Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo
Abstract
:1. Introduction
- (1)
- To investigate whether intravitreal injection of CeO2-NPs is able to modulate the VEGF expression and to counteract neovascularization in an in vivo model of AMD.
- (2)
- To highlight the antioxidant effects of CeO2-NPs on ARPE-19 cells by analyzing the modulation of specific oxidative stress-related markers.
- (3)
- To understand whether, in an in vitro model, the ARPE-19 dysfunction induced by oxidative stress could promote neovascularization in HUVEC cells and if it might be prevented by CeO2-NPs treatment.
2. Materials and Methods
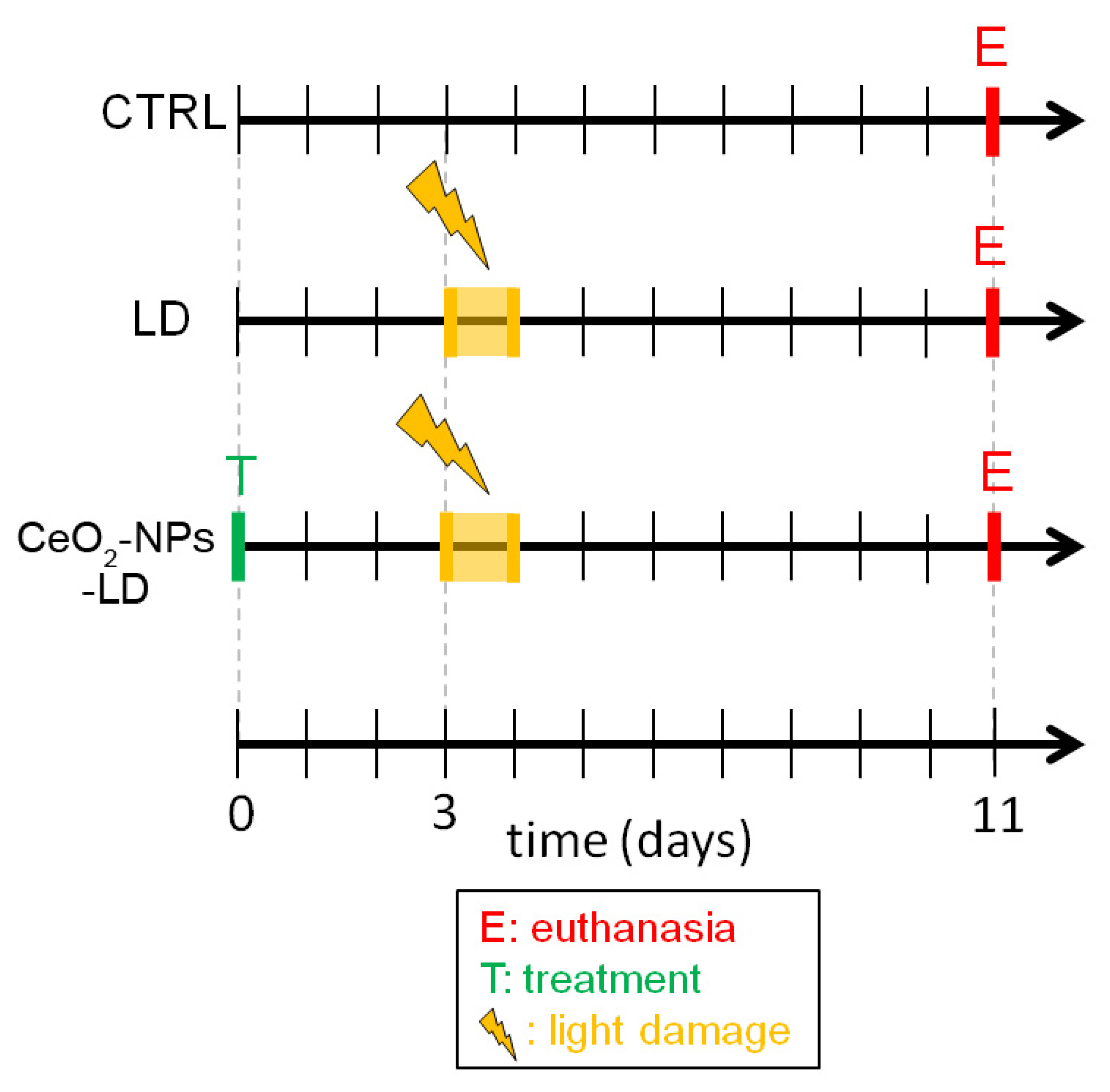
2.1. In Vivo Experimental Design
2.2. Intravitreal Injections of CeO2-NPs
2.3. In Vivo Retinal Light Damage
2.4. Retinal Samples Collection
2.5. Western Blot Analysis
2.6. Isolectin Staining and Vasculature Analysis of Rat Retinas
2.7. Cell Culture
2.8. Oxidative Stress Induction and CeO2-NPs Treatment
2.9. Preparation of Conditioned Media (CM) of ARPE-19 Cells
2.10. Enzyme Activity Measurements
2.10.1. Superoxide Dismutase 2 (SOD 2) Activity Assay
2.10.2. Glutathione Peroxidase (GPx) Activity Assay
2.10.3. Glyoxalase 1 (Glo1) Activity Assay
2.10.4. Glutathione-S-Transferase (GST) Activity Assay
2.10.5. Glutathione (GSH) Assay
2.11. Heme Oxygenase-1 (HO-1) Detection
2.12. 5-Hydro-5-Methylimidazolone (MG-H1) Protein Adducts Detection
2.13. Malondialdehyde (MDA) Detection
2.14. Phalloidin Staining
2.15. VEGF Determination
2.16. In Vitro Tubule-Like Formation
2.17. Cell Viability Assay
2.18. Tubule Formation in Co-Culture Assay
2.19. Statistical Analysis
3. Results
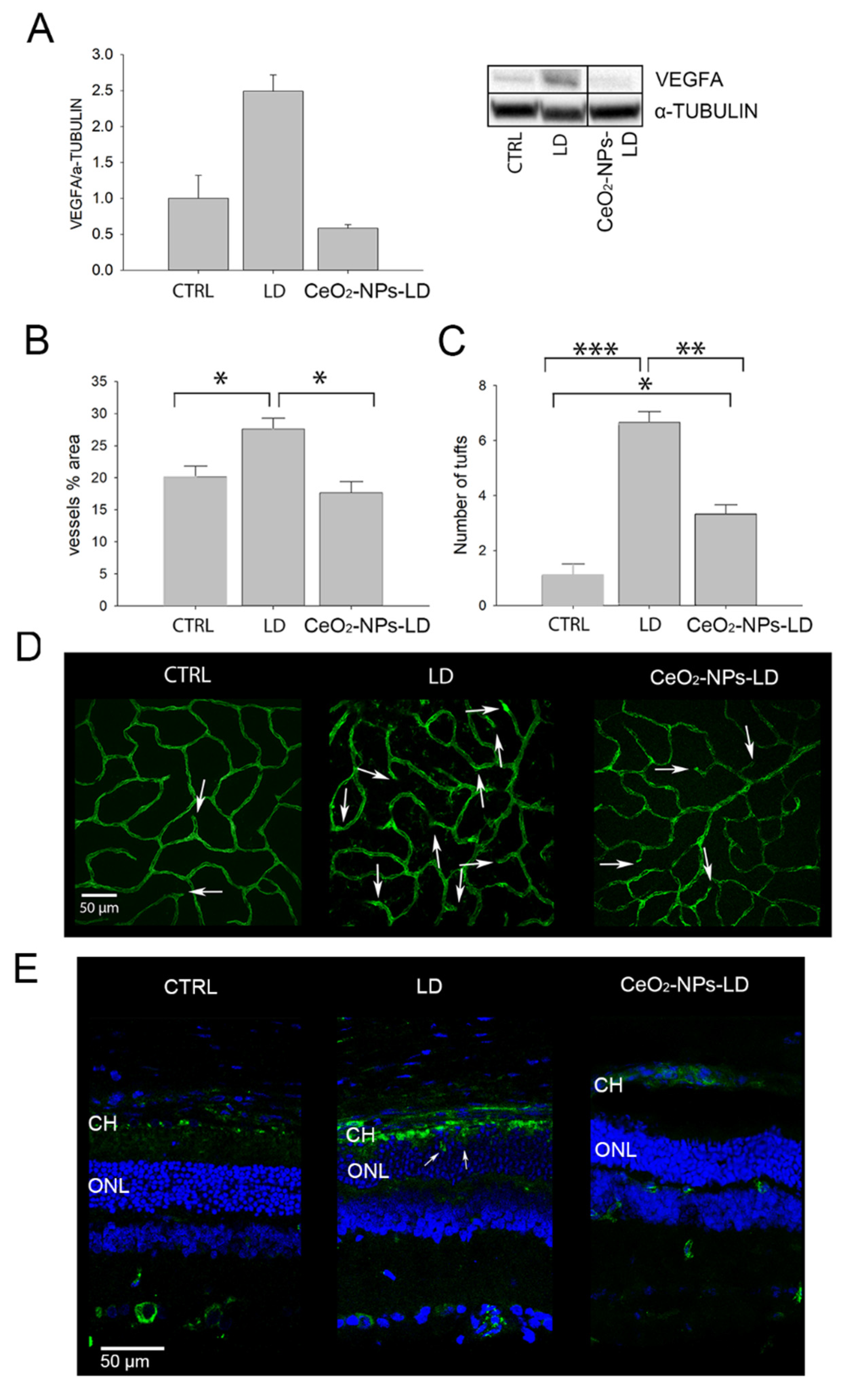
3.1. Effects of CeO2-NPs on Retinal Vasculature In Vivo
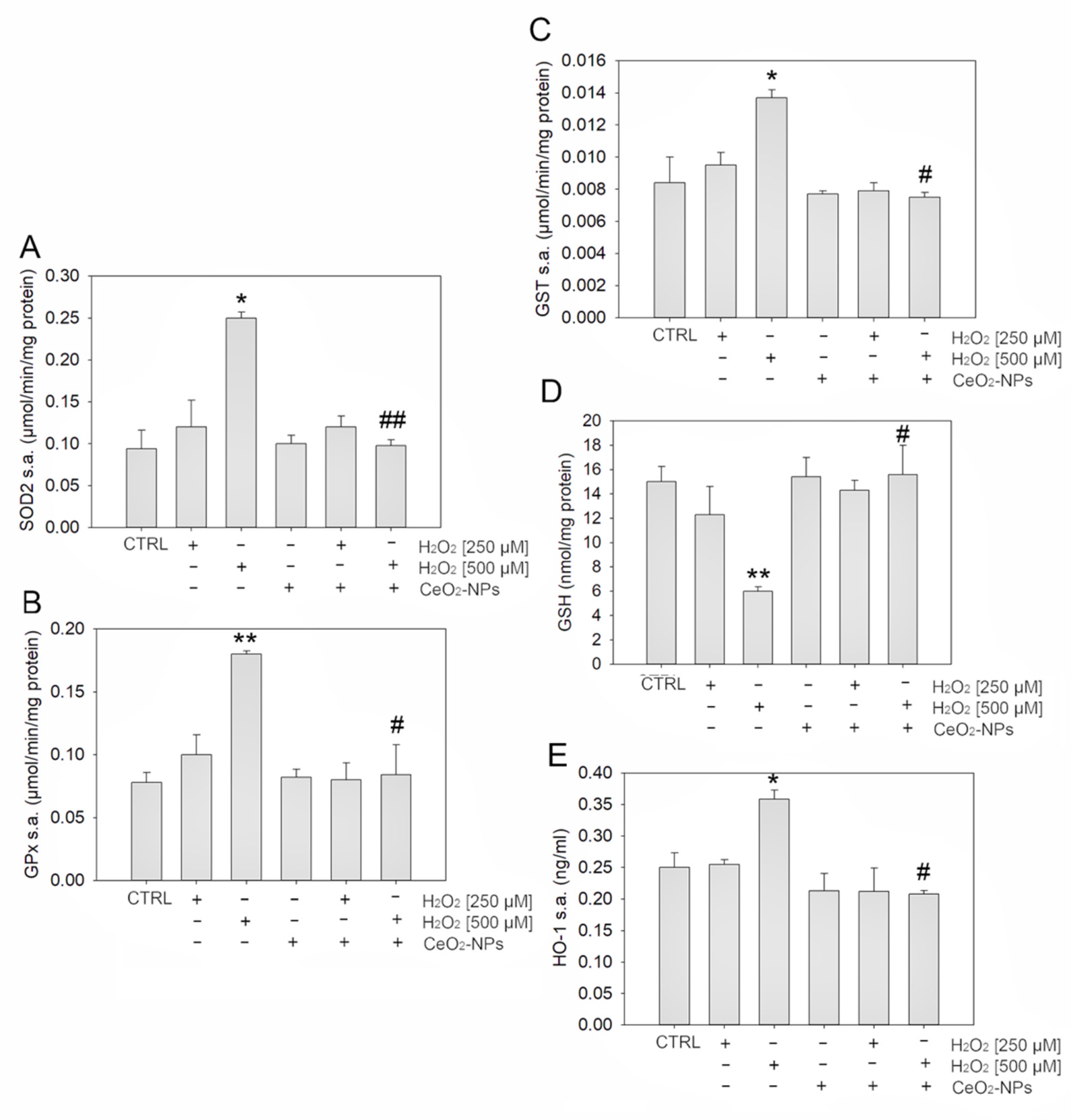
3.2. CeO2-NPs Counteract H2O2-Induced Oxidative Stress in ARPE-19
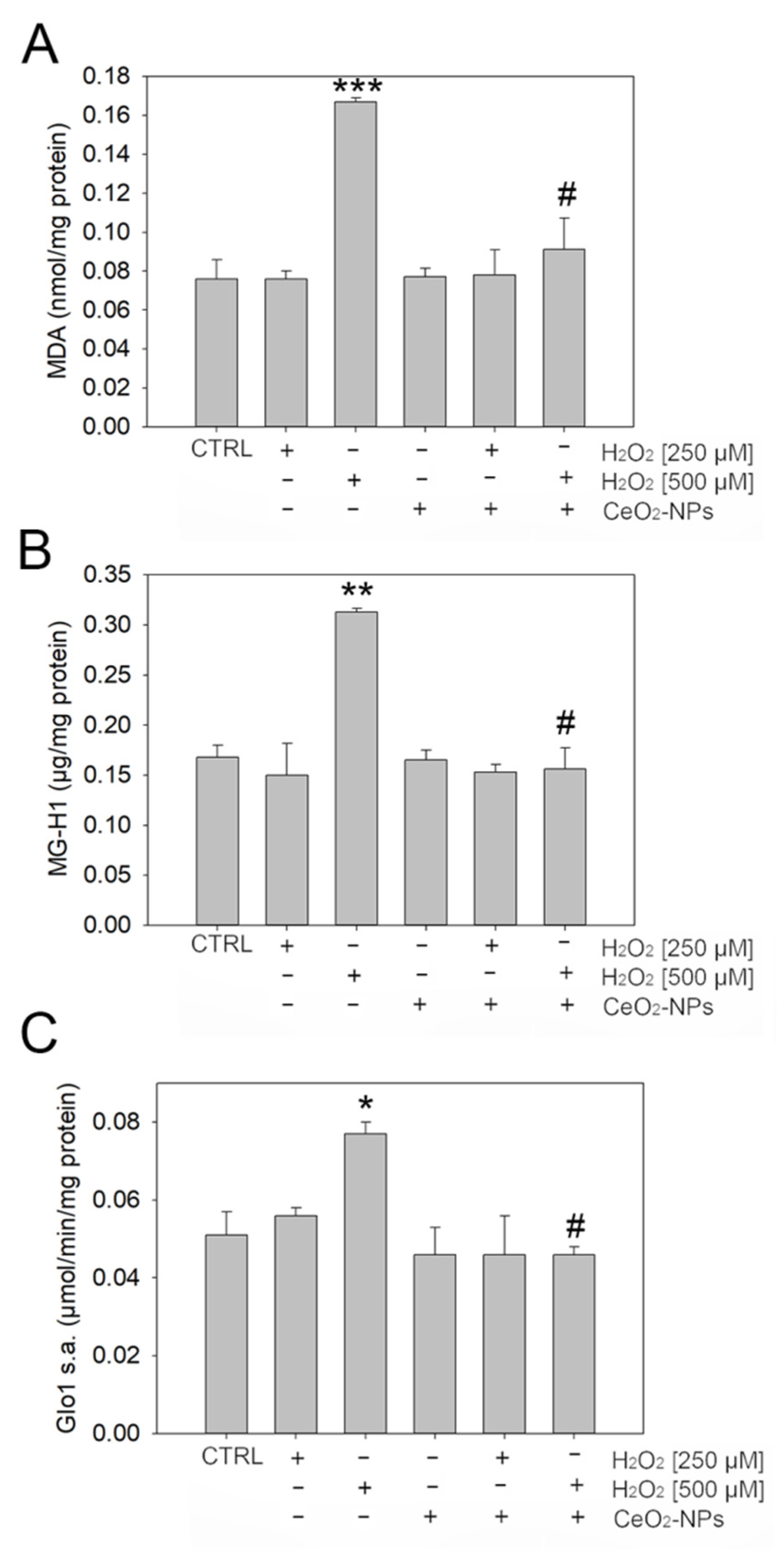
3.3. CeO2 NPs Counteract Dicarbonyl Stress in ARPE-19
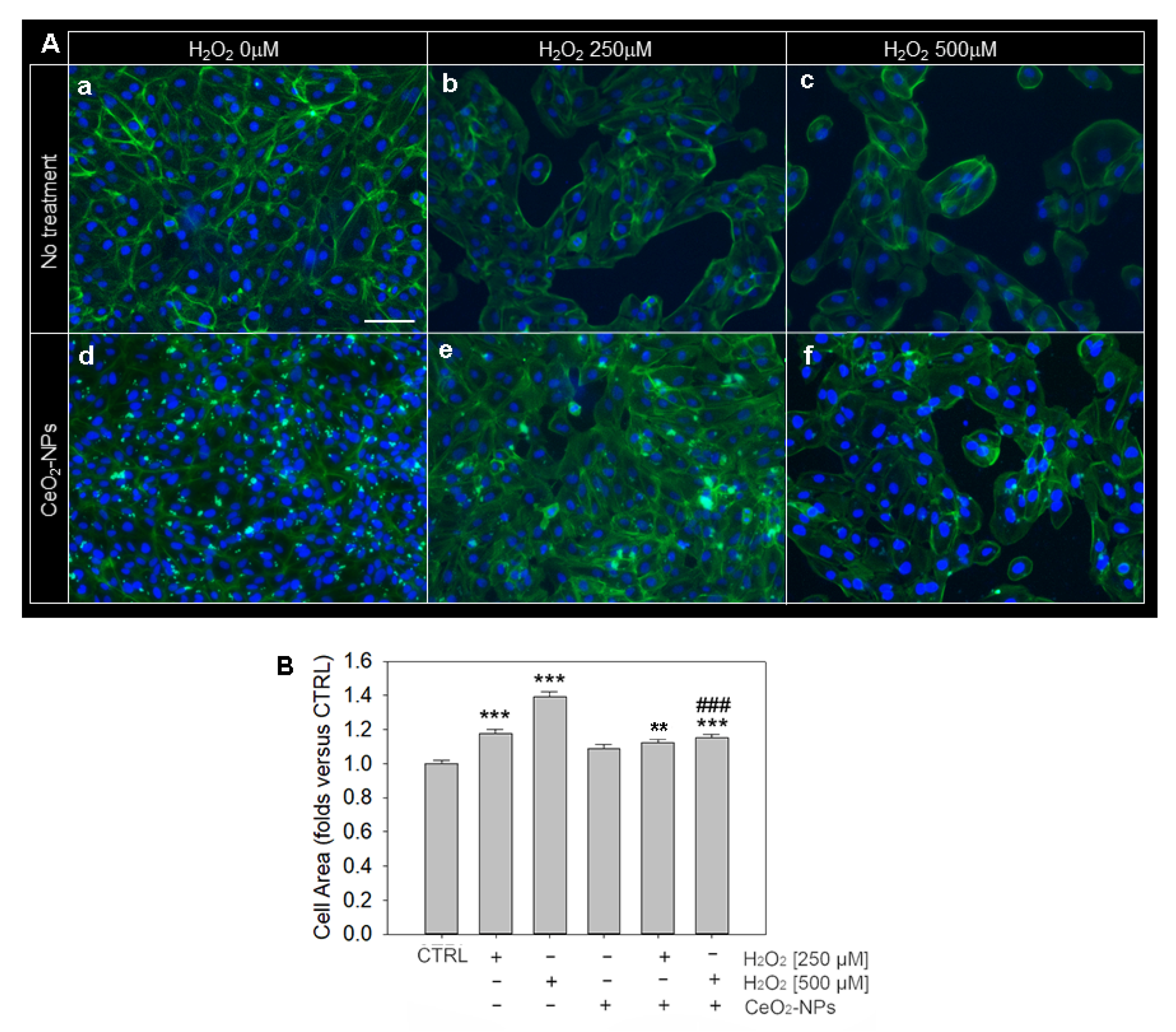
3.4. Effects of CeO2-NPs on ARPE-19 Cells Morphology and Cytoskeleton Organization
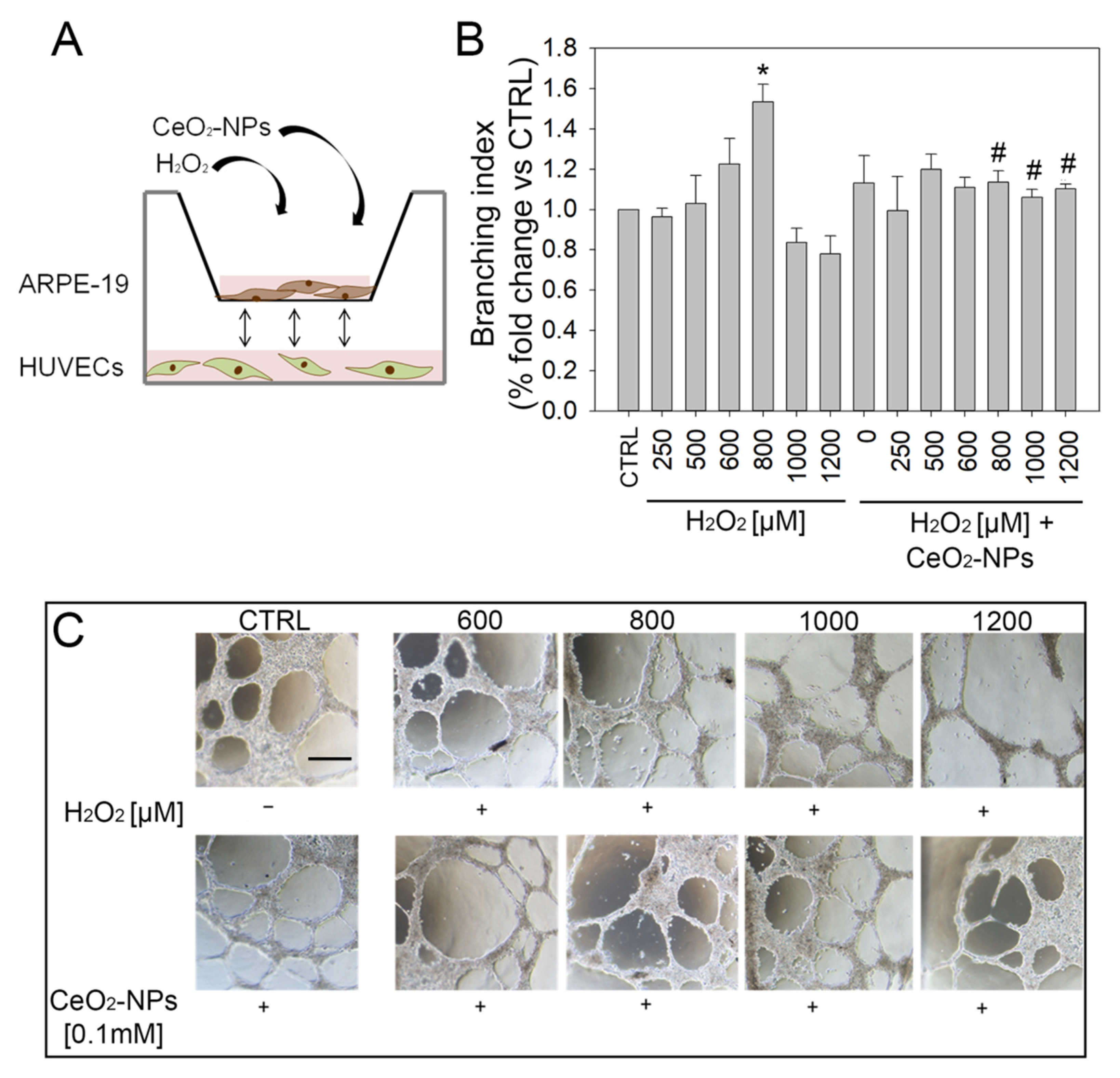
3.5. CeO2-NPs Counteract the Effects Exerted by CM from ARPE-19 Cells on Tubule Formation
4. Discussion
4.1. CeO2-NPs Inhibit Retinal Neovascularization and VEGFA Up-Regulation in the Light Damage Model of AMD
4.2. CeO2-NPs Protect ARPE-19 Cells Restoring Antioxidant Defenses
4.3. CeO2-NPs Are Able to Restore the Tubule Formation Ability of HUVECs, Protecting ARPE-19 Cells and HUVECs from Oxidative Stress-Induced Cell Damage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.M.; Arepalli, S.; Ehlers, J.P. Current and Future Anti-VEGF Agents for Neovascular Age-Related Macular Degeneration. J. Exp. Pharmacol. 2021, 13, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. Int. J. Mol. Sci. 2020, 21, 8242. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T. How does the macula protect itself from oxidative stress? Mol. Asp. Med. 2012, 33, 418–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.P. Redox theory of aging. Redox Biol. 2015, 5, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Han, X.; Wittchen, E.S.; Hartnett, M.E. TNF-α mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent β-catenin activation. Mol. Vis. 2016, 22, 116–128. [Google Scholar]
- Klettner, A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. 2012, 4, 392. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M.; John, H.; Paulson, A. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef] [PubMed]
- Farecki, M.L.; Gutfleisch, M.; Faatz, H.; Rothaus, K.; Heimes, B.; Spital, G.; Lommatzsch, A.; Pauleikhoff, D. Characteristics of type 1 and 2 CNV in exudative AMD in OCT-Angiography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Passacantando, M.; Santucci, S. Surface electronic and structural properties of CeO2 nanoparticles: A study by core-level photoemission and peak diffraction. J. Nanoparticle Res. 2013, 15, 2–7. [Google Scholar] [CrossRef]
- Tisi, A.; Flati, V.; Delle Monache, S.; Lozzi, L.; Passacantando, M.; Maccarone, R. Nanoceria Particles Are an Eligible Candidate to Prevent Age-Related Macular Degeneration by Inhibiting Retinal Pigment Epithelium Cell Death and Autophagy Alterations. Cells 2020, 9, 1617. [Google Scholar] [CrossRef]
- Fiorani, L.; Passacantando, M.; Santucci, S.; Di Marco, S.; Bisti, S.; Maccarone, R. Cerium Oxide Nanoparticles Reduce Microglial Activation and Neurodegenerative Events in Light Damaged Retina. PLoS ONE 2015, 10, e0140387. [Google Scholar] [CrossRef] [Green Version]
- Tisi, A.; Passacantando, M.; Lozzi, L.; Riccitelli, S.; Bisti, S.; Maccarone, R. Retinal long term neuroprotection by Cerium Oxide nanoparticles after an acute damage induced by high intensity light exposure. Exp. Eye Res. 2019, 182, 30–38. [Google Scholar] [CrossRef]
- Tisi, A.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. Nanoceria neuroprotective effects in the light-damaged retina: A focus on retinal function and microglia activation. Exp. Eye Res. 2019, 188, 107797. [Google Scholar] [CrossRef]
- Tisi, A.; Passacantando, M.; Lozzi, L.; Maccarone, R. Cerium oxide nanoparticles reduce the accumulation of autofluorescent deposits in light-induced retinal degeneration: Insights for age-related macular degeneration. Exp. Eye Res. 2020, 199, 108169. [Google Scholar] [CrossRef]
- Tisi, A.; Parete, G.; Flati, V.; Maccarone, R. Up-regulation of pro-angiogenic pathways and induction of neovascularization by an acute retinal light damage. Sci. Rep. 2020, 10, 6376. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Antognelli, C.; Gambelunghe, A.; Talesa, V.N.; Muzi, G. Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: Involvement of superoxide anion, hydrogen peroxide and NF-κB. Apoptosis 2014, 19, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Talesa, V.N. Glyoxalases in Urological Malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habig, W.H.; Jakoby, W.B. Glutathione S-transferases (rat and human). In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 77, pp. 218–231. [Google Scholar]
- Delle Monache, S.; Pulcini, F.; Frosini, R.; Mattei, V.; Talesa, V.N.; Antognelli, C. Methylglyoxal-Dependent Glycative Stress Is Prevented by the Natural Antioxidant Oleuropein in Human Dental Pulp Stem Cells through Nrf2/Glo1 Pathway. Antioxidants 2021, 10, 716. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Carpentier, G.; Berndt, S.; Ferratge, S.; Rasband, W.; Cuendet, M.; Uzan, G.; Albanese, P. Angiogenesis Analyzer for ImageJ—A comparative morphometric analysis of “Endothelial Tube Formation Assay” and “Fibrin Bead Assay”. Sci. Rep. 2020, 10, 11568. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Li, J.Y.; Li, L.Z.; Zhang, Y.L.; Gong, H.Y.; Cui, Y. Protective Effect of Rosamultin against H2O2-Induced Oxidative Stress and Apoptosis in H9c2 Cardiomyocytes. Oxid. Med. Cell. Longev. 2018, 2018, 8415610. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Chen, J.; Xing, Y.Q. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed. Pharmacother. 2017, 85, 136–140. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Anti-Inflammatory CeO2 Nanoparticles Prevented Cytotoxicity Due to Exogenous Nitric Oxide Donors via Induction Rather Than Inhibition of Superoxide/Nitric Oxide in HUVE Cells. Molecules 2021, 26, 5416. [Google Scholar] [CrossRef]
- Wang, C.; Blough, E.; Dai, X.; Olajide, O.; Driscoll, H.; Leidy, J.W.; July, M.; Triest, W.E.; Wu, M. Protective Effects of Cerium Oxide Nanoparticles on MC3T3-E1 Osteoblastic Cells Exposed to X-Ray Irradiation. Cell Physiol. Biochem. 2016, 38, 1510–1519. [Google Scholar] [CrossRef]
- García-Salvador, A.; Katsumiti, A.; Rojas, E.; Aristimuño, C.; Betanzos, M.; Martínez-Moro, M.; Moya, S.E.; Goñi-De-Cerio, F. A Complete In Vitro Toxicological Assessment of the Biological Effects of Cerium Oxide Nanoparticles: From Acute Toxicity to Multi-Dose Subchronic Cytotoxicity Study. Nanomaterials 2021, 11, 1577. [Google Scholar] [CrossRef] [PubMed]
- Balla, J.; Jacob, H.S.; Balla, G.; Nath, K.; Eaton, J.W.; Vercellotti, G.M. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc. Natl. Acad. Sci. USA 1993, 90, 9285–9289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vistoli, G.; de Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic. Biol. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.J.; Won, J.Y. Molecular Mechanisms of Retinal Pigment Epithelium Dysfunction in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 12298. [Google Scholar] [CrossRef]
- Rutar, M.; Provis, J.M.; Valter, K. Brief exposure to damaging light causes focal recruitment of macrophages, and long-term destabilization of photoreceptors in the albino rat retina. Curr. Eye Res. 2010, 35, 631–643. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Chen, L.; Seal, S.; McGinnis, J.F. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Exp. Eye Res. 2013, 116, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Seal, S.; McGinnis, J.F. Sustained inhibition of neovascularization in vldlr−/− mice following intravitreal injection of cerium oxide nanoparticles and the role of the ASK1-P38/JNK-NF-κB pathway. Biomaterials 2014, 35, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Mitra, R.N.; Gao, R.; Zheng, M.; Wu, M.J.; Voinov, M.A.; Smirnov, A.I.; Smirnova, T.I.; Wang, K.; Chavala, S.; Han, Z. Glycol Chitosan Engineered Autoregenerative Antioxidant Significantly Attenuates Pathological Damages in Models of Age-Related Macular Degeneration. ACS Nano 2017, 11, 4669–4685. [Google Scholar] [CrossRef]
- Wong, L.L.; Barkam, S.; Seal, S.; McGinnis, J.F. Temporal Distribution Patterns of Alexa Fluor 647-Conjugated CeNPs in the Mouse Retina After a Single Intravitreal Injection. Adv. Exp. Med. Biol. 2019, 1185, 125–130. [Google Scholar] [PubMed]
- Cai, X.; Seal, S.; McGinnis, J.F. Non-toxic retention of nanoceria in murine eyes. Mol. Vis. 2016, 22, 1176. [Google Scholar] [PubMed]
- Wong, L.L.; Hirst, S.M.; Pye, Q.N.; Reilly, C.M.; Seal, S.; McGinnis, J.F. Catalytic Nanoceria Are Preferentially Retained in the Rat Retina and Are Not Cytotoxic after Intravitreal Injection. PLoS ONE 2013, 8, e58431. [Google Scholar] [CrossRef]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; de Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Bagatini, M.D.; Jaques, J.A.D.S.; de Oliveira, C.S.; de Oliveira, G.A.; Pillat, M.M.; Mânica, A.; Moser, C.D.S.; dos Santos, L.D.; Ulrich, H. Oxidative Stress: Noxious but Also Vital. In Novel Prospects in Oxidative and Nitrosative Stress; IntechOpen: London, UK, 2018. [Google Scholar]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Tsujinaka, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Ota, H.; Takeda, M.; Fujimura, T.; Takasawa, S.; Ogata, N. Human retinal pigment epithelial cell proliferation by the combined stimulation of hydroquinone and advanced glycation end-products via up-regulation of VEGF gene. Biochem. Biophys. Rep. 2015, 2, 123–131. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, B.; Jang, Y.P.; Pachydaki, S.; Schmidt, A.M.; Sparrow, J.R. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp. Eye Res. 2005, 80, 567–580. [Google Scholar] [CrossRef]
- Chen, Q.M.; Tu, V.C.; Wu, Y.; Bahl, J.J. Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Arch. Biochem. Biophys. 2000, 373, 242–248. [Google Scholar] [CrossRef]
- Dinc, E.; Ayaz, L.; Kurt, A.H. Protective Effect of Combined Caffeic Acid Phenethyl Ester and Bevacizumab Against Hydrogen Peroxide-Induced Oxidative Stress in Human RPE Cells. Curr. Eye Res. 2017, 42, 1659–1666. [Google Scholar] [CrossRef]
- Cai, H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc. Res. 2005, 68, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, M.; Ohzeki, Y.; Shimizu, S.; Naito, S.; Ohtsuru, A.; Yamamoto, T.; Kuroiwa, Y. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999, 64, 249–258. [Google Scholar] [CrossRef]
- Shono, T.; Ono, M.; Izumi, H.; Jimi, S.I.; Matsushima, K.; Okamoto, T.; Kohno, K.; Kuwano, M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 1996, 16, 4231–4239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, P.; Liu, Q.; Zheng, R. Biphasic regulation of H2O2 on angiogenesis implicated NADPH oxidase. Cell Biol. Int. 2010, 34, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Oltra, M.; Vidal-Gil, L.; Maisto, R.; Sancho-Pelluz, J.; Barcia, J.M. Oxidative stress-induced angiogenesis is mediated by miR-205-5p. J. Cell. Mol. Med. 2020, 24, 1428–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Patel, K.D.; Mahapatra, C.; Parthiban, S.P.; Kim, T.H.; Kim, H.W. Combinatory Cancer Therapeutics with Nanoceria-Capped Mesoporous Silica Nanocarriers through pH-triggered Drug Release and Redox Activity. ACS Appl. Mater. Interfaces 2019, 11, 288–299. [Google Scholar] [CrossRef]
- Safaeian, L.; Sajjadi, S.E.; Montazeri, H.; Ohadi, F.; Javanmard, S. Citral Protects Human Endothelial Cells Against Hydrogen Peroxide-induced Oxidative Stress. Turk. J. Pharm. Sci. 2020, 17, 549–554. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisi, A.; Pulcini, F.; Carozza, G.; Mattei, V.; Flati, V.; Passacantando, M.; Antognelli, C.; Maccarone, R.; Delle Monache, S. Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo. Antioxidants 2022, 11, 1133. https://doi.org/10.3390/antiox11061133
Tisi A, Pulcini F, Carozza G, Mattei V, Flati V, Passacantando M, Antognelli C, Maccarone R, Delle Monache S. Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo. Antioxidants. 2022; 11(6):1133. https://doi.org/10.3390/antiox11061133
Chicago/Turabian StyleTisi, Annamaria, Fanny Pulcini, Giulia Carozza, Vincenzo Mattei, Vincenzo Flati, Maurizio Passacantando, Cinzia Antognelli, Rita Maccarone, and Simona Delle Monache. 2022. "Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo" Antioxidants 11, no. 6: 1133. https://doi.org/10.3390/antiox11061133
APA StyleTisi, A., Pulcini, F., Carozza, G., Mattei, V., Flati, V., Passacantando, M., Antognelli, C., Maccarone, R., & Delle Monache, S. (2022). Antioxidant Properties of Cerium Oxide Nanoparticles Prevent Retinal Neovascular Alterations In Vitro and In Vivo. Antioxidants, 11(6), 1133. https://doi.org/10.3390/antiox11061133











