Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina?
Abstract
1. Introduction
2. Materials and Methods
2.1. General Chemicals and Reagents
2.2. Preparation of Liposomes
2.3. Comparison of the Effects of Ascorbate, α-Tocopherol and Zeaxanthin on Photosensitized Oxidation of Lipids
2.4. Cell Culture
2.5. Supplementation of Cells with Lipophilic Antioxidants
2.6. Exposure of Cells to Light and Liposomes
2.7. Evaluation of Cell Viability
2.8. Statistical Analysis
3. Results
3.1. Effects of Vitamin C, Vitamin E and/or Zeaxanthin on Photosensitized Oxidation
3.2. Effects of Various Liposomal ATR Concentrations and Exposure Times in Dark and Light on RPE Cell Viability
3.3. Effects on Phototoxicity of Supplementation of RPE Cells with Lipophilic Antioxidants
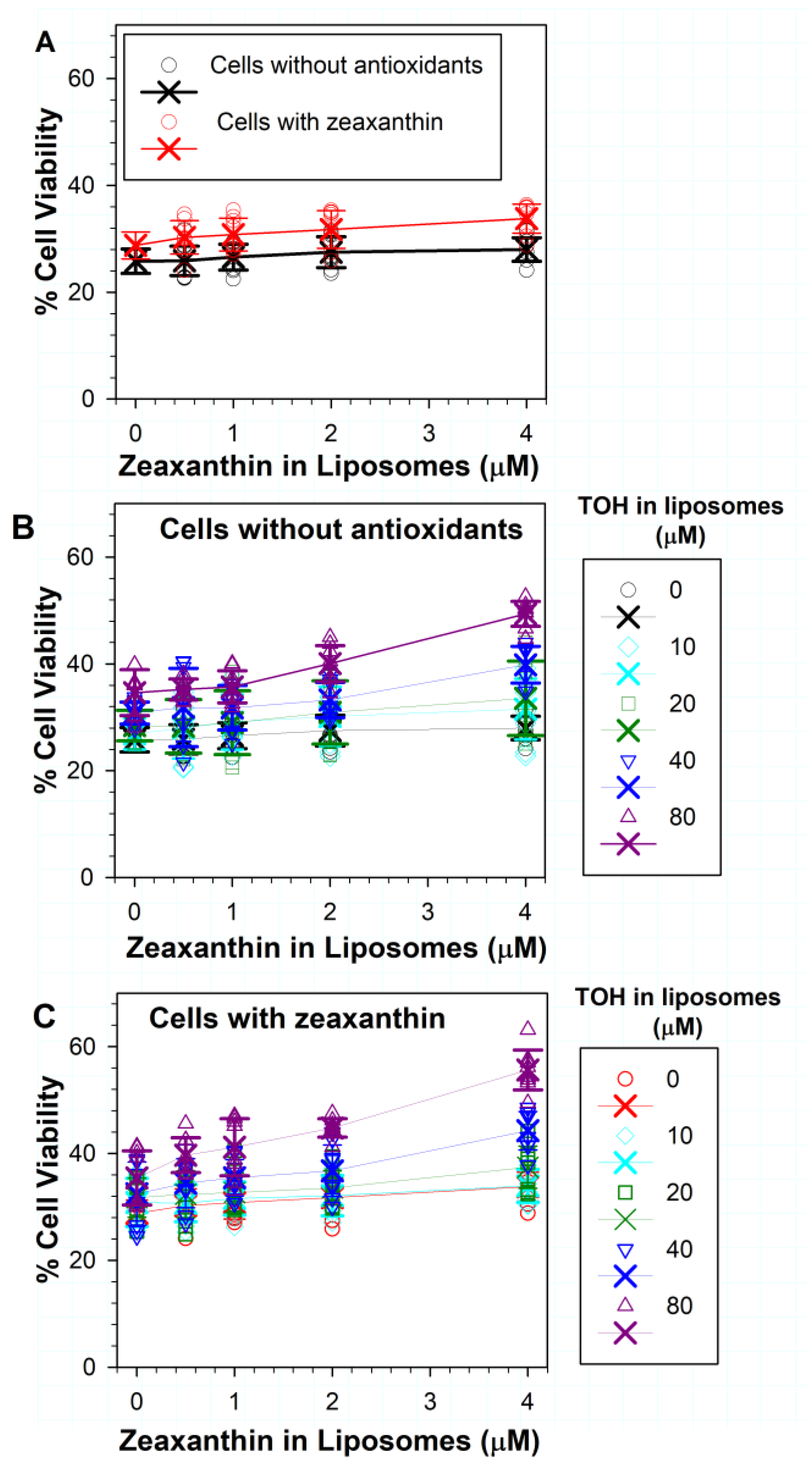
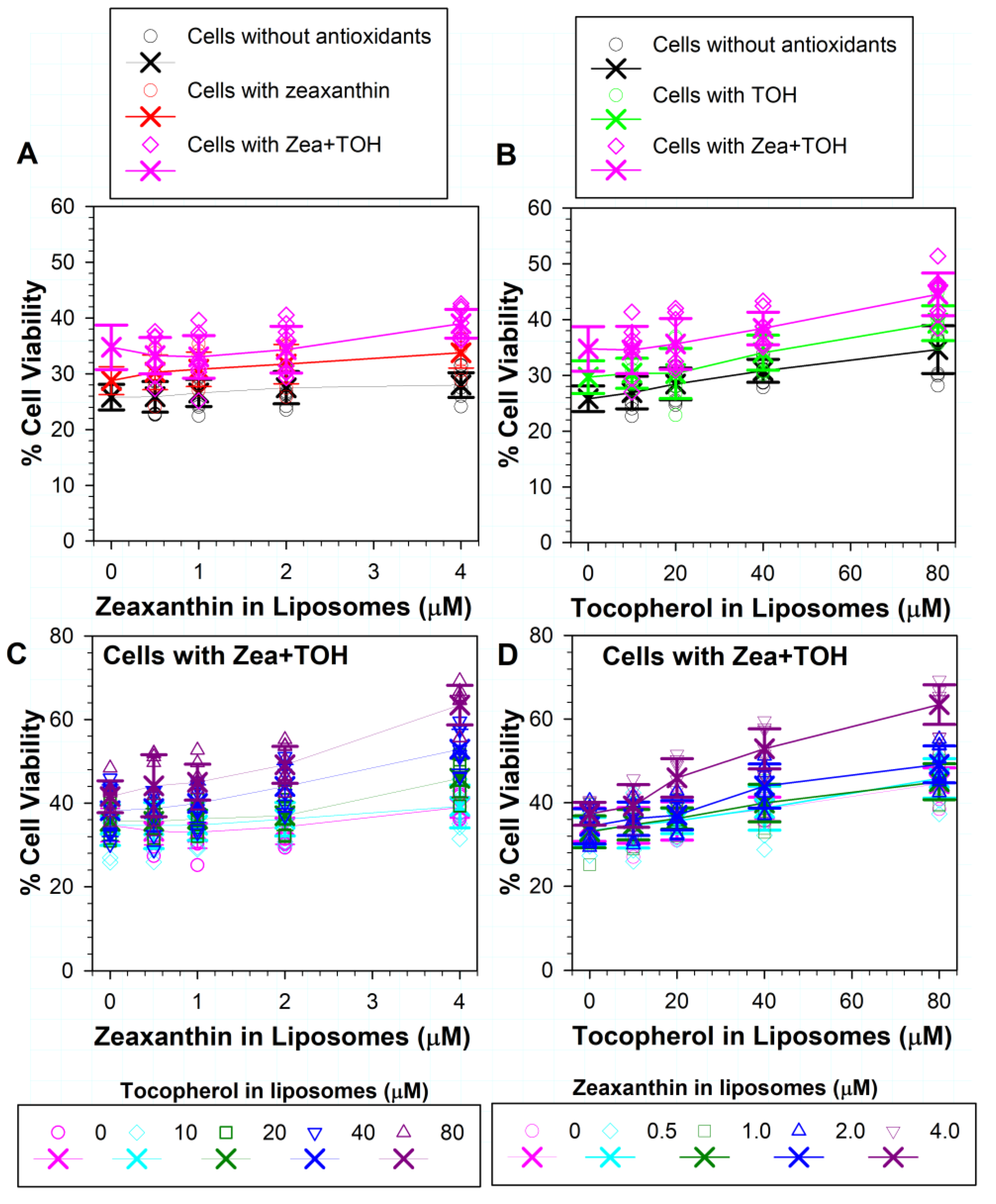
3.4. Effects on Phototoxicity to RPE Cells of Zeaxanthin Supplementation and Incorporation of Zeaxanthin and α-Tocopherol into ATR Liposomes
3.5. Effects on ATR Phototoxicity to RPE Cells of α-Tocopherol Supplementation and Incorporation of Zeaxanthin and α-Tocopherol into ATR Liposomes
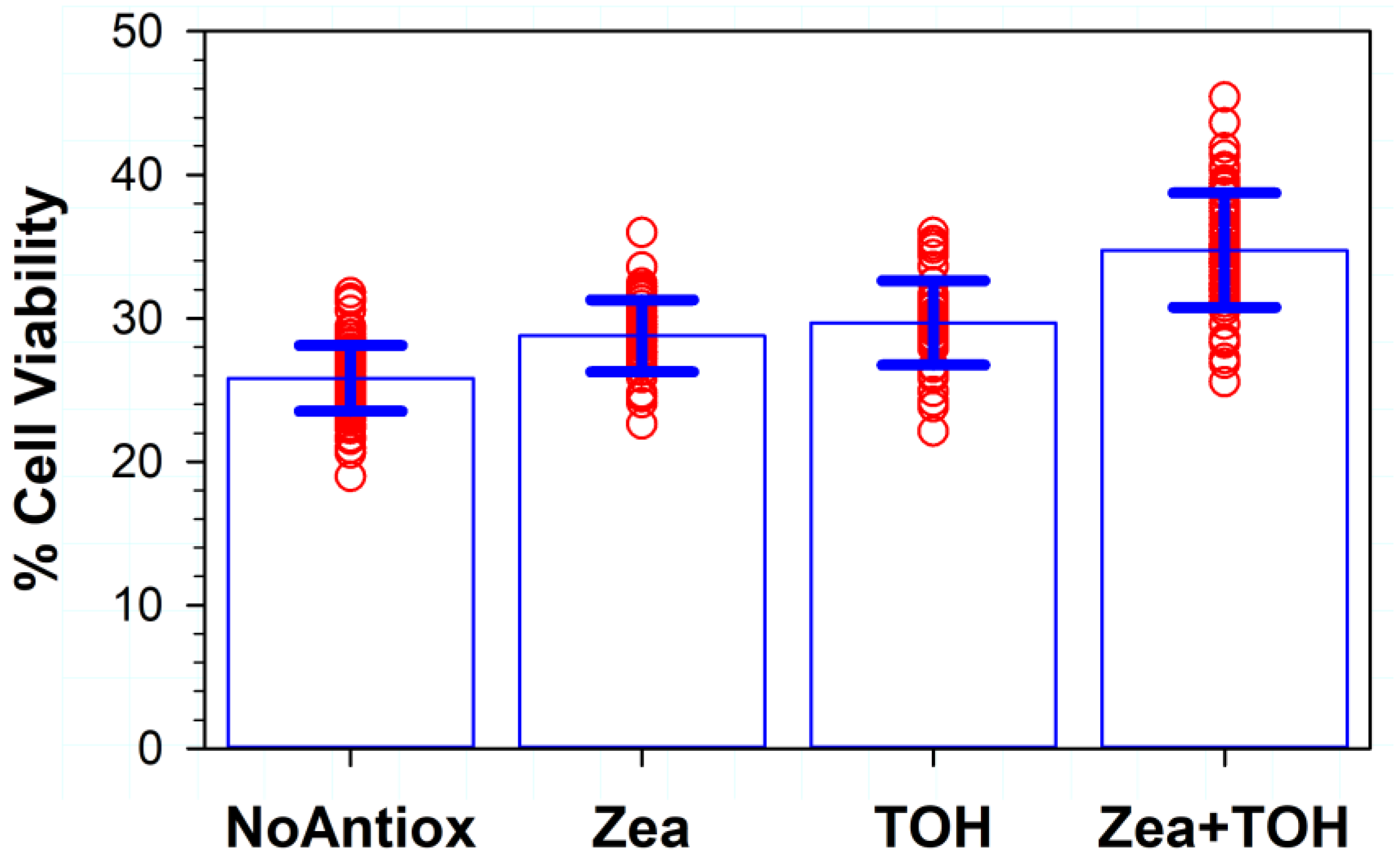
3.6. Effects of Different Combinations of Vitamin E and Zeaxanthin in Cells and/or in ATR-Containing Liposomes on Phototoxicity
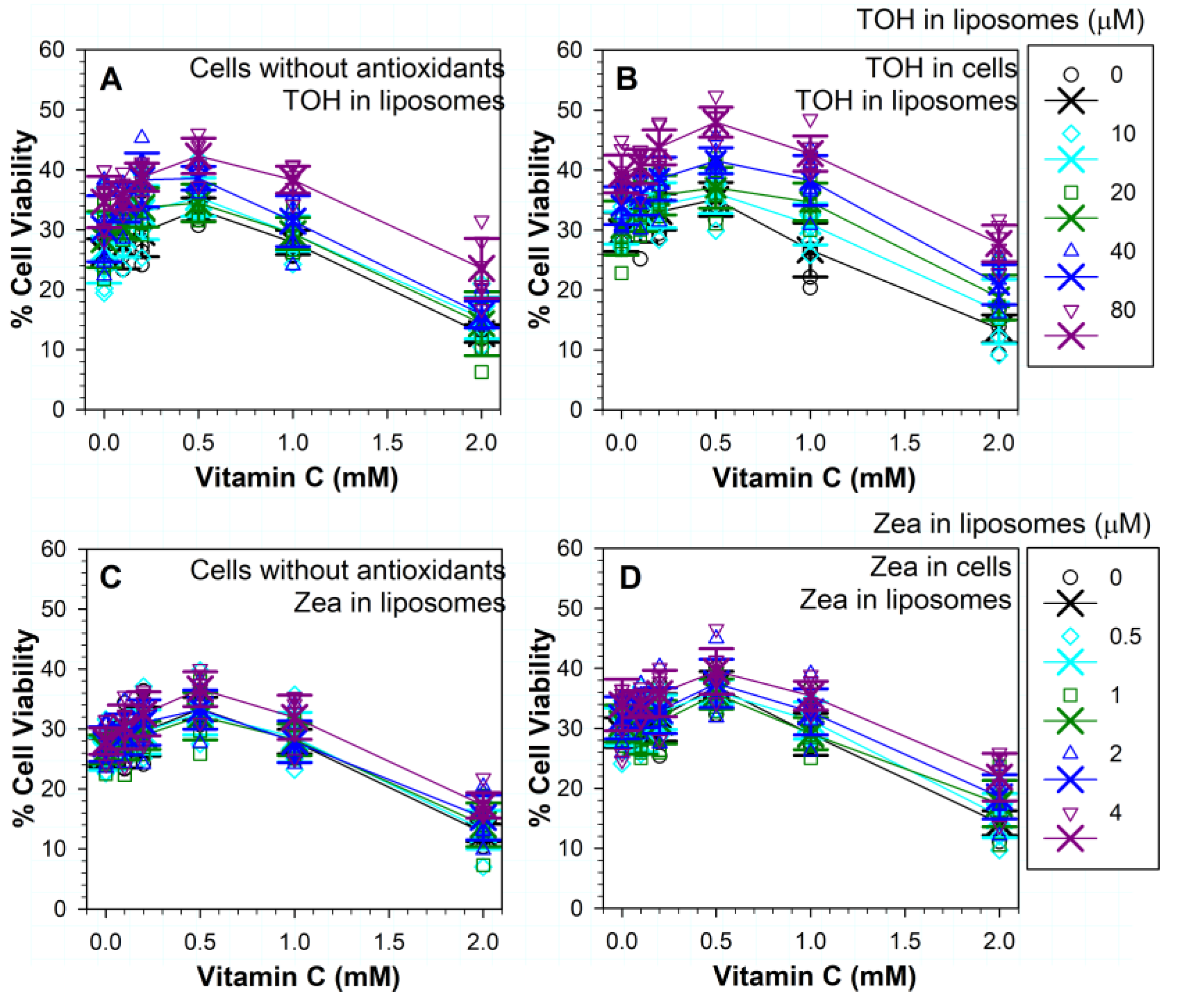
3.7. Effects of Vitamin C on (Photo) Toxicity
3.8. Effects of Different Combinations of Vitamins C and E on Phototoxicity
3.9. Effects of Different Combinations of Vitamins C and Zeaxanthin on Phototoxicity
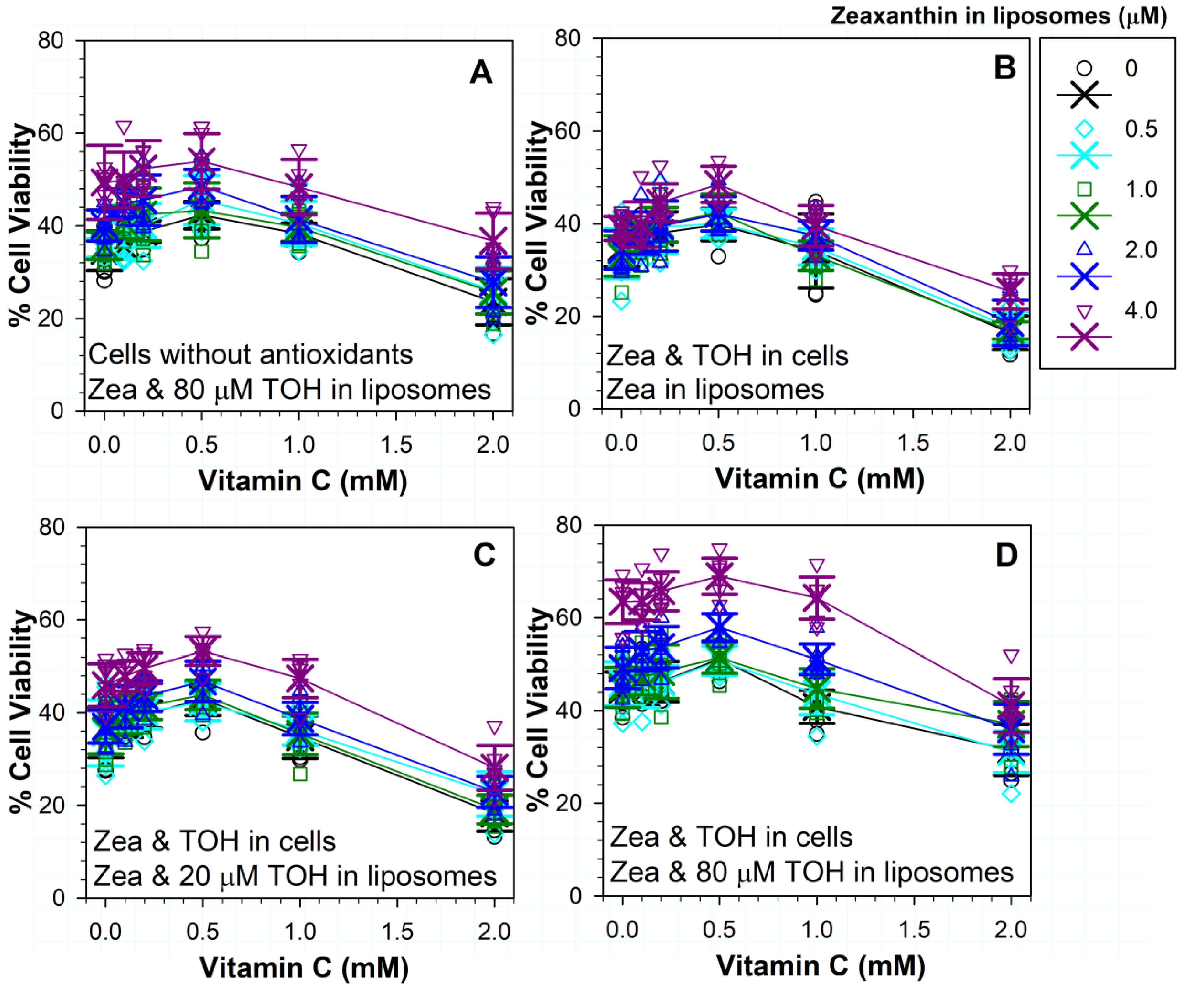
3.10. Effects on Phototoxicity of Different Combinations of Three Antioxidants: Vitamins C, E and Zeaxanthin
4. Discussion
4.1. Effects of Single Antioxidants
4.2. Effects of Combinations of Two Antioxidants: Zeaxanthin and α-Tocopherol
4.3. Effects of Combinations of Two Antioxidants: Ascorbate with Zeaxanthin or α-Tocopherol
4.4. Effects of Combinations of Three Antioxidants: Ascorbate with Zeaxanthin and α-Tocopherol
4.5. Physiological Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozanowska, M.; Sarna, T. Light-induced damage to the retina: Role of rhodopsin chromophore revisited. Photochem. Photobiol. 2005, 81, 1305–1330. [Google Scholar] [CrossRef] [PubMed]
- Różanowska, M.; Różanowski, B. Visual transduction and age-related changes in lipofuscin. In Ophthalmology Research: The Visual Transduction Cascade; Tombran-Tink, J., Barnstable, C.J., Eds.; The Humana Press Inc.: Totowa, NJ, USA, 2008; pp. 405–446. [Google Scholar]
- Rozanowska, M.; Rozanowski, B.; Boulton, M. Photobiology of the retina: Light damage to the retina. In Photobiological Sciences; Smith, K.C., Ed.; American Society for Photobiology: Herndon, VA, USA, 2009; Available online: http://www.photobiology.info (accessed on 22 January 2021).
- Maeda, T.; Golczak, M.; Maeda, A. Retinal photodamage mediated by all-trans-retinal. Photochem. Photobiol. 2012, 88, 1309–1319. [Google Scholar] [CrossRef]
- Kiser, P.D.; Golczak, M.; Palczewski, K. Chemistry of the retinoid (visual) cycle. Chem. Rev. 2014, 114, 194–232. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Golczak, M.; Chen, Y.; Okano, K.; Kohno, H.; Shiose, S.; Ishikawa, K.; Harte, W.; Palczewska, G.; Maeda, T.; et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 2011, 8, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Okano, K.; Maeda, T.; Chauhan, V.; Golczak, M.; Maeda, A.; Palczewski, K. Mechanism of all-trans-retinal toxicity with implications for Stargardt disease and age-related macular degeneration. J. Biol. Chem. 2012, 287, 5059–5069. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, K.; Payton, J.L.; Lakmal, O.H.; Karunarathne, A. Blue light excited retinal intercepts cellular signaling. Sci. Rep. 2018, 8, 10207. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, K.; Payton, J.L.; Meger, M.E.; Godage, N.H.; Gionfriddo, E.; Karunarathne, A. Blue light-triggered photochemistry and cytotoxicity of retinal. Cell Signal 2020, 69, 109547. [Google Scholar] [CrossRef]
- Rozanowska, M.; Wessels, J.; Boulton, M.; Burke, J.M.; Rodgers, M.A.J.; Truscott, T.G.; Sarna, T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic. Biol. Med. 1998, 24, 1107–1112. [Google Scholar] [CrossRef]
- Dillon, J.; Gaillard, E.R.; Bilski, P.; Chignell, C.F.; Reszka, K.J. The photochemistry of the retinoids as studied by steady-state and pulsed methods. PhotoChem. PhotoBiol. 1996, 63, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Palczewski, K. Retinal degeneration in animal models with a defective visual cycle. Drug Discov. Today Dis. Models 2013, 10, e163–e172. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Maeda, A. Retinol Dehydrogenases Regulate Vitamin A Metabolism for Visual Function. Nutrients 2016, 8, 746. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Garces, F.A.; Scortecci, J.F.; Molday, L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2021; 101036, in press. [Google Scholar] [CrossRef]
- Yu, G.; Gao, S.Q.; Dong, Z.; Sheng, L.; Sun, D.; Zhang, N.; Zhang, J.; Margeivicus, S.; Fu, P.; Golczak, M.; et al. Peptide Derivatives of Retinylamine Prevent Retinal Degeneration with Minimal Side Effects on Vision in Mice. Bioconjug Chem. 2021, 32, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Puntel, A.; Maeda, A.; Golczak, M.; Gao, S.Q.; Yu, G.; Palczewski, K.; Lu, Z.R. Prolonged prevention of retinal degeneration with retinylamine loaded nanoparticles. Biomaterials 2015, 44, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, X.; Ayat, N.; Maeda, A.; Gao, S.Q.; Golczak, M.; Palczewski, K.; Lu, Z.R. Multifunctional PEG retinylamine conjugate provides prolonged protection against retinal degeneration in mice. Biomacromolecules 2014, 15, 4570–4578. [Google Scholar] [CrossRef]
- Sawada, O.; Perusek, L.; Kohno, H.; Howell, S.J.; Maeda, A.; Matsuyama, S.; Maeda, T. All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis. Exp. Eye Res. 2014, 123, 27–36. [Google Scholar] [CrossRef]
- Maeda, A.; Palczewska, G.; Golczak, M.; Kohno, H.; Dong, Z.; Maeda, T.; Palczewski, K. Two-photon microscopy reveals early rod photoreceptor cell damage in light-exposed mutant mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1428–E1437. [Google Scholar] [CrossRef]
- Hahn, P.; Milam, A.H.; Dunaief, J.L. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch. OphthalMol. 2003, 121, 1099–1105. [Google Scholar] [CrossRef]
- Ng, K.P.; Gugiu, B.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Rozanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal pigment epithelium lipofuscin proteomics. Mol. Cell Proteom. 2008, 7, 1397–1405. [Google Scholar] [CrossRef]
- Gu, J.; Pauer, G.J.; Yue, X.; Narendra, U.; Sturgill, G.M.; Bena, J.; Gu, X.; Peachey, N.S.; Salomon, R.G.; Hagstrom, S.A.; et al. Proteomic and genomic biomarkers for age-related macular degeneration. Adv. Exp. Med. Biol. 2010, 664, 411–417. [Google Scholar] [CrossRef]
- Biesemeier, A.; Yoeruek, E.; Eibl, O.; Schraermeyer, U. Iron accumulation in Bruch’s membrane and melanosomes of donor eyes with age-related macular degeneration. Exp. Eye Res. 2015, 137, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; De Cilla, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and Nitrosative Stress in Age-Related Macular Degeneration: A Review of Their Role in Different Stages of Disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vazquez, S.; Llorens, V.; Soler-Boronat, A.; Figueras-Roca, M.; Adan, A.; Molins, B. Interlink between Inflammation and Oxidative Stress in Age-Related Macular Degeneration: Role of Complement Factor H. Biomedicines 2021, 9, 763. [Google Scholar] [CrossRef]
- Potilinski, M.C.; Tate, P.S.; Lorenc, V.E.; Gallo, J.E. New insights into oxidative stress and immune mechanisms involved in age-related macular degeneration tackled by novel therapies. Neuropharmacology 2021, 188, 108513. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Martin, P.M. Oxidative Stress and Inflammation in Retinal Degeneration. Antioxidants 2021, 10, 790. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vision Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Schalch, W.; Bone, R.A.; Landrum, J.T. The functional role of xanthophylls in the primate retina. In Carotenoids Physical, Chemichal, and Biological Functions and Properties; Landrum, J., Ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2010; pp. 257–282. [Google Scholar]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Widomska, J.; SanGiovanni, J.P.; Subczynski, W.K. Why is zeaxanthin the most concentrated xanthophyll in the central fovea? Nutrients 2020, 12, 1333. [Google Scholar] [CrossRef]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Ramkumar, S.; Imanishi, S.; Thomas, L.D.; Sawant, O.B.; Imanishi, Y.; von Lintig, J. Aster proteins mediate carotenoid transport in mammalian cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2200068119. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Moon, J.; Babino, D. Molecular components affecting ocular carotenoid and retinoid homeostasis. Prog. Retin. Eye Res. 2021, 80, 100864. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.G.; Siems, W.G.; Hurst, J.S.; Lewis, J.W.; Kliger, D.S.; van Kuijk, F.J. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr. Eye Res. 1999, 19, 491–495. [Google Scholar] [CrossRef]
- Rapp, L.M.; Maple, S.S.; Choi, J.H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1200–1209. [Google Scholar]
- Nagra, M.; Gilmartin, B.; Thai, N.J.; Logan, N.S. Determination of retinal surface area. J. Anat. 2017, 231, 319–324. [Google Scholar] [CrossRef]
- Rodieck, R.W. The First Steps in Seeing; Sinauer Associates; Oxford University Press: Sunderland, MA, USA, 1998. [Google Scholar]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet oxygen and free radical reactions of retinoids and carotenoids-A review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef]
- Rozanowska, M.B.; Czuba-Pelech, B.; Landrum, J.T.; Rozanowski, B. Comparison of Antioxidant Properties of Dehydrolutein with Lutein and Zeaxanthin, and their Effects on Cultured Retinal Pigment Epithelial Cells. Antioxidants 2021, 10, 753. [Google Scholar] [CrossRef]
- Fiedor, J.; Fiedor, L.; Haessner, R.; Scheer, H. Cyclic endoperoxides of beta-carotene, potential pro-oxidants, as products of chemical quenching of singlet oxygen. Biochim. Biophys. Acta 2005, 1709, 1–4. [Google Scholar] [CrossRef]
- Różanowska, M.; Różanowski, B. Uptake and photoprotection in cultured RPE cells. In Carotenoids: Physical, Chemical, and Biological Functions and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2010; pp. 309–364. [Google Scholar]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.; Di Mascio, P.; Murphy, M.E.; Sies, H. Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Arch. BioChem. Biophys. 1990, 277, 101–108. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Rozanowska, M.; Edge, R.; Land, E.J.; Navaratnam, S.; Sarna, T.; Truscott, T.G. Scavenging of Retinoid Cation Radicals by Urate, Trolox, and alpha-, beta-, gamma-, and delta-Tocopherols. Int. J. Mol. Sci. 2019, 20, 2799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Robison, W.G.; Kuwabara, T.; Bieri, J.G. The roles of vitamin E and unsaturated fatty acids in the visual process. Retina 1982, 2, 263–281. [Google Scholar] [CrossRef]
- Friedrichson, T.; Kalbach, H.L.; Buck, P.; van Kuijk, F.J. Vitamin E in macular and peripheral tissues of the human eye. Curr. Eye Res. 1995, 14, 693–701. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Berman, E.R.; Wang, H.M.; Feeney-Burns, L. Vitamin E in human neural retina and retinal pigment epithelium: Effect of age. Curr. Eye Res. 1987, 6, 1051–1055. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhauser-Berthold, M. Interrelation between Plasma Concentrations of Vitamins C and E along the Trajectory of Ageing in Consideration of Lifestyle and Body Composition: A Longitudinal Study over Two Decades. Nutrients 2020, 12, 2944. [Google Scholar] [CrossRef]
- Stuetz, W.; Weber, D.; Dolle, M.E.T.; Jansen, E.; Grubeck-Loebenstein, B.; Fiegl, S.; Toussaint, O.; Bernhardt, J.; Gonos, E.S.; Franceschi, C.; et al. Plasma Carotenoids, Tocopherols, and Retinol in the Age-Stratified (35–74 Years) General Population: A Cross-Sectional Study in Six European Countries. Nutrients 2016, 8, 614. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Ma, N.; Siegfried, C.; Kubota, M.; Huang, J.; Liu, Y.; Liu, M.; Dana, B.; Huang, A.; Beebe, D.; Yan, H.; et al. Expression Profiling of Ascorbic Acid-Related Transporters in Human and Mouse Eyes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Russo-Menna, I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience 1998, 82, 1213–1223. [Google Scholar] [CrossRef]
- Bisby, R.H.; Morgan, C.G.; Hamblett, I.; Gorman, A.A. Quenching of singlet oxygen by Trolox C, ascorbate, and amino acids: Effects of pH and temperature. J. Phys. Chem. A 1999, 103, 7454–7459. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Goldman, R.; Darrow, R.M.; Organisciak, D.T.; Kagan, V.E. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr. Eye Res. 1995, 14, 181–189. [Google Scholar] [CrossRef]
- Wrona, M.; Korytowski, W.; Rozanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of antioxidants in protection against photosensitized oxidation. Free Radic. Biol. Med. 2003, 35, 1319–1329. [Google Scholar] [CrossRef]
- Wrona, M.; Rozanowska, M.; Sarna, T. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef]
- Rozanowska, M.; Bakker, L.; Boulton, M.E.; Rozanowski, B. Concentration dependence of vitamin C in combinations with vitamin E and zeaxanthin on light-induced toxicity to retinal pigment epithelial cells. Photochem. Photobiol. 2012, 88, 1408–1417. [Google Scholar] [CrossRef]
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Zhao, D.Y.; Bernstein, P.S. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Investig. Ophthalmol. Vis. Sci. 2007, 48, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Mares, J.A.; Igo, R.P., Jr.; Truitt, B.; Liu, Z.; Millen, A.E.; Klein, M.; Johnson, E.J.; Engelman, C.D.; Karki, C.K.; et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Investig. Ophthalmol. Vis. Sci. 2014, 55, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.; Li, S.; Fairall, L.; Pfisterer, S.G.; Gurnett, J.E.; Xiao, X.; Weston, T.A.; Vashi, D.; Ferrari, A.; Orozco, J.L.; et al. Aster Proteins Facilitate Nonvesicular Plasma Membrane to ER Cholesterol Transport in Mammalian Cells. Cell 2018, 175, 514–529.e20. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaffarin, A.S.; Ng, S.F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Leonard, S.W.; Ebenuwa, I.; Violet, P.C.; Wang, Y.; Niyyati, M.; Padayatty, S.; Tu, H.; Courville, A.; Bernstein, S.; et al. Vitamin E absorption and kinetics in healthy women, as modulated by food and by fat, studied using 2 deuterium-labeled alpha-tocopherols in a 3-phase crossover design. Am. J. Clin. Nutr. 2019, 110, 1148–1167. [Google Scholar] [CrossRef]
- Rozanowska, M.; Handzel, K.; Boulton, M.E.; Rozanowski, B. Cytotoxicity of all-trans-retinal increases upon photodegradation. Photochem. Photobiol. 2012, 88, 1362–1372. [Google Scholar] [CrossRef]
- Rozanowska, M.; Cantrell, A.; Edge, R.; Land, E.J.; Sarna, T.; Truscott, T.G. Pulse radiolysis study of the interaction of retinoids with peroxyl radicals. Free Radic. Biol. Med. 2005, 39, 1399–1405. [Google Scholar] [CrossRef]
- Shui, Y.B.; Holekamp, N.M.; Kramer, B.C.; Crowley, J.R.; Wilkins, M.A.; Chu, F.; Malone, P.E.; Mangers, S.J.; Hou, J.H.; Siegfried, C.J.; et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: Relationship to the etiology of nuclear cataracts. Arch. OphthalMol. 2009, 127, 475–482. [Google Scholar] [CrossRef]
- Rozanowski, B.; Burke, J.; Sarna, T.; Rozanowska, M. The pro-oxidant effects of interactions of ascorbate with photoexcited melanin fade away with aging of the retina. Photochem. Photobiol. 2008, 84, 658–670. [Google Scholar] [CrossRef]
- Rozanowska, M.; Korytowski, W.; Rozanowski, B.; Skumatz, C.; Boulton, M.E.; Burke, J.M.; Sarna, T. Photoreactivity of aged human RPE melanosomes: A comparison with lipofuscin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2088–2096. [Google Scholar]
- Rozanowska, M.; Bober, A.; Burke, J.M.; Sarna, T. The role of retinal pigment epithelium melanin in photoinduced oxidation of ascorbate. PhotoChem. PhotoBiol. 1997, 65, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Maeda, A.; Matosky, M.; Okano, K.; Roos, S.; Tang, J.; Palczewski, K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Organisciak, D.T.; Bicknell, I.R.; Darrow, R.M. The effects of L-and D-ascorbic acid administration on retinal tissue levels and light damage in rats. Curr. Eye Res. 1992, 11, 231–241. [Google Scholar] [CrossRef]
- Blanks, J.C.; Pickford, M.S.; Organisciak, D.T. Ascorbate treatment prevents accumulation of phagosomes in RPE in light damage. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2814–2821. [Google Scholar]
- Organisciak, D.T.; Jiang, Y.L.; Wang, H.M.; Bicknell, I. The protective effect of ascorbic acid in retinal light damage of rats exposed to intermittent light. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1195–1202. [Google Scholar]
- Organisciak, D.T.; Wang, H.M.; Noell, W.K. Aspects of the ascorbate protective mechanism in retinal light damage of rats with normal and reduced ROS docosahexaenoic acid. Prog. Clin. Biol. Res. 1987, 247, 455–468. [Google Scholar]
- Noell, W.K.; Organisciak, D.T.; Ando, H.; Braniecki, M.A.; Durlin, C. Ascorbate and dietary protective mechanisms in retinal light damage of rats: Electrophysiological, histological and DNA measurements. Prog. Clin. Biol. Res. 1987, 247, 469–483. [Google Scholar]
- Organisciak, D.T.; Wang, H.M.; Li, Z.Y.; Tso, M.O. The protective effect of ascorbate in retinal light damage of rats. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1580–1588. [Google Scholar]
- Li, Z.Y.; Tso, M.O.; Wang, H.M.; Organisciak, D.T. Amelioration of photic injury in rat retina by ascorbic acid: A histopathologic study. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1589–1598. [Google Scholar]
- Organisciak, D.T.; Wang, H.M.; Kou, A.L. Ascorbate and glutathione levels in the developing normal and dystrophic rat retina: Effect of intense light exposure. Curr. Eye Res. 1984, 3, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Woodford, B.J.; Tso, M.O.; Lam, K.W. Reduced and oxidized ascorbates in guinea pig retina under normal and light-exposed conditions. Investig. Ophthalmol. Vis. Sci. 1983, 24, 862–867. [Google Scholar]
- Olchawa, M.M.; Furso, J.A.; Szewczyk, G.M.; Sarna, T.J. Lipofuscin-mediated photic stress inhibits phagocytic activity of ARPE-19 cells; effect of donors’ age and antioxidants. Free Radic. Res. 2017, 51, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Olchawa, M.M.; Szewczyk, G.M.; Zadlo, A.C.; Krzysztynska-Kuleta, O.I.; Sarna, T.J. The effect of aging and antioxidants on photoreactivity and phototoxicity of human melanosomes: An in vitro study. Pigment. Cell Melanoma Res. 2020, 34, 670–682. [Google Scholar] [CrossRef]
- Olchawa, M.M.; Szewczyk, G.M.; Zadlo, A.C.; Sarna, M.W.; Wnuk, D.; Sarna, T.J. The Effect of Antioxidants on Photoreactivity and Phototoxic Potential of RPE Melanolipofuscin Granules from Human Donors of Different Age. Antioxidants 2020, 9, 1044. [Google Scholar] [CrossRef]
- Burke, M.; Edge, R.; Land, E.J.; Truscott, T.G. Characterisation of carotenoid radical cations in liposomal environments: Interaction with vitamin C. J. Photochem. Photobiol. B 2001, 60, 1–6. [Google Scholar] [CrossRef]
- Marshall, J.; Grindle, J.; Ansell, P.L.; Borwein, B. Convolution in human rods: An ageing process. Br. J. OphthalMol. 1979, 63, 181–187. [Google Scholar] [CrossRef]
- Rychlicka, M.; Niezgoda, N.; Gliszczynska, A. Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Citronellic Acid. New Insight into Synthesis of Isoprenoid-Phospholipids. Molecules 2018, 23, 314. [Google Scholar] [CrossRef]
- Bazan, H.E.; Bazan, N.G.; Feeney-Burns, L.; Berman, E.R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comp. Rod Outer Segm. Neural Retina. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1433–1443. [Google Scholar]
- Naash, M.I.; Anderson, R.E. Glutathione-dependent enzymes in intact rod outer segments. Exp. Eye Res. 1989, 48, 309–318. [Google Scholar] [CrossRef]
- Ohira, A.; Tanito, M.; Kaidzu, S.; Kondo, T. Glutathione peroxidase induced in rat retinas to counteract photic injury. Investig. OphthalMol. Vis. Sci. 2003, 44, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Gosbell, A.D.; Stefanovic, N.; Scurr, L.L.; Pete, J.; Kola, I.; Favilla, I.; de Haan, J.B. Retinal light damage: Structural and functional effects of the antioxidant glutathione peroxidase-1. Investig. Ophthalmol. Vis Sci. 2006, 47, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Chucair, A.J.; Rotstein, N.P.; Sangiovanni, J.P.; During, A.; Chew, E.Y.; Politi, L.E. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: Relation with docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5168–5177. [Google Scholar] [CrossRef] [PubMed]
- Firsov, A.M.; Franco, M.S.F.; Chistyakov, D.V.; Goriainov, S.V.; Sergeeva, M.G.; Kotova, E.A.; Fomich, M.A.; Bekish, A.V.; Sharko, O.L.; Shmanai, V.V.; et al. Deuterated polyunsaturated fatty acids inhibit photoirradiation-induced lipid peroxidation in lipid bilayers. J. PhotoChem. PhotoBiol. B 2022, 229, 112425. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Ramana, K.V.; Srivastava, S.K.; van Kuijk, F.J. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp. Eye Res. 2008, 86, 70–80. [Google Scholar] [CrossRef]
- Lamberson, C.R.; Xu, L.; Muchalski, H.; Montenegro-Burke, J.R.; Shmanai, V.V.; Bekish, A.V.; McLean, J.A.; Clarke, C.F.; Shchepinov, M.S.; Porter, N.A. Unusual kinetic isotope effects of deuterium reinforced polyunsaturated fatty acids in tocopherol-mediated free radical chain oxidations. J. Am. Chem. Soc. 2014, 136, 838–841. [Google Scholar] [CrossRef]
- Burke, M.; Edge, R.; Land, E.J.; McGarvey, D.J.; Truscott, T.G. One-electron reduction potentials of dietary carotenoid radical cations in aqueous micellar environments. FEBS Lett. 2001, 500, 132–136. [Google Scholar] [CrossRef]
- Prasain, J.K.; Moore, R.; Hurst, J.S.; Barnes, S.; van Kuijk, F.J. Electrospray tandem mass spectrometric analysis of zeaxanthin and its oxidation products. J. Mass Spectrom. 2005, 40, 916–923. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różanowska, M.B.; Czuba-Pełech, B.; Różanowski, B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants 2022, 11, 1132. https://doi.org/10.3390/antiox11061132
Różanowska MB, Czuba-Pełech B, Różanowski B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants. 2022; 11(6):1132. https://doi.org/10.3390/antiox11061132
Chicago/Turabian StyleRóżanowska, Małgorzata B., Barbara Czuba-Pełech, and Bartosz Różanowski. 2022. "Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina?" Antioxidants 11, no. 6: 1132. https://doi.org/10.3390/antiox11061132
APA StyleRóżanowska, M. B., Czuba-Pełech, B., & Różanowski, B. (2022). Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants, 11(6), 1132. https://doi.org/10.3390/antiox11061132






