The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extract and Reagents
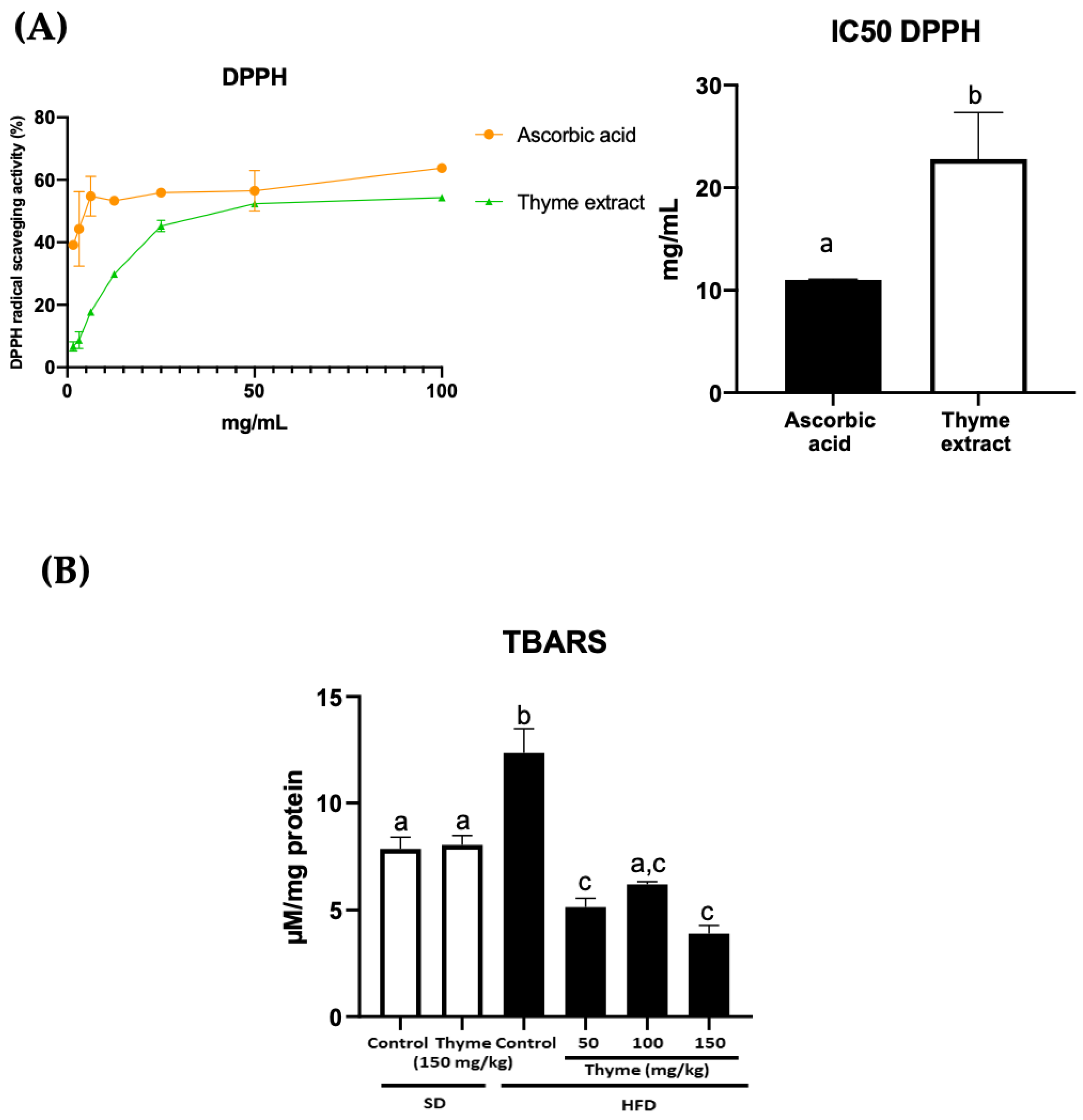
2.2. DPPH Scavenging Activity
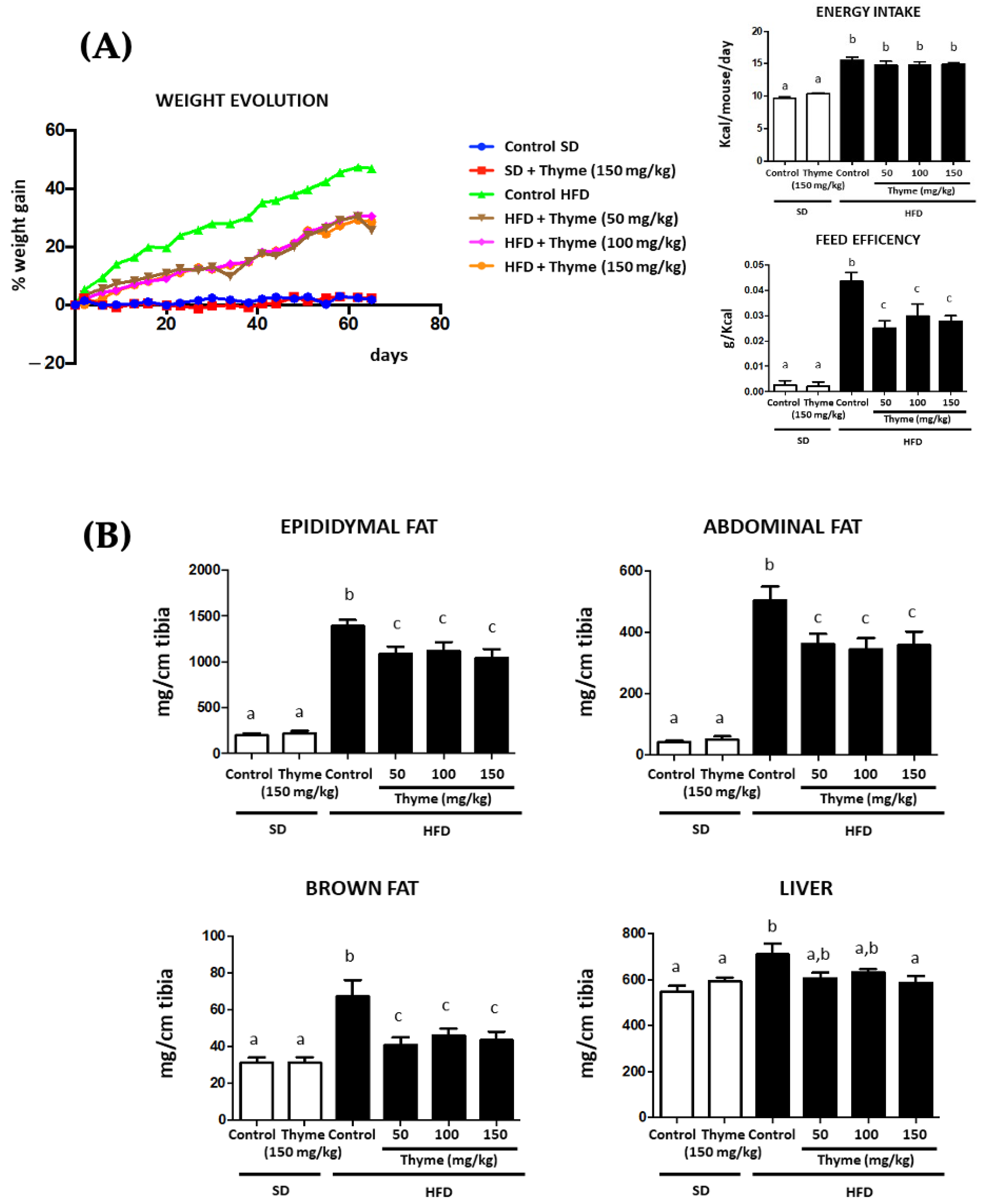
2.3. Animals, Diets and Experimental Design
2.4. Thiobarbituric Acid Reactive Substance Assay
2.5. Glucose Tolerance Test
2.6. Plasma Determinations
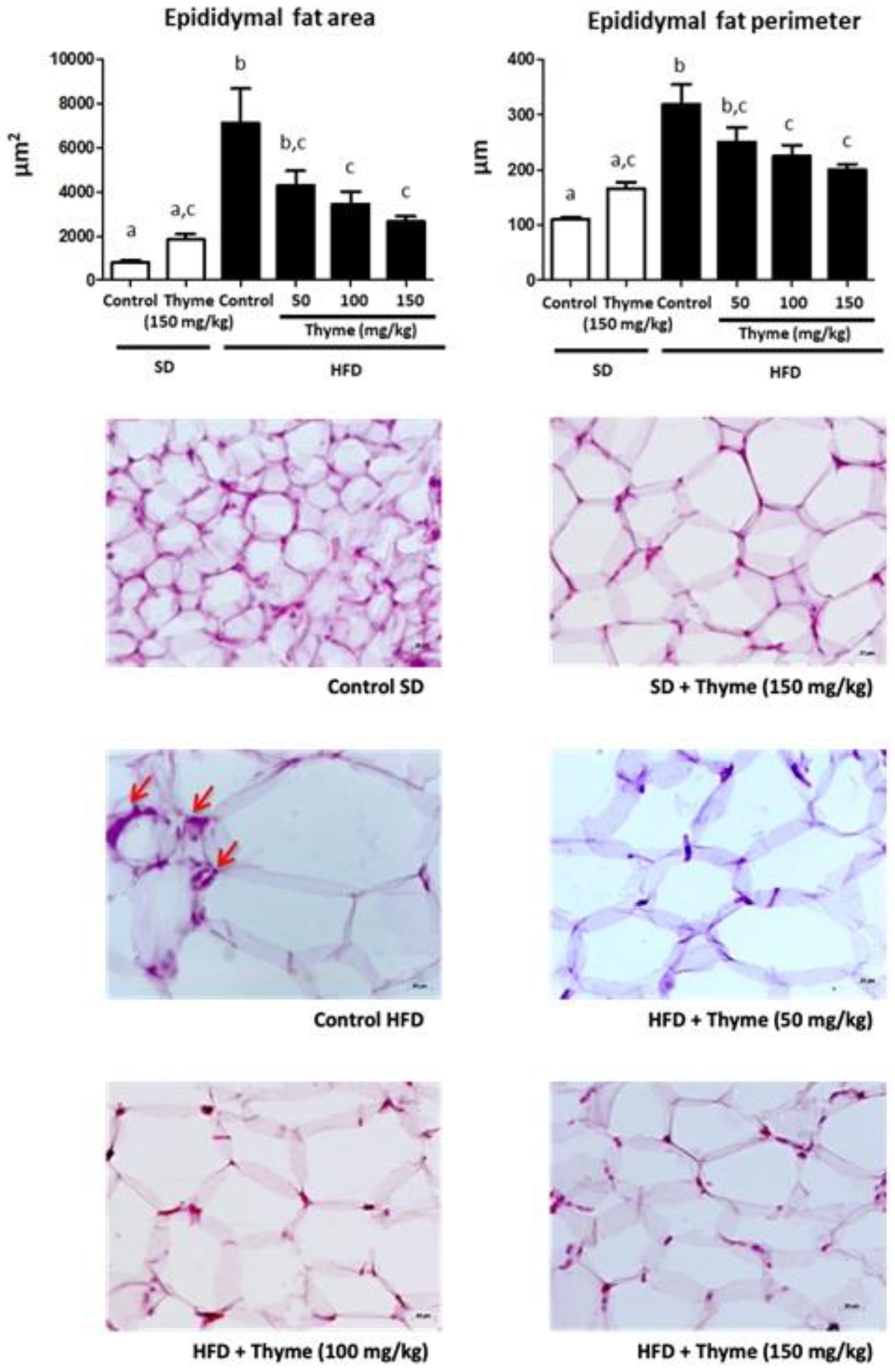
2.7. Morphological Variables
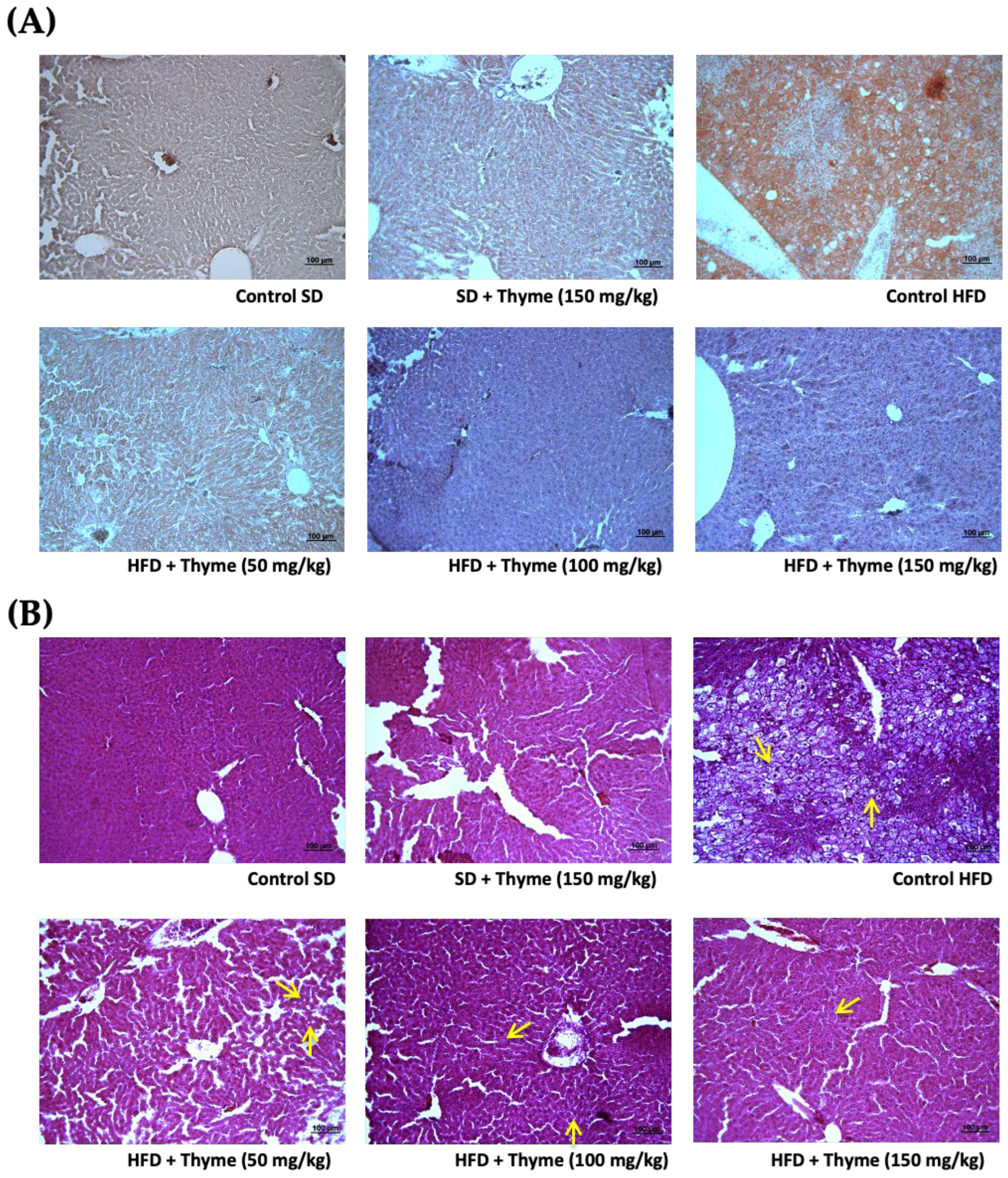
2.8. Histological Studies
2.9. Vascular Reactivity Studies and NADPH Oxidase Activity
2.10. Analysis of Gene Expression by RT-qPCR
2.11. Western Blotting Analysis
2.12. DNA Extraction and Sequencing Analysis
2.13. Quantification of the SCFA Concentrations in the Intestinal Luminal Contents
2.14. Statistical Analysis
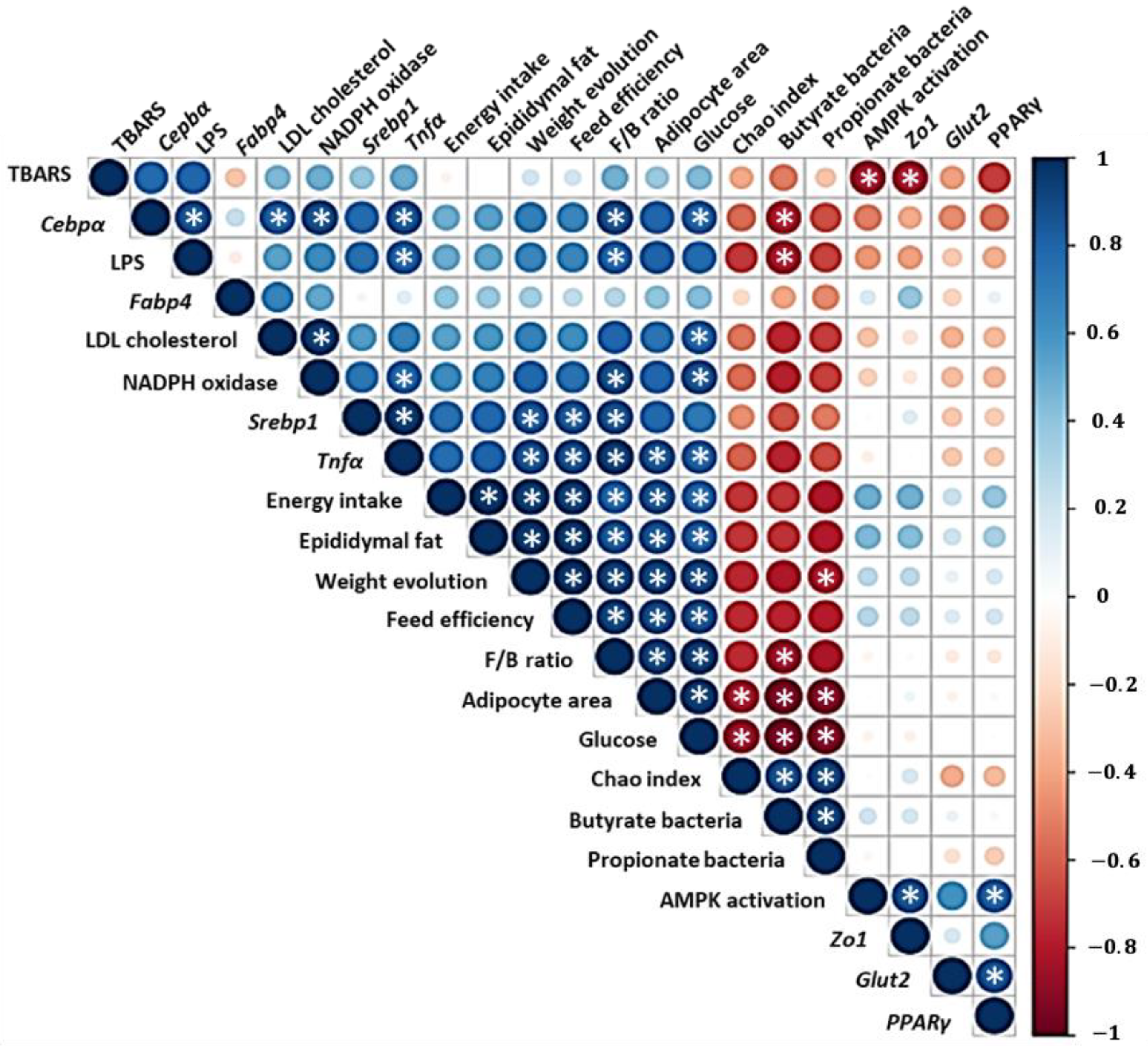
3. Results and Discussion
3.1. Thyme Extract Shows Antioxidant Effects by the Reduction of Radical Scavenging Activity In Vitro and the Mitigation of Lipid Peroxidation in HFD Fed Mice
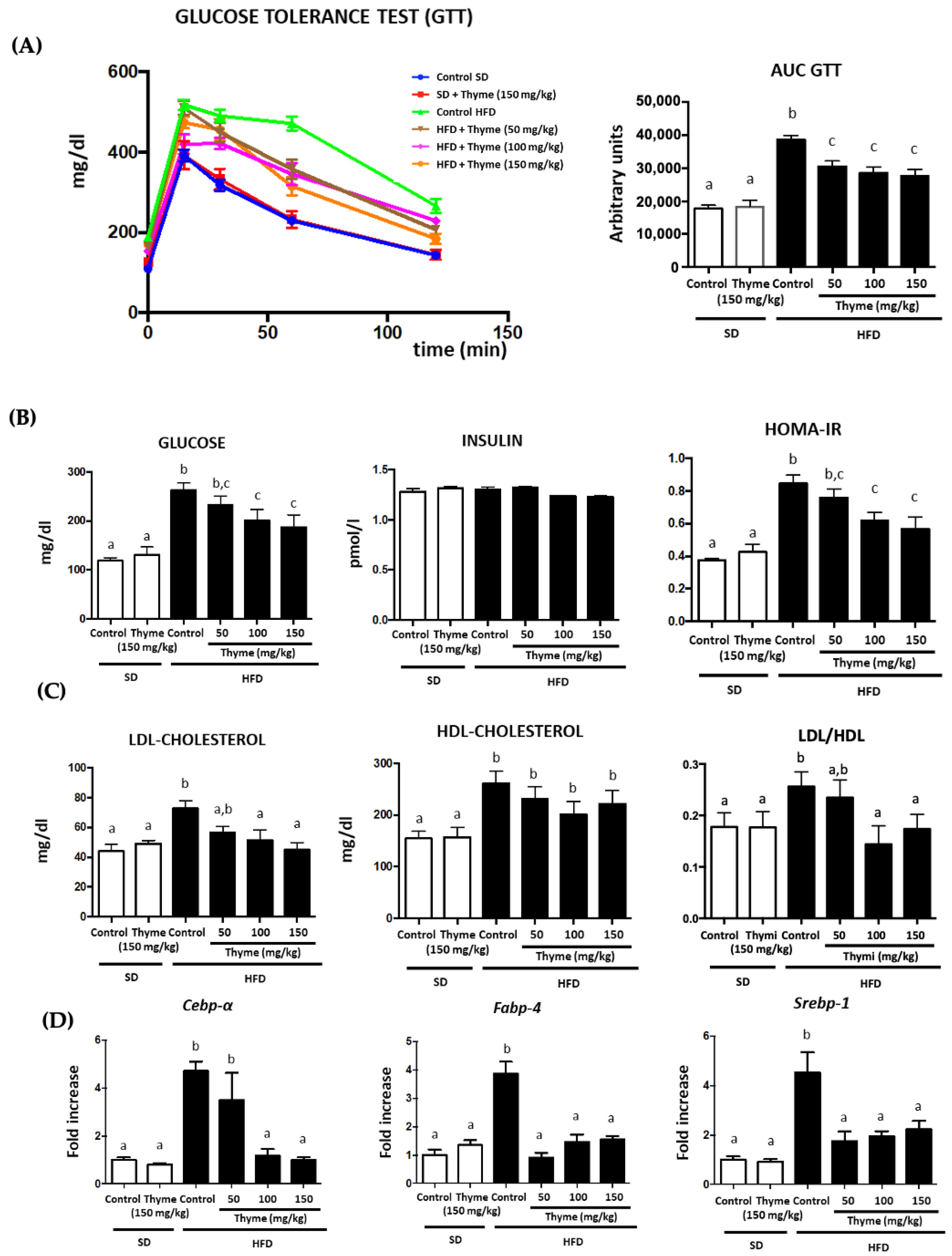
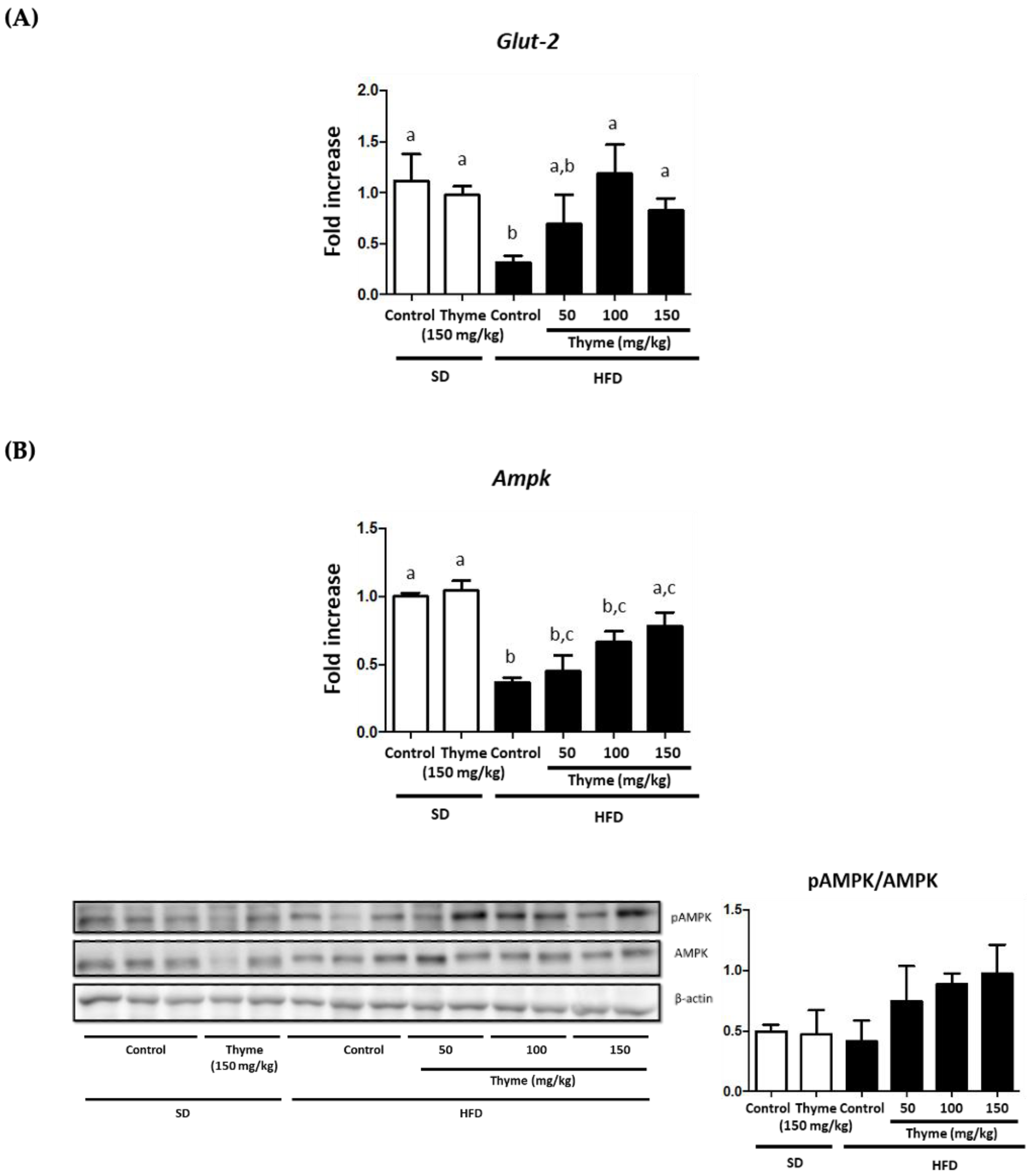
3.2. Thyme Extract Reduces Body Weight Gain in HFD Fed Mice and Improves Glucose and Lipid Metabolic Profile
3.3. Thyme Extract Ameliorates Obesity-Associated Inflammation
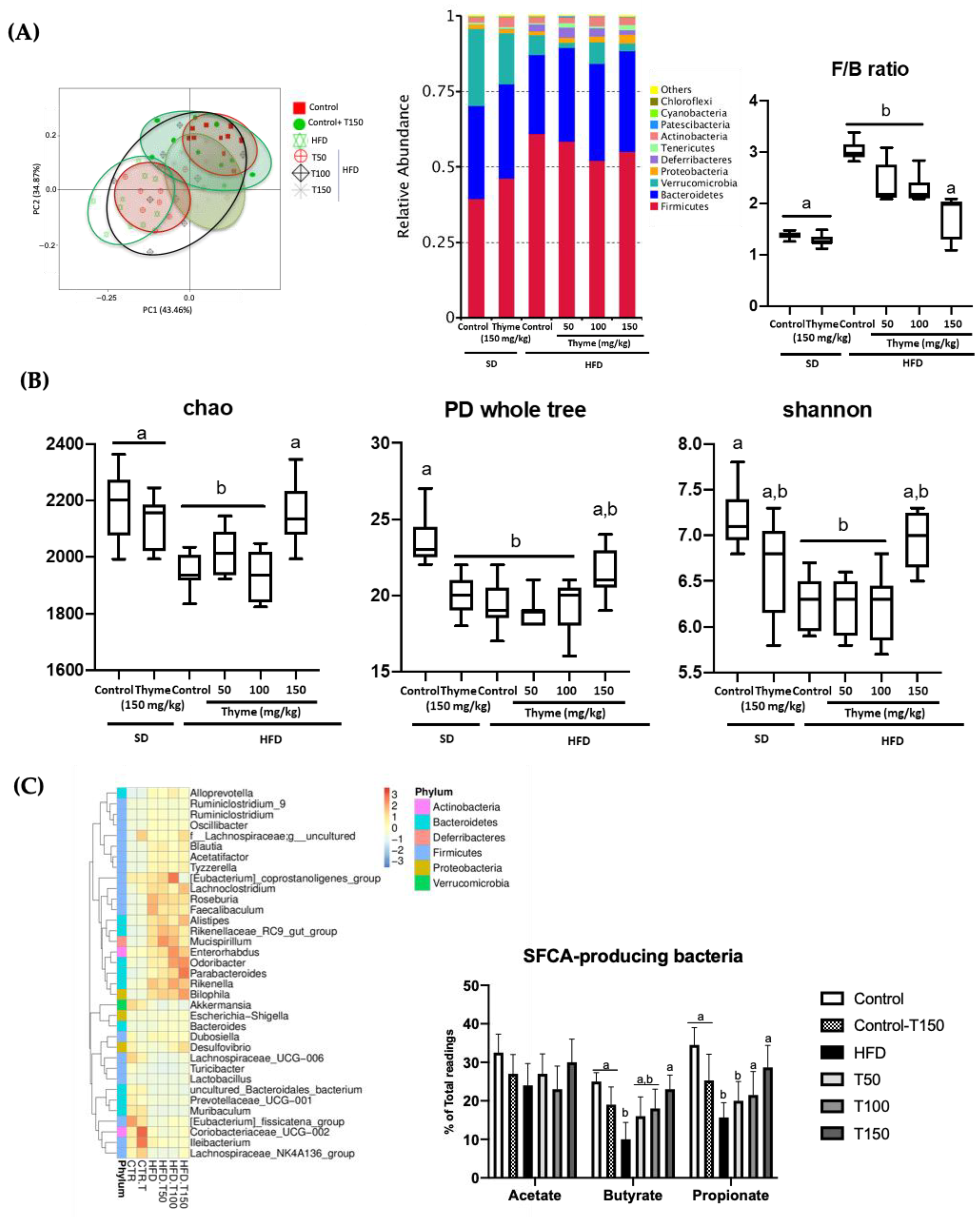
3.4. Thyme Extract Treatment Restores Gut Dysbiosis in HFD Fed Mice
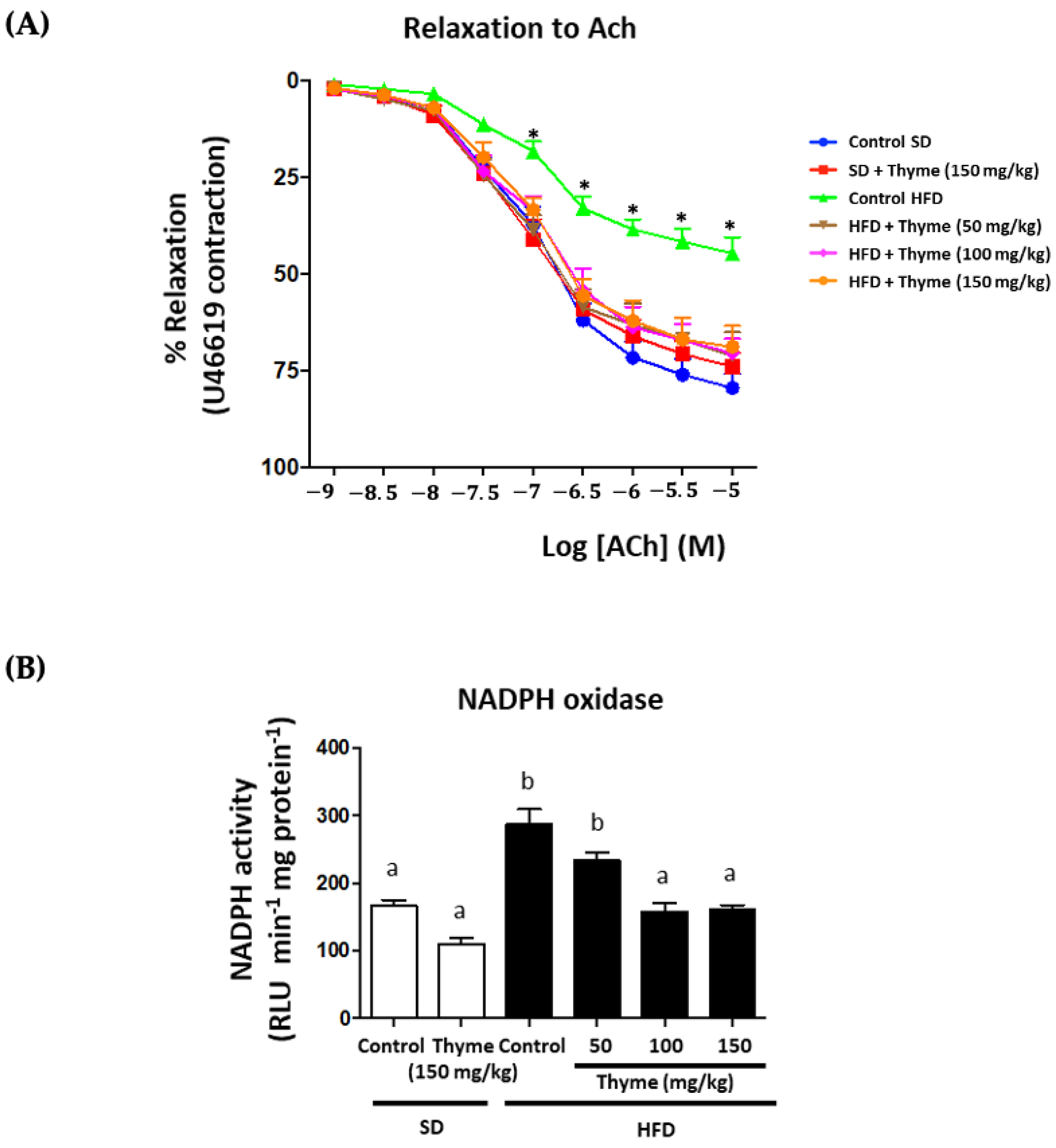
3.5. Thyme Extract Administration Enhances Endothelial Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Toshima, T.; Yoshizumi, T.; Inokuchi, S.; Kosai-Fujimoto, Y.; Kurihara, T.; Yoshiya, S.; Mano, Y.; Takeishi, K.; Itoh, S.; Harada, N.; et al. Risk factors for the metabolic syndrome components of hypertension, diabetes mellitus, and dyslipidemia after living donor liver transplantation. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2020, 22, 511–520. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. A report about Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 May 2022).
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Reviews. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Turner, A.W.; Duchez, A.-C.; Soubeyrand, S.; Rasheed, A.; Smyth, D.C.; Cook, D.P.; Nikpay, M.; Kandiah, J.W.; Pan, C.; et al. Publisher Correction: RIPK1 gene variants associate with obesity in humans and can be therapeutically silenced to reduce obesity in mice. Nat. Metab. 2020, 2, 1181. [Google Scholar] [CrossRef]
- Fain, J.N. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006, 74, 443–477. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- McLaughlin, T.; Sherman, A.; Tsao, P.; Gonzalez, O.; Yee, G.; Lamendola, C.; Reaven, G.M.; Cushman, S.W. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 2007, 50, 1707–1715. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; García, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 167, 105471. [Google Scholar] [CrossRef] [PubMed]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- de Freitas Junior, L.M.; de Almeida, E.B., Jr. Medicinal plants for the treatment of obesity: Ethnopharmacological approach and chemical and biological studies. Am. J. Transl. Res. 2017, 9, 2050–2064. [Google Scholar]
- Leyva-Jiménez, F.J.; Ruiz-Malagón, A.J.; Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-García, L.; Lozano-Sánchez, J.; Arráez-Román, D.; Cenis, J.L.; Lozano-Pérez, A.A.; et al. Comparative Study of the Antioxidant and Anti-Inflammatory Effects of Leaf Extracts from Four Different Morus alba Genotypes in High Fat Diet-Induced Obesity in Mice. Antioxidants 2020, 9, 733. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sánchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef] [PubMed]
- Diez-Echave, P.; Vezza, T.; Rodríguez-Nogales, A.; Hidalgo-Garcia, L.; Garrido-Mesa, J.; Ruiz-Malagon, A.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Leyva-Jiménez, F.J.; et al. The Beneficial Effects of Lippia Citriodora Extract on Diet-Induced Obesity in Mice Are Associated with Modulation in the Gut Microbiota Composition. Mol. Nutr. Food Res. 2020, 64, e2000005. [Google Scholar] [CrossRef] [PubMed]
- Sinisgalli, C.; Vezza, T.; Diez-Echave, P.; Ostuni, A.; Faraone, I.; Hidalgo-Garcia, L.; Russo, D.; Armentano, M.F.; Garrido-Mesa, J.; Rodriguez-Cabezas, M.E.; et al. The Beneficial Effects of Red Sun-Dried Capsicum annuum L. Cv Senise Extract with Antioxidant Properties in Experimental Obesity are Associated with Modulation of the Intestinal Microbiota. Mol. Nutr. Food Res. 2021, 65, e2000812. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. PTR 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complementary Altern. Med. Ecam. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Algieri, F.; Rodriguez-Nogales, A.; Garrido-Mesa, N.; Zorrilla, P.; Burkard, N.; Pischel, I.; Sievers, H.; Benedek, B.; Feistel, B.; Walbroel, B.; et al. Intestinal anti-inflammatory activity of the Serpylli herba extract in experimental models of rodent colitis. J. Crohn’s Colitis 2014, 8, 775–788. [Google Scholar] [CrossRef]
- European Scientific Cooperative on Phytotherapy. ESCOP Monographs The Scientific Foundation for Herbal Medicinal Products: Serpylli herba (Wild Thyme); ESCOP: Exeter, UK, 2014. [Google Scholar]
- Malterud, K.E.; Farbrot, T.L.; Huse, A.E.; Sund, R.B. Antioxidant and Radical Scavenging Effects of Anthraquinones and Anthrones. Pharmacolgy 1993, 47, 77–85. [Google Scholar] [CrossRef]
- Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Sánchez, R.; Utrilla, M.P.; Garrido-Mesa, N.; Rodríguez-Cabezas, M.E.; Olivares, M.; Gálvez, J.; Duarte, J. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin. Sci. 2014, 127, 33–45. [Google Scholar] [CrossRef]
- Zarzuelo, M.J.; Jimenez, R.; Galindo, P.; Sanchez, M.; Nieto, A.; Romero, M.; Quintela, A.M.; Lopez-Sepulveda, R.; Gomez-Guzman, M.; Bailon, E.; et al. Antihypertensive effects of peroxisome proliferator-activated receptor-beta activation in spontaneously hypertensive rats. Hypertension 2011, 58, 733–743. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Rodríguez-Cabezas, M.E.; Camuesco, D.; Arribas, B.; Garrido-Mesa, N.; Comalada, M.; Bailón, E.; Cueto-Sola, M.; Utrilla, P.; Guerra-Hernández, E.; Pérez-Roca, C.; et al. The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats. Clin. Nutr. 2010, 29, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.H.; Umashanker, D.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Obesity Pharmacotherapy. Med. Clin. North Am. 2018, 102, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Van Hul, M.; Knauf, C.; Cani, P.D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 2021, 248, R67–R82. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. Effects of Probiotics on Biomarkers of Oxidative Stress and Inflammatory Factors in Petrochemical Workers: A Randomized, Double-blind, Placebo-controlled Trial. Int. J. Prev. Med. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy Res. 2008, 75, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 827, 76–82. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Invest. 2014, 124, 2099–2112. [Google Scholar] [CrossRef]
- Roberts-Toler, C.; O’Neill, B.T.; Cypess, A.M. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity 2015, 9, 1765–1770. [Google Scholar] [CrossRef]
- Shimizu, I.; Yoshida, Y.; Suda, M.; Minamino, T. DNA damage response and metabolic disease. Cell Metab. 2014, 20, 967–977. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Mullican, S.E.; Tomaru, T.; Qatanani, M.; Schupp, M.; Lazar, M.A. Endoplasmic reticulum stress regulates adipocyte resistin expression. Diabetes 2009, 58, 1879–1886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rui, Y.; Tong, L.; Cheng, J.; Wang, G.; Qin, L.; Wan, Z. Rosmarinic acid suppresses adipogenesis, lipolysis in 3T3-L1 adipocytes, lipopolysaccharide-stimulated tumor necrosis factor-α secretion in macrophages, and inflammatory mediators in 3T3-L1 adipocytes. Food Nutr. Res. 2017, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Nagoor, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Arranz, S.; Carrión, S.; Subirana, I.; Muñoz-Aguayo, D.; Blanchart, G.; Kool, M.; Solà, R.; Motilva, M.J.; Escolà-Gil, J.C.; et al. A Functional Virgin Olive Oil Enriched with Olive Oil and Thyme Phenolic Compounds Improves the Expression of Cholesterol Efflux-Related Genes: A Randomized, Crossover, Controlled Trial. Nutrients 2019, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R.; Agarwal, A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am. J. Reprod. Immunol. 2019, 82, e13178. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Lancaster, G.I. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J. Physiol. 2016, 594, 267–279. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar] [CrossRef]
- Shao, M.; Ishibashi, J.; Kusminski, C.M.; Wang, Q.A.; Hepler, C.; Vishvanath, L.; MacPherson, K.A.; Spurgin, S.B.; Sun, K.; Holland, W.L.; et al. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab. 2016, 23, 1167–1184. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Rosmarinic acid attenuates obesity and obesity-related inflammation in human adipocytes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 149, 112002. [Google Scholar] [CrossRef] [PubMed]
- Kola, B.; Grossman, A.B.; Korbonits, M. The role of AMP-activated protein kinase in obesity. Front. Horm. Res. 2008, 36, 198–211. [Google Scholar] [PubMed]
- Jayanthy, G.; Roshana Devi, V.; Ilango, K.; Subramanian, S.P. Rosmarinic Acid Mediates Mitochondrial Biogenesis in Insulin Resistant Skeletal Muscle Through Activation of AMPK. J. Cell. Biochem. 2017, 118, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Vlavcheski, F.; Shamshoum, H.; Tsiani, E. Rosemary Extract as a Potential Anti-Hyperglycemic Agent: Current Evidence and Future Perspectives. Nutrients 2017, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Nyandwi, J.B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic Acid Exhibits a Lipid-Lowering Effect by Modulating the Expression of Reverse Cholesterol Transporters and Lipid Metabolism in High-Fat Diet-Fed Mice. Biomolecules 2021, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Kalis, C.; Kanzler, B.; Lembo, A.; Poltorak, A.; Galanos, C.; Freudenberg, M.A. Toll-like receptor 4 expression levels determine the degree of LPS-susceptibility in mice. Eur. J. Immunol. 2003, 33, 798–805. [Google Scholar] [CrossRef]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M., Jr.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 signaling in obese NAFLD. Am. J. Physiology. Gastrointest. Liver Physiol. 2015, 309, G270–G278. [Google Scholar] [CrossRef]
- Damms-Machado, A.; Louis, S.; Schnitzer, A.; Volynets, V.; Rings, A.; Basrai, M.; Bischoff, S.C. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017, 105, 127–135. [Google Scholar] [CrossRef]
- Nagpal, R.; Newman, T.M.; Wang, S.; Jain, S.; Lovato, J.F.; Yadav, H. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. J. Diabetes Res. 2018, 2018, 3462092. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Vezza, T.; Rodríguez-Nogales, A.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Garrido-Mesa, J.; Molina-Tijeras, J.A.; Romero, M.; Robles-Vera, I.; Pimentel-Moral, S.; et al. The prebiotic properties of Hibiscus sabdariffa extract contribute to the beneficial effects in diet-induced obesity in mice. Food Res. Int. 2020, 127, 108722. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, J.; Guo, Y.; Cui, H.; Lin, A.; Hu, B.; Gao, Q.; Chen, Y.; Liu, H. Improvement on metabolic syndrome in high fat diet-induced obese mice through modulation of gut microbiota by sangguayin decoction. J. Ethnopharmacol. 2020, 246, 112225. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T.; et al. Naringin Attenuates High Fat Diet Induced Non-alcoholic Fatty Liver Disease and Gut Bacterial Dysbiosis in Mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Jiménez, R.; Romero, M.; Sánchez, M.; Zarzuelo, M.J.; Gómez-Morales, M.; O’Valle, F.; López-Farré, A.J.; Algieri, F.; Gálvez, J.; et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension 2014, 64, 330–337. [Google Scholar] [CrossRef] [PubMed]


| Gen | Sequence 5′-3′ | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|
| Gapdh | F: CCATCACCATCTTCCAGGAG R: CCTGCTTCACCACCTTCTTG | 60 | NM_001289726.1 |
| Cebp-α | F: AAGGGTGTATGTAGTAGTGG R: AAAAAGAAGAGAAGGAAGCG | 55 | NM_001287523 |
| Fabp-4 | F: AGCATCATAACCCTAGATGG R: CCTTTCATAACACATTCCACC | 55 | MW286445 |
| Srebp-1 | F:CTGTGTGATCTACTTCTTGTG R: CATGTAGGAATACCCTCCTC | 55 | BC056922.1 |
| Tnf-α | F: AACTAGTGGTGCCAGCCGAT R: CTTCACAGAGCAATGACTCC | 60 | NM_001278601.1 |
| Il-6 | F: TAGTCCTTCCTACCCCAATTTCC R: TTGGTCCTTAGCCACTCCTTCC | 60 | NM_031168.2 |
| Jnk-1 | F: GATTTTGGACTGGCGAGGACT R: TAGCCCATGCCGAGAATGA | 60 | AY383616.1 |
| Jnk-2 | F: TTGTGCTGCTTTTGATACAGTTCTTGGG R: CTGGAAAGAGCTCTTCAAATTTGAT | 62 | NM_207692.2 |
| Ampk | F: GACTTCCTTCACAGCCTCATC R:CGCGCGACTATCAAAGACATACG | 60 | XM_036159053.1 |
| Glut-2 | F: TCAGAAGACAAGATCACCGGA R: GCTGGTGTGACTGTAAGTGGG | 59 | NM_031197.2 |
| Tlr-4 | F: GCCTTTCAGGGAATTAAGCTCC R: AGATCAACCGATGGACGTGTAA | 60 | NM_021297.3 |
| Muc-1 | F: GCAGTCCTCAGTGGCACCTC R: CACCGTGGGCTACTGGAGAG | 60 | NM_013605.2 |
| Muc-3 | F: CGTGGTCAACTGCGAGAATGG R: CGGCTCTATCTCTACGCTCTCC | 60 | NM_005960.1 |
| Occludin | F: ACGGACCCTGACCACTATGA R: TCAGCAGCAGCCATGTACTC | 56 | U49185.1 |
| Zo-1 | F: GGGGCCTACACTGATCAAGA R: TGGAGATGAGGCTTCTGCTT | 56 | D14340.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Malagón, A.J.; Rodríguez-Sojo, M.J.; Hidalgo-García, L.; Molina-Tijeras, J.A.; García, F.; Pischel, I.; Romero, M.; Duarte, J.; Diez-Echave, P.; Rodríguez-Cabezas, M.E.; et al. The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants 2022, 11, 1073. https://doi.org/10.3390/antiox11061073
Ruiz-Malagón AJ, Rodríguez-Sojo MJ, Hidalgo-García L, Molina-Tijeras JA, García F, Pischel I, Romero M, Duarte J, Diez-Echave P, Rodríguez-Cabezas ME, et al. The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants. 2022; 11(6):1073. https://doi.org/10.3390/antiox11061073
Chicago/Turabian StyleRuiz-Malagón, Antonio Jesús, María Jesús Rodríguez-Sojo, Laura Hidalgo-García, José Alberto Molina-Tijeras, Federico García, Ivo Pischel, Miguel Romero, Juan Duarte, Patricia Diez-Echave, María Elena Rodríguez-Cabezas, and et al. 2022. "The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice" Antioxidants 11, no. 6: 1073. https://doi.org/10.3390/antiox11061073
APA StyleRuiz-Malagón, A. J., Rodríguez-Sojo, M. J., Hidalgo-García, L., Molina-Tijeras, J. A., García, F., Pischel, I., Romero, M., Duarte, J., Diez-Echave, P., Rodríguez-Cabezas, M. E., Rodríguez-Nogales, A., & Gálvez, J. (2022). The Antioxidant Activity of Thymus serpyllum Extract Protects against the Inflammatory State and Modulates Gut Dysbiosis in Diet-Induced Obesity in Mice. Antioxidants, 11(6), 1073. https://doi.org/10.3390/antiox11061073













