Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Onion Husk Extracts
2.2. Chemicals
2.3. UPLC-ESI-Q-TOF-MS Analysis
2.4. Determination of Total Antioxidant Capacity (TAC)
2.4.1. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.2. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.5. Chemiluminescence Assay
2.6. Statistical Analyses
3. Results
3.1. Identification of Active Compounds in the Extract of Onion Peels
3.2. Total Antioxidant Capacity Analysis
3.3. Chemiluminescence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Jørgensen, S.; Pedersen, L.J.T. The Circular Rather than the Linear Economy. In RESTART Sustainable Business Model Innovation. Palgrave Studies in Sustainable Business in Association with Future Earth, 1st ed.; Jørgensen, S., Pedersen, L.J.T., Eds.; Palgrave Macmillan: Cham, Switzerland, 2018; pp. 103–120. [Google Scholar] [CrossRef]
- Morseletto, P. Targets for a circular economy. Resour. Conserv. Recycl. 2020, 153, 104553. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Purnell, P. Principles for a sustainable circular economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Aprodu, I.; Enachi, E.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Whey Protein Isolate-Xylose Maillard-Based Conjugates with Tailored Microencapsulation Capacity of Flavonoids from Yellow Onions Skins. Antioxidants 2021, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Celano, R.; Docimo, T.; Piccinelli, A.L.; Gazzerro, P.; Tucci, M.; Di Sanzo, R.; Carabetta, S.; Campone, L.; Russo, M.; Rastrelli, L. Onion Peel: Turning a Food Waste into a Resource. Antioxidants 2021, 10, 304. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Development of Optimized Ultrasound-Assisted Extraction Methods for the Recovery of Total Phenolic Compounds and Anthocyanins from Onion Bulbs. Antioxidants 2021, 10, 1755. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef] [Green Version]
- Sagar, N.A.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 1–33. [Google Scholar] [CrossRef]
- Cebin, A.V.; Šeremet, D.; Mandura, A.; Martinić, A.; Komes, D. Onion Solid Waste as a Potential Source of Functional Food Ingredients. Eng. Power 2020, 15, 7–13. [Google Scholar]
- Bedrníček, J.; Kadlec, J.; Laknerová, I.; Mráz, J.; Samková, E.; Petrášková, E.; Hasoňová, L.; Vácha, F.; Kron, V.; Smetana, P. Onion Peel Powder as an Antioxidant-Rich Material for Sausages Prepared from Mechanically Separated Fish Meat. Antioxidants 2020, 9, 974. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Olszowy-Tomczyk, M.; Garbaczewska, S. A Central Composite Design in increasing the quercetin content in the aqueous onion waste isolates with antifungal and antioxidant properties. Eur. Food Res. Technol. 2022, 248, 497–505. [Google Scholar] [CrossRef]
- Chernukha, I.; Kotenkova, E.; Derbeneva, S.; Khvostov, D. Bioactive Compounds of Porcine Hearts and Aortas May Improve Cardiovascular Disorders in Humans. Int. J. Environ. Res. Public Health 2021, 18, 7330. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Saito, K. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods 2019, 16, 295–298. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Malta, L.G.; Liu, R.H. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 305–314. [Google Scholar] [CrossRef]
- Vladimirov, G.K.; Sergunova, E.V.; Izmaylov, D.Y.; Vladimirov, Y.A. Chemiluminescent determination of total antioxidant capacity in medicinal plant material. Bull. RSMU 2016, 2, 62–68. [Google Scholar] [CrossRef]
- Siddeeg, A.; Al Kehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; Al-Farga, A.M. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. JFDA 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. eCAM 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; da Fonseca Machado, A.P.; Forster-Carneiro, T.; Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Jahromi, S.G. Extraction techniques of phenolic compounds from plants. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jokić, S.; Bucić-Kojić, A.; Planinić, M.; Velic, D.; Tomas, S.; Bilic, M.; Besvir, Z. The effect of solvent and temperature on extraction yield of phenolic compounds from soybeans, antioxidant activity and colour of extracts. In Proceedings of the 5th International Congress Flour-Bread ’09 and 7th Croatian Congress of Cereal Technologists, Opatija, Croatia, 21–23 October 2009. [Google Scholar]
- Lee, K.A.; Kim, K.T.; Kim, H.J.; Chung, M.S.; Chang, P.S.; Park, H.; Pai, H.-D. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci. Biotechnol. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Viera, V.B.; Piovesan, N.; Rodrigues, J.B.; Mello, R.d.O.; Prestes, R.C.; Santos, R.C.V.d.; Vaucher, R.d.A.; Hautrive, T.P.; Kubota, E.H. Extraction of phenolic compounds and evaluation of the antioxidant and antimicrobial capacity of red onion skin (Allium cepa L.). Int. Food Res. J. 2017, 24, 990–999. [Google Scholar]
- Shim, S.Y.; Choi, Y.S.; Kim, H.Y.; Kim, H.W.; Hwang, K.E.; Song, D.H.; Lee, M.A.; Lee, J.W.; Kim, C.J. Antioxidative properties of onion peel extracts against lipid oxidation in raw ground pork. Food Sci. Biotechnol. 2021, 21, 565–572. [Google Scholar] [CrossRef]
- Savic-Gajic, I.M.; Savic, I.M.; Nikolic, V.D. Modelling and optimization of quercetin extraction and biological activity of quercetin-rich red onion skin extract from Southeastern Serbia. J. Food Nutr. Res. 2018, 57, 15–26. [Google Scholar]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.H.; Seo, J.M.; Kim, N.H.; Arasu, M.V.; Kim, S.; Yoon, M.K.; Kim, S.-J. Variation of quercetin glycoside derivatives in three onion (Allium cepa L.) varieties. Saudi J. Biol. Sci. 2017, 24, 1387–1391. [Google Scholar] [CrossRef] [Green Version]
- Nile, A.; Nile, S.H.; Cespedes-Acuña, C.L.; Oh, J.W. Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food Chem. Toxicol. 2021, 154, 112327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.-B.; Wu, D.-T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.-H.; Gan, R.-Y. Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.S.; Pike, L.M.; Yoo, K.S. Variation in the Quercetin Content in Different Colored Onions (Allium cepa L.). J. Am. Soc. Hortic. Sci. 1995, 120, 909–913. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Sun, H.; Tang, J.; Ouyang, Z. Pharmacological activities and pharmacokinetic study of hyperoside: A short review. Trop. J. Pharm. Res. 2017, 16, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Lymperis, P.; Tomou, E.M.; De Canha, M.N.; Lall, N.; Skaltsa, H. Traditional Uses, Phytochemistry, and Pharmacology of Elegia Species: A Review. Sci. Pharm. 2021, 90, 4. [Google Scholar] [CrossRef]
- Chang, W.-A.; Hung, J.-Y.; Tsai, Y.-M.; Hsu, Y.-L.; Chiang, H.-H.; Chou, S.-H.; Huang, M.-S.; Kuo, P.-L. Laricitrin suppresses increased benzo(a)pyrene-induced lung tumor-associated monocyte-derived dendritic cell cancer progression. Oncol. Lett. 2016, 11, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Chen, X.; Gu, N.; Xue, C.; Li, B. Plant flavonoid taxifolin inhibits the growth, migration and invasion of human osteosarcoma cells. Mol. Med. Rep. 2018, 17, 3239–3245. [Google Scholar] [CrossRef] [Green Version]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.-T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Fredotović, Ž.; Puizina, J.; Nazlić, M. Phytochemical Characterization and Screening of Antioxidant, Antimicrobial and Antiproliferative Properties of Allium × cornutum Clementi and Two Varieties of Allium cepa L. Peel Extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Suwal, S.; Marciniak, A. Technologies for the Extraction, Separation and Purification of polyphenols—A Review. Nepal J. Biotechnol. 2018, 6, 74–91. [Google Scholar] [CrossRef] [Green Version]
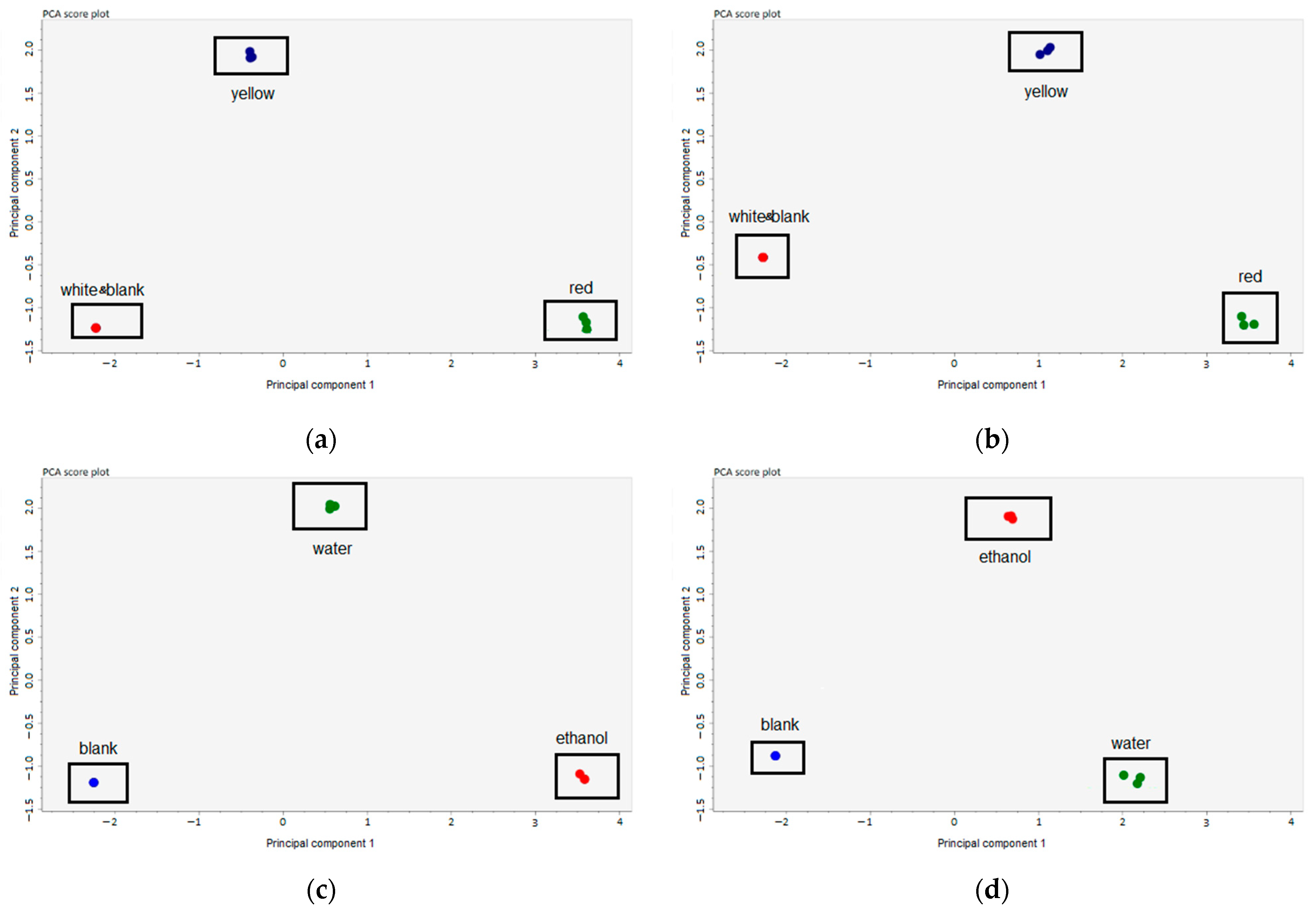
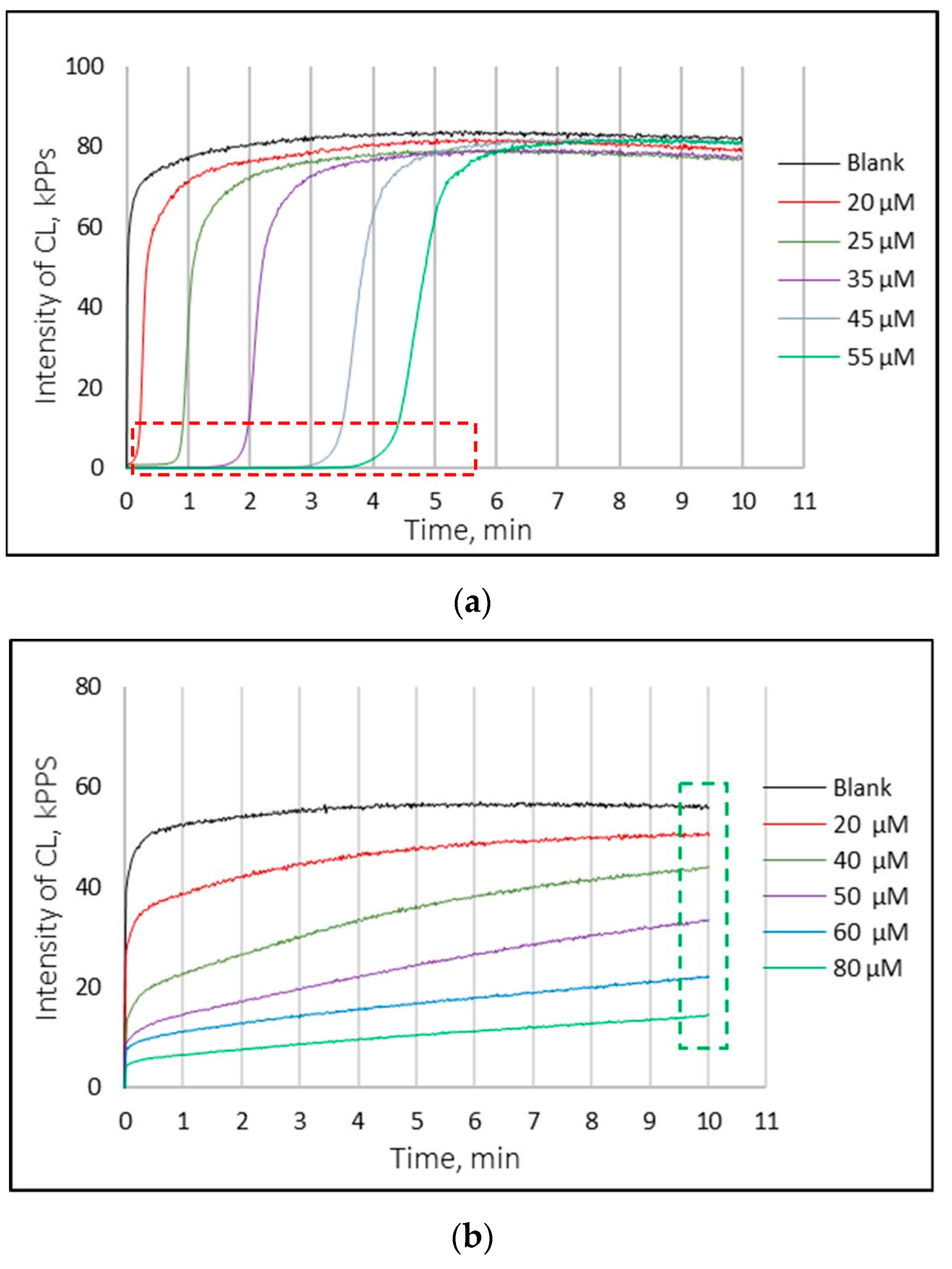

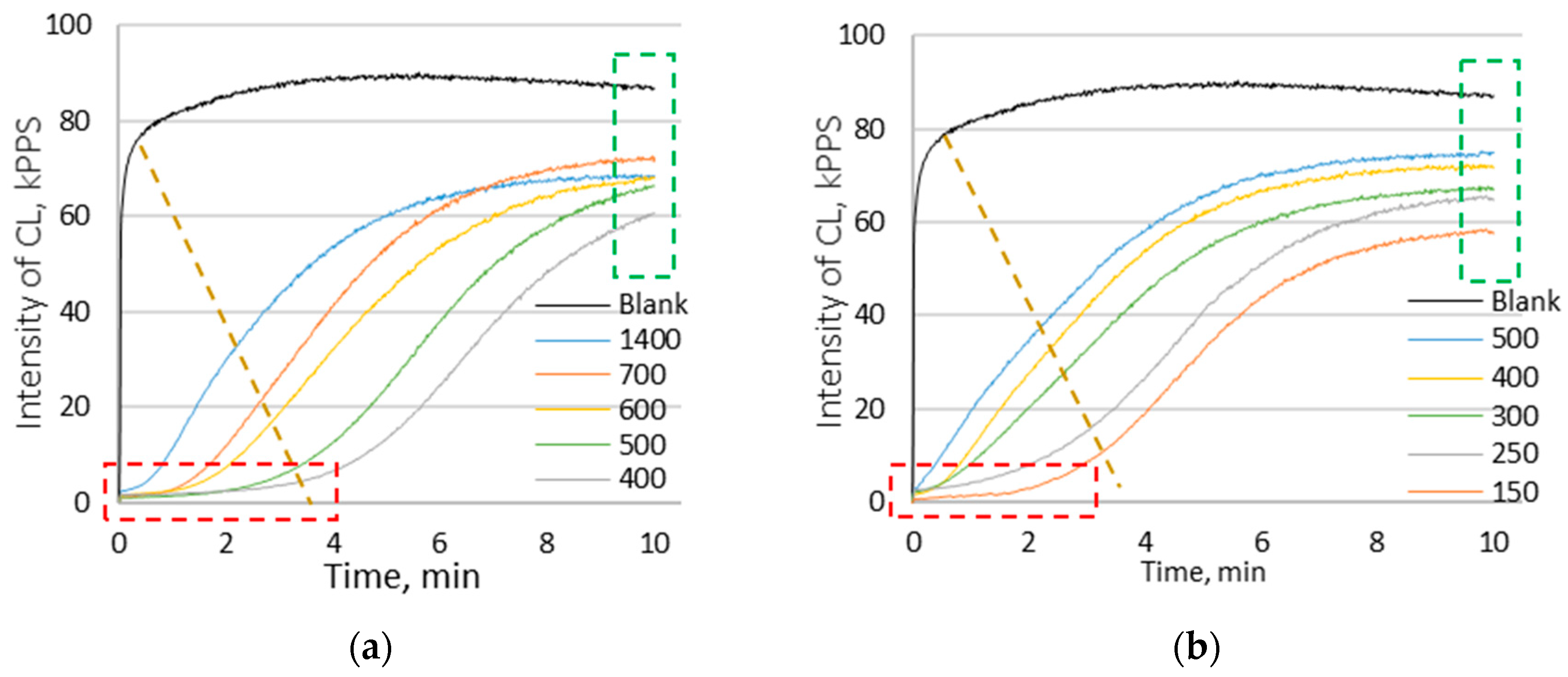

| Compounds | Average (μg/g Raw Material) ± SD | |||||
|---|---|---|---|---|---|---|
| Ethanol | Water | |||||
| Red | Yellow | White | Red | Yellow | White | |
| Flavonols | ||||||
| Quercetin | 1021.84 ± 4.07 | 320.86 ± 1.05 * | N.D. | 83.15 ± 1.14 # | 10.66 ± 2.27 *,^ | N.D. |
| 3′-Methoxy-4′,5,7-trihydroxyflavonol | 140.93 ± 2.04 | 12.05 ± 0.50 * | N.D. | 4.85 ± 0.10 # | 0.37 ± 0.04 *,^ | N.D. |
| Myricetin | 155.84 ± 2.60 | 80.77 ± 1.26 * | N.D. | 87.26 ± 1.05 # | 45.07 ± 0.23 *,^ | N.D. |
| Laricitrin | 16.66 ± 0.65 | 12.31 ± 0.15 * | N.D. | 10.43 ± 0.44 # | 9.09 ± 0.35 *,^ | N.D. |
| Flavanonols | ||||||
| Taxifolin | 18.70 ± 1.00 | 1.56 ± 0.60 * | N.D. | 10.95 ± 0.18 # | 0.53 ± 0.11 *,^ | N.D. |
| Flavonoid-O-glycosides | ||||||
| Quercetin-3,4′-O-di-beta-glucoside | 18.49 ± 1.66 | 1.50 ± 0.09 * | N.D. | 3.26 ± 1.56 # | 0.43 ± 0.20 *,^ | N.D. |
| Hyperoside | 47.26 ± 0.08 | 0.96 ± 0.07 * | N.D. | 1.06 ± 0.08 # | N.D. | N.D. |
| Isoquercetrin | 9.51 ± 0.48 | 0.92 ± 0.31 * | N.D. | 2.61 ± 0.32 # | 0.34 ± 0.15 *,^ | N.D. |
| Spiraeoside | 485.37 ± 5.26 | 112.33 ± 0.59 * | N.D. | 117.8 ± 0.86 # | 22.92 ± 0.32 *,^ | N.D. |
| Isoflavones | ||||||
| Tectorigenin | 1.31 ± 0.59 | 0.78 ± 0.27 | N.D. | N.D. | N.D. | N.D. |
| Total flavonoids | 1915.90 ± 9.92 | 544.06 ± 2.73 * | N.D. | 321.42 ± 2.61 # | 89.41 ± 2.08 *,^ | N.D. |
| Ethanol | Water | |||||
|---|---|---|---|---|---|---|
| Red | Yellow | White | Red | Yellow | White | |
| TACORAC, µmol-equiv. Trolox/g raw material | 2017.34 ± 29.52 a | 921.47 ± 63.57 b,c | 16.48 ± 0.24 b,d | 540.02 ± 12.58 e,* | 342.85 ± 39.95 f,g,* | 5.15 ± 0.14 f,h |
| TACFRAP, µmol-equiv. DQ/g raw material | 2050.23 ± 46.01 i | 757.61 ± 140.8 j,k,# | 2.03 ± 0.34 j,l | 709.17 ± 21.68 m,*,# | 282.63 ± 55.65 n,o,*,# | 2.73 ± 0.06 n,p |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernukha, I.; Kupaeva, N.; Kotenkova, E.; Khvostov, D. Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties. Antioxidants 2022, 11, 1243. https://doi.org/10.3390/antiox11071243
Chernukha I, Kupaeva N, Kotenkova E, Khvostov D. Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties. Antioxidants. 2022; 11(7):1243. https://doi.org/10.3390/antiox11071243
Chicago/Turabian StyleChernukha, Irina, Nadezhda Kupaeva, Elena Kotenkova, and Daniil Khvostov. 2022. "Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties" Antioxidants 11, no. 7: 1243. https://doi.org/10.3390/antiox11071243
APA StyleChernukha, I., Kupaeva, N., Kotenkova, E., & Khvostov, D. (2022). Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties. Antioxidants, 11(7), 1243. https://doi.org/10.3390/antiox11071243






