Peroxygenase-Catalyzed Selective Synthesis of Calcitriol Starting from Alfacalcidol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Purification of AaeUPO
2.3. Enzymatic Calcitriol Synthesis from Alfacalcidol and Vitamin D3
2.4. Enzyme Activity Assays
2.5. HPLC Analysis
2.6. LC–MS Analysis
2.7. Docking
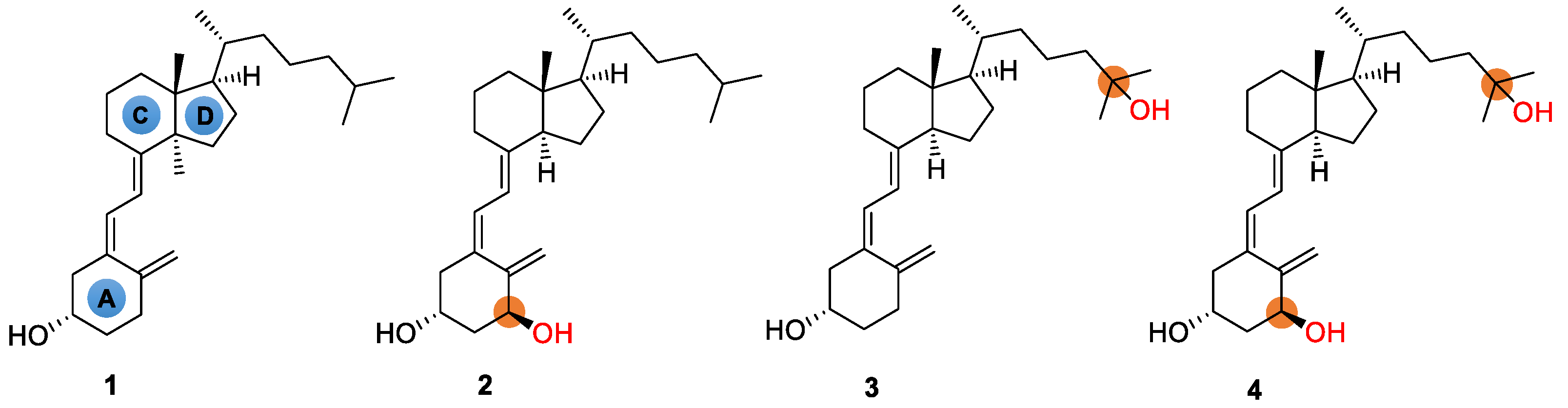
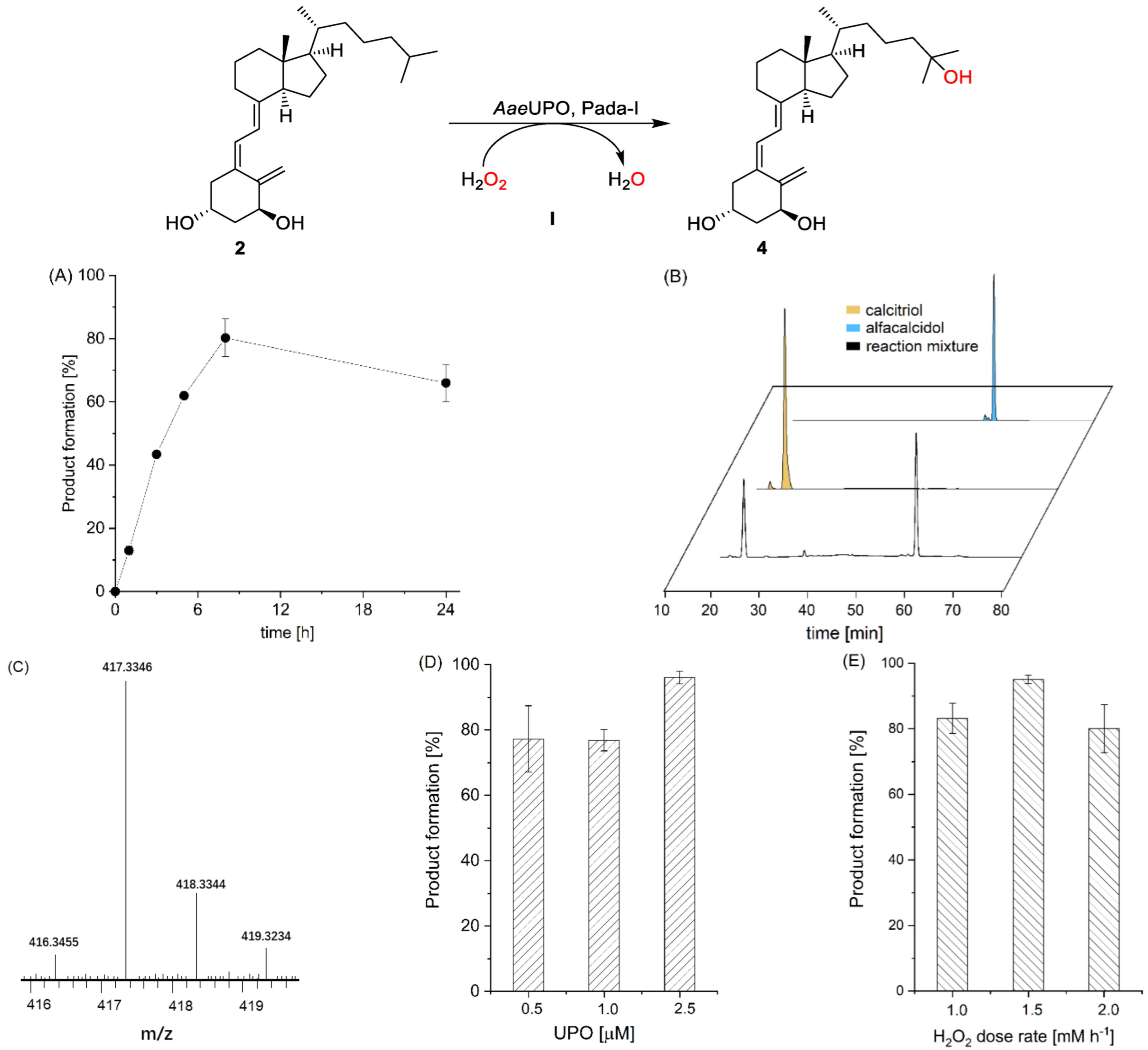
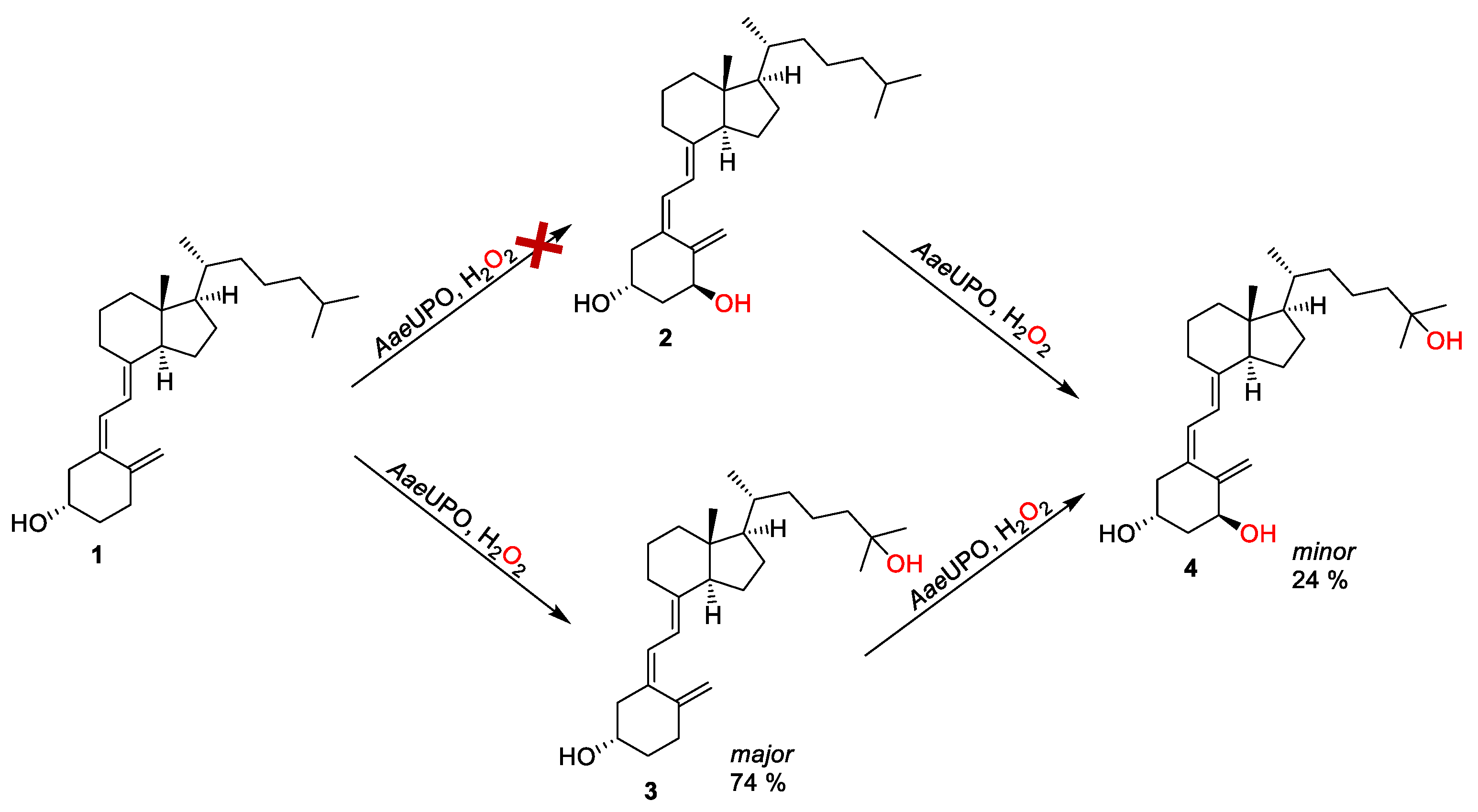
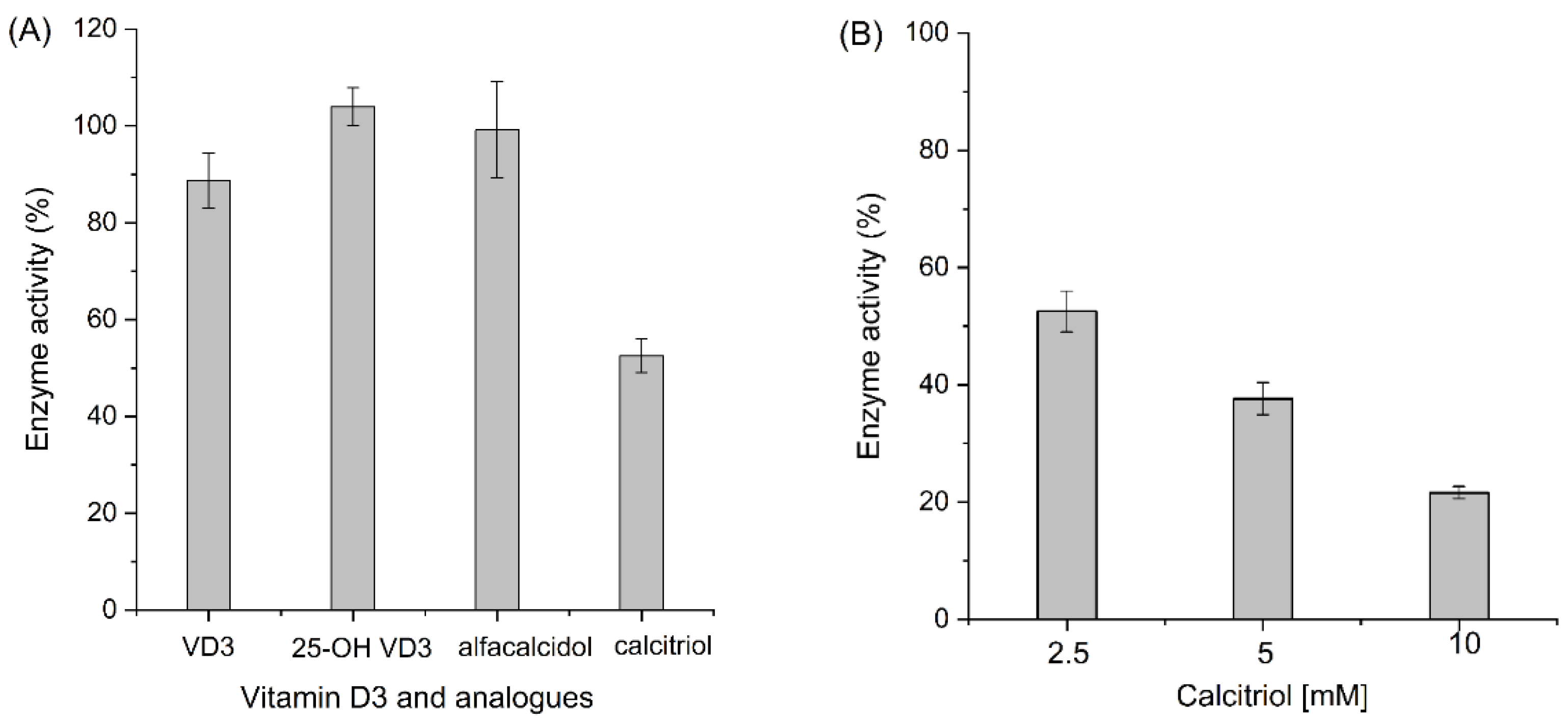
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLuca, H.F. Vitamin D: Historical Overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Nickolas, T.L.; Denburg, M.; Yarlagadda, S.; Weiner, D.E.; Gutiérrez, O.M.; Bansal, V.; Rosas, S.E.; Nigwekar, S.; Yee, J.; et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am. J. Kidney. Dis. 2017, 70, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmura, K.; Kato, M.; Watanabe, T.; Oku, K.; Bohgaki, T.; Horita, T.; Yasuda, S.; Ito, Y.M.; Sato, N.; Atsumi, T. Effect of combined treatment with bisphosphonate and vitamin D on atherosclerosis in patients with systemic lupus erythematosus: A propensity score-based analysis. Arthritis Res. Ther. 2018, 20, 72. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Tang, Z.; Slominski, A.T.; Li, W.; Żmijewski, M.A.; Liu, Y.; Chen, J. Vitamin D and its analogs as anticancer and anti-inflammatory agents. Eur. J. Med. Chem. 2020, 207, 112738. [Google Scholar] [CrossRef] [PubMed]
- Slatopolsky, E.; Dusso, A.; Brown, A. New analogs of vitamin D3. Kidney Int. 1999, 73, S46–S51. [Google Scholar] [CrossRef] [Green Version]
- Takata, S. Active vitamin D3 analog. Nihon Rinsho. 2015, 73, 1701–1705. [Google Scholar]
- Jones, G. Historical aspects of vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef]
- Nagata, A.; Akagi, Y.; Masoud, S.S.; Yamanaka, M.; Kittaka, A.; Uesugi, M.; Odagi, M.; Nagasawa, K. Stereoselective Synthesis of Four Calcitriol Lactone Diastereomers at C23 and C25. J. Org. Chem. 2019, 84, 7630–7641. [Google Scholar] [CrossRef]
- Gesmundo, I.; Silvagno, F.; Banfi, D.; Monica, V.; Fanciulli, A.; Gamba, G.; Congiusta, N.; Libener, R.; Riganti, C.; Ghigo, E.; et al. Calcitriol Inhibits Viability and Proliferation in Human Malignant Pleural Mesothelioma. Cells 2020, 11, 720. [Google Scholar] [CrossRef]
- López-Pérez, B.; Maestro, M.A.; Mouriño, A. Total synthesis of 1α,25-dihydroxyvitamin D(3) analogs modified at the side chain and D-ring. Org. Biomol. Chem. 2018, 16, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, B.; Maestro, M.A.; Mouriño, A. Total synthesis of 1α,25-dihydroxyvitamin D(3) (calcitriol) through a Si-assisted allylic substitution. Chem. Commun. 2017, 53, 8144–8147. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Püllmann, P.; Zhang, W.; Weissenborn, M.J. Enzymatic Hydroxylations of sp3-Carbons. ACS Catal. 2021, 11, 9168–9203. [Google Scholar] [CrossRef]
- Dong, J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic Oxidation Reactions: A Chemist’s Perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, J.; Qiu, X.; Jiao, N. Homogeneous Oxygenase Catalysis. Chem. Rev. 2018, 118, 4912–4945. [Google Scholar] [CrossRef]
- Fu, B.; Ren, Q.; Ma, J.; Chen, Q.; Zhang, Q.; Yu, P. Enhancing the production of physiologically active vitamin D(3) by engineering the hydroxylase CYP105A1 and the electron transport chain. World J. Microbiol. Biotechnol. 2021, 38, 14. [Google Scholar] [CrossRef]
- Gottfried, E.; Rehli, M.; Hahn, J.; Holler, E.; Andreesen, R.; Kreutz, M. Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite. Biochem. Biophys. Res. Commun. 2006, 349, 209–213. [Google Scholar] [CrossRef]
- Yasutake, Y.; Kameda, T.; Tamura, T. Structural insights into the mechanism of the drastic changes in enzymatic activity of the cytochrome P450 vitamin D(3) hydroxylase (CYP107BR1) caused by a mutation distant from the active site. Acta Cryst. F 2017, 73, 266–275. [Google Scholar] [CrossRef]
- Gilardi, G.; Di Nardo, G. Heme iron centers in cytochrome P450: Structure and catalytic activity. Rend. Fis. Acc. Lincei. 2017, 28, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Hofrichter, M.; Kellner, H.; Herzog, R.; Karich, A.; Kiebist, J.; Scheibner, K.; Ullrich, R. Peroxide-Mediated Oxygenation of Organic Compounds by Fungal Peroxygenases. Antioxidants 2022, 11, 163. [Google Scholar] [CrossRef]
- Bormann, S.; Kellner, H.; Hermes, J.; Herzog, R.; Ullrich, R.; Liers, C.; Ulber, R.; Hofrichter, M.; Holtmann, D. Broadening the Biocatalytic Toolbox—Screening and Expression of New Unspecific Peroxygenases. Antioxidants 2022, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- González-Benjumea, A.; Linde, D.; Carro, J.; Ullrich, R.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Regioselective and Stereoselective Epoxidation of n-3 and n-6 Fatty Acids by Fungal Peroxygenases. Antioxidants 2021, 10, 1888. [Google Scholar] [CrossRef] [PubMed]
- Hobisch, M.; Holtmann, D.; Gomez de Santos, P.; Alcalde, M.; Hollmann, F.; Kara, S. Recent developments in the use of peroxygenases—Exploring their high potential in selective oxyfunctionalisations. Biotechnol. Adv. 2021, 51, 107615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Lan, D.M.; Durrani, R.; Hollmann, F. Peroxygenases en route to becoming dream catalysts. What are the opportunities and challenges? Curr. Opin. Chem. Biol. 2017, 37, 1–9. [Google Scholar] [CrossRef]
- Babot, E.D.; Aranda, C.; Kiebist, J.; Scheibner, K.; Ullrich, R.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Enzymatic Epoxidation of Long-Chain Terminal Alkenes by Fungal Peroxygenases. Antioxidants 2022, 11, 522. [Google Scholar] [CrossRef]
- Biondi, D.M.; Sanfilippo, C.; Patti, A. Stereospecific Epoxidation of Limonene Catalyzed by Peroxygenase from Oat Seeds. Antioxidants 2021, 10, 1462. [Google Scholar] [CrossRef]
- Tonin, F.; Tieves, F.; Willot, S.; van Troost, A.; van Oosten, R.; Breestraat, S.; van Pelt, S.; Alcalde, M.; Hollmann, F. Pilot-Scale Production of Peroxygenase from Agrocybe aegerita. Org. Process Res. Dev. 2021, 25, 1414–1418. [Google Scholar] [CrossRef]
- Kinner, A.; Rosenthal, K.; Lütz, S. Identification and Expression of New Unspecific Peroxygenases—Recent Advances, Challenges and Opportunities. Front. Bioeng. Biotech. 2021, 9, 705630. [Google Scholar] [CrossRef]
- Aranda, C.; Carro, J.; González-Benjumea, A.; Babot, E.D.; Olmedo, A.; Linde, D.; Martínez, A.T.; Gutiérrez, A. Advances in enzymatic oxyfunctionalization of aliphatic compounds. Biotechnol. Adv. 2021, 51, 107703. [Google Scholar] [CrossRef]
- Lucas, F.; Babot, E.D.; Cañellas, M.; del Río, J.C.; Kalum, L.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Martínez, A.T.; Gutiérrez, A. Molecular determinants for selective C25-hydroxylation of vitamins D2 and D3 by fungal peroxygenases. Catal. Sci. Technol. 2016, 6, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Babot, E.D.; del Río, J.C.; Kalum, L.; Martínez, A.T.; Gutiérrez, A. Regioselective Hydroxylation in the Production of 25-Hydroxyvitamin D by Coprinopsis cinerea Peroxygenase. ChemCatChem 2015, 7, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Molina-Espeja, P.; Ma, S.; Mate, D.M.; Ludwig, R.; Alcalde, M. Tandem-yeast expression system for engineering and producing unspecific peroxygenase. Enzyme Microb. Technol. 2015, 73–74, 29–33. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System, Version 2.5; Schrödinger, LLC: New York, NY, USA, 2021.
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, B.; Sun, Z.; Zhang, W. C–H bond functionalization reactions enabled by photobiocatalytic cascades. Green Synth. Catal. 2021, 2, 267–274. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; van Schie, M.M.C.H.; Hagedoorn, P.-L.; Alcalde, M.; Denkova, A.G.; Djanashvili, K.; Hollmann, F. Nuclear Waste and Biocatalysis: A Sustainable Liaison? ACS Catal. 2020, 10, 14195–14200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Mahor, D.; Fei, Q.; Wever, R.; Alcalde, M.; Zhang, W.; Hollmann, F. Water-Soluble Anthraquinone Photocatalysts Enable Methanol-Driven Enzymatic Halogenation and Hydroxylation Reactions. ACS Catal. 2020, 10, 8277–8284. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, J.; Tieves, F.; Zhang, W.; Alcalde, M.; Hollmann, F.; Park, C.B. Piezobiocatalysis: Ultrasound-Driven Enzymatic Oxyfunctionalization of C–H Bonds. ACS Catal. 2020, 10, 5236–5242. [Google Scholar] [CrossRef]
- Bormann, S.; van Schie, M.M.C.H.; De Almeida, T.P.; Zhang, W.; Stöckl, M.; Ulber, R.; Hollmann, F.; Holtmann, D. H2O2 Production at Low Overpotentials for Electroenzymatic Halogenation Reactions. ChemSusChem 2019, 12, 4759–4763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Fernández-Fueyo, E.; Ni, Y.; van Schie, M.; Gacs, J.; Renirie, R.; Wever, R.; Mutti, F.G.; Rother, D.; Alcalde, M.; et al. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalizations. Nat. Catal. 2018, 1, 55–62. [Google Scholar] [CrossRef]
- Zhang, W.; Burek, B.O.; Fernández-Fueyo, E.; Alcalde, M.; Bloh, J.Z.; Hollmann, F. Selective Activation of C−H Bonds in a Cascade Process Combining Photochemistry and Biocatalysis. Angew. Chem. Int. Ed. 2017, 56, 15451–15455. [Google Scholar] [CrossRef]
- Freakley, S.J.; Kochius, S.; van Marwijk, J.; Fenner, C.; Lewis, R.J.; Baldenius, K.; Marais, S.S.; Opperman, D.J.; Harrison, S.T.L.; Alcalde, M.; et al. A chemo-enzymatic oxidation cascade to activate C–H bonds with in situ generated H2O2. Nat. Commun. 2019, 10, 4178. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, P.; Sun, Z.; Li, H.; Ge, R.; Sheng, X.; Zhang, W. Peroxygenase-Catalyzed Selective Synthesis of Calcitriol Starting from Alfacalcidol. Antioxidants 2022, 11, 1044. https://doi.org/10.3390/antiox11061044
Li Y, Zhang P, Sun Z, Li H, Ge R, Sheng X, Zhang W. Peroxygenase-Catalyzed Selective Synthesis of Calcitriol Starting from Alfacalcidol. Antioxidants. 2022; 11(6):1044. https://doi.org/10.3390/antiox11061044
Chicago/Turabian StyleLi, Yuanying, Pengpeng Zhang, Zhoutong Sun, Huanhuan Li, Ran Ge, Xiang Sheng, and Wuyuan Zhang. 2022. "Peroxygenase-Catalyzed Selective Synthesis of Calcitriol Starting from Alfacalcidol" Antioxidants 11, no. 6: 1044. https://doi.org/10.3390/antiox11061044
APA StyleLi, Y., Zhang, P., Sun, Z., Li, H., Ge, R., Sheng, X., & Zhang, W. (2022). Peroxygenase-Catalyzed Selective Synthesis of Calcitriol Starting from Alfacalcidol. Antioxidants, 11(6), 1044. https://doi.org/10.3390/antiox11061044





