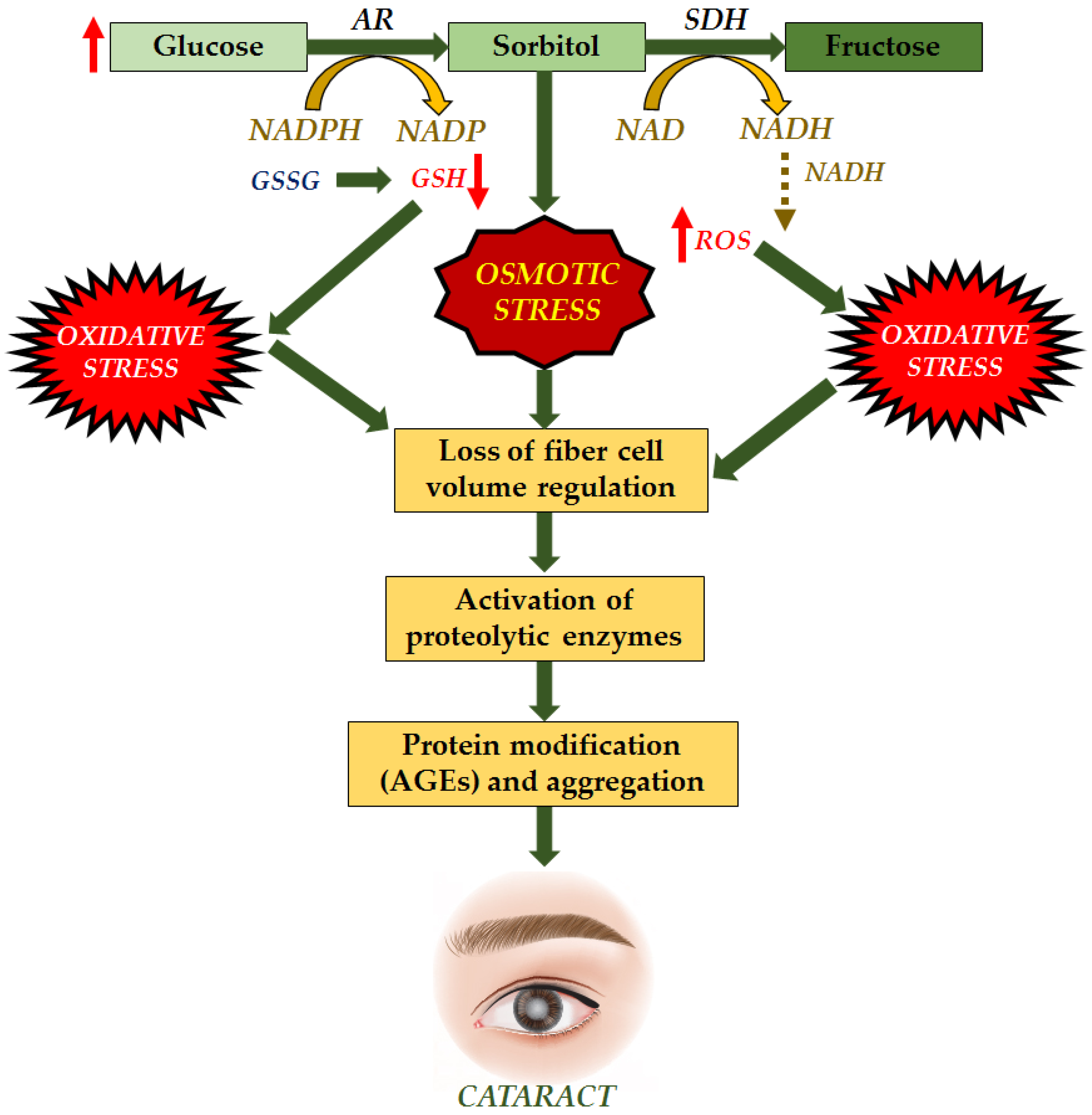
These various causes allow the development of cataracts to occur by multiple mechanisms as well. Cataracts can occur due to the accumulation of sorbitol. Extracellular glucose diffuses into the lens during hyperglycemia, causing post-translational modifications. Cataract progression is caused by excessive sorbitol synthesis and accumulates in the lens fibers, causing osmotic stress (Figure 2). Sorbitol is produced by aldose reductase using NADPH and cannot cross cell membranes. However, it can accumulate in cells and disrupt the osmotic balance, causing cell injury [24,25].
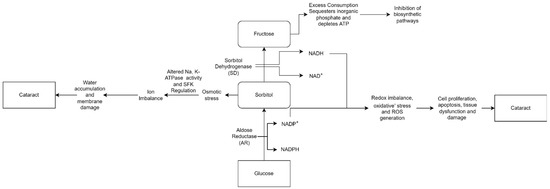
Figure 2.
“Osmotic Hypothesis” of sugar cataract formation, relating AR-mediated accumulation of polyols in lens swelling associated with complex biochemical changes, ultimately leading to cataract formation [26].
Cataract formation is also associated with hydrogen peroxide production via glucose auto-oxidation [27]. Aldose reductase, the key enzyme in the polyol pathway, catalyzes the conversion of glucose to the sugar alcohol sorbitol, which is ultimately converted to fructose by sorbitol dehydrogenase. As an osmolyte, sorbitol causes osmotic swelling, changes in membrane permeability, glutathione loss, myo-inositol loss, free radical formation, and hydrogen peroxide, all of which contribute to diabetes complications as cataracts, retinopathy, and neuropathy [28,29]. Higher concentrations of hydrogen peroxide cause tissue damage and clouding of the lens.
Special glasses, anti-glare glasses, or magnifying lenses can help with the early symptoms of cataracts, and if they are not treated, surgery is the treatment of choice for cataracts [30,31,32,33]. However, cataract surgery is costly with several consequences: endophthalmitis, posterior capsule rupture, postoperative macular edema, and posterior capsule opacification [34]. Besides that, cataract surgery changes the shape of the corneas and this treatment occasionally causes presbyopia. Presbyopia is the physiological degradation of accommodation or loss of accommodation power due to nuclear cataract. One of the disadvantages of cataract surgery is a lack of true accommodative ability. The loss of accommodative power is essentially due to the progressive failure of the capsule to mold the lens into a more spherical shape [35]. Therefore, searching for safe substances that can reduce the risk or delay the onset of cataracts is an essential step in developing cataract treatments.
2.2. Free Radicals Contribute to Cataract Formation
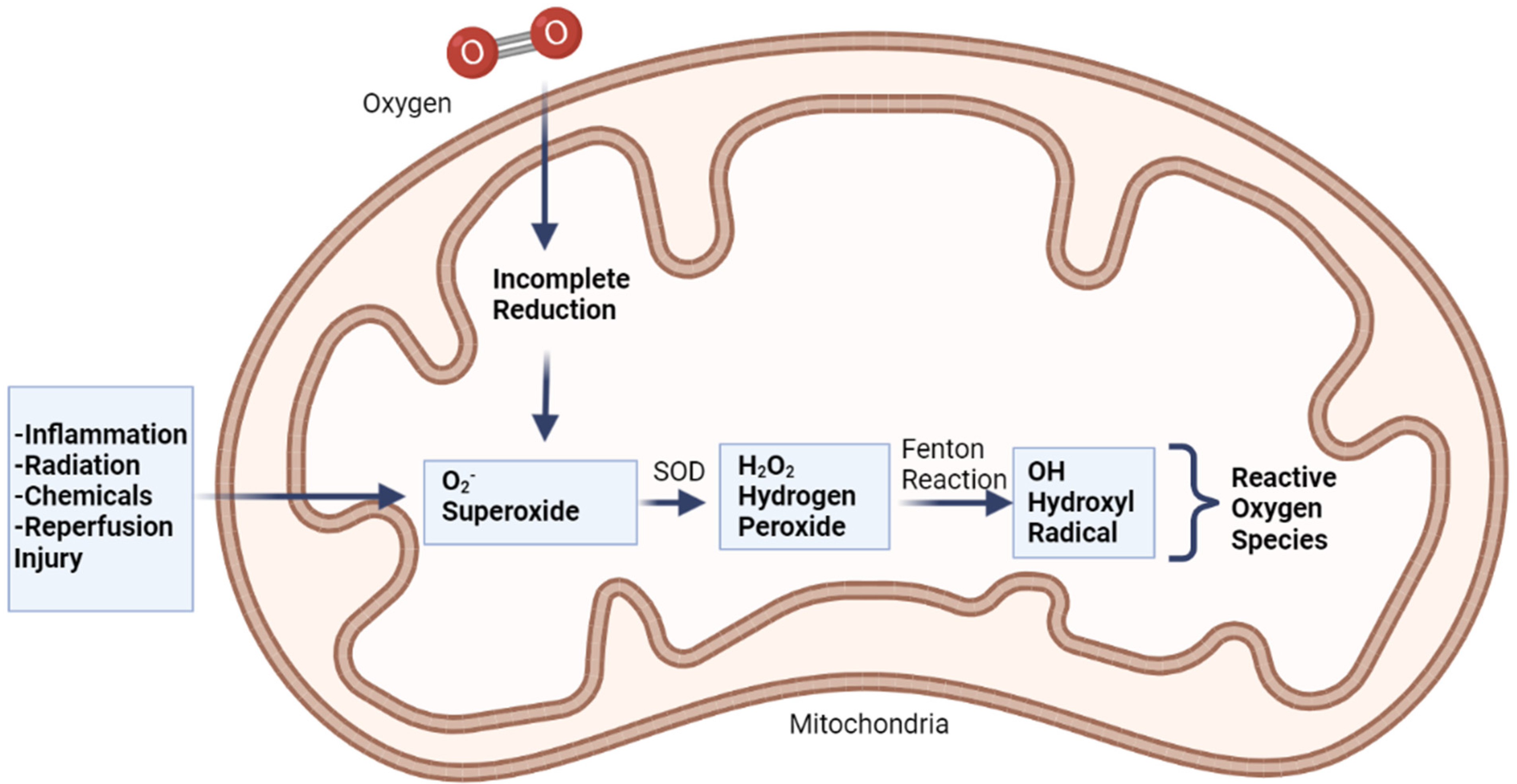
The electrons of an atom are arranged into orbitals, each of which can accommodate a different pair of electrons. Free radicals are molecules that have only one electron in their outermost orbital or an unpaired electron [36]. Free radicals will take electrons from each adjacent molecule to be stable, triggering cell damage. When each molecule gains or loses electrons, free radicals are produced. Free radicals can be created in the body in two ways: physiologically as part of normal metabolic processes, or pathologically due to illness [37,38,39].
The primary physiological source of free radicals is cellular respiration [40]. An electron transport chain carries electrons from complex to complex and ultimately to oxygen, providing a proton gradient that is utilized to make ATP. The process of generating ATP by donating electrons to the complex in the inner mitochondrial membrane is known as oxidative phosphorylation. In the last part of this process, a cytochrome c oxidase molecule transforms electrons into oxygen [40].
When oxygen accepts four electrons, it usually turns into water. If oxygen does not take all four electrons, it will have an unpaired electron in its orbital, which will cause free radicals to develop. Superoxide is produced when oxygen is supplied with only one electron (O2). It produces hydrogen peroxide (H2O2) with two electrons and hydroxyl radical with three electrons (Figure 3) [41].
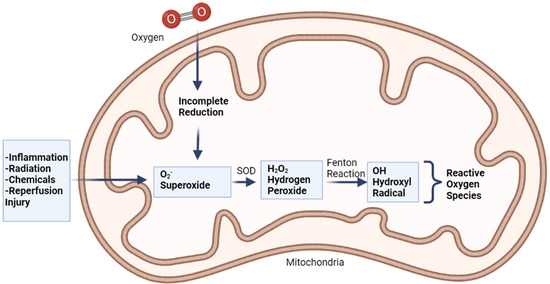
Figure 3.
Production of free radicals via Fenton reaction, adapted from Coleman (2010) [42].
Free radicals can also be produced as a result of a pathogen. First, during inflammation, phagocytes such as macrophages can produce free radicals. Phagolysosomes are formed when infections enter the body and are consumed by phagocytes. NADPH oxidase, triggered by lysosomal enzymes and causing NADPH to be oxidized, losing two electrons, is also present in these phagocytes. These electrons can be captured by nearby oxygen molecules, forming O2 ions [43]. Superoxide dismutase (SOD), another enzyme, may combine these ions with hydrogen ions to create hydrogen peroxide. A respiratory burst (also known as an oxidative burst) is a process that results in the production of superoxide ions and hydrogen peroxide. Furthermore, phagocytes include a kind of nitric oxide synthase (eNOS), an enzyme that produces nitric oxide, which aids in the killing of infections [44]. On the other hand, nitric oxide reacts with superoxide ions to produce peroxynitrite free radicals (ONOO−). These ions and chemicals kill bacteria by rupturing cell membranes and disrupting protein synthesis [45].
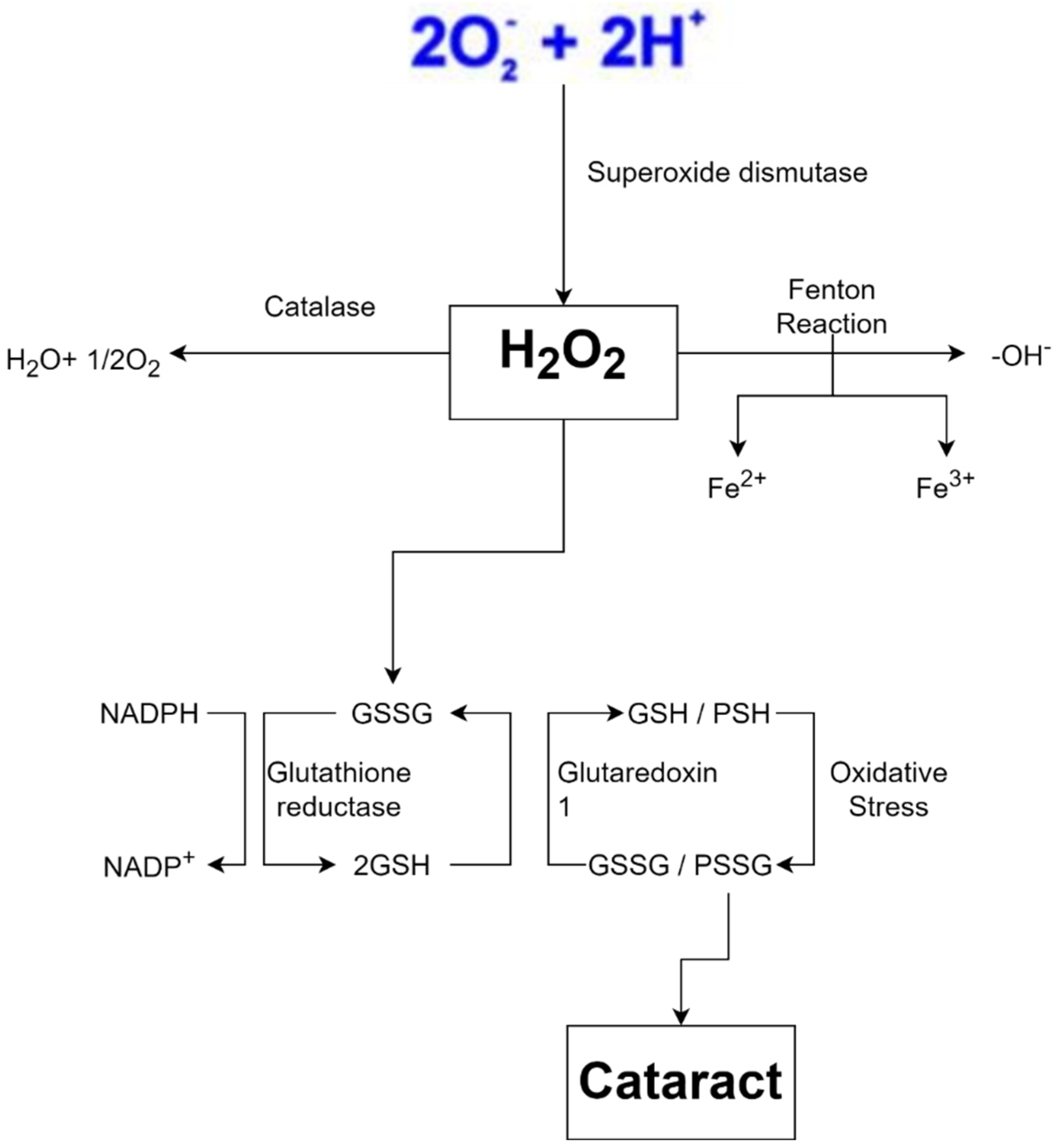
Free radicals are also produced by exposure to ionizing radiation such as X-rays. Radiation steals electrons from water in tissues, converting them into hydroxyl radicals. When metals such as copper or iron accumulate in the body, free radicals are produced. Hemochromatosis, for example, is a condition in which the body absorbs too much iron. Excess iron is oxidized by hydrogen peroxide, yielding iron 3+, hydroxyl radicals, and hydroxide ions as byproducts; iron 3+ may then be reduced to iron 2+ by hydrogen peroxide, yielding peroxide radicals and protons, and the cycle can be repeated indefinitely. As a result, the Fenton reaction can break down H2O2 to OH- in the presence of transmission metals, such as Fe2+ or Cu2 +. Fenton reaction also produces free radicals, including numerous ROS such as superoxide anion radical (•O2−), H2O2, and hydroxyl free radical (•OH), and may lead to structural damage of the crystalline lens and contribute to cataract formation (Figure 4) [4]. This harms cells in numerous organs over time, resulting in cell death and tissue fibrosis [46].
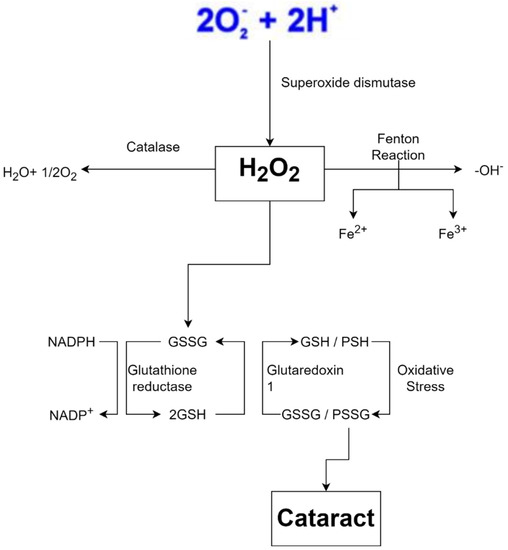
Figure 4.
Oxidative stress is a key feature of cataract formation [47,48,49].
Ischemia, or lack of blood flow to organs or tissues, also generates free radicals. Ischemic damage can cause mitochondria to produce ROS. Reperfusion occurs when blood flows back into is chemical tissue, carrying extra oxygen. When all this oxygen combines with pre-existing free radicals, it causes more cellular damage. Ischemia-reperfusion injury (IRI) is the medical term for this [50]. Free radicals are also produced when chemicals or drugs enter the body and are metabolized by the liver. Many free radicals are created when the liver metabolizes medicines such as acetaminophen or paracetamol (the primary active ingredient in TYLENOL® products), which may cause considerable liver damage [51].
Because the body creates free radicals under normal circumstances, defensive systems are in place to keep them in check. Antioxidants such as vitamin A, C, and E, for example, deliver electrons that neutralize free radicals and protect cells [52]. Glutathione, another chemical in our body, functions as an antioxidant and neutralizes H2O2. To work properly, the two glutathione must be in a reduced form, allowing them to donate electrons and protons to H2O2 and turn it into harmless water. Because this mechanism oxidizes glutathione, glutathione reductase needs reduced nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor to restore glutathione to its functional state before restarting its activity. NADPH forms nicotinamide adenine dinucleotide phosphate (NADP+) after losing electrons. To replenish the supply of NADPH, an enzyme called glucose-6-phosphate dehydrogenase (G6PD) oxidizes glucose-6-phosphate and converts NADP+ to NADPH. Since glucose-6-phosphate is a byproduct of glucose, humans usually have large amounts of this substance as long as they are not starving [53].
Metal-carrying proteins, which attach to metal ions and assist in transporting or storing them, are another protective mechanism. This mechanism fights free radicals as if the ions were hidden so they could not form free radicals. Transferrin, which binds to iron, and ceruloplasmin, which binds to copper, are two examples of proteins attaching to metals and transporting them through the bloodstream. On the other hand, free radical scavenging enzymes transform free radicals into non-toxic molecules such as water. The enzyme SOD converts superoxide to hydrogen peroxide. In peroxisomes, catalase (CAT) converts hydrogen peroxide to water, while glutathione peroxidase in the cytoplasm does the same. When the amount of free radicals created surpasses this defensive system, cell damage ensues [54,55].
2.3. Natural Ingredients’ Potential as an Alternative Cataract Treatment
There have been attempts to employ herbal medicines to prevent cataract advancement based on the model of cataract development and the mechanism of its production route. Natural antioxidant molecules have been reported to have an inevitable application in cataract prevention and control due to their easy availability and fewer complications [56]. These biomolecules are excellent at preventing other molecules from oxidizing and producing free radicals. These free radicals set off a chain reaction, causing all lens cells to be damaged. Most of these antioxidants are reducing agents, such as thiols or polyphenols, which inhibit free radical chain reactions. Flavonoids, phenolic acids, carotenoids, vitamins, and lactoferrin are natural antioxidant compounds with anticataract action [57].
In fact, many antioxidants derived from plants such as curcumin, vitamin C, and vitamin E have been well recognized as potential anticataractogenic therapeutics. Vitamin C has been shown to be effective against UV-induced cataracts and age-related cataracts. It also prevents nuclear cataract. Vitamin C also scavenges free radicals. Vitamin E has been shown to be effective against both UV-induced and age-related cataracts by postponing galactose and amino thiazole-induced cataract, inhibiting lipid peroxidation, and maintaining membrane integrity. Curcumin was discovered to be an effective free radical scavenger due to its cytoprotective effect on glutathione-S-transferase enzymes and its efficacy against hyperglycemia, galactose, and naphthalene-induced cataract. Curcumin can also inhibit NFκB [12]. This section provides an overview of the various categories of plant-derived compounds that have been evaluated for potential as anti-cataracts.
2.4. Antioxidant Activities of Plants in Preventing Cataractogenesis
2.4.1. Antioxidant Activities of Plants
The function of oxidative stress in cataract formation has been well documented [58,59]. As a result, antioxidants and free radical scavengers might be used as therapeutic techniques to treat cataracts. The study of antioxidants is growing because of their protective role in food and pharmaceutical products against oxidative damage in the body and pathological processes mediated by oxidative stress [60]. To obtain good antioxidant activity, several things need to be considered, such as using the type of solvent, as [61] reported. The antioxidant activity of the methanol extract of Torilis leptophylla L. crude and its derivative fractions was found to be varied. In addition, screening plant antioxidant properties and their derivative compounds require appropriate methods [60]. Therefore, this review examines previous studies related to antioxidant activity derived from plants.
This difference in antioxidant activity appears from the difference in the degree of polarity between the solvents used. The results of one-way analysis of variance (ANOVA) obtained in [62] showed that the extraction yield, phytochemical content, and antioxidant properties were significantly affected (p < 0.05) by the polarity of the extraction solvent. The results of other studies related to the different types of solvents on antioxidant activity were carried out by [63], who extracted Sargassum serratifolium leaves using various solvents such as ethyl acetate, ethanol, methanol, acetone, n-hexane, chloroform, and water. According to the study’s findings, ethanol is the most efficient extraction solvent and has the potential to operate as a natural antioxidant. Extraction in highly polar solvents yields high extracts but low phenolic and flavonoid content compared to non-polar ones [62]. The increase in total antioxidant activity and polarity-dependent reducing properties indicated the extraction of strong antioxidant compounds in polar solvents.
In addition to being influenced by the solvent used, antioxidant activity in several works of literature is also related to total phenolic and flavonoid levels. Research conducted by [64] has shown a strong association between antioxidant activity and total flavonoid content of many varieties of Nepalese vegetables.
Plant secondary metabolites with an aromatic ring containing at least one hydroxyl group are phenolic compounds and natural flavonoids [65]. Because their hydroxyl groups can directly contribute to antioxidant activity, phenolic substances are effective electron donors [66]. In addition, several of them promote the production of endogenous antioxidant molecules in cells [67]. According to various studies, free radical inhibition, peroxide decomposition, and metal inactivation are all properties of phenolic compounds [68]. The research conducted by [69] has shown a correlation between total phenolic content with total antioxidant capacity and lipid peroxidation inhibitory activity in in vitro studies.
Previous reports showed that Sargassum serratifolium extracted using several solvents exhibited different total phenolics and antioxidant activities [63]. In addition to differences in solvent types related to polarity, plant preparation methods were also reported to affect antioxidant activity, such as research on fresh leaves and dried leaves of Datura metel L., (Amethyst) plants extracted with several solvents. The tendency of the content is the same, but the antioxidant activity test shows a difference where the antioxidant activity of dry crude extract equivalent to DPPH is on the order of butanol > chloroform > ethyl acetate extract > methanol > hexane extract. However, the order of antioxidant activity of the fresh organic crude extract against DPPH was methanol > hexane > chloroform > ethyl acetate extract > butanol [70]. Table 1 below shows some of the plants reported to have antioxidant activity.

Table 1.
Summary of plants that have been reported to have antioxidant activities.
Table 1 shows that the strength of antioxidant activity in plants is affected by several factors such as polarity of the solvent extraction, growth location plant species, and mode of action of antioxidant compounds present in a sample. These factors need to be studied more deeply to understand the potency of plant species to obtain maximum antioxidant activity. The white horehound shows relatively high antioxidant activity. The highest EC50 is the MVA extract of Marrubium vulgare L. leaves, with an EC50 of 6.43 ± 0.0411 mg/mL. Ginger also showed promising results. The highest IC50 is the methanol extract of Nigerian Zingiber officinale with a FRAP assay result of 89.15 ± 0.29 μg/mL. Antioxidant activities of ginger extracts were also studied in acetone extract, which has a maximum IC50 value of 0.654 and 0.812 mg/mL.
2.4.2. Cataract Treatment with Herbal Plants
A cataract is a complex illness with several risk factors. Oxidative stress is a key factor in the onset and progression of cataracts [84,85]. An assessment of the contribution of this mechanism to cataract formation was carried out in a model of induced cataracts in experimental animals. A selenite-induced cataract is one of the good models of senile nuclear cataract and is very rapidly induced [86]. Degradation of calcium homeostasis increased ROS or free radical generation, calpain (calcium-activated protease) activation, insoluble protein, crystal precipitation, phase change, and cytoskeletal loss are the major causes of selenite-induced cataracts [87]. The eye lens possesses a robust antioxidant system as a defensive mechanism against harmful damage from ROS or free radicals. This system contains antioxidants such as reduced glutathione and antioxidant enzymes such as SOD, CAT, and glutathione reductase/peroxidase (GR/Gpx) [88].
Free radicals can cause gene mutations that lead to the formation of cataracts. Free radicals compete with electrons from intracellular molecules resulting in lipid peroxidation, protein modification, lesions on chromosomes, and mitochondrial DNA. This can result in impaired transmission and gene expression and react with DNA chains that also cause mitochondrial DNA (mtDNA or mDNA) damage. This DNA damage disrupts the gene regulatory system, interfering with protein regulation and expression. Mutations in the R48C gene impair A-crystallin stability, associated with lens opacities [89,90].
Meanwhile, free radicals also can cause autophagy, necrosis, and apoptosis of tissues. The regulation of the autophagic system in the body depends on the autophagic flux process, which is responsible for removing abnormal proteins. This results in impaired autophagosome binding to lysosomes, resulting in the accumulation of p62 (a classical receptor of autophagy). This accumulation activates caspases which then increase apoptosis due to the activation of factor-kappa B (NF-κB) [90,91].
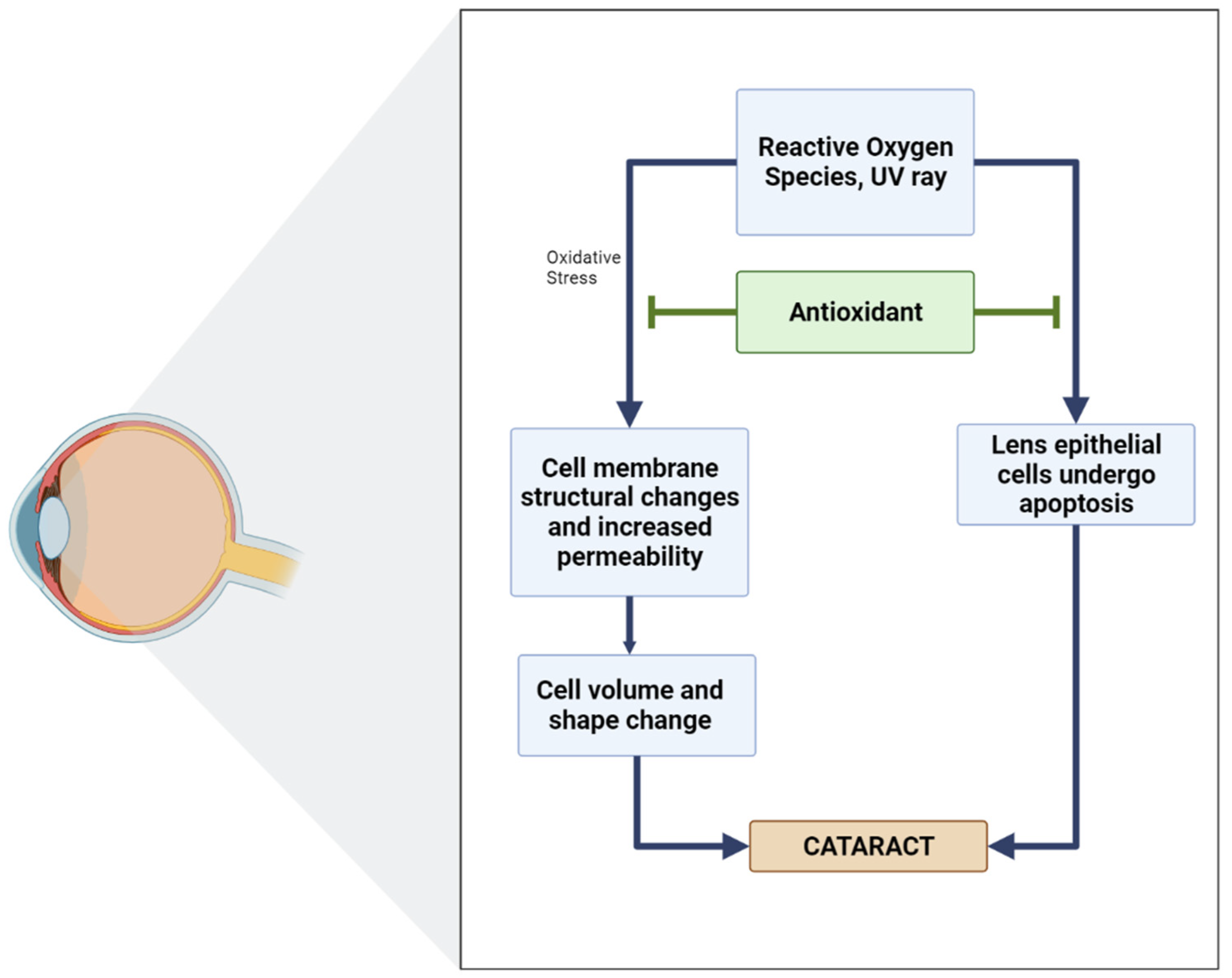
In biological systems, the balance between oxidants and antioxidants is of the utmost importance, which has both physiological relevance (beneficial) and pathological consequences (which usually lead to the formation of diseases, for example, cataracts). Several studies have shown a positive relationship between antioxidant intake and a reduction in the incidence or development of cataracts (Figure 5) [92].
In animal experiments with this condition, compounds of plant origin and herbal medicine have also been demonstrated to have anticataract potential. Quercetin, a flavonoid found in fruits and vegetables, is a potent antioxidant and free radical scavenger with various health advantages, including cardioprotective, anti-diabetic, anti-inflammatory, and anticancer properties [93]. In the study [94], in Sprague Dawley mice, quercetin reduced the onset and development of selenite-induced cataracts and maintained lens chaperone function. In another study, intraperitoneal injections of citrus flavonoids prevented selenite-induced lenticular opacities in Wistar rats, with a corresponding increase in antioxidant enzyme activity, CAT, SOD, glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), and glutathione reductase (GSH-Rx), as well as a reduction in lipid peroxidation, when compared to lenses treated only with selenite [95].
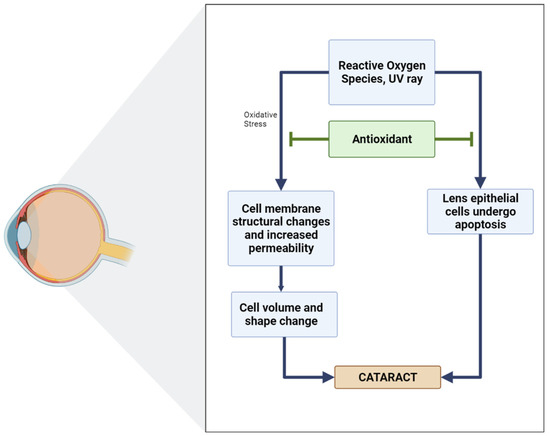
Figure 5.
Diagram of the role of antioxidants in inhibiting cataracts [96].
Figure 5.
Diagram of the role of antioxidants in inhibiting cataracts [96].
Curcumin is a brilliant yellow chemical with antioxidant qualities that is derived from the Zingiberaceae family’s Curcuma longa plant. Curcumin inhibits the formation of cataracts produced by galactose, oxidative stress, and streptozotocin by inhibiting lenticular antioxidants, lipid peroxidation, and the maintenance of soluble protein content. In Sprague Dawley mice, Nakazawa et al. (2017) found that both oil-soluble antioxidant compounds and water-soluble antioxidants may prevent the onset and progression of selenite-induced cataracts while still maintaining lens chaperone activity [97,98,99].
The report [100] stated that the ethanolic extract of the leaves and stems of Cineraria maritima showed promising results in treating cataracts in the eye lens of goats. According to the ethanol extract of the leaves of the binahong plant, the lens group of the goat lens induced with glucose and the addition of the binahong (Anredera cordifolia (Tenore) Steenis) extract exhibited more transparent results than the lens group induced with 55 mM glucose concentration. Binahong can suppress malondialdehyde generation at doses of 100 or 200 μg/mL [101]. In another study, it was stated that Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinereal, was effective in the treatment of cataracts in the eye lens of Sprague Dawley rats induced by selenite from the results of testing biochemical parameters such as activity of SOD, CAT, GPx, GR, GST, Ca2+ ATPase, glutathione, ROS, and lipid peroxidation product (malondialdehyde) were found to be effective in the treatment of cataracts with lupeol [100,102].
Another study found that the root extract had more antioxidant activity than the leaf extract of the two extracts tested. This conclusion was corroborated by the presence of more apparent antioxidant components in the ethanolic extract of L. aspera root. The root extract of aspera root was tested in the lenses of cultured Wistar rats for probable anticataractogenic potential. The results showed that when the extract was combined with the extract aspera root ethanol in the lenses of selenite-induced Wistar rats, mean enzymatic antioxidant activity, mean levels of reduced glutathione, and mean malondialdehyde expression levels of genes encoding A- and B1-crystalline proteins were kept close to normal, and mean levels of crystalline proteins themselves were kept close to normal [103]. Kaemoferol, for example, is a natural flavonol, a secondary metabolite found in many plants, reveals effectiveness for anti-inflammatory and antioxidant properties. This compound also demonstrated therapeutic antiglaucoma efficacy through suppressing ocular hypertension, inflammation, and oxidative stress [104]. Table 2 shows the results of the analysis of several types of plants that are reported to be able to be used in cataracts management.

Table 2.
A list of plants and parts of plants used to prevent cataractogenesis.
Based on several references, as shown in Table 2, it can be seen that the use of plant extracts shows promising results in overcoming the problem of cataracts. The induction cataract model can show the effectiveness of the extracts given. In addition to plant extracts, nanoparticles synthesized from plants have also demonstrated effectiveness in treating cataracts, as reported by [109], where the nanoparticles synthesized from shallots showed good anticataract activity compared to shallot extracts that were not synthesized into nanoparticles. Another study investigated the antioxidant capacity and efficiency of silver nanoparticles (AgNPs) biosynthesized using an ethanolic extract of Tabernaemontana divaricata leaf in preventing selenite-induced opacification of the ocular lens in vitro (cataractogenesis). The activity of CAT, SOD, GPx, and GST, as well as levels of reduced glutathione and malondialdehyde, were measured in this investigation. The ethanolic extract of T. divaricata and AgNPs biosynthesized using T. divaricata extracts exhibit excellent in vitro antioxidant activity and the capacity to inhibit experimental selenite-induced opacification in Wistar mice’s lenses, according to the findings [114].
Several in-vivo studies have also proved the ability of plant products to have a positive effect on cataract [11]. Streptozotocin (STZ)-induced diabetic rats were used in the in vivo experiment by Chung et al. At 11 weeks following STZ injection, diabetic control rats acquired cataracts, but oral Aralia elata extract provided at 300 and 600 mg/kg body weight for 11 weeks decreased cataract formation by 15% and 12%, respectively [115].
The research looked at whether highbush blueberry leaf polyphenols could help prevent cataracts and the reasons behind it. HPLC-DAD was used to measure chlorogenic acid, quercetin, rutin, isoquercetin, and hyperoside in Vaccinium corymbosum leaf decoction (BBL). On postnatal days 11 and 12, Wistar rats were administered subcutaneously with 20 μmol selenite (Na2SeO3)/kg body weight or intraperitoneally with 100 mg dry BBL/kg body weight. Only normal saline was given to the control group. BBL considerably reduced lens opacification, according to a cataract examination. It also protected the lens from oxidative selenite assault, calpain activation, and protein loss and aggregation [116]. In model rats, rosmarinic acid, a polyphenol found in rosemary (Rosmarinus officinalis), was confirmed to delay cataract development and lower the degree of lens opacification [116].
Natural substances containing antioxidants or secondary anti-inflammatory metabolites may serve as anticataract agents in modern herbal medicine, which has played a significant role in treating oxidative stress and its consequences [117]. In most instances, free radicals cause lens opacity [12], and protein alteration by free radicals is also a result of extreme oxidative stress. Some plant-based substances can inhibit protein insolubilization, delaying lens opacification [12]. Natural chemicals that are antioxidants or secondary anti-inflammatory metabolites have the potential to be the most effective anticataract treatments. Antioxidant effects are one of the primary mechanisms for cataract prevention in most instances. However, not all plants with antioxidant potential can have anticataract properties. Plant polyphenols have been known to have an anticataractogenic effect has been thoroughly investigated in vitro and in animals [118,119].
As reported in the literature, the chemical structure of many antioxidants plays an important role in preventing ocular disease progression. The effect of aromatic ring number in phenolic compound-conjugated chitosan injectables was investigated with the purpose of developing a more sophisticated drug carrier with significant anti-inflammatory and antioxidant characteristics. Low and high numbers of aromatic rings might have negative effects on injectables’ pharmaceutical uses; however, a molecule with a moderate ring number has been shown to be the most effective agent for improving drug delivery and giving chitosan injectables medicinal qualities. The intracameral infusion of kaempferol-conjugated pilocarpine, which can treat progressive glaucoma by concurrently exerting various pharmacological actions to decrease ocular hypertension, inflammation, and oxidative stress, shows extraordinary efficacy [104].
2.5. Other Natural Ingredients Besides Antioxidants That Can Inhibit Cataracts
2.5.1. Natural Antioxidant as Antiglycation Agent
Glycation is a phenomenon which is caused by increased glucose level in skin fibers. Glycation, also known as Maillard reaction, is a non-enzymatic reaction adduct formation between amino groups and carbonyl compounds. Glycation process occurs through oxidation, dehydration and cyclization reactions, and irreversible compounds, called advanced glycation end products (AGEs). During healthy aging, AGEs are formed at accelerated rates in diabetes, and also as causative factors for pathogenesis of diabetes, neurodegenerative disease, and cataracts [120].
Protein glycation changes the biological activity of proteins and starts the breakdown process; therefore, stopping it can help people with diabetes avoid significant consequences. With aging, advanced glycation end products (AGEs) build up in the lens, causing opacities [121]. Non-enzymatic interactions between the amino groups of proteins and the carbonyl-reducing sugars create the primary problems of diabetes (one of which is cataracts). Attempts to impact protein glycation have been made in a variety of ways. Various natural and synthetic substances, including flavonoids, phenol derivatives, imidazoles, Schiff bases, thiazolidines, and sulfates, have been shown to suppress protein glycation and the formation of AGE products. There are several mechanisms involved, including capturing reactive amino groups and preventing them from reacting with glucose or capturing carbonyl compounds, chelation with glycation-catalyzing trace metal ions, radical scavenging, and inhibition of oxidative degradation of metal catalysts for glucose or various glycated protein intermediates. By avoiding AGEs buildup, antiglycation treatment will become a feasible approach for managing advanced diabetic complications [122].
Other natural substances such as quinic acid from Erigeron annuus was reported to exhibit the most potent inhibitory activity against AGE formation and prevented opacification of rat lenses. This compound also has been reported to act as an inhibitor of RLAR (rat lens aldose reductase), AGE formation, AGEs–BSA cross-linking, and cataractogenesis. The molecular mechanisms of AGEs in the formation of cataracts are presented in Figure 6 [123,124].
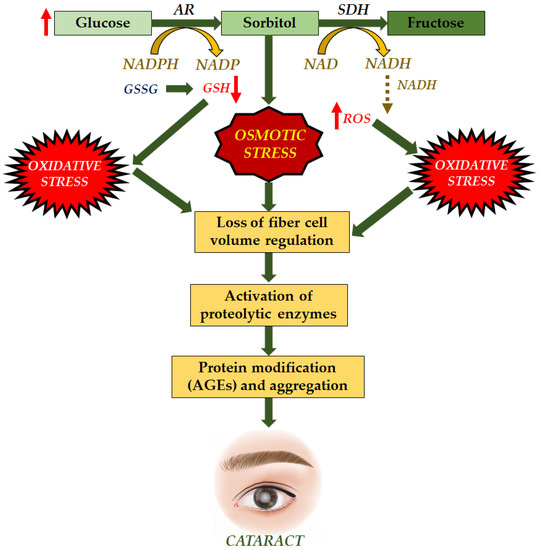
Figure 6.
Molecular mechanisms of AGEs for formation of cataract. Increase in glucose led to decrease in glutathione and increase in ROS induced osmotic stress and oxidative stress, synergistically inhibiting the ability of fiber cell results in the activation of enzymes and leading to formation of AGEs and formation of cataract [125].
2.5.2. Natural Antioxidant of Plant as Aldose Reductase Inhibitors in Cataractogenesis
In diabetes, chronically elevated blood glucose plays a crucial role in determining complications such as cataracts. Aldose reductase converts glucose to sorbitol during hyperglycemia, while sorbitol dehydrogenase catalyzes the conversion of sorbitol to fructose via sorbitol dehydrogenase. Because the polyol pathway is involved in the etiology of diabetic cataracts and AR is the rate-limiting enzyme of the polyol pathway, sorbitol cannot cross cell membranes, causing cell swelling, degeneration, and necrosis. Therefore, it has been hypothesized that AR inhibition could be a pharmaceutical target in managing diabetic cataracts. AR has a role in various disease pathological processes by regulating cytokines, growth factors, oxidative stress, and other intracellular signal transduction pathways. The binding site of the AR inhibitor is a large hydrophobic pocket that serves as the target [126]. As a result of the polar and non-polar interactions between the inhibitor and the complementary residue corresponding to the enzyme-binding pocket, binding of the inhibitor occurs. The selectivity of the inhibitor is thought to be mainly due to the interaction of the inhibitor enzyme in the non-polar domain [127,128].
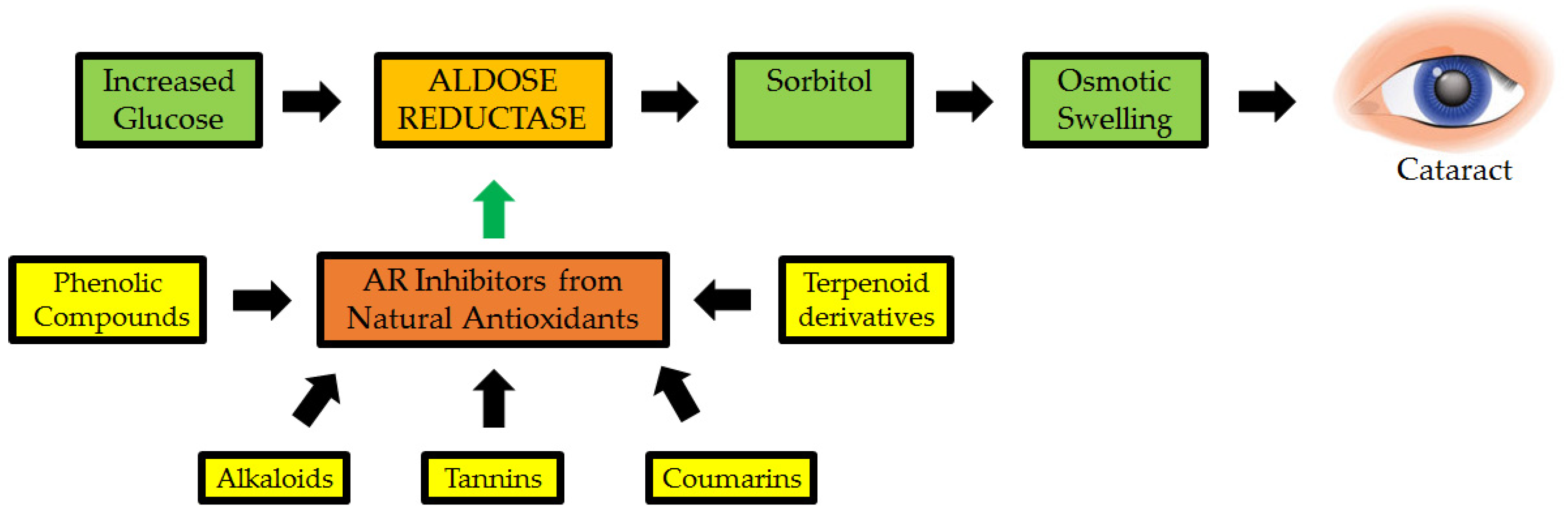
Polyphenols in Eleusine coracana are an important anti-diabetic and natural antioxidant component. They were tested for their ability to suppress AR in a study of cataractogenesis (Figure 7). Syringic, ferulic, trans-cinnamic acids, p-hydroxy benzoic, p-coumaric, gallic, protocatechuic, vanillic, and quercetin, among other phenolic elements in Eleusine coracana, significantly suppressed cataract eye lens, with the latter being more active, with an IC50 of 14.8 nM. Polyphenols present in the seed coats of Eleusine coracana plants have been reported to suppress AR in a reversible, non-competitive manner. As a result, the findings add to the body of evidence supporting Eleusine coracana ability to suppress cataractogenesis in people [129].
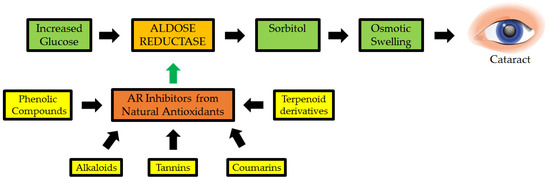
Figure 7.
Natural antioxidants as possible inhibitors of aldose reductase (AR: a key enzyme implicated in cataractogenesis) [130].
In Chinese traditional medicine, Chrysanthemum indicum L. blooms are used to treat eye diseases. On rat lens AR, the inhibitory activity of components extracted from this plant’s active fractions was investigated. Luteolin, acacetin-7-O-(600-a-L-rhamopyranosyl)-b-D-glucopyranoside, and chlorogenic acid were found to be effective inhibitors [11]. Isolated from the methanolic extract of the dried leaves of Manilkara indica, the C-glucosyl flavone, isoaffinetin, inhibited AR in bovine lens, rat lens, and human recombinant. Isoaffinetin, like many other flavonoids, works by inhibiting dl-glyceraldehyde and NADPH in a noncompetitive manner. The quantity of hydroxyl groups in ring B increases C-glucosyl flavone inhibition, according to a structure–activity connection study [131].
Another study created curcumin analogues and tested their potential to block the enzyme. Curcumin analogues with ortho-dihydroxyl groups create a tighter binding to AR, allowing them to display strong action, according to structure–activity relationship studies [132]. The OH group at position 4 was found to be crucial for AR inhibitory property in a structure–activity connection investigation. AR action is also inhibited by the presence of an O-methyl group close to the carbon bearing the phenolic OH moiety. The noncompetitive inhibition of AR by phenolic acids was discovered to be reversible [129].
2.5.3. The Potential of Natural Antioxidant as Antiapoptotic against Cataractogenesis
Apoptosis by ocular lens epithelial cells also contributes significantly to cataract progression. There are many mechanisms of cataracts that ultimately lead to lens cell apoptosis and impair vision. For this reason, one of the benefits of natural compounds in plants against cataracts is the inhibition of the epithelial cells of the eye lens to perform apoptosis. Many pathways involved in apoptosis are classified as intrinsic and extrinsic pathways, depending on different apoptotic triggers. Lens opacity is related to mitochondria-dependent processes. Radiation, drugs, toxins, and free radicals cause mitochondrial damage and malfunction. These lead to the release of pro-apoptotic proteins (such as cytochrome c and second mitochondrial activator of caspases, SMAC) from the inner mitochondrial surface into the cytosol, resulting in programmed cell death. Oxidative stress in cataract formation has been identified as a critical mediator of apoptosis in lens epithelial cells [133,134].
Green tea’s most prevalent component, epigallocatechin gallate (EGCG), is a powerful antioxidant. In HLEB-3 cells, EGCG was found to protect against cell death caused by H2O2. H2O2-induced formation of ROS, loss of mitochondrial membrane potential (m), and cytochrome c release from the mitochondria into the cytoplasm were all reduced by EGCG. The H2O2-stimulated rise in caspase-9 and caspase-3 expression, as well as the drop in the Bcl-2/Bax ratio, were both suppressed by EGCG. Furthermore, EGCG prevented H2O2 from reducing the activation and expression of ERK, p38 MAPK, and Akt. These data imply that EGCG protects HLE cells against H2O2-induced mitochondrial apoptosis by modulating caspases, the Bcl-2 family, as well as the MAPK and Akt pathways [135].
Myricetin, a flavonoglycoside, is isolated from the stem, bark, branches, and fruits of Myrica rubra or other plant sources, with antioxidant properties have been known to act as a scavenger of ROS molecules. The antioxidant and anti-apoptotic role of myricetin has already been proven to decrease the level of ROS significantly. Myricetin can also inhibit the apoptosis of epithelial cells by increasing the levels of SOD, CAT, and glutathione through the Bax/Bcl-2 signaling pathway. Myricetin also inhibited the apoptosis of H2O2 stressed lens epithelial cells and through its anti-apoptotic potential, this compound is effective in preventing apoptosis-driven cataractogenesis of the human eye lens [136].
3. Conclusions
Currently, cataracts are still the leading cause of visual impairment. Cataracts are caused by a variety of factors, including tissue changes caused by aging in which proteins and lens fibers begin to break down, resulting in blurred or unclear vision, diabetes complications that cause high sugar levels in the aqueous humor, and oxidative stress caused by free radicals such as ROS. The way to neutralize ROS and other free radicals is with natural antioxidants. Antioxidants can donate electrons to make ROS and other free radicals less reactive. An online literature review revealed that many medicinal plants contain high antioxidant activity, such as amethyst leaves, passion fruit leaves, and ginger. Based on some literature that has been studied, it can be seen that many plants have bioactivity as anticataracts. Therefore, it is concluded that plants with high levels of antioxidants can be incorporated into cataract prevention efforts, and further research on cataract treatment can incorporate plants as a natural source of antioxidants that inhibit the progression of cataracts.
Author Contributions
Conceptualization, R.I., K.K. and R.R.L.; methodology, E.I.; software, A.H.A.; validation, R.I., K.K., R.R.L. and T.E.T.; formal analysis, A.J.N.; investigation, E.I.; resources, E.I., R.I., K.K. and R.R.L.; data curation, M.R.; writing—original draft preparation, E.I.; writing—review and editing, R.I., K.K. and R.R.L.; visualization, K.K.; supervision, R.I., K.K. and R.R.L.; project administration, E.I.; funding acquisition, E.I. and R.I.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanwar Hossain, M.; Khanom, T.; Mazaharul islam, M. A Study on Prevalence of Cataract and Importance of Cataract Surgery at Tertiary Care Hospital in Bangladesh. SAS J. Med. 2021, 7, 12–14. [Google Scholar] [CrossRef]
- Wale, M.Z.; Derbew, M.; Tilahun, M.; Terefe, M. Cataract and Associated Factors among Adults Visiting Ophthalmic Clinic at Debre Markos Comprehensive Specialized Hospital, Northwest Ethiopia, 2020. SAGE Open Med. 2021, 9, 205031212198963. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Lambert, S. Referral Basis for Congenital and Juvenile Cataracts. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus {JAAPOS} 2019, 23, e32. [Google Scholar] [CrossRef]
- Ho, M.-C.; Peng, Y.-J.; Chen, S.-J.; Chiou, S.-H. Senile Cataracts and Oxidative Stress. J. Clin. Gerontol. Geriatr. 2010, 1, 17–21. [Google Scholar] [CrossRef]
- Made, N.; Suryathi, A.; Jayanegara, W.G.; Bagus, I.; Manuaba, P. Characteristics Retinometry Pre and Post Cataract Surgery on Senile Cataract Patients in Sanglah Hospital, Bali Indonesia. Intisari Sains Medis 2020, 11, 1504–1509. [Google Scholar] [CrossRef]
- Muliani, R.; Simanjuntak, R.; Jundiah, S. Hubungan Tingkat Kebiasaan Merokok Dengan Stadium Katarak Senilis Di Poliklinik Katarak Dan Bedah Refraktif (KBR) Rumah Sakit Mata Cicendo Bandung. J. Med. Health 2020, 2, 5. [Google Scholar] [CrossRef]
- Brad, H.; Feldman, M.D.; Sebastian Heersink, M. Cataract. In Basic Clinical Science Course (BCSC); American Academy of Ophthalmology: San Francisco, CA, USA, 2021; pp. 1–8. [Google Scholar]
- Jiang, J.; Xiong, Y.L. Natural Antioxidants as Food and Feed Additives to Promote Health Bene Fi Ts and Quality of Meat Products: A Review. MESC 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Mold, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Costa, R.; Lima, S.A.C.; Gameiro, P. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef]
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef]
- Thiagarajan, R.; Manikandan, R. Antioxidants and Cataract. Free Radic. Res. 2013, 47, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kukreja, S.; Kaur, A.; Malhotra, N.; Kaur, R. The Oxidative Stress in Cataract Patients. J. Clin. Diagn. Res. JCDR 2012, 6, 1629. [Google Scholar] [CrossRef] [PubMed]
- Dahm, R.; van Marle, J.; Quinlan, R.A.; Prescott, A.R.; Vrensen, G.F.J.M. Homeostasis in the Vertebrate Lens: Mechanisms of Solute Exchange. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1265–1277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lou, M.F. Redox Regulation in the Lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- El-Sayyad, H.; Bakr, E.; El-Ghawet, H.; El-Desoky, T. Overview of Congenital, Senile and Metabolic Cataract. J. Ocul. Biol. 2015, 3, 12. [Google Scholar]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in Diabetes Mellitus. World J. Diabetes 2019, 10, 140. [Google Scholar] [CrossRef]
- Domínguez-Calva, J.A.; Pérez-Vázquez, M.L.; Serebryany, E.; King, J.A.; Quintanar, L. Mercury-Induced Aggregation of Human Lens γ-Crystallins Reveals a Potential Role in Cataract Disease. JBIC J. Biol. Inorg. Chem. 2018, 23, 1105–1118. [Google Scholar] [CrossRef]
- Janzen, N.; Illsinger, S.; Meyer, U.; Shin, Y.S.; Sander, J.; Lücke, T.; Das, A.M. Early Cataract Formation Due to Galactokinase Deficiency: Impact of Newborn Screening. Arch. Med. Res. 2011, 42, 608–612. [Google Scholar] [CrossRef]
- Self, J.E.; Taylor, R.; Solebo, A.L.; Biswas, S.; Parulekar, M.; Dev Borman, A.; Ashworth, J.; McClenaghan, R.; Abbott, J.; O’Flynn, E. Cataract Management in Children: A Review of the Literature and Current Practice across Five Large UK Centres. Eye 2020, 34, 2197–2218. [Google Scholar] [CrossRef]
- Ye, J.; He, J.; Wang, C.; Wu, H.; Shi, X.; Zhang, H.; Xie, J.; Lee, S.Y. Smoking and Risk of Age-Related Cataract: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3885–3895. [Google Scholar] [CrossRef]
- Kamari, F.; Hallaj, S.; Dorosti, F.; Alinezhad, F.; Taleschian-Tabrizi, N.; Farhadi, F.; Aslani, H. Phototoxicity of Environmental Radiations in Human Lens: Revisiting the Pathogenesis of UV-Induced Cataract. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
- Haeck, I.M.; Rouwen, T.J.; Timmer-de Mik, L.; de Bruin-Weller, M.S.; Bruijnzeel-Koomen, C.A. Topical Corticosteroids in Atopic Dermatitis and the Risk of Glaucoma and Cataracts. J. Am. Acad. Dermatol. 2011, 64, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Z.; Zarina, S. Osmotic Stress Induced Oxidative Damage: Possible Mechanism of Cataract Formation in Diabetes. J. Diabetes Complicat. 2012, 26, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xing, K.; Randazzo, J.; Blessing, K.; Lou, M.F.; Kador, P.F. Osmotic Stress, Not Aldose Reductase Activity, Directly Induces Growth Factors and MAPK Signaling Changes during Sugar Cataract Formation. Exp. Eye Res. 2012, 101, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A. Diabetic Cataract: Pathogenesis and Management with Focus on Potential Pharmacotherapeutics. SIES J. Pharma. Bio. Manag. 2013, 1, 1–13. [Google Scholar]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. Cataract: A Major Secondary Complication of Diabetes, Its Epidemiology and an Overview on Major Medicinal Plants Screened for Anticataract Activity. Asian Pac. J. Trop. Dis. 2011, 1, 323–329. [Google Scholar] [CrossRef]
- Yan, L. Redox Imbalance Stress in Diabetes Mellitus: Role of the Polyol Pathway. Anim. Model. Exp. Med. 2018, 1, 7–13. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Chung, S.S.M.; Kador, P.F. Diabetic Cataracts: Mechanisms and Management. Diabetes Metab. Res. Rev. 2010, 26, 172–180. [Google Scholar] [CrossRef]
- Neale, R.E.; Purdie, J.L.; Hirst, L.W.; Green, A.C. Sun Exposure as a Risk Factor for Nuclear Cataract. Epidemiology 2003, 14, 701–702. [Google Scholar] [CrossRef]
- Chan, E.; Mahroo, O.A.R.; Spalton, D.J. Complications of Cataract Surgery. Clin. Exp. Optom. 2010, 93, 379–389. [Google Scholar] [CrossRef]
- Bandello, F.; Coassin, M.; Di Zazzo, A.; Rizzo, S.; Biagini, I.; Pozdeyeva, N.; Sinitsyn, M.; Verzin, A.; De Rosa, P.; Calabrò, F.; et al. One Week of Levofloxacin plus Dexamethasone Eye Drops for Cataract Surgery: An Innovative and Rational Therapeutic Strategy. Eye 2020, 34, 2112–2122. [Google Scholar] [CrossRef]
- Zheng Selin, J.; Orsini, N.; Ejdervik Lindblad, B.; Wolk, A. Long-Term Physical Activity and Risk of Age-Related Cataract: A Population-Based Prospective Study of Male and Female Cohorts. Ophthalmology 2015, 122, 274–280. [Google Scholar] [CrossRef]
- Tabin, G.; Chen, M.; Espandar, L. Cataract Surgery for the Developing World. Curr. Opin. Ophthalmol. 2008, 19, 55–59. [Google Scholar] [CrossRef]
- Alió, J.L.; Alió del Barrio, J.L.; Vega-Estrada, A. Accommodative Intraocular Lenses: Where Are We and Where We Are Going. Eye Vis. 2017, 4, 16. [Google Scholar] [CrossRef]
- Staveness, D.; Bosque, I.; Stephenson, C.R.J. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res. 2016, 49, 2295–2306. [Google Scholar] [CrossRef]
- Cadenas, E. Mitochondrial Free Radical Production and Cell Signaling. Mol. Asp. Med. 2004, 25, 17–26. [Google Scholar] [CrossRef]
- Verhaar, M.C.; Westerweel, P.E.; van Zonneveld, A.J.; Rabelink, T.J. Free Radical Production by Dysfunctional ENOS. Heart 2004, 90, 494–495. [Google Scholar] [CrossRef][Green Version]
- Maeda, H.; Akaike, T. Oxygen Free Radicals as Pathogenic Molecules in Viral Diseases. Proc. Soc. Exp. Biol. Med. 1991, 198, 721–727. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Wiley, C.D.; Velarde, M.C. Mitochondrial Effectors of Cellular Senescence: Beyond the Free Radical Theory of Aging. Aging Cell 2015, 14, 1–7. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Reactive Oxygen Species Production by Mitochondria. Mitochondrial DNA 2009, 554, 165–181. [Google Scholar]
- Coleman, J.F. Robbins and Cotran’s Pathologic Basis of Disease. J. Neuropathol. Exp. Neurol. 2010, 69, 214. [Google Scholar]
- Supinski, G.S.; Callahan, L.A. Free Radical-Mediated Skeletal Muscle Dysfunction in Inflammatory Conditions. J. Appl. Physiol. 2007, 102, 2056–2063. [Google Scholar] [CrossRef]
- Thomas, D.C. The Phagocyte Respiratory Burst: Historical Perspectives and Recent Advances. Immunol. Lett. 2017, 192, 88–96. [Google Scholar] [CrossRef]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling Peroxynitrite Formation in Biological Systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Contreras, D.; Moreno, Y.; Mansilla, H.D. Fenton Reaction Driven by Iron Ligands. J. Chil. Chem. Soc. 2013, 58, 2096–2101. [Google Scholar] [CrossRef]
- Al-Dalaen, S.M.; Al-Qtaitat, A.I. Review Article: Oxidative Stress Versus Antioxidants. Am. J. Biosci. Bioeng. 2014, 2, 60. [Google Scholar] [CrossRef]
- Granger, D.N. Role of Xanthine Oxidase and Granulocytes in Ischemia-Reperfusion Injury. Am. J. Physiol. Circ. Physiol. 1988, 255, H1269–H1275. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Sun, M.-S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.-L.; Guo, Z.-N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef]
- Dou, X.; Li, J.; Danelisen, I.; Trush, M.A.; Misra, H.P.; Zhu, H.; Jia, Z.; Li, Y. Acetaminophen, the Active Ingredient of Tylenol, Protects against Peroxynitrite-Induced DNA Damage: A Chemiluminometric and Electron Paramagnetic Resonance Spectrometric Study. React. Oxyg. Species 2017, 3, 127–134. [Google Scholar] [CrossRef]
- Rutkowski, M.; Matuszewski, T.; Kedziora, J.; Paradowski, M.; Kłos, K.; Zakrzewski, A. Vitamins E, A and C as Antioxidatives. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2010, 29, 377–381. [Google Scholar]
- Iskusnykh, I.Y.; Popova, T.N.; Agarkov, A.A.; Pinheiro de Carvalho, M.Â.A.; Rjevskiy, S.G. Expression of Glutathione Peroxidase and Glutathione Reductase and Level of Free Radical Processes under Toxic Hepatitis in Rats. J. Toxicol. 2013, 2013, 870628. [Google Scholar] [CrossRef]
- Ganini, D.; Canistro, D.; Jang, J.; Stadler, K.; Mason, R.P.; Kadiiska, M.B. Ceruloplasmin (Ferroxidase) Oxidizes Hydroxylamine Probes: Deceptive Implications for Free Radical Detection. Free Radic. Biol. Med. 2012, 53, 1514–1521. [Google Scholar] [CrossRef]
- Takami, T.; Sakaida, I. Iron Regulation by Hepatocytes and Free Radicals. J. Clin. Biochem. Nutr. 2011, 48, 103–106. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural Antioxidants from Residual Sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Sunkireddy, P.; Jha, S.N.; Kanwar, J.R.; Yadav, S.C. Natural Antioxidant Biomolecules Promises Future Nanomedicine Based Therapy for Cataract. Colloids Surf. B Biointerfaces 2013, 112, 554–562. [Google Scholar] [CrossRef]
- Spector, A. Oxidative Stress-Induced Cataract: Mechanism of Action. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 1173–1182. [Google Scholar] [CrossRef]
- Truscott, R.J.W. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus Vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of Antioxidant Activities of Various Solvent Extract from Sargassum Serratifolium and Its Major Antioxidant Components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An Appraisal of Eighteen Commonly Consumed Edible Plants as Functional Food Based on Their Antioxidant and Starch Hydrolase Inhibitory Activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and Structure—Activity Relationships (SARs) of Some Phenolic and Anilines Compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive Compounds in Cranberries and Their Biological Properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues. Crit. Rev. Food Sci. Nutr. 2015, 55, 319–337. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of Antioxidant Activity and Total Phenol in Different Varieties of Lantana Camara Leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef]
- Alabri, T.H.A.; Al Musalami, A.H.S.; Hossain, M.A.; Weli, A.M.; Al-Riyami, Q. Comparative Study of Phytochemical Screening, Antioxidant and Antimicrobial Capacities of Fresh and Dry Leaves Crude Plant Extracts of Datura metel L. J. King Saud Univ.-Sci. 2014, 26, 237–243. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Lawal, B.; Abubakar, A.N.; Berinyuy, E.B.; Omonije, Y.O.; Umar, S.I.; Shebe, M.N.; Alhaji, Y.M. In-Vitro Antioxidants, Antimicrobial and Toxicological Evaluation of Nigerian Zingiber Officinale. Clin. Phytosci. 2018, 4, 12. [Google Scholar] [CrossRef]
- Chaphalkar, R.; Apte, K.G.; Talekar, Y.; Ojha, S.K.; Nandave, M. Antioxidants of Phyllanthus Emblica L. Bark Extract Provide Hepatoprotection against Ethanol-Induced Hepatic Damage: A Comparison with Silymarin. Oxid. Med. Cell. Longev. 2017, 2017, 3876040. [Google Scholar] [CrossRef]
- Martiningsih, N.W.; Mudianta, I.W.; Suryanti, I.A.P. Phytochemical Screening and Antioxidant Activity of Hippobroma Longiflora Extracts. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Sanya, China, 12–14 November 2021; Volume 1115, p. 12078. [Google Scholar]
- Baba, S.A.; Malik, S.A. Determination of Total Phenolic and Flavonoid Content, Antimicrobial and Antioxidant Activity of a Root Extract of Arisaema Jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C. In Vitro and in Vivo Antioxidant Activity of Ethanolic Extract of White Button Mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- da Silva, J.K.; Cazarin, C.B.B.; Colomeu, T.C.; Batista, Â.G.; Meletti, L.M.M.; Paschoal, J.A.R.; Bogusz Júnior, S.; Furlan, M.F.; Reyes, F.G.R.; Augusto, F.; et al. Antioxidant Activity of Aqueous Extract of Passion Fruit (Passiflora edulis) Leaves: In Vitro and in Vivo Study. Food Res. Int. 2013, 53, 882–890. [Google Scholar] [CrossRef]
- Mansour, R.B.; Jilani, I.B.H.; Bouaziz, M.; Gargouri, B.; Elloumi, N.; Attia, H.; Ghrabi-Gammar, Z.; Lassoued, S. Phenolic Contents and Antioxidant Activity of Ethanolic Extract of Capparis Spinosa. Cytotechnology 2016, 68, 135–142. [Google Scholar] [CrossRef]
- Abu, F.; Mat Taib, C.N.; Mohd Moklas, M.A.; Mohd Akhir, S. Antioxidant Properties of Crue Extract, Partition Extract, and Fermented Medium of Dendrobium Sabin Flower. Evid.-Based Complement. Altern. Med. 2017, 2017, 2907219. [Google Scholar] [CrossRef]
- Ezez, D.; Tefera, M. Effects of Solvents on Total Phenolic Content and Antioxidant Activity of Ginger Extracts. J. Chem. 2021, 2021, 6635199. [Google Scholar] [CrossRef]
- Bruck de Souza, L.; Leitão Gindri, A.; de Andrade Fortes, T.; Felli Kubiça, T.; Enderle, J.; Roehrs, R.; Moura e Silva, S.; Manfredini, V.; Gasparotto Denardin, E.L. Phytochemical Analysis, Antioxidant Activity, Antimicrobial Activity, and Cytotoxicity of Chaptalia Nutans Leaves. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 3260745. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; Hamamouch, N.; Lyoussi, B.; Derwich, E. Chemical Composition and In Vitro Antioxidant and Antimicrobial Activities of Marrubium Vulgare L. Sci. World J. 2021, 2021, 7011493. [Google Scholar] [CrossRef]
- Syed Salleh, S.N.A.; Mohd Hanapiah, N.A.; Ahmad, H.; Wan Johari, W.L.; Osman, N.H.; Mamat, M.R. Determination of Total Phenolics, Flavonoids, and Antioxidant Activity and GC-MS Analysis of Malaysian Stingless Bee Propolis Water Extracts. Scientifica (Cairo) 2021, 2021, 3789351. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Rishmawi, S.; Ariqat, S.H.; Khalid, M.F.; Warad, I.; Salah, Z. Anticancer Activity, Antioxidant Activity, and Phenolic and Flavonoids Content of Wild Tragopogon Porrifolius Plant Extracts. Evid.-Based Complement. Altern. Med. 2016, 2016, 9612490. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.-B. Oxidative Damage and the Prevention of Age-Related Cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, V.M.; Beyer, E.C. Oxidative Stress, Lens Gap Junctions, and Cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, M.; Elanchezhian, R.; Ramesh, E.; Isai, M.; Jesudasan, C.N.; Thomas, P.A.; Geraldine, P. Prevention of Selenite-Induced Cataractogenesis in Wistar Rats by the Polyphenol, Ellagic Acid. Exp. Eye Res. 2008, 86, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Biju, P.G.; Rooban, B.N.; Lija, Y.; Devi, V.G.; Sahasranamam, V.; Abraham, A. Drevogenin D Prevents Selenite-Induced Oxidative Stress and Calpain Activation in Cultured Rat Lens. Mol. Vis. 2007, 13, 1121–1129. [Google Scholar] [PubMed]
- Wojcik, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A. A Review of Natural and Synthetic Antioxidants Important for Health and Longevity. Curr. Med. Chem. 2010, 17, 3262–3288. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, W.; Wu, W.; Xu, J.; Zheng, S.; Shentu, X.; Chen, X. Cataract-Causing Mutation R48C Increases ΓA-Crystallin Susceptibility to Oxidative Stress and Ultraviolet Radiation. Int. J. Biol. Macromol. 2022, 194, 688–694. [Google Scholar] [CrossRef]
- Hsueh, Y.-J.; Chen, Y.-N.; Tsao, Y.-T.; Cheng, C.-M.; Wu, W.-C.; Chen, H.-C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Huang, J.; Yu, W.; He, Q.; He, X.; Yang, M.; Chen, W.; Han, W. Autophagy Facilitates Age-Related Cell Apoptosis—A New Insight from Senile Cataract. Cell Death Dis. 2022, 13, 37. [Google Scholar] [CrossRef]
- Kyselova, Z. Different Experimental Approaches in Modelling Cataractogenesis: An Overview of Selenite-Induced Nuclear Cataract in Rats. Interdiscip. Toxicol. 2010, 3, 3–14. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Shetty, L.; Harikiran, H.; Sharma, A. In Vitro Prophylactic Cataract Prevention Study on Glucose Induced Cataract by Quercetin and Alpha Tocopherol. Int. J. Pharm. Sci. Res. 2010, 1, 41–45. [Google Scholar]
- Isai, M.; Sakthivel, M.; Ramesh, E.; Thomas, P.A.; Geraldine, P. Prevention of Selenite-Induced Cataractogenesis by Rutin in Wistar Rats. Mol. Vis. 2009, 15, 2570–2577. [Google Scholar]
- Nakazawa, Y.; Nagai, N.; Ishimori, N.; Oguchi, J.; Tamura, H. Administration of Antioxidant Compounds Affects the Lens Chaperone Activity and Prevents the Onset of Cataracts. Biomed. Pharmacother. 2017, 95, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, S.; Raju, T.N. Antioxidant Effect of Curcumin in Selenium Induced Cataract of Wistar Rats. Indian J. Exp. Biol. 2004, 42, 601–603. [Google Scholar] [PubMed]
- Suryanarayana, P.; Krishnaswamy, K.; Reddy, G.B. Effect of Curcumin on Galactose-Induced Cataractogenesis in Rats. Mol. Vis. 2003, 9, 223–230. [Google Scholar] [PubMed]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Sudhandiran, G.; Arumugam, M. Curcumin Prevents Free Radical-Mediated Cataractogenesis through Modulations in Lens Calcium. Free Radic. Biol. Med. 2010, 48, 483–492. [Google Scholar] [CrossRef]
- Durgapal, S.; Juyal, V.; Verma, A. In Vitro Antioxidant and Ex Vivo Anti-Cataract Activity of Ethanolic Extract of Cineraria Maritima: A Traditional Plant from Nilgiri Hills. Futur. J. Pharm. Sci. 2021, 7, 105. [Google Scholar] [CrossRef]
- Feriyani, F.; Maulanza, H.; Lubis, R.R.; Balqis, U.; Darmawi, D. Effects of Binahong (Anredera Cordifolia (Tenore) Steenis) Extracts on the Levels of Malondialdehyde (MDA) in Cataract Goat Lenses. Sci. World J. 2021, 2021, 6617292. [Google Scholar] [CrossRef]
- Asha, R.; Gayathri Devi, V.; Abraham, A. Lupeol, a Pentacyclic Triterpenoid Isolated from Vernonia Cinerea Attenuate Selenite Induced Cataract Formation in Sprague Dawley Rat Pups. Chem. Biol. Interact. 2016, 245, 20–29. [Google Scholar] [CrossRef]
- Sundararajan, M.; Thomas, P.A.; Babyshalini, K.; Geraldine, P. Identification of Phytoconstituents and In-Vitro Evaluation of the Putative Anticataractogenic Effect of an Ethanolic Root Extract of Leucas Aspera. Biomed. Pharmacother. 2017, 85, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.-J.; Lue, S.J.; Lai, J.-Y. The Role of Aromatic Ring Number in Phenolic Compound-Conjugated Chitosan Injectables for Sustained Therapeutic Antiglaucoma Efficacy. Carbohydr. Polym. 2020, 231, 115770. [Google Scholar] [CrossRef] [PubMed]
- Kyei, S.; Koffuor, G.A.; Ramkissoon, P.; Afari, C.; Asiamah, E.A. The Claim of Anti-Cataract Potential of Heliotropium Indicum: A Myth or Reality? Ophthalmol. Ther. 2015, 4, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Dongare, V.; Kulkarni, C.; Kondawar, M.; Magdum, C.; Haldavnekar, V.; Arvindekar, A. Inhibition of Aldose Reductase and Anti-Cataract Action of Trans-Anethole Isolated from Foeniculum Vulgare Mill. Fruits. Food Chem. 2012, 132, 385–390. [Google Scholar] [CrossRef]
- Onkaramurthy, M.; Veerapur, V.P.; Thippeswamy, B.S.; Madhusudana Reddy, T.N.; Rayappa, H.; Badami, S. Anti-Diabetic and Anti-Cataract Effects of Chromolaena Odorata Linn., in Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol. 2013, 145, 363–372. [Google Scholar] [CrossRef]
- Mestry, S.N.; Juvekar, A.R. Aldose Reductase Inhibitory Potential and Anti-Cataract Activity of Punica Granatum Linn. Leaves against Glucose-Induced Cataractogenesis in Goat Eye Lens. Orient. Pharm. Exp. Med. 2017, 17, 277–284. [Google Scholar] [CrossRef]
- Sruthi, T.; Sasikala, V.; Vangalapati, M. Anti Cataract Activity of Synthesized Silver Nano Particles from Skin Of Allium Cepa Species. In Proceedings of the Journal of Physics, Xi’an, China, 18–19 October 2020; Volume 1455, p. 12017. [Google Scholar]
- Zhang, X.; Hu, Y. Inhibitory Effects of Grape Seed Proanthocyanidin Extract on Selenite-Induced Cataract Formation and Possible Mechanism. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012, 32, 613–619. [Google Scholar] [CrossRef]
- Bhadada, S.V.; Goyal, R.K. Effect of Aqueous Extract of Tephrosia Purpurea on Cardiovascular Complications and Cataract Associated with Streptozotocin-Induced Diabetes in Rats. Indian J. Pharm. Sci. 2015, 77, 522–529. [Google Scholar] [CrossRef]
- Bhadada, S.V.; Bhadada, V.J.; Goyal, R.K. Preventive Effect of Tephrosia Purpurea on Selenite-Induced Experimental Cataract. Curr. Eye Res. 2016, 41, 222–231. [Google Scholar] [CrossRef]
- Kim, J.; Choung, S.-Y. Pinus Densiflora Bark Extract Prevents Selenite-Induced Cataract Formation in the Lens of Sprague Dawley Rat Pups. Mol. Vis. 2017, 23, 638–648. [Google Scholar]
- Anbukkarasi, M.; Thomas, P.A.; Sheu, J.-R.; Geraldine, P. In Vitro Antioxidant and Anticataractogenic Potential of Silver Nanoparticles Biosynthesized Using an Ethanolic Extract of Tabernaemontana Divaricata Leaves. Biomed. Pharmacother. 2017, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-S.; Choi, Y.-H.; Lee, S.-J.; Choi, S.; Lee, J.; Kim, H.; Hong, E.-K. Water Extract of Aralia Elata Prevents Cataractogenesis in Vitro and in Vivo. J. Ethnopharmacol. 2005, 101, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.-V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin Glycosides and Chlorogenic Acid in Highbush Blueberry Leaf Decoction Prevent Cataractogenesis in Vivo and in Vitro: Investigation of the Effect on Calpains, Antioxidant and Metal Chelating Properties. Exp. Eye Res. 2016, 145, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Heruye, S.H.; Maffofou Nkenyi, L.N.; Singh, N.U.; Yalzadeh, D.; Ngele, K.K.; Njie-Mbye, Y.-F.; Ohia, S.E.; Opere, C.A. Current Trends in the Pharmacotherapy of Cataracts. Pharmaceuticals 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Analysis on the Alterations of Lens Proteins by Vitex Negundo in Selenite Cataract Models. Mol. Vis. 2011, 17, 1239–1248. [Google Scholar] [PubMed]
- Sasikala, V.; Rooban, B.N.; Sahasranamam, V.; Abraham, A. Rutin Ameliorates Free Radical Mediated Cataract by Enhancing the Chaperone Activity of α-Crystallin. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albr. von Graefes Arch. fur Klin. Exp. Ophthalmol. 2013, 251, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Odjakova, M.; Popova, E.; Al, M.; Mironov, R. Plant-Derived Agents with Anti-Glycation Activity. In Glycosylation; InTech: London, UK, 2012. [Google Scholar]
- Hashim, Z.; Zarina, S. Advanced Glycation End Products in Diabetic and Non-Diabetic Human Subjects Suffering from Cataract. Age (Omaha) 2011, 33, 377–384. [Google Scholar] [CrossRef]
- Abbas, G.; Al-Harrasi, A.S.; Hussain, H.; Hussain, J.; Rashid, R.; Choudhary, M.I. Antiglycation Therapy: Discovery of Promising Antiglycation Agents for the Management of Diabetic Complications. Pharm. Biol. 2016, 54, 198–206. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, Y.J.; Yoon, N.Y.; Jeong, D.M.; Bae, H.J.; Kim, D.-W.; Na, D.H.; Choi, J.S. Inhibitory Effects of Nelumbo Nucifera Leaves on Rat Lens Aldose Reductase, Advanced Glycation Endproducts Formation, and Oxidative Stress. Food Chem. Toxicol. 2008, 46, 3818–3826. [Google Scholar] [CrossRef]
- Jang, D.S.; Yoo, N.H.; Kim, N.H.; Lee, Y.M.; Kim, C.-S.; Kim, J.; Kim, J.-H.; Kim, J.S. 3,5-Di-O-Caffeoyl-Epi-Quinic Acid from the Leaves and Stems of Erigeron Annuus Inhibits Protein Glycation, Aldose Reductase, and Cataractogenesis. Biol. Pharm. Bull. 2010, 33, 329–333. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Donaldson, C.I.; Lim, J.C.; Donaldson, P.J. Nutritional Strategies to Prevent Lens Cataract: Current Status and Future Strategies. Nutrients 2019, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- El-Kabbani, O.; Ruiz, F.; Darmanin, C.; Chung, R.-T. Aldose Reductase Structures: Implications for Mechanism and Inhibition. Cell. Mol. Life Sci. C 2004, 61, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Reddy, A.; Yadav, U. Aldose Reductase Inhibitors in the Functional Foods: Regulation of Diabetic Complications. In Functional Food and Human Health; Springer: New York, NY, USA, 2018; pp. 555–574. [Google Scholar]
- Quattrini, L.; La Motta, C. Aldose Reductase Inhibitors: 2013-Present. Expert Opin. Ther. Pat. 2019, 29, 199–213. [Google Scholar] [CrossRef]
- Chethan, S.; Dharmesh, S.M.; Malleshi, N.G. Inhibition of Aldose Reductase from Cataracted Eye Lenses by Finger Millet (Eleusine coracana) Polyphenols. Bioorg. Med. Chem. 2008, 16, 10085–10090. [Google Scholar] [CrossRef]
- Grewal, A.S.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S. Natural Compounds as Source of Aldose Reductase (AR) Inhibitors for the Treatment of Diabetic Complications: A Mini Review. Curr. Drug Metab. 2020, 21, 1091–1116. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Hayashi, R.; Ishizu, T.; Yagi, A. A Flavone from Manilkara Indica as a Specific Inhibitor against Aldose Reductase In Vitro. Planta Med. 2003, 69, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Bao, Y.-D.; Liu, Z.; Qiao, W.; Ma, L.; Huang, Z.-S.; Gu, L.-Q.; Chan, A.S.C. Curcumin Analogs as Potent Aldose Reductase Inhibitors. Arch. Pharm. 2006, 339, 123–128. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, T.; Liang, Y.; Duan, L.; Zhang, Y.; Wang, L.-J.; He, G.; Xiao, H. P-Coumaric Acid Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by MAPK Signaling. Oxid. Med. Cell. Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef]
- Gong, W.; Zhu, G.; Li, J.; Yang, X. LncRNA MALAT1 Promotes the Apoptosis and Oxidative Stress of Human Lens Epithelial Cells via P38MAPK Pathway in Diabetic Cataract. Diabetes Res. Clin. Pract. 2018, 144, 314–321. [Google Scholar] [CrossRef]
- Yao, K.; Ye, P.; Zhang, L.; Tan, J.; Tang, X.; Zhang, Y. Epigallocatechin Gallate Protects against Oxidative Stress-Induced Mitochondria-Dependent Apoptosis in Human Lens Epithelial Cells. Mol. Vis. 2008, 14, 217–223. [Google Scholar]
- Yang, Y.; Xu, X.; Liu, Q.; Huang, H.; Huang, X.; Lv, H. Myricetin Prevents Cataract Formation by Inhibiting the Apoptotic Cell Death Mediated Cataractogenesis. Med. Sci. Monit. 2020, 26, e922519-1. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).