Abstract
Glaucoma is the leading cause of irreversible blindness worldwide, and the burden of the disease continues to grow as the global population ages. Currently, the only treatment option is to lower intraocular pressure. A better understanding of glaucoma pathogenesis will help us to develop novel therapeutic options. Oxidative stress has been implicated in the pathogenesis of many diseases. Oxidative stress occurs when there is an imbalance in redox homeostasis, with reactive oxygen species producing processes overcoming anti-oxidant defensive processes. Oxidative stress works in a synergistic fashion with endoplasmic reticulum stress, to drive glaucomatous damage to trabecular meshwork, retinal ganglion cells and the optic nerve head. We discuss the oxidative stress and endoplasmic reticulum stress pathways and their connections including their key intermediary, calcium. We highlight therapeutic options aimed at disrupting these pathways and discuss their potential role in glaucoma treatment.
1. Introduction
Glaucoma, an age-dependent disease which is more common in the elderly population, is a common cause of visual impairment, with a prevalence of 3.5% in those over the age of 40 and 9% in those over 80 [1,2]. It is the leading cause of irreversible blindness worldwide, with an estimated 79.6 million people suffering the effects of glaucoma in 2020, a number which is expected to increase to 111 million by 2040 [3]. It can be defined as an irreversible and progressive optic neuropathy [4]. Glaucoma is associated with visual field loss, primarily from damage to retinal ganglion cell (RGC) axons as they exit the eye via the optic nerve head (ONH). Glaucoma may be classified as either open, in which the anterior chamber angle remains open, or closed, whereby the aqueous outflow is impaired due to appositional closure of the drainage angle. Both forms may be primary or secondary processes. While the majority of patients with glaucoma have an elevated intraocular pressure (IOP), there are patients with glaucomatous optic neuropathy whose pressures lie within the normal range (normal tension glaucoma), as well as patients whose pressures are higher than normal values, yet have no damage to their ONH (ocular hypertension) [5,6]. These exceptions to the rule would suggest that raised IOP is not the only factor contributing to ONH damage; however, current therapeutic options in the management of glaucoma revolve around IOP reduction either via reduced aqueous production or increased outflow. A better understanding of the molecular pathogenesis of glaucoma will allow for the development of novel therapeutic options in the future.
Oxidative stress occurs when there is an excess of reactive oxygen species (ROS), the partially reduced metabolites of oxygen molecules [7]. These are primarily derived from electron leak in the mitochondrial electron transport chain [8]. This surpasses the buffering effect of the body’s anti-oxidative capacity, comprised of enzymes and nutrients [9]. Oxidative stress is a harmful state that leads to DNA, protein and lipid damage in addition to mitochondrial dysfunction which induces further ROS generation in a vicious cycle [10]. Oxidative stress is heavily implicated and studied in glaucoma at multiple levels and contributes to pro-fibrotic remodelling, IOP dysregulation and impeded RGC axoplasmic transport [10].
The endoplasmic reticulum (ER) is an organelle with a wide variety of functions—it is critical to protein folding and transportation, as well as functioning as a sensor of unfolded or misfolded proteins, which it retains within the ER for eventual degradation [11,12]. Its myriad other roles include lipid synthesis, steroid synthesis, carbohydrate metabolism and drug metabolism [13]. The ER is, in addition, a calcium store, with a balance of intraluminal calcium being key to proper functioning [14]. Normal ER function can also be perturbed by changes in glycosylation, nutrient deprivation, oxidative stress, and hypoxia, any of which can lead to the aggregation of misfolded and unfolded proteins in the ER, a condition known as ER stress [15]. ER stress triggers signalling cascades in a bid to restore homeostasis and a return to normal functioning. These include the less well-known ER overload response (EOR), which promotes proliferation and inflammation [16]. The better understood cascade is that of the unfolded protein response (UPR). The UPR coordinates an extensive response to decrease protein translation, increase protein folding and upregulate protein degradation [17]. It can also direct misfolded and unfolded proteins through the ubiquitin proteasome system, also called ER associated degradation (ERAD) [18]. As well as increasing apoptosis and general autophagy, the UPR also induces a selective form of autophagy, namely, ‘ER-phagy’—the removal of damaged ER [19].
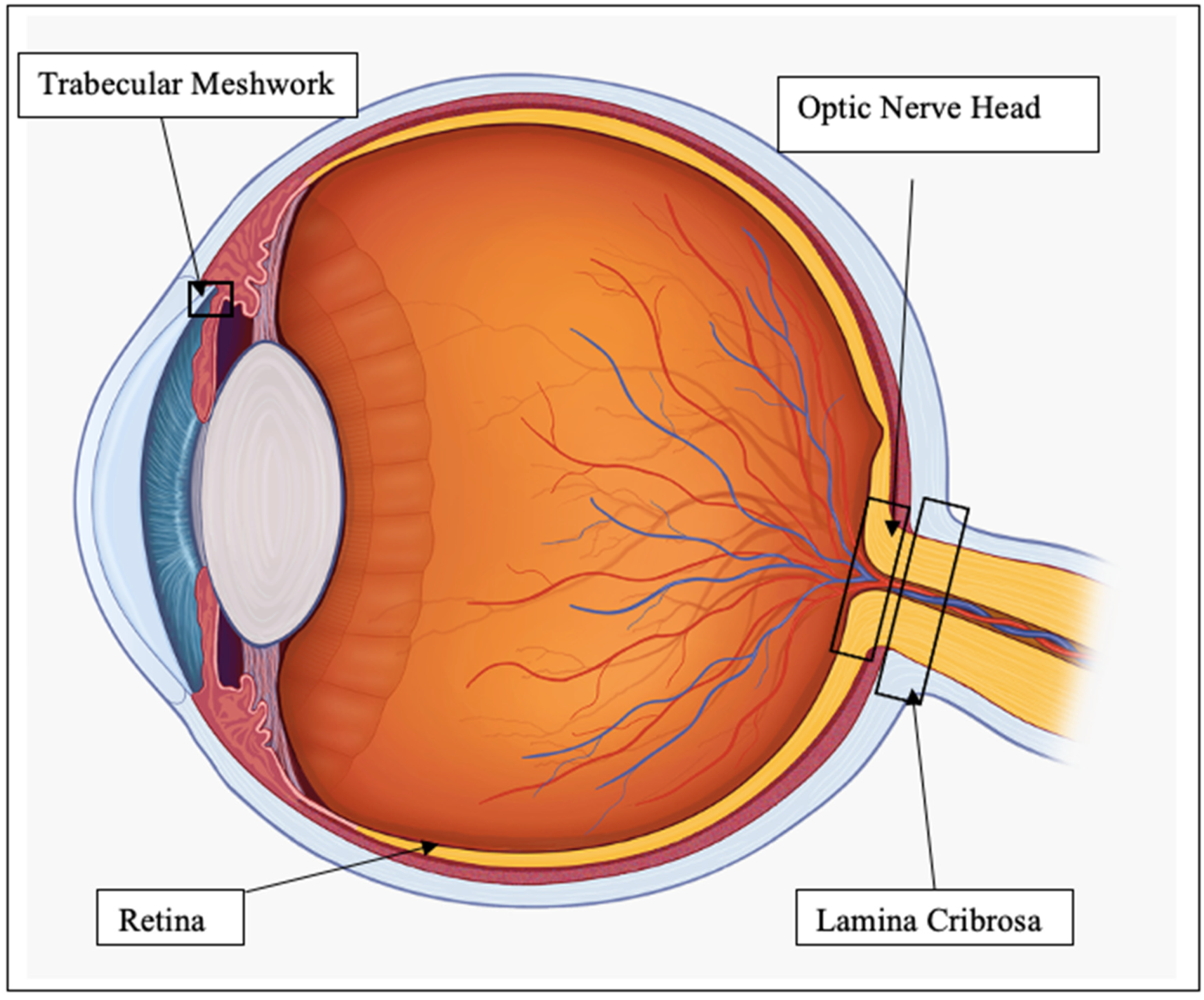
The many links between oxidative stress and ER stress are yet to be fully understood. In the ER, redox homeostasis is critical for correct protein folding [20]. Several oxidants can pathologically initiate the UPR, such as ketocholesterol, but its effect can be reversed by treatment with N-acetyl-cysteine, an antioxidant [21,22]. It has been proposed that redox based amplification loops contribute to the switch between adaptive and apoptotic UPR [23]. To discuss in detail the effect of oxidative and ER stress on the eye, we will focus on the regions of the eye most relevant to glaucoma, namely, the trabecular meshwork (TM), RGC, ONH and lamina cribrosa (LC), as shown in Figure 1.
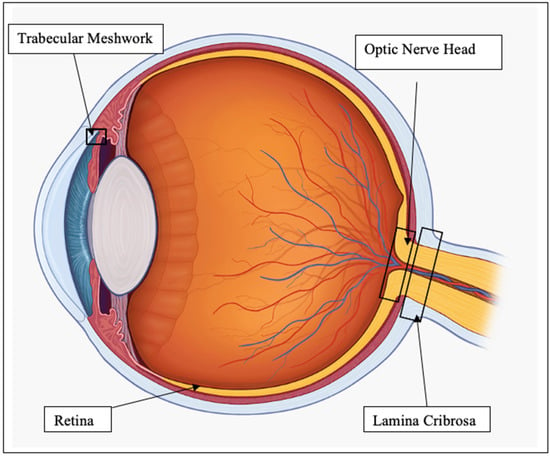
Figure 1.
Diagram of eye regions of interest.
2. Oxidative Stress
Oxidative stress occurs when there is an imbalance in redox homeostasis, with pro-oxidative processes that generate reactive oxygen species (ROS) overcoming intrinsic anti-oxidant defence mechanisms [7]. ROS are the partially reduced metabolites of oxygen molecules [9]. They are a subset of free radicals which are substances with unpaired electrons [24].
2.1. Pro-Oxidants
ROS may derive from endogenous or exogenous sources. The primary endogenous source of ROS is generated as a by-product in the electron transport chain, involved in aerobic respiration in the mitochondria [25]. This process, known as oxidative phosphorylation (OXPHOS), involves the transfer of electrons through protein complexes (C1–5) coupled to the pumping of hydrogen ions across the mitochondrial inner membrane [26]. This generates a proton gradient that is utilised by ATP synthase to generate ATP [26]. Oxygen serves as the terminal electron acceptor in this pathway, and it binds to hydrogen ions and is reduced to H2O as a by-product [8]; however, electron leakage occurs, primarily at the flavin mononucleotide group of complex I (NADH-ubiquinone oxidoreductase) or the ubiquinone site of complex III (ubiquinone-cytochrome c oxidoreductase), and this may reduce oxygen to generate superoxide (O2−), a potent ROS [27]. This in turn can generate hydrogen peroxide (H2O2), hydroxyl radicals (OH) and other ROS. Mitochondrial dysfunction results in an excessive release of ROS [28].
Other endogenous sources of ROS include endoplasmic reticulum stress, nitric oxide synthase reaction [29], Fenton reaction [30], polynuclear cells involved in pro-inflammatory responses [31] and the cytochrome p450 system [32]. Exogenous sources that increase ROS production include ionizing and ultraviolet radiation [33], pollutants [34], toxins (including alcohol and smoking) [35], intense physical exercise [36] and polymicrobial infections [37].
2.2. Anti-Oxidants
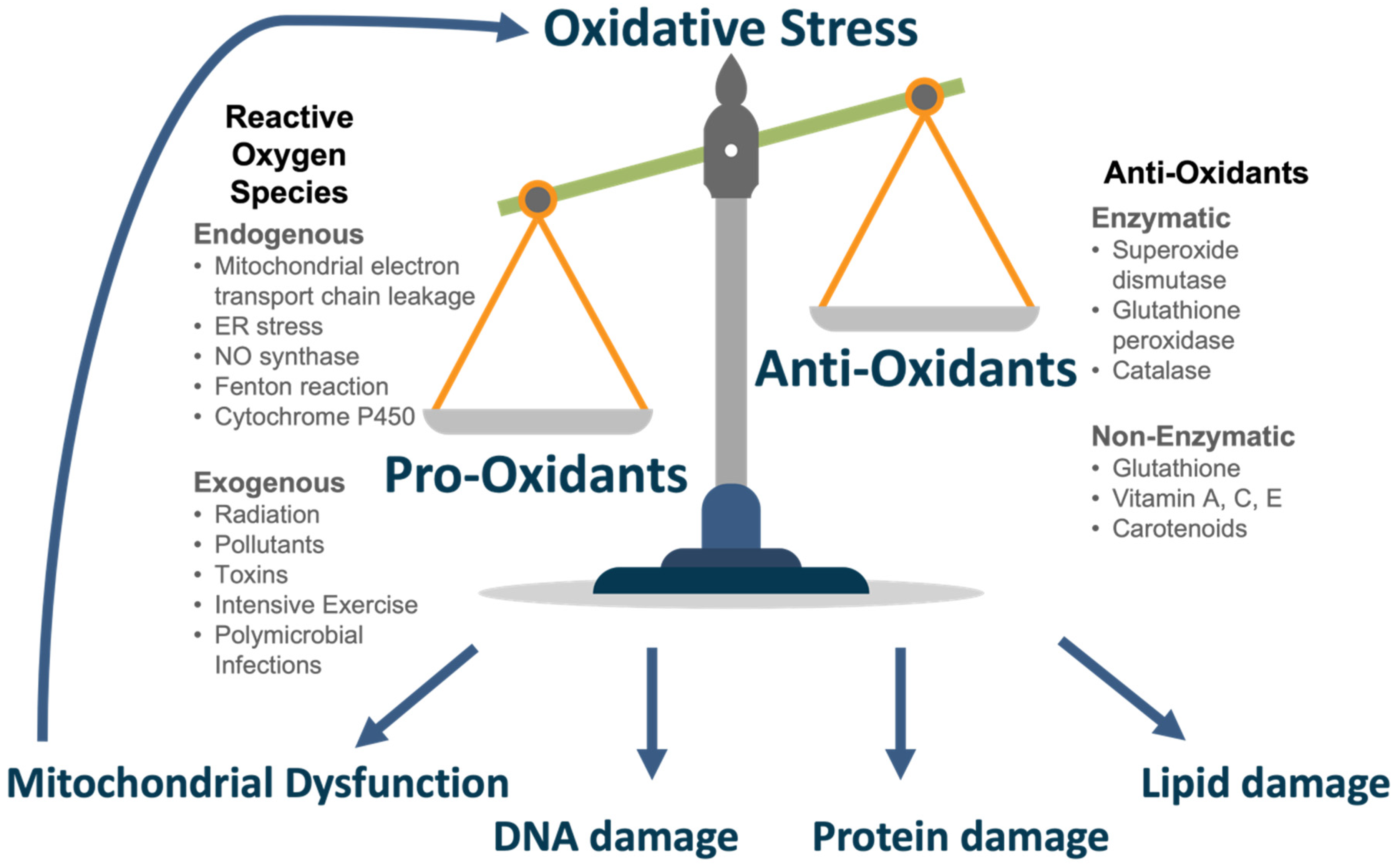
The body possesses an anti-oxidant defence system to an appropriate level of ROS in normal conditions. This is achieved primarily by enzymes. Superoxide dismutase (SOD) is involved in the conversion of hydrogen peroxide to oxygen (oxidation) and hydrogen peroxide (reduction) [38]. Glutathione peroxidase (GPx) reduces hydrogen peroxide to water or lipid hydroperoxides to their corresponding alcohols [39]. It achieves this using reduced glutathione (GSH), the most abundant anti-oxidant, as a co-factor [39,40]. Catalase is involved in degrading hydrogen peroxide to water and oxygen [41]. Further anti-oxidants include glutathione, ascorbic acid (Vitamin C), α-tocopherol (Vitamin E) and carotenoids which may be synthesised or consumed through diet [42]. In conditions of oxidative stress, the anti-oxidant capacity becomes overwhelmed and insufficient to buffer ROS levels. The delicate balance of pro- and anti-oxidants is seen in Figure 2.
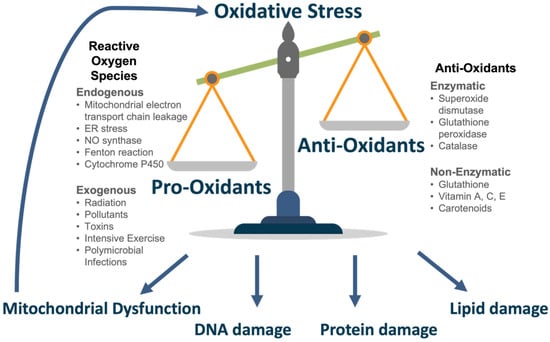
Figure 2.
Oxidative Stress.
2.3. Effects of ROS
ROS act as a direct cytotoxic stimulus, in a caspase dependent or independent manner [43]. They can induce apoptosis [44], programmed cell death or autophagy [45], and lysosomal degradation of a cell’s own constituents. They may induce potent secondary oxidants, such as peroxynitrite, formed from the interaction of superoxide radicals with nitric oxide [46]. ROS may also act as a 2nd messenger, modulating protein function. These oxidative protein modulations increase neuronal susceptibility to damage and result in glial dysfunction in neurodegenerative conditions [47,48]. Another mechanism of ROS damage, of particular concern in neurodegenerative disease, is the accumulation of advanced glycation end products (AGEs). AGEs are non-degradable, detergent insoluble and protease-resistant aggregates that impair tissue function leading to fibrosis [49] and protein function resulting in faulty gene transcription [50]. ROS may stimulate antigen presenting cells (APCs) and activate an autoimmune or inflammatory response [51]. Oxidative stress acts as a deleterious cyclical process with ROS impairing mitochondrial electron transport resulting in further ROS production and oxidative damage [10].
Excessive ROS cause oxidative damage to DNA, proteins and lipids and these may serve as markers for oxidative stress, for example, 8′-hydroxy-2′-deoxyguanosine (8-OHdG) is a DNA oxidative marker [52], while lipid peroxidation is identified by malondialdehyde (MDA) [53]. Finally, Nitrotyrosine, a nitrated amino acid that is formed by peroxynitrite, is a marker of protein oxidative damage [54].
3. Oxidative Stress in Glaucoma
Oxidative stress is found in a vast array of pathologies. It is a key feature in neurodegenerative conditions, such as Alzheimer’s [55] and Parkinson’s [56]. Axons in the central nervous system (CNS) are highly susceptible to oxidative damage owing to their high metabolic needs, high lipid and glutamate content and limited regenerative abilities [57]. It is also involved in a number of ocular conditions, such as keratoconus [58], diabetic retinopathy [59], age-related macular degeneration [60] and non-glaucomatous optic neuropathies [61]. It is heavily implicated in primary open angle glaucoma (POAG), a neurodegenerative condition affecting RGC axons. We will examine the role and evidence of oxidative stress in POAG at different ocular tissue levels and systemically.
3.1. Trabecular Meshwork
The TM, as the site of aqueous drainage in the anterior chamber, is a key regulator of intra-ocular pressure. Oxidative stress in TM cells contributes to increased aqueous outflow resistance and resultant elevation in IOP. TM cells are the most ROS-sensitive cells of the anterior chamber as they are not sensitised to direct UV radiation [62]. Evidence of oxidative stress is seen in human glaucomatous TM cells with multiple studies showing raised 8-OHdG and a positive correlation with worsening visual fields [63,64]. Nitrotyrosine, a marker for peroxynitrite-mediated injury, shows higher immunoreactivity in TM cells of POAG and this correlates with increased levels of IOP [65]. Peroxynitrite, formed in conditions of oxidative stress, depletes the bioactivity of NO, a free radical that normally relaxes the TM to promote aqueous humour drainage [66]. Reduced anti-oxidant capacity in TM cells is related to progression of POAG [67], and as with all aspects of oxidative stress, this a detrimental cyclical process with excess ROS contributing to elevated IOP which then results in further induction of oxidative free radicals.
ROS promote a pro-fibrotic remodelling of glaucomatous TM tissue [68]. Excessive oxidative stress decreases the function of miRNA-29b, which normally acts to reduce the expression of extra-cellular matrix (ECM) genes [69]. This reduced miRNA-29b function may act to promote ECM deposition in the TM and physically impede aqueous drainage. An accumulation of AGEs has been shown to increase the markers of oxidative stress in human TM cells [70]. They showed that this results in cellular senescence which would lead to further stiffening of the TM cytoskeleton.
Studies have shown evidence of mitochondrial dysfunction in human glaucomatous TM cells [71,72]. He et al. found evidence that mitochondrial complex 1 defects in human glaucomatous TM cells induce ROS release and lead to decreased ATP levels [72]. A study by He et al. found increased levels of calcium (Ca2+) in glaucomatous TM cells [71]. This induces the mitochondrial permeability transition pore (MPTP) opening resulting in mitochondrial dysfunction and ROS production.
3.2. Optic Nerve Head and Lamina Cribrosa
Oxidative stress at the LC, a mesh-like connective tissue structure at the optic nerve head through which the RGC axons pass, contributes to impeded axoplasmic transport, reduced ocular perfusion and visual field loss. Our lab has previously shown evidence of oxidative stress in human glaucomatous LC (GLC) fibroblast cells in vitro [73]. We found a significant increase in MDA and reduced anti-oxidants, aldo-keto reductase family 1 member C1 (AKR1C1) and glutamate—cysteine ligase catalytic subunit (GCLC), in GLC cells. In the same study, we showed evidence of mitochondrial dysfunction and raised calcium levels in GLC cells [73]. We found a mechano-sensitive pathway of calcium release in LC cells whereby cell membrane stretching, representative of raised IOP, resulted in excessive Ca2+ release which may have detrimental effects on mitochondrial functioning [73].
Chidlow et al. found that induced ocular hypertension increased the formation of superoxide radicals and up-regulated ROS-generating NADPH oxidase in ONH cells and RGCs in murine models [74]. ROS stimulates pathological glial activity in the ONH which results in secondary apoptosis of RGCs. This occurs through major histocompatibility complex (MHC) class II upregulation with resultant increased secretion of tumour necrosis factor-α (TNF-α) [75], in addition to activation of the AGE/RAGE (AGE receptors) signalling pathway [57]. There is evidence of oxidative stress in the ONH of POAG patients with higher nitrotyrosine immunoreactivity being noted in the vasculature and glia of the pre-laminar optic nerve head in glaucomatous patients [76]. Untreated glaucomatous ONH astrocytes also exhibit depleted levels of anti-oxidants, such as glutathione [77]. Human ONH astrocytes also display decreased mitochondrial fission and volume density [78] and elevated intracellular cyclic adenosine 3′,5′-monophosphate (cAMP) [79], which promotes further oxidative stress and axonal damage.
The accumulation of AGEs in the LC and vasculature surrounding the ONH increases tissue rigidity and impairs microcirculation [80]. ROS also impact ocular hemodynamics through NO scavenging. This removes a potent vasodilator and has been shown to increase vascular tone in porcine posterior ciliary arteries [81]. This environment makes RGC axons more susceptible to ischaemic damage caused by increased IOP in glaucoma [57]. Excessive ECM deposition in the ONH also stimulates metalloproteinases (MMPs). These proteolytic enzymes contribute to optic nerve head remodelling and may account for the appearance of optic disc cupping [82].
3.3. Retinal Ganglion Cells
Oxidative stress leads to apoptosis when ROS production chronically surpasses antioxidant capacity. In glaucoma, RGC apoptosis clinically manifests as optic nerve atrophy and a corresponding visual field loss. Mitochondrial dysfunction and subsequent oxidative stress have been shown to play a key role in RGC death in glaucoma [83,84,85,86]. Mittag et al. found that RGC death occurred after three to four months of elevated intraocular pressure with the surrounding areas of focal loss showing an 18% reduction in mitochondrial membrane potential [83]. RGC death may occur in a caspase-dependent [87] or independent [84] manner during the conditions of oxidative stress. Elevated levels of calcium, indicative of altered mitochondrial bioenergetics, have been shown in pressure-induced RGC apoptosis [88].
Glutamate is the most abundant excitatory neurotransmitter in the human central nervous system [89]. Excessive glutamate levels have been shown to induce oxidative stress and are implicated in numerous neurodegenerative conditions [90]. Vorwerk et al. showed that intravitreal injections of glutamate in rats resulted in apoptosis of RGCs [91]. They subsequently showed further evidence of this relationship as they found that the inhibition of glutamate transporters also leads to RGC death [92]. Glutamate-induced oxidative stress in RGCs is of particular importance as acute elevations of IOP have been shown to elevate glutamate levels [93]. The glutamate analog, N-methyl-D-aspartate (NMDA), has been used to induce oxidative stress in mice leading to raised calcium and ROS levels and ultimately RGC death [94,95]. Studies have also shown that H202, a potent ROS, leads to apoptosis of RGCs through the induction of nuclear factor-kappa B (NF-κB) [96,97].
3.4. Other Ocular Tissue and Systemic Oxidative Stress in Glaucoma
Patients with POAG are highly susceptible to oxidative damage as their aqueous and systemic anti-oxidant capacity is 60–70% lower than normal [98,99]. Studies have shown lower serum levels of antioxidant markers, total antioxidant status (TAS) and biologic antioxidant potential (BAP), in patients with POAG [99,100,101,102]. This correlates with higher intraocular pressures [99], higher cup-to-disc ratios [100], worse visual field (VF) loss [101] and lower numbers of RGCs [102]. Total oxidant status (TOS), a marker of oxidative stress, has also been shown to be raised in the serum of POAG patients [103]. Additionally, raised plasma markers of DNA (8-OHdG) [104] and lipid (MDA) [103] oxidation are seen. Serum levels of glutathione show a higher ratio of the oxidised form compared to its usual reduced form [105]. This lower redox index correlated with worse VF loss. Finally, systemic oxidative stress levels have been found to be significantly higher in African-American individuals compared to Caucasians [106,107] and this mirrors their 5–6× increased risk of glaucoma [108].
Research on oxidative stress in aqueous humor samples has yielded similar results to serum samples indicating that local redox status closely mirrors that of systemic antioxidant capacity. A study by Nucci et al. found raised aqueous humor levels of MDA and reduced levels of total antioxidant capacity (TAC) in patients with POAG [109]. In this study, POAG patients also showed evidence of mitochondrial dysfunction with significantly less ATP production versus normal. Interestingly, patients on IOP lowering medications have been shown to have higher aqueous humor levels of protective TAS compared to those not on medication [110]. In an effort to combat oxidative stress, SOD and GPx activity have been shown to be increased in aqueous samples of POAG patients [98,111,112]; however, their protective capacity can be overwhelmed and we see a cumulative rise in ROS as indicated by raised aqueous levels of 8-OHdG and a reduced expression of base excision repair (BER) markers, poly (ADP-ribose) polymerase 1 (PARP1) and oxoguanine DNA glycosylase 1 (OGG1) [113]. Aqueous markers of oxidative stress are also seen in pseudoexfoliation (PXF) glaucoma as demonstrated by the large body of work from Ursula Schlötzer-Schrehardt, [114,115]. She has found greatly reduced levels of ascorbic acid, the most effective free radical scavenger in the eye, in addition to raised markers of oxidative stress in patients with PXF glaucoma, suggestive of a faulty antioxidative defence system [114].
4. Endoplasmic Reticulum Stress
4.1. Overview
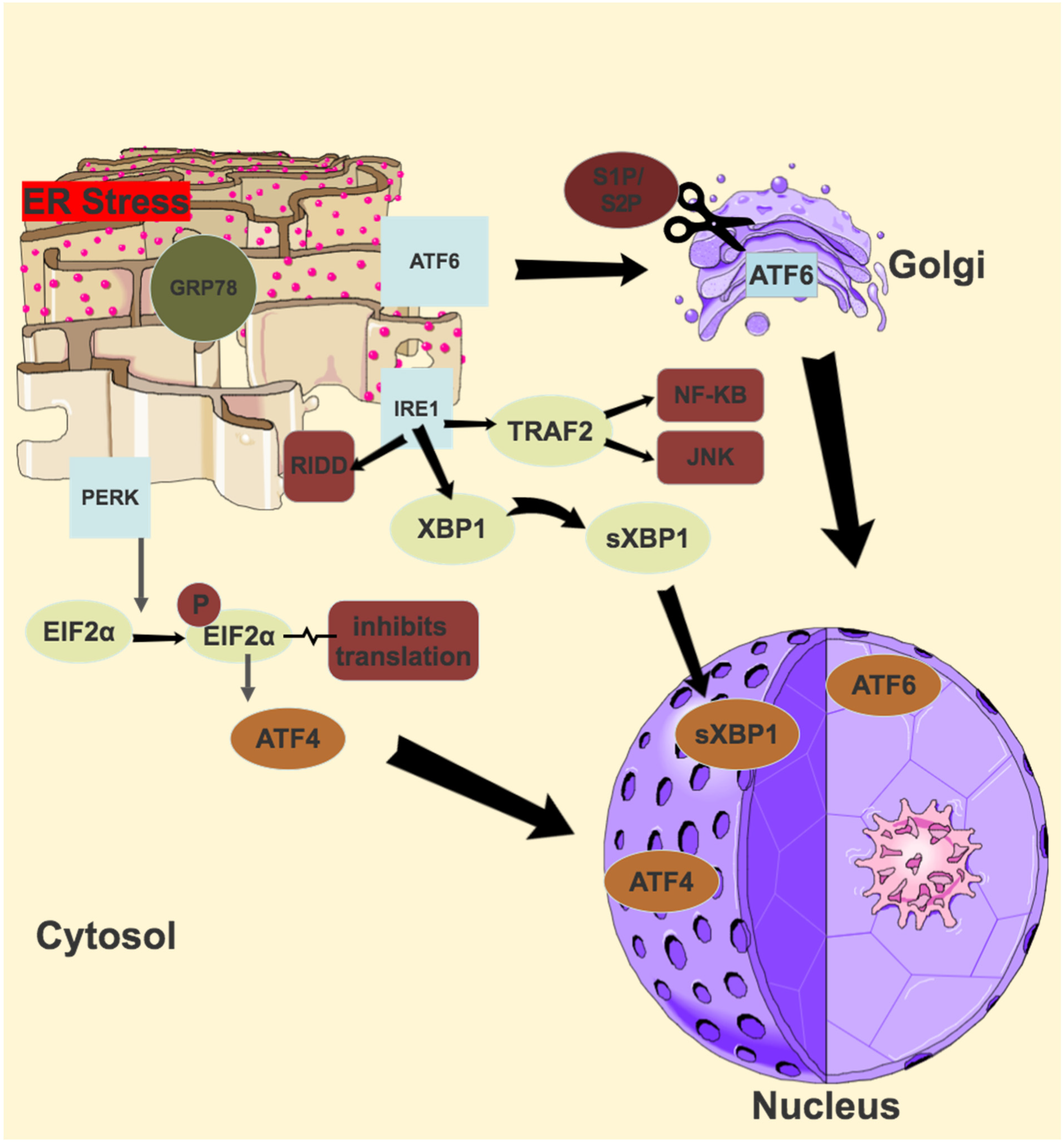
ER stress occurs when the capacity of the ER to correctly fold proteins is saturated. This stress sets off various signal transduction pathways in a bid to either return the ER to its normal equilibrium or to induce apoptosis [116]. Three of these pathways include the unfolded protein response (UPR), the ER overload response (EOR) and the ER associated degradation (ERAD). Three transmembrane ER resident proteins govern three signalling pathways in the UPR, and these are inositol requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6) and pancreatic ER kinase-like ER kinase (PERK). All three have ER lumen domains which sense ER stress, coupled to cytosolic effector domains. In a normally functioning ER, the ER lumen domains of IRE1, ATF6 and PERK are bound by glucose regulating protein 78 (GRP78) [117]. In a stressed ER, GRP 78 is titrated away, activating the UPR. The primary goal of these pathways is to restore ER protein homeostasis and ensure cell survival, but in the face of persistent activation from unmitigated ER stress, a signalling switch will occur, favouring apoptosis.
4.2. IRE1
The branch of the UPR which is most conserved, and most extensively investigated, is that of IRE1. First identified as a component of the UPR in yeast in 1993, there are actually 2 IRE1 genes in mammals—IRE1α and IRE1β [118]. IRE1α is ubiquitously expressed, while IRE1 β has only been found in respiratory and intestinal epithelial cells [119,120]. IRE1 is a type 1 transmembrane protein with three domains—an N-terminal domain in the ER lumen, and an endoribonuclease domain and serine/threonine kinase domain, both in the cytosol [121]. Once activated by ER stress, the luminal domain dimerises and trans- autophosphorylates, leading to the activation of its cytosolic domains [122]. Activated IRE1α excises a 26 base intron from an mRNA that encodes X-box-binding protein 1 (XBP1), which, due to this translational frameshift, becomes the potent transcription factor spliced XBP1 (sXBP1) [123]. sXBP1 upregulates the genes involved in ER protein folding and ERAD [124]. The endoribonuclease domain of activated IRE1α also degrades several ER-localised mRNAs, an activity known as regulated IRE1-dependent decay or RIDD [125]. The activated kinase domain interacts with TNF receptor associated factor 2 (TRAF2) to eventually activate the JNK and NF-κB pathways, integrating ER stress with pro–inflammatory responses [126,127]. Finally, sustained IRE1 activation causes the decay of several microRNAs which eventually leads to activation of the NLRP3 (the NACHT, LRR and PYD domains containing-3) inflammasome [128,129].
4.3. ATF6
A type II transmembrane glycoprotein and member of the basic leucine zipper (bZIP) transcription factor family, ATF6 also has two mammalian genes: ATF6α and ATF6β, with the former a potent transcriptional activator, and the latter a poor transcriptional activator [130,131]. Deletion of both causes early embryonic lethality [132]. ATF6 has a C-terminal domain which is in the ER lumen, and an N-terminal domain in the cytosol [133]. In response to ER stress, GRP78 release of the luminal domain allows the transport of ATF6 to the Golgi, where ATF6 is cleaved by site-1 protease (S1P) and site-2 protease (S2P) [134,135]. This releases the cytosolic domain of ATF6 (which contains the bZIP domain), which migrates to the nucleus. In the nucleus it interacts with the ER stress response element (ERSE1) and the ATF/cAMP response element (CRE) to activate numerous target genes, including GRP78 and XBP1, and some components of ERAD [136,137].
4.4. PERK
The final ER stress sensor, PERK, is a type 1 transmembrane protein with an N-terminal luminal domain and a C-terminal serine/threonine kinase domain in the cytosol [138]. In response to ER stress, similarly to IRE1, PERK dimerises and trans-autophosphorylates [138]. Activated PERK phosphorylates eIF2α at Serine5, and this inhibits eIF2β, which normally recycles the eIF2 complex to its active form [139]. Lower levels of this active complex result in lower rates of translation initiation of most mRNA, and a reduced protein synthesis, thereby reducing the protein-folding burden of the ER [140]. Phosphorylated eIF2α selectively translates several mRNAs, including the key transcription factor activating transcription factor 4 (ATF4) [141]. Growth arrest and DNA damage-inducible 34 (GADD34) and transcription factor C/EBP homologous protein (CHOP) are two key genes activated by ATF4 [142,143]. GADD34 is a regulatory targeting subunit of protein phosphatase PPP1, which counteracts PERK by dephosphorylating eIF2α, thereby restoring protein synthesis once the acute phase of ER stress has passed [144]. CHOP, in the setting of chronic or overwhelming ER stress, is a transcription factor which promotes apoptosis [145]. An illustration of the UPR pathways is shown in Figure 3.
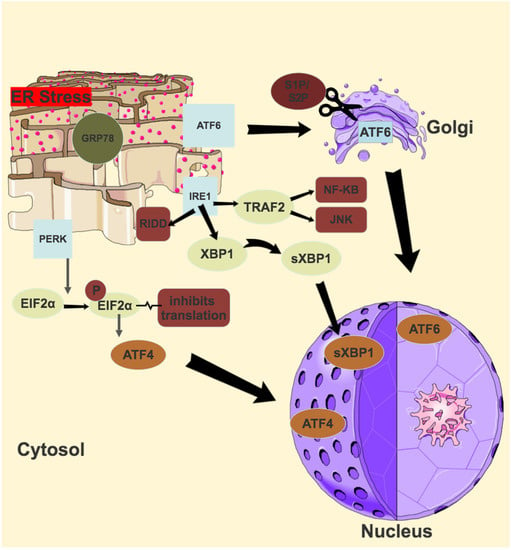
Figure 3.
Unfolded protein response (UPR) Pathways.
4.5. Endoplasmic Reticulum Overload Response + Oxidative Stress
Although much less is understood about the EOR than the UPR, it is an important link between ER stress and oxidative stress. The EOR is activated by the several stimuli that also activate the UPR, but the common signal is the accumulation of protein in the ER [16]. Calcium is then released from the ER and subsequently ROS are produced, both of which then result in the activation of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) [20].
NF-κB is a transcription factor which is key to cell proliferation and inflammation. In comparison to the UPR, the EOR is also more specialised in detecting calcium perturbations in the ER. Transmembrane and Coiled-Coil Domains 1 (TMCO1), encodes for an ER transmembrane protein that functions as a calcium load-activated calcium channel and, in the event of ROS overproduction, can perturb calcium equilibrium and activate the EOR [146].
4.6. Endoplasmic Reticulum Associated Protein Degradation
The ERAD is a pathway that targets misfolded proteins in the ER, which, once selected for ERAD, are retro-translocated to the cytosol, where they undergo ubiquitination and subsequent degradation by the proteasome [147,148]. It is implicated in several protein folding diseases, as if ERAD efficiency is compromised or overwhelmed, misfolded proteins will aggregate unless targeted for autophagy. Myocilin, a key gene in glaucoma, when mutated can overwhelm the ERAD and build up in the TM. Glucose regulated protein 94 (GRP94) triages mutant myocilin to ERAD, and studies of GRP94 deletion in mice have shown that subsequent shunting of myocilin from ERAD to autophagy causes less protein aggregation in the TM suggesting autophagy is a more robust pathway to deal with misfolded proteins [149].
5. ER Stress in Glaucoma
5.1. Overview
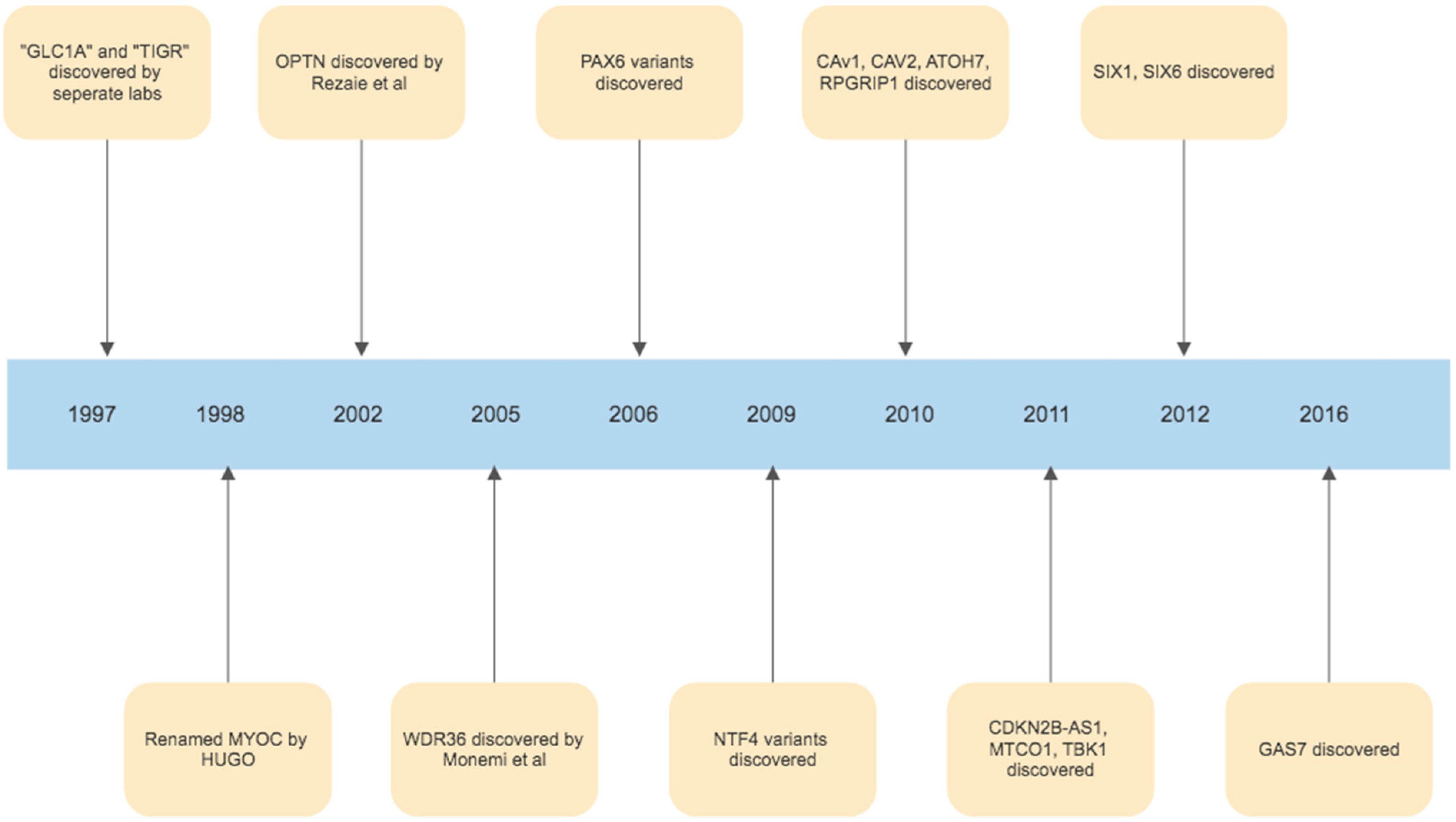
The foundations of our understanding of the role of ER stress in glaucoma were laid in 1997, with the discovery of the myocilin (MYOC) gene. The initial discovery was that an area of chromosome 1q was harbouring a gene associated with juvenile onset POAG, and this area was initially called GLC1A (picked to denote glaucoma, POAG and the fact that this was the first gene linkage site found in glaucoma) [150]. In the same year, it was then realised, that Polansky et al. had previously found, sequenced and named the gene trabecular meshwork inducible glucocorticoid response or TIGR, during studies of corticosteroid induced glaucoma [151,152]. Simultaneously, Kubota et al. in Japan had been studying retinal photoreceptors, and finding a protein similar to myosin, calling it myocilin [153]. In 1998, the Human Genome Organization stepped in and chose the name MYOC, by which the gene has been referred to as since [154]. While MYOC remains the best understood and most investigated gene linked to glaucoma, in the years since many other gene linkages have been identified. (Figure 3). The second gene identified was Optineurin (OPTN), by Rezaie et al., which is a variant with rare frequency but with a high effect size correlated with glaucoma pathogenesis [155,156]. Other similarly rare variants with high effect size in the development of POAG include WD Repeat Domain 36 (WDR36), TANK Binding Kinase 1(TBK1), Neurotrophin 4 (NTF4) and Paired Box 6 (PAX6) [157,158,159,160]. Variants of genes identified in the pathogenesis of glaucoma which are common but have a low effect size in the development of glaucoma include TMCO1, Cyclin-dependent kinase inhibitor 2B (CDKN2B-AS1 or ANRIL), Caveolin 1 (CAV1), Caveolin 2 (CAV2), Sine Oculis Homeobox Homolog 1 (SIX1), Sine Oculis Homeobox Homolog 6 (SIX6), Growth Arrest Specific Protein 7 (GAS7), Atonal BHLH Transcription Factor 7 (ATOH7) and RPGR Interacting Protein 1 (RPGRIP1) [161,162,163,164,165,166,167,168,169]. Identifying these genes not only allows us to potentially target these pathways using novel treatments, but in the future may also allow us to create DNA based tests that could identify those at risk of developing glaucoma, and potentially assist in evaluating who is at a higher risk of progression. Furthermore, identifying these genes and understanding their functions has been critical in allowing us to better understand the pathogenesis of glaucoma in their identification of key biological signalling pathways, such as those involved in ER stress, which play a role in glaucoma development and progression. The timeline of milestone gene discoveries in glaucoma is shown in Figure 4.
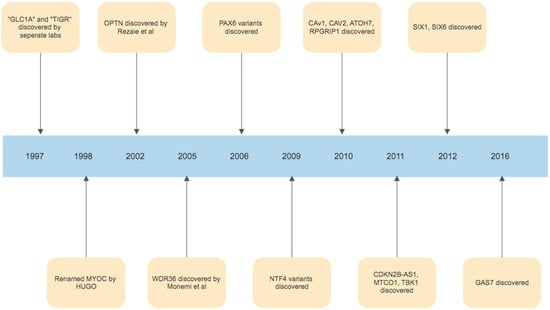
Figure 4.
Timeline of gene discoveries linked to the development of glaucoma.
5.2. Trabecular Meshwork
The TM is a key part of the aqueous humour outflow pathway of the eye. It is a complex tissue consisting of connective tissue beams and lamellae covered by TM cells [170]. In the TM, several studies have shown that the interplay between ECM accumulation and ER stress leads to increased outflow resistance and increased IOP [171,172]. In 2012, Suntharalingam showed that GRP94 depletion reduced mutant myocilin build up in human embryonic kidney by preferentially using autophagy rather than ERAD [149]. A large amount of our knowledge of ER stress in TM comes from the work of Zode et al., who in 2015, went on to look at glaucomatous TM (GTM) and normal TM (NTM), and showed that GTM cells had an increased GRP78 and GRP94 expression [173]. They also demonstrated that markers of chronic ER stress such as CHOP had a 3-fold increase in GTM, with ATF4 also being significantly enriched. This group, in 2017, showed that the increased ECM accumulation in the TM in glaucoma led to ER stress, in the TM more so than in other ocular tissues [174]. In 2018 they showed that in glaucoma induced by glucocorticoid use, increased extracellular matrix (ECM) deposition and ER stress were induced through TGFβ signalling, and interestingly, knockdown of ATF4 or CHOP completely prevented this signalling, thereby preventing ECM deposition and ER stress in mouse models of TM [175]. Most recently in 2020, Zode’s group showed that GTM cells had a significantly increased level of protein synthesis as well as induction of the chronic ER stress pathway ATF4-CHOP-GADD34, and demonstrated that in a mouse model, inhibition of this pathway prevented TM cell death and reduced protein synthesis and ER client protein load in TM cells [176]. Meanwhile, through in vitro studies of human TM cells and in vivo studies of mice TM, Ying et al. showed that ATF4 is a crucial mediator of both oxidative stress and ER stress-induced TM cell dysfunction and apoptosis [177].
5.3. Optic Nerve Head
The ONH is a structure in the posterior pole of the eye which allows the exit of retinal ganglion cell (RGC) axons and the entry and exit of blood vessels. This can be divided into three layers—the opening in Bruch’s membrane, the choroidal opening and the scleral opening [178]. This scleral opening is covered by the lamina cribrosa, a multi-layered network of load-bearing trabeculae that provide structural support to the optic nerve head and supply nutrients to the retinal ganglion cell axons as they leave the eye, as well as serving as a pressure barrier between the intravitreal and retrobulbar spaces [179]. Damage at the optic nerve head is the end result of glaucoma, and as such the optic nerve head is a key focus of glaucoma research. In 2012, Shimazawa et al. showed that RGC death led to an increase in GRP78, GRP 94 and CHOP, key markers of ER stress, in the optic nerve head 14 days later [180]. Ojino et al., in 2015 using the DBA/2J mouse, investigated the relationship between ER stress and the ONH in glaucoma and showed that ER stress may be related to astrocyte activation, playing a role in optic nerve degeneration in the setting of chronic ocular hypertension [181]. Stowell et al., using a primate model, demonstrated that several key ER chaperone proteins including GRP78, were significantly increased in the ONH in early experimental glaucoma [182]. In 2020, Mesentier-Louro et al. demonstrated that systemic hypoxia caused increased CHOP expression in astrocytes, and a loss of oligodendrocytes [183].
5.4. Retinal Ganglion Cells
RGCs are neurons which connect the retinal input to visual processing centres. Their axons converge at the optic disc and become myelinated, forming the optic nerve [184]. RGC loss is the hallmark of many optic neuropathies, including glaucoma, where loss occurs at the level of the optic nerve head [185]. While this review focuses on RGC and ER stress in glaucoma, there are many studies implicating ER stress in retinal diseases such as age-related macular degeneration, retinitis pigmentosa and Stargardt disease [186,187,188]. The ATF6 pathway has also been intensely studied in relation to RGC due to its genetic link to achromatopsia [189,190]. Hata et al., in 2008, induced retinal ischemia in rats, and showed that IRE1α was significantly increased in ganglion cells post ischaemia and subsequent reperfusion [191]. Doh et al., in 2010, in a rat model of glaucoma, tested the third ER stress pathway, the PERK pathway, and demonstrated a significant increase in GRP78 and CHOP expression, and a decrease in the number of ganglion cells compared to controls [192]. Hu et al. showed that inhibition of the PERK-eIF2α-CHOP pathway and activation of XBP1 can protect RGC soma and axons in mouse models of glaucoma, but in 2019, Marola et al. demonstrated that CHOP deletion alone, while conferring mild protection to RGCs, did not prevent axonal degeneration [193,194]. Yang et al., building on their previous work showing that optic nerve injury induced neuronal ER stress plays an important role in RGC death, used mouse models of both traumatic and glaucomatous optic neuropathies to show that manipulation of the UPR promotes RGC survival [195]. In 2021, Hetzer et al. demonstrated that in traumatic optic neuropathy, elevated ER stress markers were found in RGCs, as well as increased RGC death, astrogliosis and microgliosis [196].
6. Potential Therapeutic Options
6.1. Targeting Oxidative Stress
6.1.1. Naturally Occurring Anti-Oxidants
Anti-oxidants, which scavenge and neutralise free radicals, offer a promising avenue for glaucoma therapeutics. Whilst anti-oxidants, such as glutathione and vitamin C, are found in high levels in the vitreous humour, their ability to buffer ROS is exceeded and oxidative stress occurs as a result [197,198]. Multiple naturally occurring anti-oxidants have shown encouraging results at a laboratory level that may replete this defence system. Maher et al. showed that flavonoids, low molecular weight phenol metabolites with anti-oxidative properties, prevented RGC-5 cell death in various oxidative stress conditions [199]. Flavonoids achieved this protective effect through the synthesis transcription factor NF-E2-related factor 2 (Nrf2) and antioxidant enzymes such as heme oxygenase 1 (HMOX1). Li et al. also found lutein, a carotenoid, to be protective against RGC-5 cell death induced by oxidative stress (H2O2) and hypoxia (cobalt chloride) [200]. Inman et al. examined the effects of an oral intake of α-lipoic acid (ALA)—a disulfide compound synthesised in the liver—in mouse models and found it improved RGC survival whilst reducing all markers of oxidative stress [201]. A study on 45 human POAG patients found that an oral anti-oxidant supplement, which included ginkgo biloba, significantly increased blood flow velocities in all retrobulbar blood vessels leading to improved ocular perfusion.
6.1.2. Synthetic Anti-Oxidants
Exogenous anti-oxidative therapies also may offer protective effects against glaucomatous neurodegeneration. Rapamycin, an mTOR inhibitor which alleviates oxidative stress, has been shown to suppress apoptosis of RGCs in rats with chronic ocular hypertension [202]. It also been found to have a protective effect on TM cells in steroid-induced glaucoma in mouse models [203].
Metformin, a commonly used medication for type 2 diabetes mellitus, has emerged as a drug of interest in combating the fibrotic changes and mitochondrial dysfunction seen in glaucomatous eyes. In a large 150,016 patient retrospective cohort study in the United States of patients with type 2 diabetes mellitus (T2DM), aged ≥ 40 and with no pre-existing POAG, Li et al. found up to a 25% reduced risk of POAG amongst diabetics taking a high dose of metformin (>1110 g in 2 years) compared to those who took no metformin after adjusting for confounding factors [204]. It has potent anti-oxidative effects, increasing anti-oxidant capacity [205,206] and decreasing ROS production through the inhibition of protein kinase C activity [207,208].
Netarsudil is a Rho kinase (ROCK) inhibitor with anti-oxidative effects that has been approved for glaucoma treatment [209]. A similar ROCK inhibitor, Y-26732, has been shown to downregulate ROS production and upregulate anti-oxidants such as catalase in cynomolgus monkey TM cells [210]. Other anti-oxidants that have been shown to be protective in lab-based glaucoma studies include nicotinamide (vitamin B3) [211], N-acetylcysteine [212] and edaravone [213].
The systemic administration of medications may offer good local protection as the redox status of ocular tissues closely mirrors that of systemic capacity. Intravitreal injections of antioxidants, resveratrol [214] and ubiquinone (Coenzyme Q10) [215], have shown excellent results in alleviating RGC damage in murine models but have not been tested in a human population.
6.2. Targeting Endoplasmic Reticulum Stress
6.2.1. 4-Phenylbutyric Acid (4-PBA)
The most widely studied novel potential treatment of ER stress in glaucoma is 4-PBA. Initially, 4-PBA was used as a treatment for urea cycle disorders in the early 1990s [216]. In 1996, it was identified as a potential treatment for cystic fibrosis, through its action as a chemical chaperone [217]. Roth et al. hypothesised in 2007, that as myocilin- associated glaucoma is essentially a protein misfolding disease, treatment with a chemical chaperone represented a novel therapeutic option [218]. They demonstrated that the treatment of human TM cells (that had been previously transfected to express mutant myocilin) with 4-PBA significantly relieved ER stress and decreased the rate of apoptosis, while the treatment of TM cells with other chemical chaperones such as glycerol, did not. In 2011, Zode et al. demonstrated that 4-PBA treatment of mice (through the addition of 4-PBA to drinking water) with myocilin mutations, decreased ER stress and prevented glaucoma phenotypes [219]. They went on, in 2012, to prove that topical application of 4-PBA eyedrops also prevented the development of glaucoma phenotypes [220]. In 2021, Zode et al. showed that the benefit of 4-PBA is not restricted to its role as a chemical chaperone alone, but that it also prevents the synthesis and deposition of glucocorticoid induced ECM in TM, as well as degrading existing abnormal ECM by inducing matrix metalloproteinase 9 expression and activity [221]. In 2019, Kumar et al. showed that treatment with 4-PBA post non-arteritic anterior ischaemic optic neuropathy rescued RGCs and oligodendrocytes, and in 2020, Mesentier-Louro et al. showed that the pre-treatment of mice with 4 -PBA pre systemic hypoxia significantly reduced ER stress and rescued mature oligodendrocytes [183,222]. Given its previous approval for oral use for the treatment of urea cycle disorders and good safety profile, 4-PBA is an exciting potential glaucoma treatment.
6.2.2. Salubrinal
First identified by Boyce et al. in 2005, in a screen for small molecules that protect cells from ER stress, salubrinal is a selective inhibitor of cellular complexes that dephosphorylate eIF2α [223]. This means the PERK pathway remains active. Tested in human TM cells by Wang et al. in 2019, salubrinal was shown to be protective against cell death [224].
6.2.3. CRISPR-Cas9
CRISPR-Cas9 is a unique and exciting technology that enables editing of the genome through altering sections of the DNA sequence [225]. The discovery that CRISPR-Cas 9 could be programmed with RNA to edit genomic DNA won Doudna and Charpentier the Nobel Prize in Chemistry in 2020 [226]. We have already discussed the many genes that have been linked to glaucoma, and in particular, MYOC. In 2017, Sheffield et al. used CRISPR-Cas9 to knock down the expression of mutant MYOC in an in vivo mouse model, showing relief of ER stress, reduction in IOP and prevention of further glaucomatous damage [227]. They also showed its utility in human cultured TM cells, as well as ex vivo perfusion cultured human eyes. Wu et al., in 2020, used a mouse model and ex vivo human ciliary body model to demonstrate the efficacy of the CRISPR-Cas9 system in disrupting the Aquaporin 1 gene to treat glaucoma, with no off target changes to the retina or cornea identified [228].
7. Conclusions
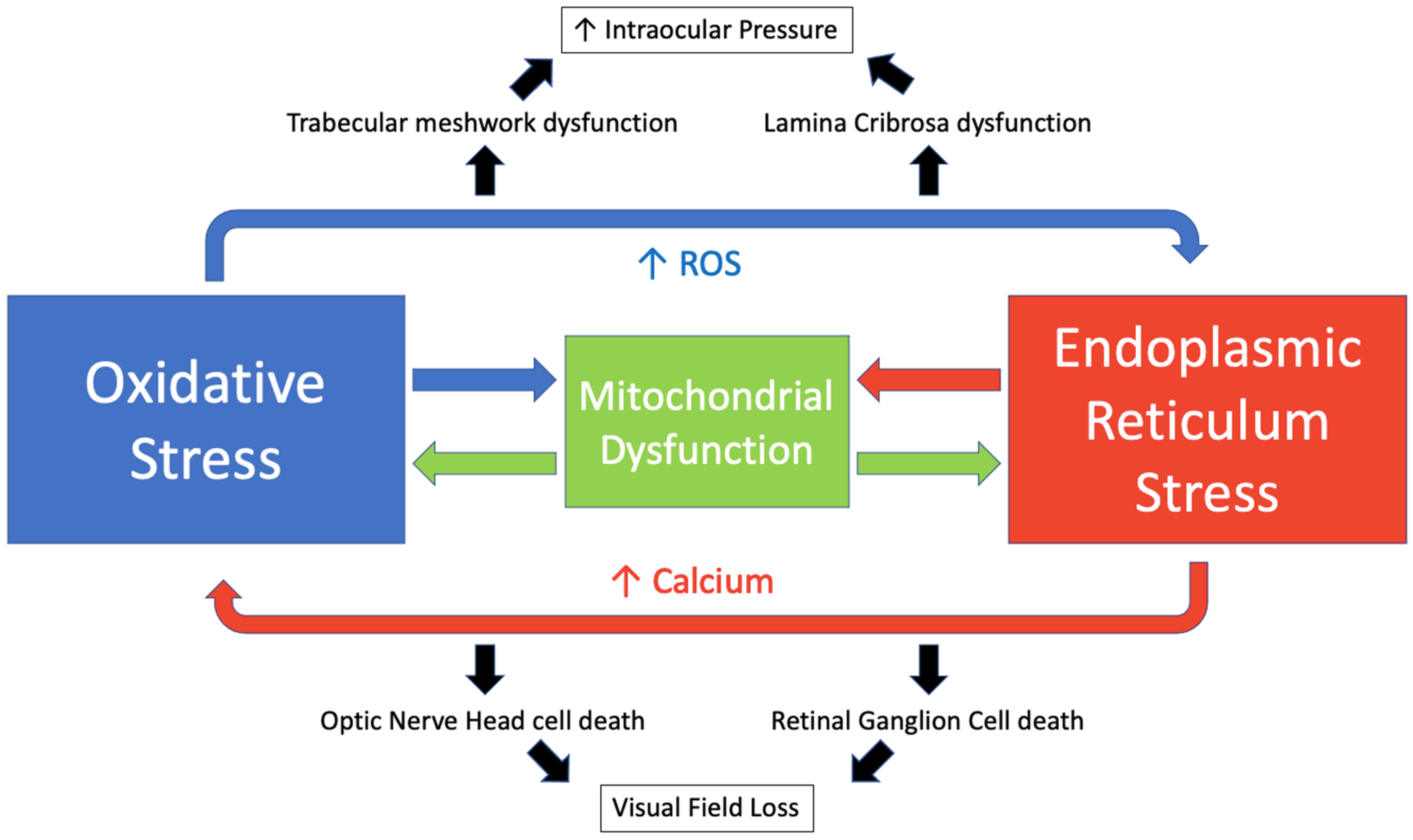
Oxidative and endoplasmic reticulum stress are key drivers of glaucoma, and their mechanisms act in an interconnected manner with calcium (Ca2+) acting as a key intermediary. Ca2+ and ROS act in a reciprocal manner. Increased calcium levels activate the ROS-generating enzymes and formation of free radicals [229]. Meanwhile, increased ROS stimulates an increase in intracellular calcium concentration [230]. The endoplasmic reticulum is the main intracellular calcium store with approximately 2 mM total Ca2+ [230]. During states of ER stress, calcium is released via inositol 1,4,5-trisphosphate (IP3) receptors and ryanodine receptors and accumulates in the mitochondrial matrix [231]. This promotes mitochondrial permeability transition pore opening, ROS accumulation and ATP depletion [232]. ROS may then disrupt the protein folding mechanism, which is dependent on redox homeostasis [21]. This perpetuates a cycle resulting in a further increase in oxidative and ER stress, mitochondrial dysfunction and the induction of apoptosis [233]. We have illustrated this cycle in Figure 5.
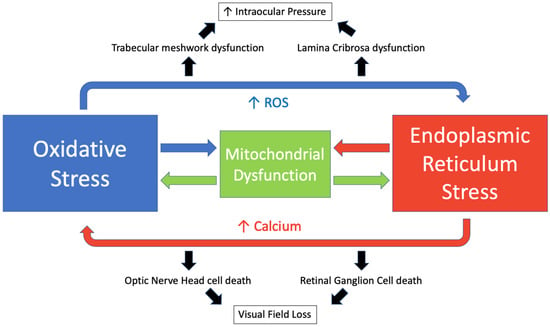
Figure 5.
The interplay of oxidative stress and endoplasmic reticulum stress in glaucoma.
Approximately 25% of ROS generated in the cell are derived from the ER [234]. An increase in ROS may also lead to activation of the UPR [235]. ATF4, which promotes aberrant protein synthesis, has been linked to oxidative stress in several studies [236,237]. Kasetti et al. have shown that induced over-expression of ATF4 leads to increased ROS expression in human TM cells [176], while a study by Kuk Joe et al. found that the expression of myocilin mutants in human TM cells resulted in ER stress which sensitised cells to oxidative stress-induced apoptosis [238]. This interplay has also been described in multiple neurodegenerative diseases [239], cancer [240,241], diabetes mellitus [242], cardiovascular disease [243] and other diseases.
The evidence of oxidative and ER stress in glaucoma, at multiple sites, represents a new avenue of therapeutic potential. Given the cyclical nature of the processes, both may be targeted by one agent as evidenced by a study showing that an overexpression of the anti-oxidant superoxide dismutase resulted in attenuated ER stress and subsequent prevention of neuronal damage in ischaemic conditions [244]. The use of such therapies that target both pathways may allow clinicians to treat patients with normal tension glaucoma or those failing to respond to conventional pressure-lowering therapies. Glaucoma is a multifactorial disease process, in which oxidative stress and endoplasmic reticulum stress play a key role, thus, there is a clear unmet clinical need to address a disease modifying agent and this requires tackling a mechanism beyond IOP regulation.
Author Contributions
Conceptualization, C.O. and M.I.; writing—Oxidative Stress, D.J.H.; writing—Endoplasmic Reticulum Stress, C.N.; supervision, C.O.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: A meta-analysis and systematic review. Sci. Rep. 2021, 11, 13762. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Pena, J.D. The optic nerve head in glaucomatous optic neuropathy. Arch. Ophthalmol. 1997, 115, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Bathija, R.; Weinreb, R.N. The definition of normal-tension glaucoma. J. Glaucoma 1998, 7, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Wang, N.; Wang, Y.X.; You, Q.S.; Yang, D.; Xu, L. Ocular hypertension: General characteristics and estimated cerebrospinal fluid pressure. The Beijing Eye Study 2011. PLoS ONE 2014, 9, e100533. [Google Scholar] [CrossRef]
- Garza-Lombo, C.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Redox homeostasis, oxidative stress and mitophagy. Mitochondrion 2020, 51, 105–117. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Diaz-Villanueva, J.F.; Diaz-Molina, R.; Garcia-Gonzalez, V. Protein Folding and Mechanisms of Proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Treiman, M. Regulation of the endoplasmic reticulum calcium storage during the unfolded protein response--significance in tissue ischemia? Trends Cardiovasc. Med. 2002, 12, 57–62. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L.; Baeuerle, P.A. The ER-overload response: Activation of NF-kappa B. Trends Biochem. Sci. 1997, 22, 63–67. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Meusser, B.; Hirsch, C.; Jarosch, E.; Sommer, T. ERAD: The long road to destruction. Nat. Cell Biol. 2005, 7, 766–772. [Google Scholar] [CrossRef]
- Yang, M.; Luo, S.; Wang, X.; Li, C.; Yang, J.; Zhu, X.; Xiao, L.; Sun, L. ER-Phagy: A New Regulator of ER Homeostasis. Front. Cell Dev. Biol. 2021, 9, 684526. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Chevet, E.; Argon, Y.; Appenzeller-Herzog, C. Redox controls UPR to control redox. J. Cell Sci. 2014, 127, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Klotz, L.O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Sun, J.; Druhan, L.J.; Zweier, J.L. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch. Biochem. Biophys. 2010, 494, 130–137. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.; Moorthy, B. Role of Cytochrome P450s in the Generation and Metabolism of Reactive Oxygen Species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Nuszkiewicz, J.; Wozniak, A.; Szewczyk-Golec, K. Ionizing Radiation as a Source of Oxidative Stress-The Protective Role of Melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cederbaum, A.I. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef]
- Spooner, R.; Yilmaz, O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011, 12, 334–352. [Google Scholar] [CrossRef]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients 2020, 12, 3224. [Google Scholar] [CrossRef] [PubMed]
- Moungjaroen, J.; Nimmannit, U.; Callery, P.S.; Wang, L.; Azad, N.; Lipipun, V.; Chanvorachote, P.; Rojanasakul, Y. Reactive oxygen species mediate caspase activation and apoptosis induced by lipoic acid in human lung epithelial cancer cells through Bcl-2 down-regulation. J. Pharmacol. Exp. Ther. 2006, 319, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Nitric oxide and cell death. Biochim. Biophys. Acta 1999, 1411, 401–414. [Google Scholar] [CrossRef]
- Wall, S.B.; Oh, J.Y.; Diers, A.R.; Landar, A. Oxidative modification of proteins: An emerging mechanism of cell signaling. Front. Physiol. 2012, 3, 369. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Cai, J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3177–3187. [Google Scholar] [CrossRef]
- Yan, H.D.; Li, X.Z.; Xie, J.M.; Li, M. Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells. Chin. Med. J. 2007, 120, 787–793. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The biology of the receptor for advanced glycation end products and its ligands. Biochim. Biophys. Acta 2000, 1498, 99–111. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71. [Google Scholar] [CrossRef]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef] [PubMed]
- Chwa, M.; Atilano, S.R.; Reddy, V.; Jordan, N.; Kim, D.W.; Kenney, M.C. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1902–1910. [Google Scholar] [CrossRef]
- Calderon, G.D.; Juarez, O.H.; Hernandez, G.E.; Punzo, S.M.; De la Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Sanz-Morello, B.; Ahmadi, H.; Vohra, R.; Saruhanian, S.; Freude, K.K.; Hamann, S.; Kolko, M. Oxidative Stress in Optic Neuropathies. Antioxidants 2021, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Sacca, S.C.; Longobardi, M.; Cartiglia, C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5251–5258. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Sacca, S.C.; Longobardi, M.; Cartiglia, C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch. Ophthalmol. 2010, 128, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Sacca, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA damage in the human trabecular meshwork: Clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005, 123, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Durango, R.; Fernandez-Martinez, A.; Garcia-Feijoo, J.; Castillo, A.; de la Casa, J.M.; Garcia-Bueno, B.; Perez-Nievas, B.G.; Fernandez-Cruz, A.; Leza, J.C. Expression of nitrotyrosine and oxidative consequences in the trabecular meshwork of patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2506–2511. [Google Scholar] [CrossRef]
- Muenster, S.; Lieb, W.S.; Fabry, G.; Allen, K.N.; Kamat, S.S.; Guy, A.H.; Dordea, A.C.; Teixeira, L.; Tainsh, R.E.; Yu, B.; et al. The Ability of Nitric Oxide to Lower Intraocular Pressure Is Dependent on Guanylyl Cyclase. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Ammar, D.A.; Hamweyah, K.M.; Kahook, M.Y. Antioxidants Protect Trabecular Meshwork Cells From Hydrogen Peroxide-Induced Cell Death. Transl. Vis. Sci. Technol. 2012, 1, 4. [Google Scholar] [CrossRef]
- Sacca, S.C.; Izzotti, A.; Rossi, P.; Traverso, C. Glaucomatous outflow pathway and oxidative stress. Exp. Eye Res. 2007, 84, 389–399. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009, 15, 2488–2497. [Google Scholar]
- Park, C.H.; Kim, J.W. Effect of advanced glycation end products on oxidative stress and senescence of trabecular meshwork cells. Korean J. Ophthalmol. 2012, 26, 123–131. [Google Scholar] [CrossRef]
- He, Y.; Ge, J.; Tombran-Tink, J. Mitochondrial defects and dysfunction in calcium regulation in glaucomatous trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4912–4922. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Leung, K.W.; Zhang, Y.H.; Duan, S.; Zhong, X.F.; Jiang, R.Z.; Peng, Z.; Tombran-Tink, J.; Ge, J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: Protection by antioxidants. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- McElnea, E.M.; Quill, B.; Docherty, N.G.; Irnaten, M.; Siah, W.F.; Clark, A.F.; O’Brien, C.J.; Wallace, D.M. Oxidative stress, mitochondrial dysfunction and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol. Vis. 2011, 17, 1182–1191. [Google Scholar] [PubMed]
- Chidlow, G.; Wood, J.P.M.; Casson, R.J. Investigations into Hypoxia and Oxidative Stress at the Optic Nerve Head in a Rat Model of Glaucoma. Front. Neurosci. 2017, 11, 478. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Peng, Y.; Sun, S.L.; Sun, D. Mechanisms of immune system activation in glaucoma: Oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 705–714. [Google Scholar] [CrossRef]
- Feilchenfeld, Z.; Yucel, Y.H.; Gupta, N. Oxidative injury to blood vessels and glia of the pre-laminar optic nerve head in human glaucoma. Exp. Eye Res. 2008, 87, 409–414. [Google Scholar] [CrossRef]
- Malone, P.E.; Hernandez, M.R. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp. Eye Res. 2007, 84, 444–454. [Google Scholar] [CrossRef]
- Ju, W.K.; Kim, K.Y.; Noh, Y.H.; Hoshijima, M.; Lukas, T.J.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A. Increased mitochondrial fission and volume density by blocking glutamate excitotoxicity protect glaucomatous optic nerve head astrocytes. Glia 2015, 63, 736–753. [Google Scholar] [CrossRef]
- Shim, M.S.; Kim, K.Y.; Bu, J.H.; Nam, H.S.; Jeong, S.W.; Park, T.L.; Ellisman, M.H.; Weinreb, R.N.; Ju, W.K. Elevated intracellular cAMP exacerbates vulnerability to oxidative stress in optic nerve head astrocytes. Cell Death Dis. 2018, 9, 285. [Google Scholar] [CrossRef]
- Tezel, G.; Luo, C.; Yang, X. Accelerated aging in glaucoma: Immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1201–1211. [Google Scholar] [CrossRef]
- Zeitz, O.; Wagenfeld, L.; Wirtz, N.; Galambos, P.; Matthiesen, N.; Wiermann, A.; Richard, G.; Klemm, M. Influence of oxygen free radicals on the tone of ciliary arteries: A model of vasospasms of ocular vasculature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tezel, G.; Wax, M.B.; Edward, D.P. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch. Ophthalmol. 2000, 118, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Mittag, T.W.; Danias, J.; Pohorenec, G.; Yuan, H.M.; Burakgazi, E.; Chalmers-Redman, R.; Podos, S.M.; Tatton, W.G. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3451–3459. [Google Scholar] [PubMed]
- Tezel, G.; Yang, X. Caspase-independent component of retinal ganglion cell death, in vitro. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Trounce, I.A.; Crowston, J.G. Mechanisms of retinal ganglion cell injury in aging and glaucoma. Ophthalmic Res. 2010, 44, 173–178. [Google Scholar] [CrossRef]
- Kong, G.Y.; Van Bergen, N.J.; Trounce, I.A.; Crowston, J.G. Mitochondrial dysfunction and glaucoma. J. Glaucoma 2009, 18, 93–100. [Google Scholar] [CrossRef]
- Thomas, C.N.; Berry, M.; Logan, A.; Blanch, R.J.; Ahmed, Z. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Sappington, R.M.; Sidorova, T.; Long, D.J.; Calkins, D.J. TRPV1: Contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 717–728. [Google Scholar] [CrossRef]
- Bleich, S.; Romer, K.; Wiltfang, J.; Kornhuber, J. Glutamate and the glutamate receptor system: A target for drug action. Int. J. Geriatr. Psychiatry 2003, 18, S33–S40. [Google Scholar] [CrossRef]
- Cassano, T.; Pace, L.; Bedse, G.; Lavecchia, A.M.; De Marco, F.; Gaetani, S.; Serviddio, G. Glutamate and Mitochondria: Two Prominent Players in the Oxidative Stress-Induced Neurodegeneration. Curr. Alzheimer Res. 2016, 13, 185–197. [Google Scholar] [CrossRef]
- Vorwerk, C.K.; Lipton, S.A.; Zurakowski, D.; Hyman, B.T.; Sabel, B.A.; Dreyer, E.B. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1618–1624. [Google Scholar] [PubMed]
- Vorwerk, C.K.; Naskar, R.; Schuettauf, F.; Quinto, K.; Zurakowski, D.; Gochenauer, G.; Robinson, M.B.; Mackler, S.A.; Dreyer, E.B. Depression of retinal glutamate transporter function leads to elevated intravitreal glutamate levels and ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3615–3621. [Google Scholar] [PubMed]
- Nucci, C.; Tartaglione, R.; Rombola, L.; Morrone, L.A.; Fazzi, E.; Bagetta, G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology 2005, 26, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.; Lu, B.; Yang, N.; Huang, K.; Wang, P.; Tian, N. The Susceptibility of Retinal Ganglion Cells to Glutamatergic Excitotoxicity Is Type-Specific. Front. Neurosci. 2019, 13, 219. [Google Scholar] [CrossRef]
- Seitz, R.; Tamm, E.R. N-methyl-D-aspartate (NMDA)-mediated excitotoxic damage: A mouse model of acute retinal ganglion cell damage. Methods Mol. Biol. 2013, 935, 99–109. [Google Scholar] [CrossRef]
- Jia, W.C.; Liu, G.; Zhang, C.D.; Zhang, S.P. Formononetin attenuates hydrogen peroxide (H2O2)-induced apoptosis and NF-kappaB activation in RGC-5 cells. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2191–2197. [Google Scholar]
- Lv, B.; Chen, T.; Xu, Z.; Huo, F.; Wei, Y.; Yang, X. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-kappaB. Int. J. Mol. Med. 2016, 37, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Evelson, P.A.; Llesuy, S.F. Oxidative stress markers in aqueous humor of glaucoma patients. Am. J. Ophthalmol. 2004, 137, 62–69. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Correlation between Systemic Oxidative Stress and Intraocular Pressure Level. PLoS ONE 2015, 10, e0133582. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Mousa, A.; Osman, E.A.; Al-Obeidan, S.A. Decreased total antioxidants in patients with primary open angle glaucoma. Curr. Eye Res. 2013, 38, 959–964. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef]
- Asano, Y.; Himori, N.; Kunikata, H.; Yamazaki, M.; Shiga, Y.; Omodaka, K.; Takahashi, H.; Nakazawa, T. Age- and sex-dependency of the association between systemic antioxidant potential and glaucomatous damage. Sci. Rep. 2017, 7, 8032. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, W.; Zalejska-Fiolka, J.; Pojda-Wilczek, D.; Hampel, A.; Majewski, W.; Ogultekin, S.; Mrukwa-Kominek, E. Differences in serum oxidative status between glaucomatous and.d nonglaucomatous cataract patients. BMC Ophthalmol. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Kondkar, A.A.; Azad, T.A.; Sultan, T.; Osman, E.A.; Almobarak, F.A.; Al-Obeidan, S.A. Elevated Plasma Level of 8-Hydroxy-2′-deoxyguanosine Is Associated with Primary Open-Angle Glaucoma. J. Ophthalmol. 2020, 2020, 6571413. [Google Scholar] [CrossRef]
- Yabana, T.; Sato, K.; Shiga, Y.; Himori, N.; Omodaka, K.; Nakazawa, T. The relationship between glutathione levels in leukocytes and ocular clinical parameters in glaucoma. PLoS ONE 2019, 14, e0227078. [Google Scholar] [CrossRef] [PubMed]
- Feairheller, D.L.; Park, J.Y.; Sturgeon, K.M.; Williamson, S.T.; Diaz, K.M.; Veerabhadrappa, P.; Brown, M.D. Racial differences in oxidative stress and inflammation: In vitro and in vivo. Clin. Transl. Sci. 2011, 4, 32–37. [Google Scholar] [CrossRef]
- Morris, A.A.; Zhao, L.; Patel, R.S.; Jones, D.P.; Ahmed, Y.; Stoyanova, N.; Gibbons, G.H.; Vaccarino, V.; Din-Dzietham, R.; Quyyumi, A.A. Differences in systemic oxidative stress based on race and the metabolic syndrome: The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab. Syndr. Relat. Disord. 2012, 10, 252–259. [Google Scholar] [CrossRef]
- Khachatryan, N.; Pistilli, M.; Maguire, M.G.; Salowe, R.J.; Fertig, R.M.; Moore, T.; Gudiseva, H.V.; Chavali, V.R.M.; Collins, D.W.; Daniel, E.; et al. Primary Open-Angle African American Glaucoma Genetics (POAAGG) Study: Gender and risk of POAG in African Americans. PLoS ONE 2019, 14, e0218804. [Google Scholar] [CrossRef]
- Nucci, C.; Di Pierro, D.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Pinazo Duran, M.D.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013, 19, 1841–1846. [Google Scholar]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Gallego-Pinazo, R.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Antioxidant status modifications by topical administration of dorzolamide in primary open-angle glaucoma. Eur. J. Ophthalmol. 2009, 19, 565–571. [Google Scholar] [CrossRef]
- Ghanem, A.A.; Arafa, L.F.; El-Baz, A. Oxidative stress markers in patients with primary open-angle glaucoma. Curr. Eye Res. 2010, 35, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Dada, R.; Dada, T. Oxidative DNA damage and reduced expression of DNA repair genes: Role in primary open angle glaucoma (POAG). Ophthalmic Genet. 2017, 38, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Koliakos, G.G.; Konstas, A.G.; Schlotzer-Schrehardt, U.; Hollo, G.; Katsimbris, I.E.; Georgiadis, N.; Ritch, R. 8-Isoprostaglandin F2a and ascorbic acid concentration in the aqueous humour of patients with exfoliation syndrome. Br. J. Ophthalmol. 2003, 87, 353–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlotzer-Schrehardt, U.; Naumann, G.O. Ocular and systemic pseudoexfoliation syndrome. Am. J. Ophthalmol. 2006, 141, 921–937. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.R.; Lee, A.S. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008, 15, 1460–1471. [Google Scholar] [CrossRef]
- Mori, K.; Ma, W.; Gething, M.J.; Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 1993, 74, 743–756. [Google Scholar] [CrossRef]
- Martino, M.B.; Jones, L.; Brighton, B.; Ehre, C.; Abdulah, L.; Davis, C.W.; Ron, D.; O’Neal, W.K.; Ribeiro, C.M. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal. Immunol. 2013, 6, 639–654. [Google Scholar] [CrossRef]
- Tsuru, A.; Fujimoto, N.; Takahashi, S.; Saito, M.; Nakamura, D.; Iwano, M.; Iwawaki, T.; Kadokura, H.; Ron, D.; Kohno, K. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2864–2869. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, F.; van Lith, M.; Sweeney, B.; Cain, K.; Bulleid, N.J. Inhibition of IRE1alpha-mediated XBP1 mRNA cleavage by XBP1 reveals a novel regulatory process during the unfolded protein response. Wellcome Open Res. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Hosokawa, N.; Kaufman, R.J.; Nagata, K.; Mori, K. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell 2003, 4, 265–271. [Google Scholar] [CrossRef]
- Coelho, D.S.; Domingos, P.M. Physiological roles of regulated Ire1 dependent decay. Front. Genet. 2014, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Kaneko, M.; Niinuma, Y.; Nomura, Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 2003, 26, 931–935. [Google Scholar] [CrossRef]
- Upton, J.P.; Wang, L.; Han, D.; Wang, E.S.; Huskey, N.E.; Lim, L.; Truitt, M.; McManus, M.T.; Ruggero, D.; Goga, A.; et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 2012, 338, 818–822. [Google Scholar] [CrossRef]
- Bronner, D.N.; Abuaita, B.H.; Chen, X.; Fitzgerald, K.A.; Nunez, G.; He, Y.; Yin, X.M.; O’Riordan, M.X. Endoplasmic Reticulum Stress Activates the Inflammasome via NLRP3- and Caspase-2-Driven Mitochondrial Damage. Immunity 2015, 43, 451–462. [Google Scholar] [CrossRef]
- Thuerauf, D.J.; Morrison, L.; Glembotski, C.C. Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J. Biol. Chem. 2004, 279, 21078–21084. [Google Scholar] [CrossRef]
- Thuerauf, D.J.; Marcinko, M.; Belmont, P.J.; Glembotski, C.C. Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. J. Biol. Chem. 2007, 282, 22865–22878. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Yoshida, H.; Okada, T.; Haze, K.; Yanagi, H.; Yura, T.; Negishi, M.; Mori, K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell Biol. 2000, 20, 6755–6767. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Kondoh, N.; Imazeki, N.; Tanaka, K.; Okada, T.; Mori, K.; Hada, A.; Arai, M.; Wakatsuki, T.; Matsubara, O.; et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: A possible involvement of the ER stress pathway in hepatocarcinogenesis. J. Hepatol. 2003, 38, 605–614. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Ron, D.; Sha, B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 423–428. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Kojima, E.; Takeuchi, A.; Haneda, M.; Yagi, A.; Hasegawa, T.; Yamaki, K.; Takeda, K.; Akira, S.; Shimokata, K.; Isobe, K. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: Elucidation by GADD34-deficient mice. FASEB J. 2003, 17, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Pitale, P.M.; Gorbatyuk, O.; Gorbatyuk, M. Neurodegeneration: Keeping ATF4 on a Tight Leash. Front. Cell Neurosci. 2017, 11, 410. [Google Scholar] [CrossRef]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.C.; Zheng, Q.; Tan, H.; Zhang, B.; Li, X.; Yang, Y.; Yu, J.; Liu, Y.; Chai, H.; Wang, X.; et al. TMCO1 Is an ER Ca(2+) Load-Activated Ca(2+) Channel. Cell 2016, 165, 1454–1466. [Google Scholar] [CrossRef]
- Lemus, L.; Goder, V. Regulation of Endoplasmic Reticulum-Associated Protein Degradation (ERAD) by Ubiquitin. Cells 2014, 3, 824–847. [Google Scholar] [CrossRef]
- Vembar, S.S.; Brodsky, J.L. One step at a time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008, 9, 944–957. [Google Scholar] [CrossRef]
- Suntharalingam, A.; Abisambra, J.F.; O’Leary, J.C., 3rd; Koren, J., 3rd; Zhang, B.; Joe, M.K.; Blair, L.J.; Hill, S.E.; Jinwal, U.K.; Cockman, M.; et al. Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism. J. Biol. Chem. 2012, 287, 40661–40669. [Google Scholar] [CrossRef]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Chen, P.; Huang, W.D.; Chen, H.; Johnson, D.; Polansky, J.R. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 1998, 273, 6341–6350. [Google Scholar] [CrossRef] [PubMed]
- Polansky, J.R.; Fauss, D.J.; Zimmerman, C.C. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye 2000, 14 (Pt. 3B), 503–514. [Google Scholar] [CrossRef]
- Kubota, R.; Noda, S.; Wang, Y.; Minoshima, S.; Asakawa, S.; Kudoh, J.; Mashima, Y.; Oguchi, Y.; Shimizu, N. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: Molecular cloning, tissue expression, and chromosomal mapping. Genomics 1997, 41, 360–369. [Google Scholar] [CrossRef]
- Kubota, R.; Kudoh, J.; Mashima, Y.; Asakawa, S.; Minoshima, S.; Hejtmancik, J.F.; Oguchi, Y.; Shimizu, N. Genomic organization of the human myocilin gene (MYOC) responsible for primary open angle glaucoma (GLC1A). Biochem. Biophys. Res. Commun. 1998, 242, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Shen, X.; Samaraweera, M.; Yue, B.Y. Studies of optineurin, a glaucoma gene: Golgi fragmentation and cell death from overexpression of wild-type and mutant optineurin in two ocular cell types. Am. J. Pathol. 2006, 169, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek-Kaminska, W.; Wojtczak, R.; Szaflik, J.P.; Szaflik, J.; Majsterek, I. The Genetic and Endoplasmic Reticulum-Mediated Molecular Mechanisms of Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2020, 21, 4171. [Google Scholar] [CrossRef]
- Skarie, J.M.; Link, B.A. The primary open-angle glaucoma gene WDR36 functions in ribosomal RNA processing and interacts with the p53 stress-response pathway. Hum. Mol. Genet. 2008, 17, 2474–2485. [Google Scholar] [CrossRef]
- Ritch, R.; Darbro, B.; Menon, G.; Khanna, C.L.; Solivan-Timpe, F.; Roos, B.R.; Sarfarzi, M.; Kawase, K.; Yamamoto, T.; Robin, A.L.; et al. TBK1 gene duplication and normal-tension glaucoma. JAMA Ophthalmol. 2014, 132, 544–548. [Google Scholar] [CrossRef]
- Pasutto, F.; Matsumoto, T.; Mardin, C.Y.; Sticht, H.; Brandstatter, J.H.; Michels-Rautenstrauss, K.; Weisschuh, N.; Gramer, E.; Ramdas, W.D.; van Koolwijk, L.M.; et al. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am. J. Hum. Genet. 2009, 85, 447–456. [Google Scholar] [CrossRef]
- Wiggs, J.L. Glaucoma Genes and Mechanisms. Prog. Mol. Biol. Transl. Sci. 2015, 134, 315–342. [Google Scholar] [CrossRef]
- Micheal, S.; Ayub, H.; Khan, M.I.; Bakker, B.; Schoenmaker-Koller, F.E.; Ali, M.; Akhtar, F.; Khan, W.A.; Qamar, R.; den Hollander, A.I. Association of known common genetic variants with primary open angle, primary angle closure, and pseudoexfoliation glaucoma in Pakistani cohorts. Mol. Vis. 2014, 20, 1471–1479. [Google Scholar] [PubMed]
- Burdon, K.P.; Macgregor, S.; Hewitt, A.W.; Sharma, S.; Chidlow, G.; Mills, R.A.; Danoy, P.; Casson, R.; Viswanathan, A.C.; Liu, J.Z.; et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat. Genet. 2011, 43, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, W.D.; van Koolwijk, L.M.; Lemij, H.G.; Pasutto, F.; Cree, A.J.; Thorleifsson, G.; Janssen, S.F.; Jacoline, T.B.; Amin, N.; Rivadeneira, F.; et al. Common genetic variants associated with open-angle glaucoma. Hum. Mol. Genet. 2011, 20, 2464–2471. [Google Scholar] [CrossRef]
- Bagetta, G.; Nucci, C. Preface: New trends in basic and clinical research of glaucoma: A neurodegenerative disease of the visual system part B. Prog. Brain Res. 2015, 221, xxiii–xxiv. [Google Scholar] [CrossRef]
- Ramdas, W.D.; van Koolwijk, L.M.; Ikram, M.K.; Jansonius, N.M.; de Jong, P.T.; Bergen, A.A.; Isaacs, A.; Amin, N.; Aulchenko, Y.S.; Wolfs, R.C.; et al. A genome-wide association study of optic disc parameters. PLoS Genet. 2010, 6, e1000978. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.L.; Kanekar, S.; Vetter, M.L.; Tucker, P.K.; Gemza, D.L.; Glaser, T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development 1998, 125, 4821–4833. [Google Scholar] [CrossRef]
- van Koolwijk, L.M.; Ramdas, W.D.; Ikram, M.K.; Jansonius, N.M.; Pasutto, F.; Hysi, P.G.; Macgregor, S.; Janssen, S.F.; Hewitt, A.W.; Viswanathan, A.C.; et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012, 8, e1002611. [Google Scholar] [CrossRef]
- Zukerman, R.; Harris, A.; Oddone, F.; Siesky, B.; Verticchio Vercellin, A.; Ciulla, T.A. Glaucoma Heritability: Molecular Mechanisms of Disease. Genes 2021, 12, 1135. [Google Scholar] [CrossRef]
- Fernandez-Martinez, L.; Letteboer, S.; Mardin, C.Y.; Weisschuh, N.; Gramer, E.; Weber, B.H.; Rautenstrauss, B.; Ferreira, P.A.; Kruse, F.E.; Reis, A.; et al. Evidence for RPGRIP1 gene as risk factor for primary open angle glaucoma. Eur. J. Hum. Genet. 2011, 19, 445–451. [Google Scholar] [CrossRef][Green Version]
- Abu-Hassan, D.W.; Acott, T.S.; Kelley, M.J. The Trabecular Meshwork: A Basic Review of Form and Function. J. Ocul. Biol. 2014, 2. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.M.; Murphy-Ullrich, J.E.; Downs, J.C.; O’Brien, C.J. The role of matricellular proteins in glaucoma. Matrix. Biol. 2014, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.C.; Bhattacharya, S.; Clark, A.F.; Zode, G.S. Increased Endoplasmic Reticulum Stress in Human Glaucomatous Trabecular Meshwork Cells and Tissues. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3860–3868. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Maddineni, P.; Millar, J.C.; Clark, A.F.; Zode, G.S. Increased synthesis and deposition of extracellular matrix proteins leads to endoplasmic reticulum stress in the trabecular meshwork. Sci. Rep. 2017, 7, 14951. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Maddineni, P.; Patel, P.D.; Searby, C.; Sheffield, V.C.; Zode, G.S. Transforming growth factor beta2 (TGFbeta2) signaling plays a key role in glucocorticoid-induced ocular hypertension. J. Biol. Chem. 2018, 293, 9854–9868. [Google Scholar] [CrossRef]
- Kasetti, R.B.; Patel, P.D.; Maddineni, P.; Patil, S.; Kiehlbauch, C.; Millar, J.C.; Searby, C.C.; Raghunathan, V.; Sheffield, V.C.; Zode, G.S. ATF4 leads to glaucoma by promoting protein synthesis and ER client protein load. Nat. Commun. 2020, 11, 5594. [Google Scholar] [CrossRef]
- Ying, Y.; Xue, R.; Yang, Y.; Zhang, S.X.; Xiao, H.; Zhu, H.; Li, J.; Chen, G.; Ye, Y.; Yu, M.; et al. Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG. Aging 2021, 13, 8628–8642. [Google Scholar] [CrossRef]
- Wang, Y.X.; Yang, H.; Luo, H.; Hong, S.W.; Gardiner, S.K.; Jeoung, J.W.; Hardin, C.; Sharpe, G.P.; Nouri-Mahdavi, K.; Caprioli, J.; et al. Peripapillary Scleral Bowing Increases with Age and Is Inversely Associated with Peripapillary Choroidal Thickness in Healthy Eyes. Am. J. Ophthalmol. 2020, 217, 91–103. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5189–5195. [Google Scholar] [CrossRef]
- Shimazawa, M.; Miwa, A.; Ito, Y.; Tsuruma, K.; Aihara, M.; Hara, H. Involvement of endoplasmic reticulum stress in optic nerve degeneration following N-methyl-D-aspartate-induced retinal damage in mice. J. Neurosci. Res. 2012, 90, 1960–1969. [Google Scholar] [CrossRef]
- Ojino, K.; Shimazawa, M.; Izawa, H.; Nakano, Y.; Tsuruma, K.; Hara, H. Involvement of endoplasmic reticulum stress in optic nerve degeneration after chronic high intraocular pressure in DBA/2J mice. J. Neurosci. Res. 2015, 93, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Stowell, C.; Jang, G.; Zhang, L.; Crabb, J.; Reynaud, J.; Gardiner, S.K.; Willard, B.; Marsh-Armstrong, N.; Crabb, J.W.; Burgoyne, C.F. Alterations in Optic Nerve Head (ONH) Endoplasmic Reticulum (ER) Stress Response Proteins in Non-Human Primate (NHP) Early Experimental Glaucoma (EG). Investig. Ophthalmol. Vis. Sci. 2018, 59, 3514. [Google Scholar]
- Mesentier-Louro, L.A.; Shariati, M.A.; Dalal, R.; Camargo, A.; Kumar, V.; Shamskhou, E.A.; de Jesus Perez, V.; Liao, Y.J. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp. Eye Res. 2020, 193, 107957. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J.; Balaratnasingam, C.; Morgan, W.H.; Yu, P.K.; Su, E.N. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog. Retin. Eye Res. 2013, 36, 217–246. [Google Scholar] [CrossRef]
- Smith, C.A.; Vianna, J.R.; Chauhan, B.C. Assessing retinal ganglion cell damage. Eye 2017, 31, 209–217. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Hyttinen, J.M.; Toropainen, E.; Kaarniranta, K. Endoplasmic reticulum stress in age-related macular degeneration: Trigger for neovascularization. Mol. Med. 2010, 16, 535–542. [Google Scholar] [CrossRef]
- Griciuc, A.; Aron, L.; Ueffing, M. ER stress in retinal degeneration: A target for rational therapy? Trends Mol. Med. 2011, 17, 442–451. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Kanuga, N.; Adamson, P.; Cheetham, M.E. The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Hum. Mol. Genet. 2017, 26, 4896–4905. [Google Scholar] [CrossRef]
- Mastey, R.R.; Georgiou, M.; Langlo, C.S.; Kalitzeos, A.; Patterson, E.J.; Kane, T.; Singh, N.; Vincent, A.; Moore, A.T.; Tsang, S.H.; et al. Characterization of Retinal Structure in ATF6-Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2631–2640. [Google Scholar] [CrossRef]
- Ansar, M.; Santos-Cortez, R.L.; Saqib, M.A.; Zulfiqar, F.; Lee, K.; Ashraf, N.M.; Ullah, E.; Wang, X.; Sajid, S.; Khan, F.S.; et al. Mutation of ATF6 causes autosomal recessive achromatopsia. Hum. Genet. 2015, 134, 941–950. [Google Scholar] [CrossRef]
- Hata, N.; Oshitari, T.; Yokoyama, A.; Mitamura, Y.; Yamamoto, S. Increased expression of IRE1alpha and stress-related signal transduction proteins in ischemia-reperfusion injured retina. Clin. Ophthalmol. 2008, 2, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Doh, S.H.; Kim, J.H.; Lee, K.M.; Park, H.Y.; Park, C.K. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010, 1308, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y. Axon injury induced endoplasmic reticulum stress and neurodegeneration. Neural Regen. Res. 2016, 11, 1557–1559. [Google Scholar] [CrossRef]
- Marola, O.J.; Syc-Mazurek, S.B.; Libby, R.T. DDIT3 (CHOP) contributes to retinal ganglion cell somal loss but not axonal degeneration in DBA/2J mice. Cell Death Discov. 2019, 5, 140. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, S.; Miao, L.; Huang, H.; Liang, F.; Teng, X.; Xu, L.; Wang, Q.; Xiao, W.; Ridder, W.H., 3rd; et al. Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. J. Neurosci. 2016, 36, 5891–5903. [Google Scholar] [CrossRef]
- Hetzer, S.M.; Guilhaume-Correa, F.; Day, D.; Bedolla, A.; Evanson, N.K. Traumatic Optic Neuropathy Is Associated with Visual Impairment, Neurodegeneration, and Endoplasmic Reticulum Stress in Adolescent Mice. Cells 2021, 10, 996. [Google Scholar] [CrossRef]
- Takano, S.; Ishiwata, S.; Nakazawa, M.; Mizugaki, M.; Tamai, M. Determination of ascorbic acid in human vitreous humor by high-performance liquid chromatography with UV detection. Curr. Eye Res. 1997, 16, 589–594. [Google Scholar] [CrossRef]
- Cicik, E.; Tekin, H.; Akar, S.; Ekmekci, O.B.; Donma, O.; Koldas, L.; Ozkan, S. Interleukin-8, nitric oxide and glutathione status in proliferative vitreoretinopathy and proliferative diabetic retinopathy. Ophthalmic Res. 2003, 35, 251–255. [Google Scholar] [CrossRef]
- Maher, P.; Hanneken, A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4796–4803. [Google Scholar] [CrossRef]
- Li, S.Y.; Lo, A.C. Lutein protects RGC-5 cells against hypoxia and oxidative stress. Int. J. Mol. Sci. 2010, 11, 2109–2117. [Google Scholar] [CrossRef]
- Inman, D.M.; Lambert, W.S.; Calkins, D.J.; Horner, P.J. alpha-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS ONE 2013, 8, e65389. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Li, Z.; Jia, Y.; Zhuo, Y. Rapamycin is neuroprotective in a rat chronic hypertensive glaucoma model. PLoS ONE 2014, 9, e99719. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, S.; Zeng, W.; Chen, X.; Zheng, T.; Ren, J.; Ke, M. Protective Effects of Rapamycin on Trabecular Meshwork Cells in Glucocorticoid-Induced Glaucoma Mice. Front. Pharmacol. 2020, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Stein, J.D.; Nan, B.; Childers, D.; Newman-Casey, P.A.; Thompson, D.A.; Richards, J.E. Association of Geroprotective Effects of Metformin and Risk of Open-Angle Glaucoma in Persons With Diabetes Mellitus. JAMA Ophthalmol. 2015, 133, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Chukwunonso Obi, B.; Chinwuba Okoye, T.; Okpashi, V.E.; Nonye Igwe, C.; Olisah Alumanah, E. Comparative Study of the Antioxidant Effects of Metformin, Glibenclamide, and Repaglinide in Alloxan-Induced Diabetic Rats. J. Diabetes Res. 2016, 2016, 1635361. [Google Scholar] [CrossRef]
- Diniz Vilela, D.; Gomes Peixoto, L.; Teixeira, R.R.; Belele Baptista, N.; Carvalho Caixeta, D.; Vieira de Souza, A.; Machado, H.L.; Pereira, M.N.; Sabino-Silva, R.; Espindola, F.S. The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxid. Med. Cell. Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef]
- Mahrouf, M.; Ouslimani, N.; Peynet, J.; Djelidi, R.; Couturier, M.; Therond, P.; Legrand, A.; Beaudeux, J.L. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem. Pharmacol. 2006, 72, 176–183. [Google Scholar] [CrossRef]
- Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M.; Wronski, S.; Tarasiuk, J.; Maredziak, M. Metformin Decreases Reactive Oxygen Species, Enhances Osteogenic Properties of Adipose-Derived Multipotent Mesenchymal Stem Cells In Vitro, and Increases Bone Density In Vivo. Oxid. Med. Cell. Longev. 2016, 2016, 9785890. [Google Scholar] [CrossRef]
- Hoy, S.M. Netarsudil Ophthalmic Solution 0.02%: First Global Approval. Drugs 2018, 78, 389–396. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inoue, T.; Ohira, S.; Awai-Kasaoka, N.; Kameda, T.; Inoue-Mochita, M.; Tanihara, H. Inhibition of Rho Kinase Induces Antioxidative Molecules and Suppresses Reactive Oxidative Species in Trabecular Meshwork Cells. J. Ophthalmol. 2017, 2017, 7598140. [Google Scholar] [CrossRef]
- Chou, T.H.; Romano, G.L.; Amato, R.; Porciatti, V. Nicotinamide-Rich Diet in DBA/2J Mice Preserves Retinal Ganglion Cell Metabolic Function as Assessed by PERG Adaptation to Flicker. Nutrients 2020, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Tolun, F.I.; Gul, M.; Imrek, S. Retinal oxidative stress induced by intraocular hypertension in rats may be ameliorated by brimonidine treatment and N-acetyl cysteine supplementation. J. Glaucoma 2009, 18, 662–665. [Google Scholar] [CrossRef]
- Akaiwa, K.; Namekata, K.; Azuchi, Y.; Guo, X.; Kimura, A.; Harada, C.; Mitamura, Y.; Harada, T. Edaravone suppresses retinal ganglion cell death in a mouse model of normal tension glaucoma. Cell Death Dis. 2017, 8, e2934. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Ishida, T.; Fang, Y.; Shinohara, K.; Li, X.; Nagaoka, N.; Ohno-Matsui, K.; Yoshida, T. Protection of the Retinal Ganglion Cells: Intravitreal Injection of Resveratrol in Mouse Model of Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Tartaglione, R.; Cerulli, A.; Mancino, R.; Spano, A.; Cavaliere, F.; Rombola, L.; Bagetta, G.; Corasaniti, M.T.; Morrone, L.A. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int. Rev. Neurobiol. 2007, 82, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Feillet, F.; Leonard, J.V. Alternative pathway therapy for urea cycle disorders. J. Inherit. Metab. Dis 1998, 21 (Suppl. 1), 101–111. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; Hong-Brown, L.Q.; Biwersi, J.; Verkman, A.S.; Welch, W.J. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1996, 1, 117–125. [Google Scholar] [CrossRef]
- Yam, G.H.; Gaplovska-Kysela, K.; Zuber, C.; Roth, J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1683–1690. [Google Scholar] [CrossRef]
- Zode, G.S.; Kuehn, M.H.; Nishimura, D.Y.; Searby, C.C.; Mohan, K.; Grozdanic, S.D.; Bugge, K.; Anderson, M.G.; Clark, A.F.; Stone, E.M.; et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Investig. 2011, 121, 3542–3553. [Google Scholar] [CrossRef]
- Zode, G.S.; Bugge, K.E.; Mohan, K.; Grozdanic, S.D.; Peters, J.C.; Koehn, D.R.; Anderson, M.G.; Kardon, R.H.; Stone, E.M.; Sheffield, V.C. Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1557–1565. [Google Scholar] [CrossRef]
- Maddineni, P.; Kasetti, R.B.; Kodati, B.; Yacoub, S.; Zode, G.S. Sodium 4-Phenylbutyrate Reduces Ocular Hypertension by Degrading Extracellular Matrix Deposition via Activation of MMP9. Int. J. Mol. Sci. 2021, 22, 10095. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mesentier-Louro, L.A.; Oh, A.J.; Heng, K.; Shariati, M.A.; Huang, H.; Hu, Y.; Liao, Y.J. Increased ER Stress After Experimental Ischemic Optic Neuropathy and Improved RGC and Oligodendrocyte Survival After Treatment With Chemical Chaperon. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef]
- Wang, Y.; Osakue, D.; Yang, E.; Zhou, Y.; Gong, H.; Xia, X.; Du, Y. Endoplasmic Reticulum Stress Response of Trabecular Meshwork Stem Cells and Trabecular Meshwork Cells and Protective Effects of Activated PERK Pathway. Investig. Ophthalmol. Vis. Sci. 2019, 60, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H.; Callaway, E. Pioneers of revolutionary CRISPR gene editing win chemistry Nobel. Nature 2020, 586, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef]
- Wu, J.; Bell, O.H.; Copland, D.A.; Young, A.; Pooley, J.R.; Maswood, R.; Evans, R.S.; Khaw, P.T.; Ali, R.R.; Dick, A.D.; et al. Gene Therapy for Glaucoma by Ciliary Body Aquaporin 1 Disruption Using CRISPR-Cas9. Mol. Ther. 2020, 28, 820–829. [Google Scholar] [CrossRef]
- Gordeeva, A.V.; Zvyagilskaya, R.A.; Labas, Y.A. Cross-talk between reactive oxygen species and calcium in living cells. Biochem. (Mosc.) 2003, 68, 1077–1080. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Deniaud, A.; Sharaf el dein, O.; Maillier, E.; Poncet, D.; Kroemer, G.; Lemaire, C.; Brenner, C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 2008, 27, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.I.; Jou, M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Raturi, A.; Simmen, T. Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM). Biochim. Biophys. Acta 2013, 1833, 213–224. [Google Scholar] [CrossRef]
- Tu, B.P.; Weissman, J.S. Oxidative protein folding in eukaryotes: Mechanisms and consequences. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bauereis, B.; Haskins, W.E.; Lebaron, R.G.; Renthal, R. Proteomic insights into the protective mechanisms of an in vitro oxidative stress model of early stage Parkinson’s disease. Neurosci. Lett. 2011, 488, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.S.; Chavez, J.C.; Pinto, J.T.; Coppola, G.; Sun, C.W.; Townes, T.M.; Geschwind, D.H.; Ratan, R.R. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J. Exp. Med. 2008, 205, 1227–1242. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Meng, Q.; Du, Y.; Xiao, F.; Zhang, Q.; Yu, J.; Li, K.; Chen, S.; Huang, Z.; et al. ATF4 deficiency protects hepatocytes from oxidative stress via inhibiting CYP2E1 expression. J. Cell Mol. Med. 2014, 18, 80–90. [Google Scholar] [CrossRef]
- Joe, M.K.; Tomarev, S.I. Expression of myocilin mutants sensitizes cells to oxidative stress-induced apoptosis: Implication for glaucoma pathogenesis. Am. J. Pathol. 2010, 176, 2880–2890. [Google Scholar] [CrossRef]
- Yokota, T.; Sugawara, K.; Ito, K.; Takahashi, R.; Ariga, H.; Mizusawa, H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem. Biophys. Res. Commun. 2003, 312, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Rico-Bautista, E.; Zhu, W.; Kitada, S.; Ganapathy, S.; Lau, E.; Krajewski, S.; Ramirez, J.; Bush, J.A.; Yuan, Z.; Wolf, D.A. Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling. Oncotarget 2013, 4, 1212–1229. [Google Scholar] [CrossRef]
- Jo, G.H.; Kim, G.Y.; Kim, W.J.; Park, K.Y.; Choi, Y.H. Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: The involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway. Int. J. Oncol. 2014, 45, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Maamoun, H.; Benameur, T.; Pintus, G.; Munusamy, S.; Agouni, A. Crosstalk Between Oxidative Stress and Endoplasmic Reticulum (ER) Stress in Endothelial Dysfunction and Aberrant Angiogenesis Associated With Diabetes: A Focus on the Protective Roles of Heme Oxygenase (HO)-1. Front. Physiol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Murugan, D.D.; Khan, H.; Huang, Y.; Cheang, W.S. Roles and Therapeutic Implications of Endoplasmic Reticulum Stress and Oxidative Stress in Cardiovascular Diseases. Antioxidants 2021, 10, 1167. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Saito, A.; Okuno, S.; Ferrand-Drake, M.; Dodd, R.L.; Nishi, T.; Maier, C.M.; Kinouchi, H.; Chan, P.H. Oxidative damage to the endoplasmic reticulum is implicated in ischemic neuronal cell death. J. Cereb. Blood Flow Metab. 2003, 23, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).