Neuroprotective Profile of Edible Flowers of Borage (Borago officinalis L.) in Two Different Models: Caenorhabditis elegans and Neuro-2a Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Material and Preparation of Extract
2.3. Fatty Acid Composition
2.4. In Vitro Antioxidant Activity Assays
2.4.1. DPPH Scavenging Activity
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.3. Oxygen Radical Antioxidant Capacity (ORAC) Assay
2.5. Neuro-2a Cell Assays
2.5.1. Cell Culture and Treatments
2.5.2. Cell Viability Assay
2.5.3. Intracellular ROS Production Assay
2.5.4. Endogenous Antioxidant Enzymes
SOD activity = (% Inhibition × 2 × F)/(50 × Vs × C)
2.6. C. elegans Assays
2.6.1. Nematode Strain and Maintenance
2.6.2. Oxidative Stress Resistance Assay
Assays were performed with at least 120 nematodes per treatment.
2.6.3. Lifespan Assay
2.6.4. Paralysis Assay
2.7. Statistics
3. Results
3.1. Extract Yield
3.2. Fatty Acid Composition
3.3. In Vitro Antioxidant Activity Assays (DPPH·Assay, FRAP, and ORAC)
3.4. Neuro-2a Cells Subjected to Oxidative Stress
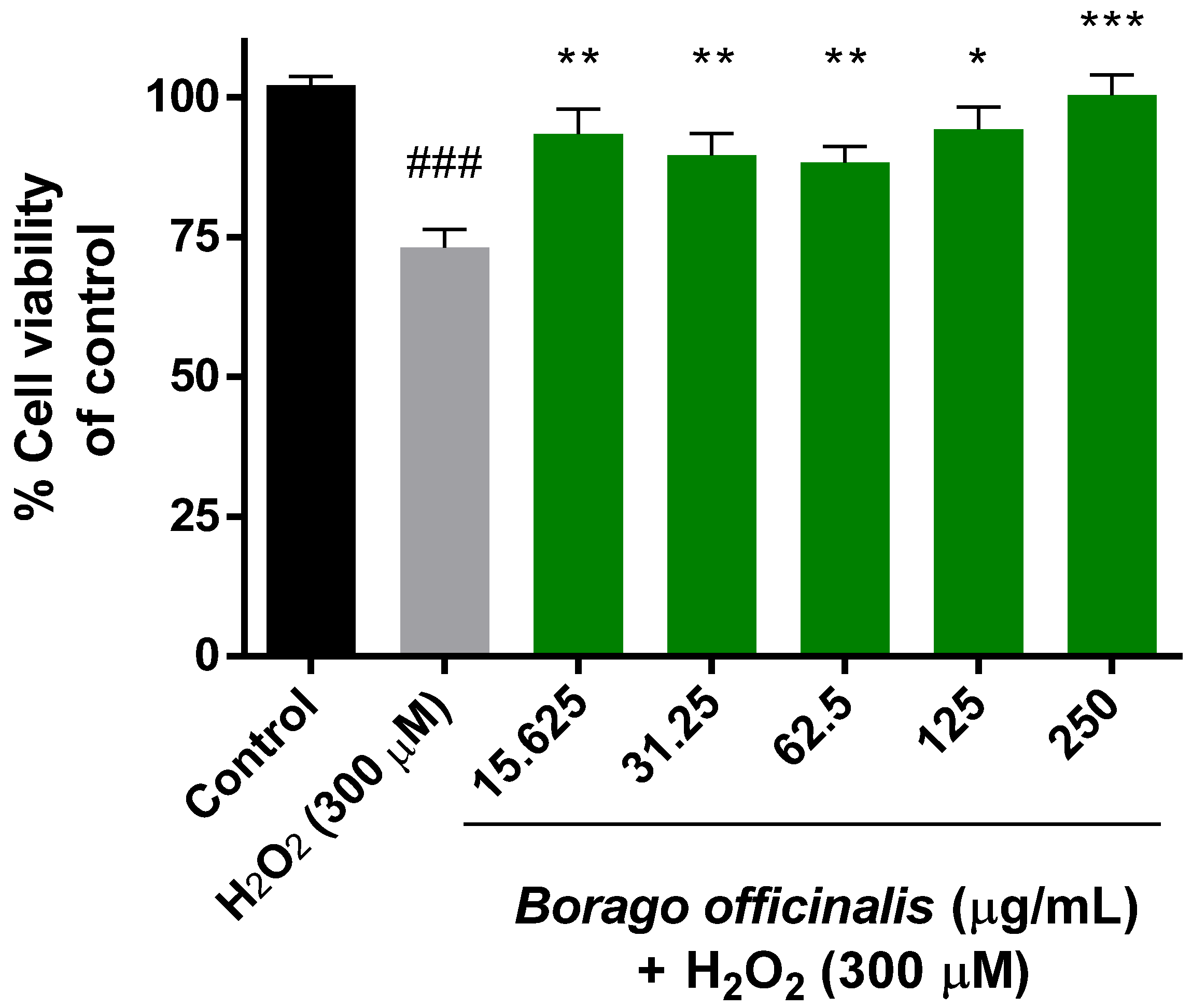
3.4.1. Neuroprotective Effect of B. Officinalis (MTT Assay)
3.4.2. Reactive Oxygen Species (ROS) Production Decreases When Neuro-2a Is Supplemented with B. officinalis
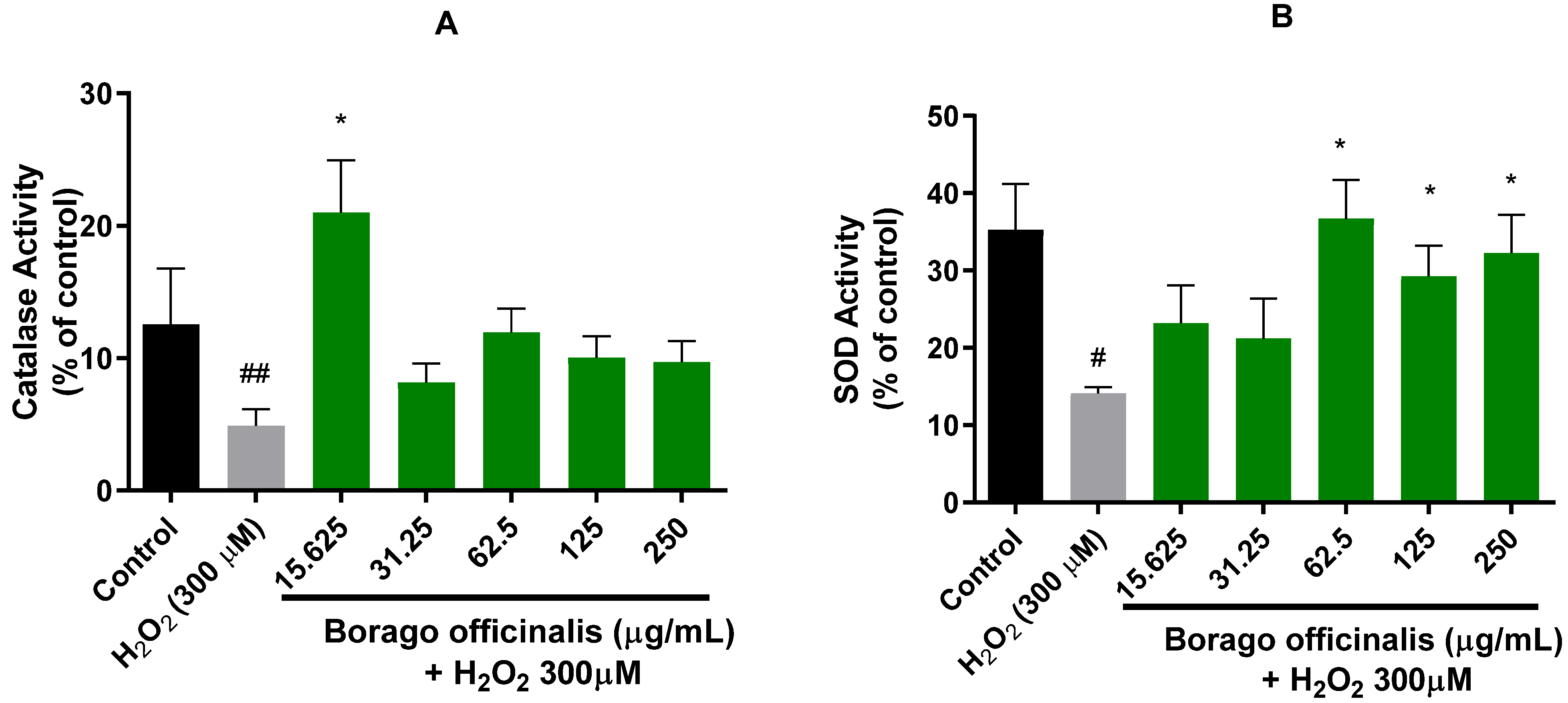
3.4.3. B. officinalis Ameliorates Catalase and Superoxide Dismutase (SOD) Activities
3.5. Neurodegenerative Model of C. elegans
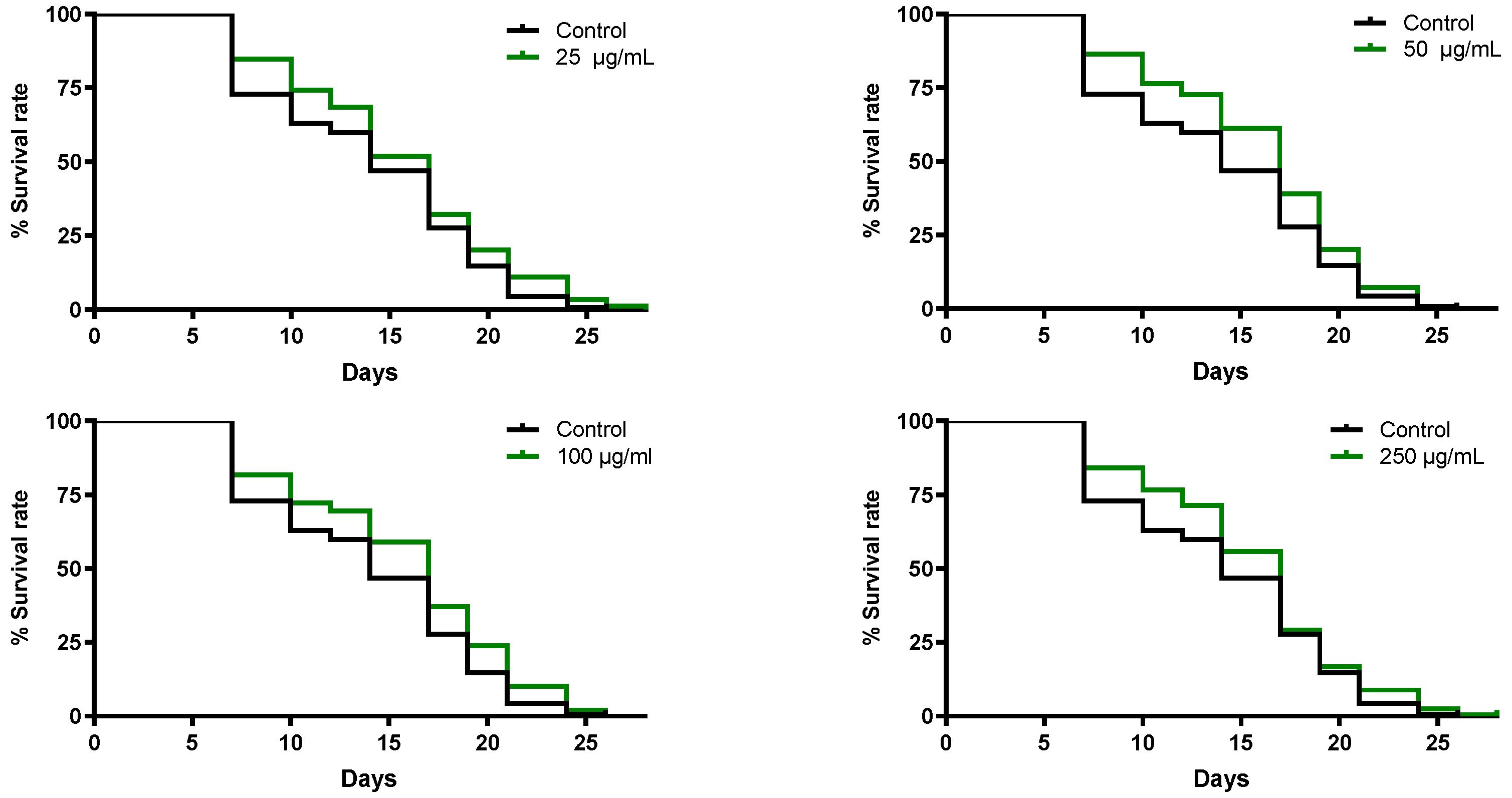
3.5.1. Extract of B. officinalis Enhances Survival Rate in C. elegans Induced with Lethal Oxidative Stress
3.5.2. B. officinalis Flower Extract Extends the Lifespan of C. elegans
3.5.3. Neuroprotective Effect of B. officinalis Flower Extract in C. elegans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramezani, M.; Amiri, M.S.; Zibaee, E.; Boghrati, Z.; Ayati, Z.; Sahebkar, A.; Emami, S.A. A Review on the Phytochemistry, Ethnobotanical Uses and Pharmacology of Borago Species. Curr. Pharm. Des. 2019, 26, 110–128. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef]
- El-Shazly, A.; Sarg, T.; Ateya, A.; Abdel Aziz, A.; El-Dahmy, S.; Witte, L.; Wink, M. Pyrrolizidine Alkaloids from Echium setosum and Echium vulgare. J. Nat. Prod. 1996, 59, 310–313. [Google Scholar] [CrossRef]
- Bruneton, J. Toxic Plants Dangerous to Humans and Animals; É ditions Tec & Doc; Intercept Limited: Huddersfield, UK, 1999; ISBN 1898298629. [Google Scholar]
- Avila, C.; Breakspear, I.; Hawrelak, J.; Salmond, S.; Evans, S. A systematic review and quality assessment of case reports of adverse events for borage (Borago officinalis), coltsfoot (Tussilago farfara) and comfrey (Symphytum officinale). Fitoterapia 2020, 142, 104519. [Google Scholar] [CrossRef]
- Lozano-Baena, M.-D.; Tasset, I.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; de Haro-Bailón, A. Cancer Prevention and Health Benefices of Traditionally Consumed Borago officinalis Plants. Nutrients 2016, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Djaoudene, O.; López, V.; Cásedas, G.; Les, F.; Schisano, C.; Bachir Bey, M.; Tenore, G.C. Phoenix dactylifera L. seeds: A by-product as a source of bioactive compounds with antioxidant and enzyme inhibitory properties. Food Funct. 2019, 10, 4953–4965. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; González-Burgos, E.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Evaluation of the antioxidant capacities and cytotoxic effects of ten parmeliaceae lichen species. Evid. Based Complement. Altern. Med. 2016, 201, 11. [Google Scholar] [CrossRef] [Green Version]
- Bandoniene, D.; Murkovic, M. The detection of radical scavenging compounds in crude extract of borage (Borago officinalis L.) by using an on-line HPLC-DPPH method. J. Biochem. Biophys. Methods 2002, 53, 45–49. [Google Scholar] [CrossRef]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F.; Loggia, R. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J. Ethnopharmacol. 2008, 116, 144–151. [Google Scholar] [CrossRef]
- Seo, S.A.; Park, B.; Hwang, E.; Park, S.Y.; Yi, T.H. Borago officinalis L. attenuates UVB-induced skin photodamage via regulation of AP-1 and Nrf2/ARE pathway in normal human dermal fibroblasts and promotion of collagen synthesis in hairless mice. Exp. Gerontol. 2018, 107, 178–186. [Google Scholar] [CrossRef]
- Barros, L.; Alves, C.T.; Dueñas, M.; Silva, S.; Oliveira, R.; Carvalho, A.M.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds in wild medicinal flowers from Portugal by HPLC–DAD–ESI/MS and evaluation of antifungal properties. Ind. Crops Prod. 2013, 44, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casanova, E.; García-mina, J.; Cavero, R.; Calvo, M. Screening of Spanish Medicinal Plants for Antioxidant and Antifungal Activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Dávalos, A.; Gómez-Cordovéz, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Cásedas, G.; González-burgos, E.; Smith, C.; López, V.; Pilar, M. Regulation of redox status in neuronal SH-SY5Y cells by blueberry (Vaccinium myrtillus L.) juice, cranberry (Vaccinium macrocarpon A.) juice and cyanidin. Food Chem. Toxicol. 2018, 118, 572–580. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; López, V.; Choya-Foces, C.; Hugo, M. The metabolite urolithin-a ameliorates oxidative stress in neuro-2a cells, becoming a potential neuroprotective agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- Stiernagle, T. Maintenance of C. elegans. WormBook 1999, 2, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Virk, B.; Jia, J.; Maynard, C.A.; Helliwell, N.; Cipinska, M.; Weinkove, D.; Virk, B.; Jia, J.; Maynard, C.A.; Raimundo, A.; et al. Folate Acts in E. coli to Accelerate C. elegans Aging Independently of Bacterial Biosynthesis. Cell Rep. 2016, 14, 1611–1620. [Google Scholar] [CrossRef] [Green Version]
- Dostal, V.; Link, C.D. Assaying β-amyloid Toxicity using a Transgenic C. elegans Model. J. Vis. Exp. 2010, 3791, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Pluci, B.; Kuczy, P. The oxidative stress in allelopathy: Participation of prenyllipid antioxidants in the response to juglone in Chlamydomonas reinhardtii. Phytochemistry 2017, 144, 171–179. [Google Scholar] [CrossRef]
- Zargooshnia, S.; Shahidi, S.; Ghahremanitamadon, F.; Nikkhah, A.; Mehdizadeh, M.; Soleimani Asl, S. The protective effect of Borago officinalis extract on amyloid β (25-35)-induced long term potentiation disruption in the dentate gyrus of male rats. Metab. Brain Dis. 2015, 30, 151–156. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Damiri, B.; Abualhasan, M.N. Antioxidant Evaluation for Urtica urens, Rumex cyprius and Borago officinalis edible Wild Plants in Palestine. Available online: https://pubmed.ncbi.nlm.nih.gov/27005499 (accessed on 12 August 2021).
- Abu-Qaoud, H.; Shawarb, N.; Hussen, F.; Jaradat, N.; Shtaya, M. Report: Comparison of qualitative, quantitative analysis and antioxidant potential between wild and cultivated Borago officinalis leaves from palestine. Pak. J. Pharm. Sci. 2018, 31, 953–959. [Google Scholar]
- Wang, F.; Miao, M.; Xia, H.; Yang, L.; Wang, S.; Sun, G. Antioxidant activities of aqueous extracts from 12 Chinese edible flowers in vitro and in vivo. Food Nutr. Res. 2017, 61, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, L.A.C.; Karp, S.G.; Vendruscolo, F.; Kanno, K.Y.F.; Zoz, L.I.C.; Carvalho, J.C. Biotechnological Production of Carotenoids and Their Applications in Food and Pharmaceutical Products. Carotenoids 2017, 8, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Beaudoin-Chabot, C.; Wang, L.; Smarun, A.V.; Vidović, D.; Shchepinov, M.S.; Thibault, G. Deuterated polyunsaturated fatty acids reduce oxidative stress and extend the lifespan of C. elegans. Front. Physiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghahremanitamadon, F.; Shahidi, S.; Zargooshnia, S.; Nikkhah, A.; Ranjbar, A.; Soleimani Asl, S. Protective effects of Borago officinalis extract on amyloid β-peptide(25-35)-induced memory impairment in male rats: A behavioral study. Biomed Res. Int. 2014, 2014, 798535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, D.R.; Martorell, P.; Genovés, S.; Gosálbez, L. Development of novel functional ingredients: Need for testing systems and solutions with Caenorhabditis elegans. Trends Food Sci. Technol. 2016, 54, 197–203. [Google Scholar] [CrossRef]
- Przybysz, A.J.; Choe, K.P.; Roberts, L.J.; Strange, K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech. Ageing Dev. 2009, 130, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, F.; Adam, R.H.I.; Broersen, K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 981–1017. [Google Scholar] [CrossRef] [Green Version]
- Volkert, M.R.; Crowley, D.J. Preventing Neurodegeneration by Controlling Oxidative Stress: The Role of OXR1. Front. Neurosci. 2020, 14, 611904. [Google Scholar] [CrossRef]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42 ) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.). Oils 2018, 8, 2606. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Purohit, S.; Kalita, D.; Barik, C.R.; Sahoo, L.; Goud, V.V. Evaluation of thermophysical, biochemical and antibacterial properties of unconventional vegetable oil from Northeast India. Mater. Sci. Energy Technol. 2021, 4, 81–91. [Google Scholar] [CrossRef]
- Hosseini, M.; Poljak, A.; Braidy, N.; Crawford, J.; Sachdev, P. Blood fatty acids in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res. Rev. 2020, 60, 101043. [Google Scholar] [CrossRef]
- Montaner, C.; Zufiaurre, R.; Movila, M.; Mallor, C. Evaluation of Borage (Borago officinalis L.) Genotypes for Nutraceutical Value Based on Leaves Fatty Acids Composition. Foods 2021, 11, 16. [Google Scholar] [CrossRef]



| Fatty Acids | Shorthand Nomenclature | Relative Percentage (%) |
|---|---|---|
| Caproic acid | 6:0 | 0.24 ± 0.04 |
| Caprylic acid | 8:0 | 0.0802 ± 0.0001 |
| Capric acid | 10:0 | 0.041 ± 0.003 |
| Docosanoic acid | 22:0 | 1.39 ± 0.001 |
| Dodecanoic acid | 12:0 | 0.114 ± 0.002 |
| Heptadecanoic acid | 17:0 | 0.48 ± 0.01 |
| Lignoceric acid | 24:0 | 0.732 ± 0.001 |
| Myristic acid | 14:0 | 0.45 ± 0.04 |
| Pentadecanoic acid | 15:0 | 2.48 ± 0.03 |
| Palmitic acid | 16:0 | 17.17 ± 0.04 |
| Stearic acid | 18:0 | 3.8 ± 0.1 |
| Tricosylic acid | 23:0 | 0.32 ± 0.01 |
| Eicosenoic acid | 20:1 | 0.49 ± 0.02 |
| Erucic acid | 22:1 ω 9 | 0.16 ± 0.01 |
| Myristoleic acid | 14:1 | 0.24 ± 0.01 |
| Nervonic acid | 24:1 | 1.05 ± 0.01 |
| Oleic acid | 18:1 ω 9 | 15.93 ± 0.04 |
| Palmitoleic acid | 16:1 | 0.19 ± 0.01 |
| Eicosadienoic acid | 20:2 | 0.42 ± 0.04 |
| Eicosatrienoic acid | 20:3 ω 3 + 21:0 | 0.276 ± 0.004 |
| Eicosapentaenoic acid | 20:5 ω 3 | 0.12 ± 0.01 |
| γ-Linolenic acid | 18:3 ω 6 | 0.13 ± 0.01 |
| α-Linolenic acid | 18:3 ω 3 | 0.07 ± 0.01 |
| Linoleic acid | 18:2 ω 6 | 53.7 ± 0.2 |
| Σ Saturated fatty acids (SFA) | 27.27 | |
| Σ Monounsaturated fatty acids (MUFA) | 18.05 | |
| Σ Polyunsaturated fatty acids (PUFA) | 54.68 | |
| Σ ω 6 | 53.83 | |
| Σ ω 3 | 0.59 | |
| Cox value | 5.73 | |
| Atherogenicity Index (AI) | 0.26 | |
| DPPH IC50 (µg/mL) | FRAP µmol Fe2+/g Extract | ORAC µmol TE/mg Extract | |
|---|---|---|---|
| B. officinalis | 646 ± 35 | 4 ± 2 | 0.54 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moliner, C.; Cásedas, G.; Barros, L.; Finimundy, T.C.; Gómez-Rincón, C.; López, V. Neuroprotective Profile of Edible Flowers of Borage (Borago officinalis L.) in Two Different Models: Caenorhabditis elegans and Neuro-2a Cells. Antioxidants 2022, 11, 1244. https://doi.org/10.3390/antiox11071244
Moliner C, Cásedas G, Barros L, Finimundy TC, Gómez-Rincón C, López V. Neuroprotective Profile of Edible Flowers of Borage (Borago officinalis L.) in Two Different Models: Caenorhabditis elegans and Neuro-2a Cells. Antioxidants. 2022; 11(7):1244. https://doi.org/10.3390/antiox11071244
Chicago/Turabian StyleMoliner, Cristina, Guillermo Cásedas, Lillian Barros, Tiane C. Finimundy, Carlota Gómez-Rincón, and Víctor López. 2022. "Neuroprotective Profile of Edible Flowers of Borage (Borago officinalis L.) in Two Different Models: Caenorhabditis elegans and Neuro-2a Cells" Antioxidants 11, no. 7: 1244. https://doi.org/10.3390/antiox11071244
APA StyleMoliner, C., Cásedas, G., Barros, L., Finimundy, T. C., Gómez-Rincón, C., & López, V. (2022). Neuroprotective Profile of Edible Flowers of Borage (Borago officinalis L.) in Two Different Models: Caenorhabditis elegans and Neuro-2a Cells. Antioxidants, 11(7), 1244. https://doi.org/10.3390/antiox11071244










