Local and Systemic Oxidative Stress Biomarkers for Male Infertility: The ORION Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Parameters and Laboratory Procedures
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Cohort Demographics and Characteristics
3.2. Laboratory Results
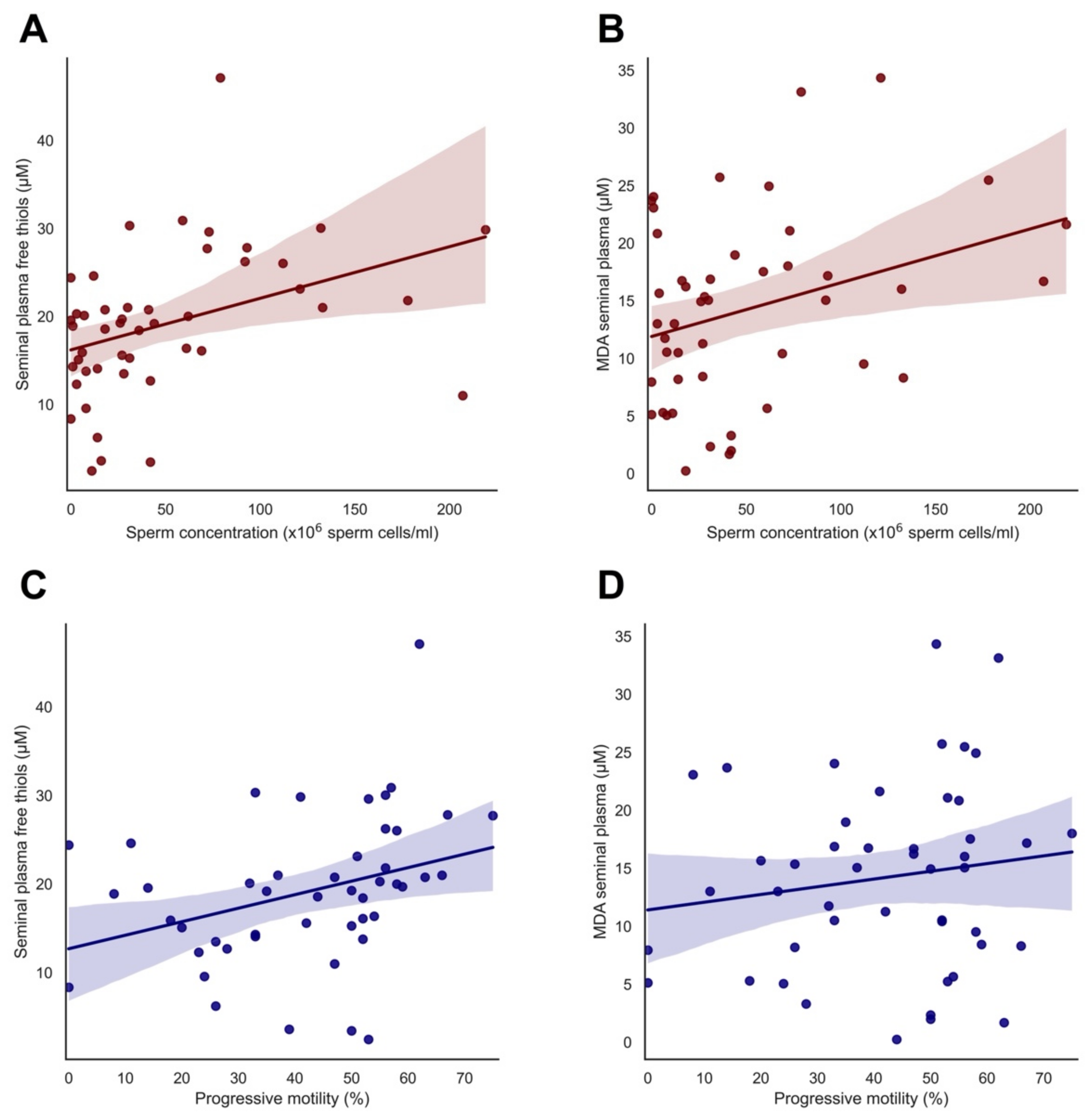
3.3. Significant Correlations of Free Thiol and MDA Levels with Semen Parameters
3.4. Systemic versus Local Oxidative Stress Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. International Classification of Diseases; 11th revision (ICD-11); World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Agarwal, A.; Baskaran, S.; Parekh, N. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Adonakis, G.; Kaponis, A.; Mamoulakis, C.; Takenaka, A.; Sofikitis, N. Pre-Testicular, Testicular, and Post-Testicular Causes of Male Infertility. In Endocrinology of the Testis and Male Reproduction; Simoni, M., Huhtaniemi, I., Eds.; Springer: Cham, Germany, 2017; pp. 981–1027. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.L.H.; Gadella, B.M.; Sutherland, J.M.; Nixon, B.; Bromfield, E.G. Male infertility: Shining a light on lipids and lipid-modulating enzymes in the male germline. J. Clin. Med. 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundhun, P.K.; Janoo, G.; Bhurtu, A.; Teeluck, A.R.; Soogund, M.Z.S.; Pursun, M.; Huang, F. Tobacco smoking and semen quality in infertile males: A systematic review and meta-analysis. BMC Public Health 2019, 19, 36. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The reactive species interactome: Evolutionary emergence, biological significance and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Mulgund, A.; Alshahrani, S.; Assidi, M.; Abuzenadah, A.M.; Sharma, R.; Sabanegh, E. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod. Biol. Endocrinol. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Fraczek, M.; Kurpisz, M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J. Androl. 2007, 28, 325–333. [Google Scholar] [CrossRef]
- Ritchie, C.; Ko, E.Y. Oxidative stress in the pathophysiology of male infertility. Andrologia 2021, 53, e13581. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Gabriëls, R.Y.; de Borst, M.H.; Bulthuis, M.L.C.; Faber, K.N.; van Goor, H.; Dijkstra, G. Serum free thiols are superior to fecal calprotectin in reflecting endoscopic disease activity in inflammatory bowel disease. Antioxidants 2019, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Eren, Y.; Dirik, E.; Neşelioğlu, S.; Erel, Ö. Oxidative stress and decreased thiol level in patients with migraine: Cross-sectional study. Acta Neurol. Belg. 2015, 115, 643–649. [Google Scholar] [CrossRef]
- Adamczyk-Sowa, M.; Bieszczad-Bedrejczuk, E.; Galiniak, S.; Rozmiłowska, I.; Czyżewski, D.; Bartosz, G.; Sadowska-Bartosz, I. Oxidative modifications of blood serum proteins in myasthenia gravis. J. Neuroimmunol. 2017, 305, 145–153. [Google Scholar] [CrossRef]
- Chen, S.S.; Chang, L.S.; Wei, Y.H. Oxidative damage to proteins and decrease of antioxidant capacity in patients with varicocele. Free Radic. Biol. Med. 2001, 30, 1328–1334. [Google Scholar] [CrossRef]
- Lewis, S.E.; Sterling, E.S.L.; Young, I.S.; Thompson, W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil. Steril. 1997, 67, 142–147. [Google Scholar] [CrossRef]
- Chen, S.S.; Huang, W.J.; Chang, L.S.; Wei, Y.H. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J. Urol. 2008, 179, 639–642. [Google Scholar] [CrossRef]
- Shiva, M.; Gautam, A.K.; Verma, Y.; Shivgotra, V.; Doshi, H.; Kumar, S. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin. Biochem. 2011, 44, 319–324. [Google Scholar] [CrossRef]
- Ottolenghi, S.; Rubino, F.M.; Sabbatini, G.; Coppola, S.; Veronese, A.; Chiumello, D.; Paroni, R. Oxidative stress markers to investigate the effects of hyperoxia in anasthesia. Int. J. Mol. Sci. 2019, 20, 5492. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Micheli, L.; Menchiari, A.; Moltoni, L.; Cerretani, D. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology 2015, 3, 280–286. [Google Scholar] [CrossRef]
- Micheli, L.; Collodel, G.; Cerretani, D.; Menchiari, A.; Noto, D.; Signorini, C.; Moretti, E. Relationships between ghrelin and obstatin with MDA, proinflammatory cytokines, GSH/GSSG ratio, catalase activity, and semen parameters in infertile patients with leukocytospermia and varicocele. Oxid. Med. Cell. Longev. 2019, 1. [Google Scholar] [CrossRef]
- Sexual and Reproductive Health and Research Team. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Barratt, C.L.R.; Björndahl, L.; Menkveld, R.; Mortimer, D. ESHRE special interest group for andrology basic semen analysis course: A continued focus on accuracy, quality, efficiency and clinical relevance. Hum. Reprod. 2011, 26, 3207–3212. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwell, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Alkan, I.; Simsek, F.; Haklar, G.; Kervancioglu, E.; Ozyeri, H.; Yalcin, S.; Akdas, A. Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility; relationship to seminal plasma antioxidants. J. Urol. 1997, 157, 140–143. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.K.; Gottschau, M.; Madsen, J.O.; Andersson, A.M.; Lassen, T.H.; Skakkebæk, N.E.; Swan, S.H.; Priskorn, L.; Juul, A.; Jørgensen, N. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open 2014, 4, e005462. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 328. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef] [PubMed]

| Total n = 50 | |
|---|---|
| Age (years) | 35.4 ± 4.9 |
| Duration of subfertility (months) | 15 [12.0–19.0] |
| BMI (kg/m2) | 25.2 ± 4.2 |
| BMI groups | |
| Underweight (BMI < 20) | 3 (6) |
| Healthy (BMI 20–25) | 25 (50) |
| Overweight (BMI 25–30) | 16 (32) |
| Obese (BMI ≥ 30) | 6 (12) |
| Educational level | |
| Low | 4 (8) |
| Average | 14 (28) |
| High | 32 (64) |
| Medication use | |
| Type of medication (1) | 10 (20) |
| Type of subfertility | |
| Primary | 35 (70) |
| Secondary | 15 (30) |
| SA type (2) | |
| Normozoospermia | 30 (60) |
| Oligozoospermia | 6 (12) |
| Asthenozoospermia | 2 (4) |
| Oligoasthenozoospermia | 12 (24) |
| Smoking | |
| Never | 23 (46) |
| Past | |
| 1–9 cigarettes/day | 7 (14) |
| ≥10 cigarettes/day | 10 (20) |
| Current | |
| 1–9 cigarettes/day | 4 (8) |
| ≥10 cigarettes/day | 8 (16) |
| Smoking years of past and present users | 10.0 [2.5–15.5] |
| Alcohol use | 40 (76.9) |
| Units per week | 3.3 [2.0–7.5] |
| Drug use | |
| Never | 30 (60) |
| Soft drugs | |
| Past | 19 (38) |
| Current | 5 (10) |
| Hard drugs | |
| Past | 8 (16) |
| Current | 0 |
| Exposure to toxic substances at work (3) | 13 (74) |
| Experience stress at work | 4 (8) |
| Warm baths 1 time per week | 6 (12) |
| Mean testicular volume (mL) (4) | 20.6 (11.2) |
| Total n = 50 | Low Seminal Plasma-Free Thiol Levels n = 22 | High Seminal Plasma-Free Thiol Levels n = 25 | p-Value | |
|---|---|---|---|---|
| Seminal plasma-free thiols (1) (µM) | 19.1 ± 8.4 | 12.5 ± 4.9 | 24.9 ± 6.2 | <0.001 * |
| Blood serum-free thiols (µmol/g) | 7.3 ± 0.6 | 7.3 ± 0.6 | 7.3 ± 0.6 | 0.923 |
| MDA seminal plasma (1) (µM) | 15.1 [8.2–19.0] | 10.5 [5.2–15.9] | 16.9 [12.4–21.4] | 0.007 * |
| MDA blood serum (µM) | 5.0 [4.2–7.4] | 4.7 [4.1–6.8] | 5.5 [4.4–9.9] | 0.090 |
| Period of abstinence (days) | 3.1 ± 1.3 | 3.4 ± 1.5 | 3.0 ± 1.0 | 0.334 |
| Sperm concentration (×106 sperm cells/mL) | 29.2 [7.4–72.3] | 15.2 [5.8–37.5] | 59.0 [21.8–102.5] | 0.010 * |
| Progressive motility (%) | 40.9 ± 18.4 | 35.2 ± 15.8 | 47.7 ± 18.4 | 0.017 * |
| Consistency of semen | 0.722 | |||
| Moderately mucous | 47 (94) | 21 (95.5) | 24 (96) | |
| Highly mucous | 3 (6) | 1 (4.5) | 1 (4) | |
| pH (1) | 7.6 ± 0.2 | 7.6 ± 0.1 | 7.6 ± 0.2 | 0.750 |
| Round cells (1) | 0.5 [0.2–1.0] | 0.6 [0.2–1.3] | 0.4 [0.2–1.0] | 0.666 |
| Total semen volume (mL) | 3.3 ± 1.6 | 3.3 ± 1.6 | 3.6 ± 1.5 | 0.587 |
| Albumin concentration in blood serum (g/L) | 47.0 [45.8–48.0] | 47.0 [45.0–48.0] | 47.0 [46.0–48.0] | 0.559 |
| Albumin concentration in seminal plasma (1) (g/L) | 0.7 [0.6–0.9] | 0.7 [0.6–0.8] | 0.7 [0.5–0.9] | 0.779 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergsma, A.T.; Li, H.T.; Eliveld, J.; Bulthuis, M.L.C.; Hoek, A.; van Goor, H.; Bourgonje, A.R.; Cantineau, A.E.P. Local and Systemic Oxidative Stress Biomarkers for Male Infertility: The ORION Study. Antioxidants 2022, 11, 1045. https://doi.org/10.3390/antiox11061045
Bergsma AT, Li HT, Eliveld J, Bulthuis MLC, Hoek A, van Goor H, Bourgonje AR, Cantineau AEP. Local and Systemic Oxidative Stress Biomarkers for Male Infertility: The ORION Study. Antioxidants. 2022; 11(6):1045. https://doi.org/10.3390/antiox11061045
Chicago/Turabian StyleBergsma, Anna T., Hui Ting Li, Jitske Eliveld, Marian L. C. Bulthuis, Annemieke Hoek, Harry van Goor, Arno R. Bourgonje, and Astrid E. P. Cantineau. 2022. "Local and Systemic Oxidative Stress Biomarkers for Male Infertility: The ORION Study" Antioxidants 11, no. 6: 1045. https://doi.org/10.3390/antiox11061045
APA StyleBergsma, A. T., Li, H. T., Eliveld, J., Bulthuis, M. L. C., Hoek, A., van Goor, H., Bourgonje, A. R., & Cantineau, A. E. P. (2022). Local and Systemic Oxidative Stress Biomarkers for Male Infertility: The ORION Study. Antioxidants, 11(6), 1045. https://doi.org/10.3390/antiox11061045







