Abstract
The critical factors for regulating cancer metabolism are oxidative stress and phosphoinositide-3-kinase/AKT serine-threonine kinase/mechanistic target of the rapamycin kinase (PI3K/AKT/mTOR). However, the metabolic impacts of oxidative stress and PI3K/AKT/mTOR on individual mechanisms such as glycolysis (Warburg effect), pentose phosphate pathway (PPP), fatty acid synthesis, tricarboxylic acid cycle (TCA) cycle, glutaminolysis, and oxidative phosphorylation (OXPHOS) are complicated. Therefore, this review summarizes the individual and interacting functions of oxidative stress and PI3K/AKT/mTOR on metabolism. Moreover, natural products providing oxidative stress and PI3K/AKT/mTOR modulating effects have anticancer potential. Using the example of brown algae-derived fucoidan, the roles of oxidative stress and PI3K/AKT/mTOR were summarized, although their potential functions within diverse metabolisms were rarely investigated. We propose a potential application that fucoidan may regulate oxidative stress and PI3K/AKT/mTOR signaling to modulate their associated metabolic regulations. This review sheds light on understanding the impacts of oxidative stress and PI3K/AKT/mTOR on metabolism and the future direction of metabolism-based cancer therapy of fucoidan.
1. Introduction
Diverse metabolisms are essential for cancer cell proliferation by regulating redox homeostasis, energy, and biosynthesis [1,2,3,4]. Several factors that modulate metabolism may improve the anticancer therapeutic effects. Oxidative stress and phosphoinositide-3-kinase/AKT serine-threonine kinase/mechanistic target of the rapamycin kinase (PI3K/AKT/mTOR) are important in regulating several kinds of metabolisms in cancer cells [5,6,7,8,9,10].
In this review, we focus on glycolysis (Warburg effect), pentose phosphate pathway (PPP), fatty acid metabolism, tricarboxylic acid cycle (TCA) cycle, glutaminolysis, and oxidative phosphorylation (OXPHOS). The relationship and interaction between oxidative stress, PI3K/AKT/mTOR, and associated metabolisms are summarized.
Several natural products show differential responses to cancer and normal cells and cause selective killing effects on cancer cells [11,12,13,14]. These drug-induced selective killing effects are associated with elevated oxidative stress generation in cancer cells compared to normal cells. Moreover, the PI3K-AKT-mTOR pathway exhibits a diverse function for regulating proliferation, metabolism, and metastasis [15,16,17,18,19]. Several cancer cells show higher expressions of AKT than normal cells [20,21,22,23,24], suggesting that PI3K-AKT-mTOR may have differential responses between cancer and normal cells. Accordingly, natural products with oxidative stress or PI3K-AKT-mTOR modulating ability are expected to provide potent anticancer candidates.
Fucoidan, a brown alga-derived polysaccharide, is a safe food supplement with suitable nutraceutical characteristics [25]. Recently, several chemopreventive and antiproliferation effects of fucoidan were reported [25], but their mechanisms and connections to metabolism have not been fully investigated yet. The functions of oxidative stress and PI3K/AKT/mTOR in fucoidan are summarized here. Although their impacts on metabolism remain unclear, the potential application of fucoidan-modulated metabolism is discussed.
This review aims to illustrate existing knowledge of individual (Section 2 and Section 3) and interacting (Section 4) effects of oxidative stress and PI3K-AKT-mTOR as well as their impact on fucoidan treatments (Section 5). We also hypothesize that these metabolic regulations may act on fucoidan treatment (Section 6). Finally, we provide a novel rationale that oxidative stress and PI3K/AKT/mTOR signaling may play a vital role in metabolism-related cancer therapy using fucoidan.
2. Oxidative Stress and Its Associated Metabolisms
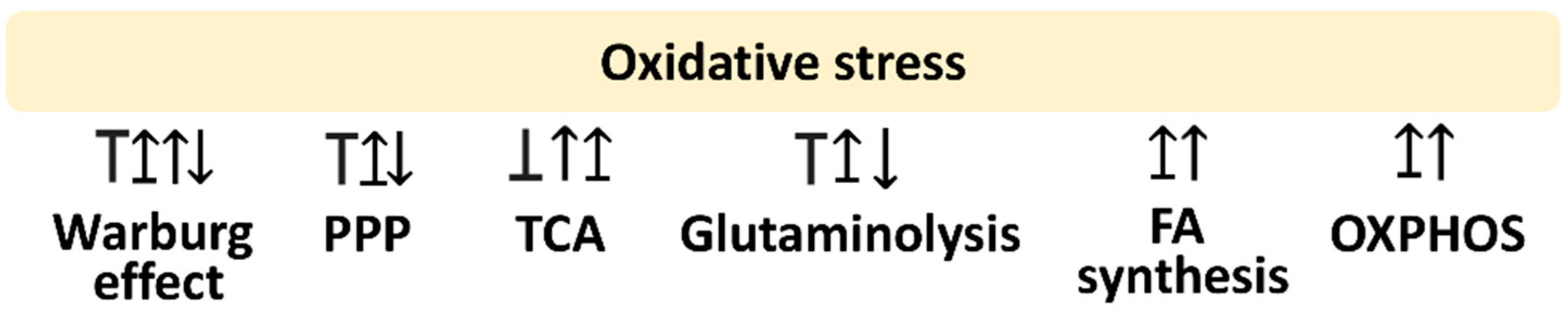
Oxidative stress is a modulator for metabolism. This review is mainly concerned with glycolysis, PPP, fatty acid synthesis, TCA cycle, glutaminolysis, and OXPHOS. The following sections provide the impact of oxidative stress on regulating these metabolisms, which is summarized in Figure 1.
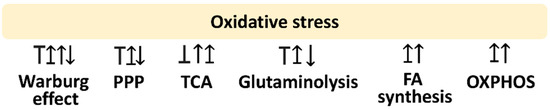
Figure 1.
Oxidative stress and its associated metabolisms. Arrow indicates activation; T indicates inhibition; T with arrow indicates inhibition leading to activation. Abbreviations: PPP, pentose phosphate pathway; TCA, tricarboxylic acid cycle; FA, fatty acid; OXPHOS, oxidative phosphorylation. These effects are summarized in the reports mentioned in Section 2.1, Section 2.2, Section 2.3, Section 2.4, Section 2.5, Section 2.6. Different studies reported differential regulations to these metabolisms by modulating oxidative stress. Various reports show different responses to oxidative stress for the same metabolism.
2.1. Relationship between the Warburg Effect and Oxidative Stress
Cancer cells prefer aerobic glycolysis for lactate production (namely Warburg effect) over oxidative phosphorylation because lactate generates several metabolites for supporting cancer cell proliferation [26]. Moreover, cancer cells take up more glucose by upregulating glucose transporting receptors such as glucose transporter (GLUT) [27].
The Warburg effect shows the crosstalk relationship with oxidative stress. Oxidative stress may activate the Warburg effect, while the Warburg effect may activate or inactivate oxidative stress. Several reports concerning these relationships were described as follows (Figure 1).
2.1.1. Warburg Effect May Inhibit Oxidative Stress
The Warburg effect may benefit cancer cell proliferation by reducing cytotoxic oxidative stress generated from aerobic respiration [5]. This oxidative stress-reducing ability is proposed to inhibit the energy dependence on mitochondrial OXPHOS, the main pool of oxidative stress. The end product of glycolysis is pyruvate; it converts to acetyl-CoA to enter the TCA cycle and processes OXPHOS. In comparison, pyruvate is converted to lactate by the Warburg effect and bypasses OXPHOS, decreasing oxidative stress [6].
In the case of Warburg effect inhibition, OXPHOS is activated. By the example of glycolysis inhibition by targeting glucose-6-phosphate isomerase (GPI; glucose-6-phosphate → fructose-6-phosphate), the energy flow is mainly attributed to OXPHOS [28]. Dichloroacetate (DCA), a mitochondrial pyruvate dehydrogenase kinase 1 (PDK1) inhibitor, also shows similar results in inhibiting the Warburg effect and switching to OXPHOS [29]. Subsequently, OXPHOS produces more oxidative stress and induces apoptosis in cancer cells [30]. Accordingly, the Warburg effect can inhibit oxidative stress-associated responses such as apoptosis of cancer cells. These results emphasize the rationale that suppressing the Warburg effect and switching to OXPHOS offers oxidative stress to inhibit the proliferation of cancer cells [31] (Figure 1).
2.1.2. The Warburg Effect May Induce Oxidative Stress
In normal cells, glycolysis generates pyruvate to enter the TCA cycle, producing several antioxidant metabolites such as citrate, malate, and oxaloacetate. Moreover, fumarate, one of the TCA metabolites, can enhance NFE2-related factor 2 (NRF2) antioxidant signaling [32]. During this processing, NADH is generated, and it is converted to nicotinamide adenine dinucleotide phosphate (NADPH) by nicotinamide nucleotide transhydrogenase (NNT). Subsequently, NADPH is converted to the antioxidant glutathione (GSH) by the catalyzation of glutathione reductase (GSR) [32]. Accordingly, these signaling pathways contribute to the antioxidant potential of normal cells.
In contrast, cancer cells skip the TCA and OXPHOS pathways but favor the Warburg effect to downregulate antioxidant signaling [32]. As a result, cancer cells may show high oxidative stress. Therefore, the Warburg effect induces oxidative stress in cancer cells (Figure 1).
2.1.3. Oxidative Stress May Induce the Warburg Effect
Cancer cells may show adaptation to high oxidative stress. In response to oxidative stress, the Warburg effect of cancer cells is activated [6]. The oxidative stress may be induced by downregulating antioxidant signaling such as AMP-activated protein kinase (AMPK)-responsive antioxidant response [33]. Mitochondrial reactive oxygen species (ROS) may influence the Warburg effect of AMPK-defective cancer cells [34]. Therefore, the ROS-modulating ability on the Warburg effect shows a bidirectional regulation (Figure 1).
2.2. Relationship between the PPP and Oxidative Stress
In addition to activating the Warburg effect, high oxidative stress of cancer cells may exhibit an alternative choice. When oxidative stress affects cancer cells, the Warburg effect is initially induced, and then it switches to the pentose phosphate pathway (PPP) if the oxidative stress is prolonged. Consequently, the NADPH generated from PPP scavenges ROS and reduces oxidative stress [6]. PPP generates NADPH to mitigate oxidative stress mainly derived from oxidative phosphorylation [35], but cancer cells still maintain high non-toxic oxidative stress for their malignant proliferation [6,36].
In the example of thyroid cancer cells, metabolomic results show high expressions of the PPP signaling pathway [37]. Inhibitors for the key enzymes of PPP (glucose-6-phosphate dehydrogenase (G6PD) and transketolase), such as 6-aminonicotinamide and oxythiamine, exhibit antiproliferation, accompanied by inducing ROS, apoptosis, and endoplasmatic reticulum stress [37]. Accordingly, PPP exhibits an oxidative stress-suppressing function in cancer cells. Targeting PPP causes oxidative stress to kill cancer cells.
In contrast, PPP may induce oxidative stress generation of drug treatment. The example of itaconic acid shows that PPP induction enhances oxidative stress and suppresses inflammation and bacterial growth [38]. Therefore, the ROS-modulating effects on PPP demonstrate a complex regulation (Figure 1).
2.3. Relationship between the TCA Cycle and Oxidative Stress
The TCA cycle exhibits oxidative stress-modulating functions. TCA cycle enzymes are sensitive to ROS [39]. ROS mainly targets aconitase and α-ketoglutarate dehydrogenase (oxoglutarate dehydrogenase; OGDH) to inhibit the TCA cycle [40]. ROS can suppress aconitase to pause α-ketoglutarate generation [41]. ROS can also inactivate OGDH to shut down the TCA cycle [40]. In contrast, malate dehydrogenase (MDH) inhibition induces ROS generation in breast cancer cells kept under hypoxia [42].
Moreover, oxidative stress regulation on the TCA cycle was demonstrated in breast cancer cells and tumor tissues exhibiting low aconitase 2 (ACO2) [43]. ACO2-overexpression causes antiproliferation to breast cancer cells [43], accompanied by a decreasing lactate level, increasing acetyl-CoA level, activating citrate synthase (CS), rising levels of TCA cycle metabolites for citrate, α-ketoglutarate, fumarate, and inducing mitochondrial superoxide [43]. Therefore, the ROS-modulating effects on the TCA cycle are complex and show a complex regulation (Figure 1).
2.4. Relationship between Glutaminolysis and Oxidative Stress
Glutaminolysis is a supporting step for the anabolic pathway to replenish the TCA metabolite α-ketoglutarate by converting glutamine to glutamate and becoming α-ketoglutarate, which is catalyzed by glutaminase (GLS) and glutamate dehydrogenase (GLUD1, GDH1) [44,45].
The function of glutaminolysis is to maintain redox homeostasis [46] by participating in antioxidant production to reduce oxidative stress [44,47,48]. Glutaminolysis provides several reducing powers such as reduced glutathione (GSH), NADPH, and α-ketoglutarate and decreases oxidative stress [46]. Moreover, glutaminolysis-derived α-ketoglutarate can run the TCA cycle and become fumarate, which controls oxidative stress scavenging enzymes such as glutathione peroxidase 1 (GPX1) and NRF2 signaling [46].
Inhibiting glutaminolysis enhances oxidative stress in combined treatment for cancer [49]. In contrast, in the Th17-skewing test, rosiglitazone and pioglitazone inhibit glutaminolysis but not glycolysis, decreasing GSH level and increasing ROS generation [49]. Additionally, oxidative stress may enhance glutaminolysis [41] to synthesize GSH to reduce oxidative stress. Therefore, glutaminolysis and oxidative stress provide a reciprocal regulation for each other (Figure 1).
2.5. Relationship between Fatty Acid Metabolism and Oxidative Stress
Modulating fatty acid metabolism can regulate oxidative stress. Mitochondrial fatty acid oxidation, a non-electron transfer chain (ETC) reaction, is accompanied by inducing oxidative stress [50,51]. Overexpressing acetyl-CoA carboxylase 1 (ACC1), a priming enzyme for fatty acid synthesis, shows antiproliferation and oxidative stress induction in a primary bone marrow culture [52]. In contrast, inhibiting ACC1 causes NADPH accumulation and decreases oxidative stress.
Different fatty acid metabolic enzymes have different responses or effects on oxidative stress. Inhibition of fatty acid synthase (FASN) stimulates oxidative stress to cause antiproliferation of breast cancer cells [51]. Inhibiting fatty acid transport protein 2 (FATP2) induces lipid production, decreases oxidative stress, and inhibits cancer stem cell proliferation [53]. Therefore, fatty acid metabolism and oxidative stress are related to reciprocal regulation (Figure 1).
2.6. Relationship between OXPHOS and Oxidative Stress
During OXPHOS, electron transfer occurs in the ETC, but it is usually accompanied by the leakage of mitochondrial superoxide, which is the main pool for oxidative stress in many cell types [54]. Inhibition of OXPHOS induces more oxidative stress attributed to electron accumulation in ETC, causing electron leakage, ROS production [55,56,57,58], and ATP depletion [59]. Examples of ETC inhibitors (rotenone, antimycin A, and carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP)) show ROS and mitochondrial superoxide generation [60]. Therefore, OXPHOS and oxidative stress offer reciprocal regulation between each other (Figure 1).
3. PI3K/AKT/mTOR and Its Associated Metabolisms
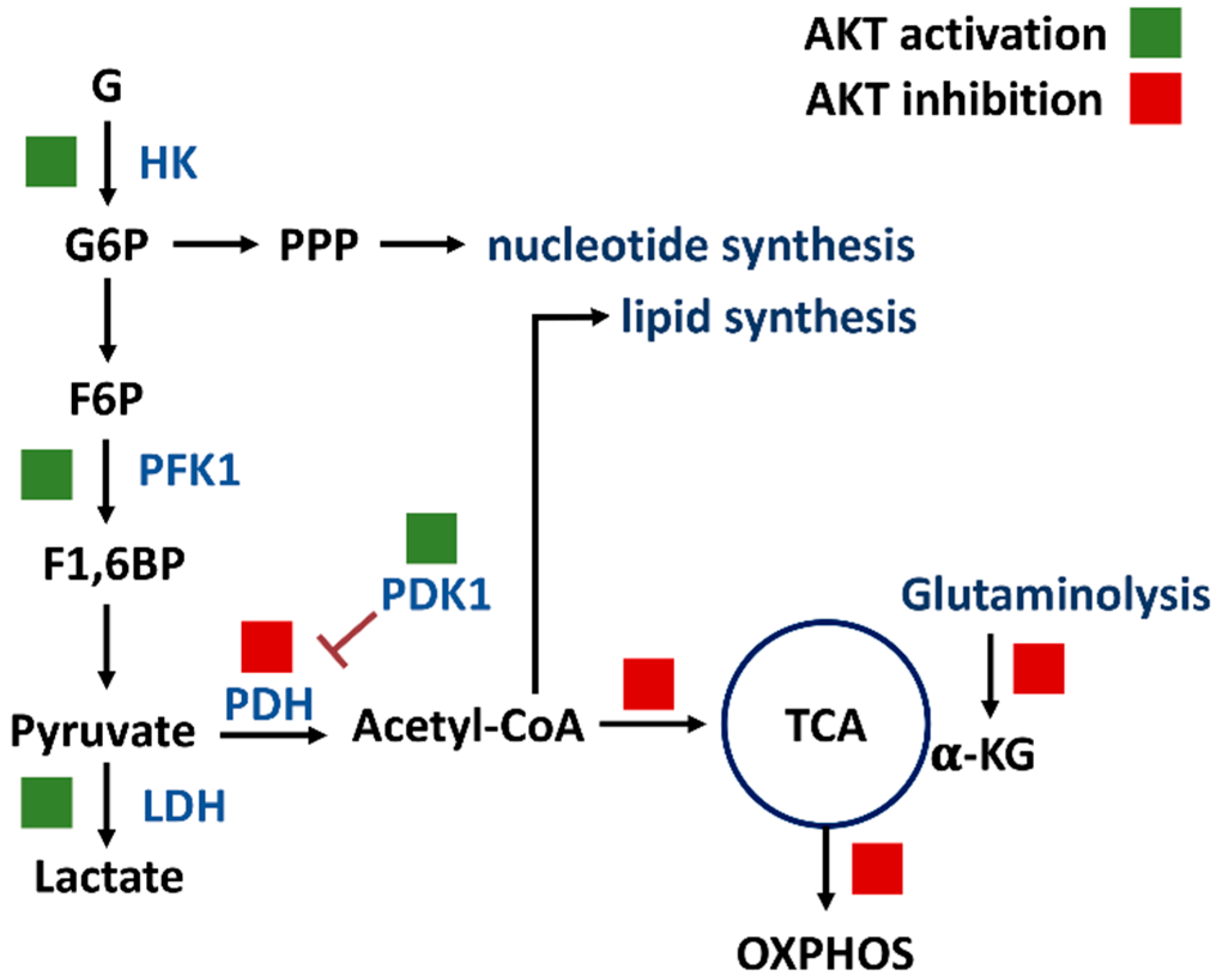
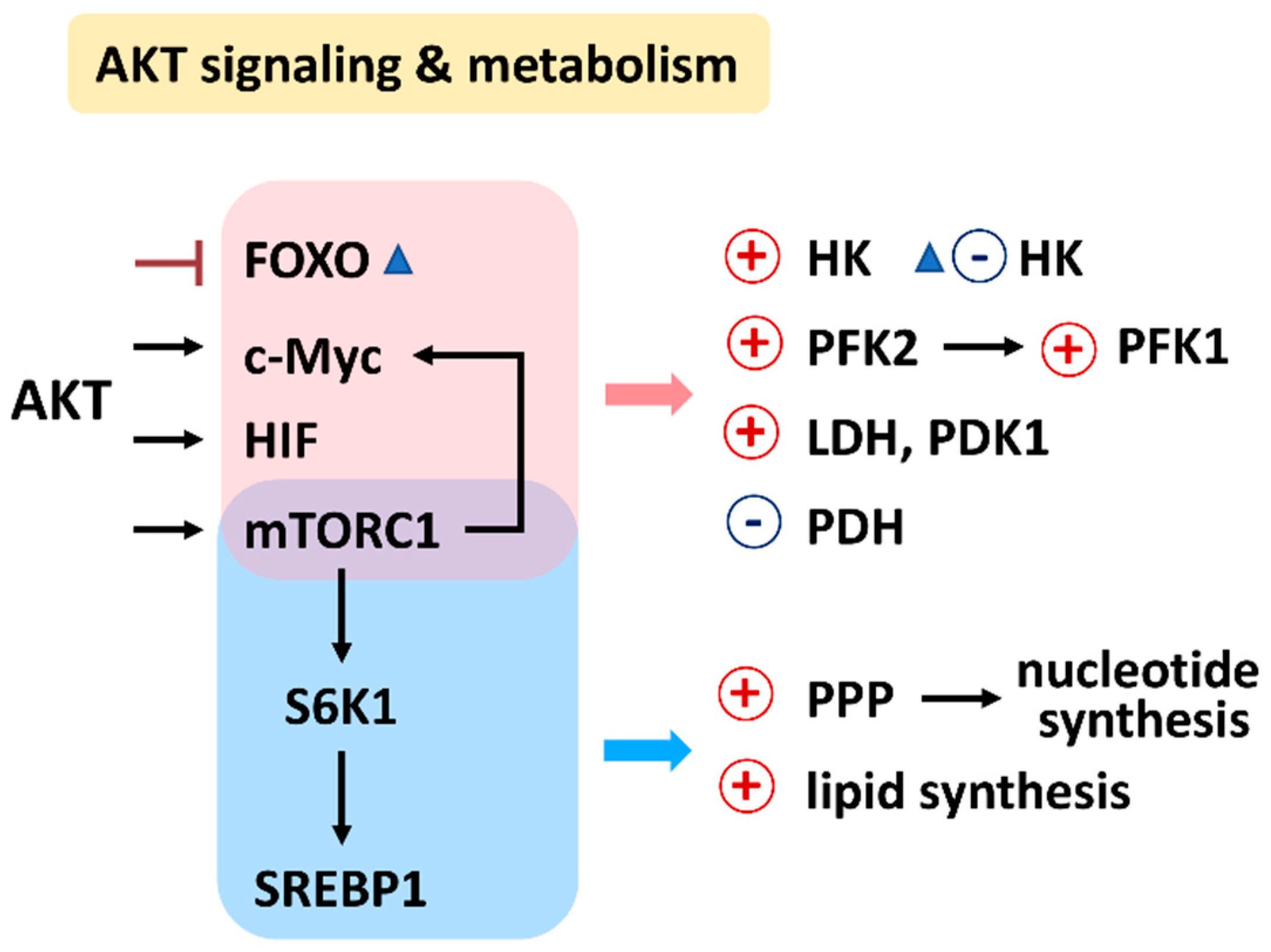
PI3K/AKT/mTOR signaling regulates a group of metabolisms such as glycolysis, PPP, nucleotide synthesis, lipid synthesis, TCA cycle, glutaminolysis, and OXPHOS (Figure 2) [7]. Moreover, the downstream effectors of the PI3K/AKT/mTOR, such as forkhead box transcription factors (FOXO), c-Myc, hypoxia-inducible factor (HIF), mechanistic target of rapamycin complex 1 (mTORC1), mTOR substrate S6 kinase 1 (S6K1), and sterol regulatory element-binding protein 1 (SREBP1), were reported. The target metabolic enzymes of PI3K/AKT/mTOR effectors were provided (Figure 3). In general, c-Myc, HIF, and mTORC1 activate hexokinase (HK), phosphofructokinase-2 (PFK2), PFK1, and lactate dehydrogenase (LDH) but inactivate pyruvate dehydrogenase (PDH) [7]. In contrast, AKT suppresses FOXO to inactivate HK, glucose-6-phosphate isomerase (GPI), aldolase, enolase, and pyruvate kinase (PK), leading to activate glycolysis and generating pyruvate. Moreover, mTORC1, S6K1, and SREBP1 activate the PPP pathway and lipid synthesis [7]. Therefore, PI3K/AKT/mTOR signaling is crucial for regulating different metabolisms.
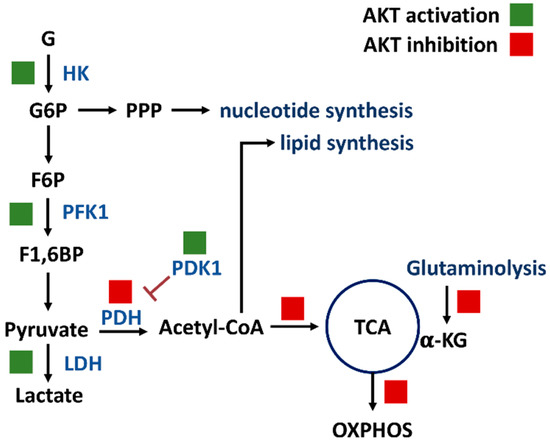
Figure 2.
PI3K/AKT/mTOR signaling regulates metabolisms of glycolysis, PPP, nucleotide synthesis, lipid synthesis, TCA cycle, glutaminolysis, and OXPHOS. Solid and blank boxes indicate activation and inactivation by AKT. Abbreviations: G, glucose; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; F1,6BP, fructose-1,6-bisphosphate; HK, hexokinase; PFK1, phosphofructokinase 1; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; α-KG, α-ketoglutarate; TCA, tricarboxylic acid cycle; OXPHOS, oxidative phosphorylation.
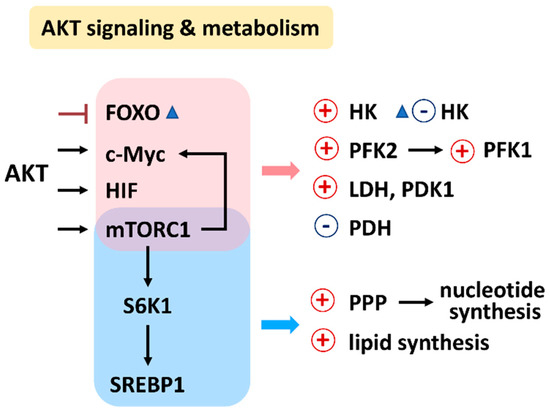
Figure 3.
Relationship between AKT signaling, target enzymes, and their affected metabolisms. Abbreviations: FOXO, forkhead box transcription factors; HIF, hypoxia-inducible factor; mTORC1, mechanistic target of rapamycin complex 1; S6K1, mTOR substrate S6 kinase 1; sterol regulatory element-binding transcription factor 1; SREBP1; HK, hexokinase; PFK1/2, phosphofructokinase 1/2; PDK1, pyruvate dehydrogenase kinase 1; LDH, lactate dehydrogenase; PDH, pyruvate dehydrogenase; PPP, pentose phosphate pathway.
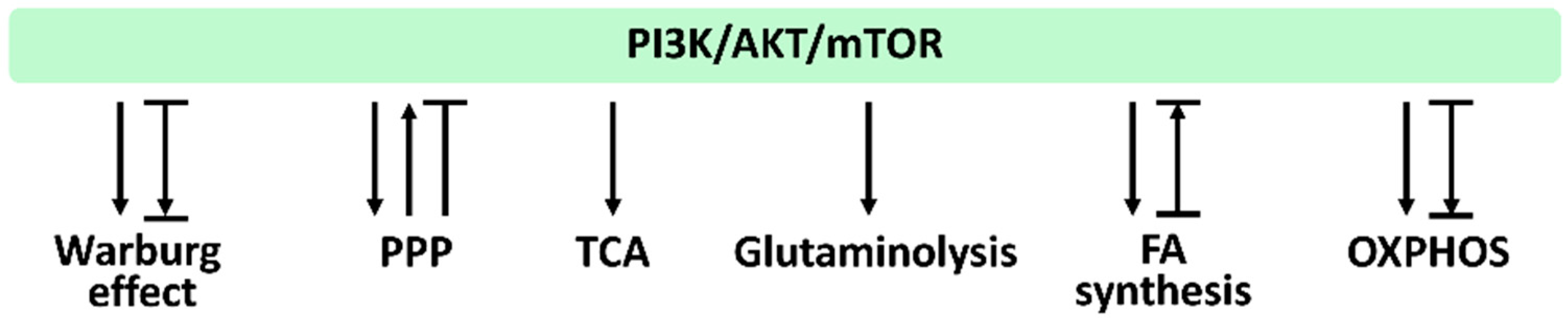
In Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6, we provide the impact of PI3K/AKT/mTOR signaling on regulating their associated metabolisms, such as the Warburg effect, PPP, TCA cycle, glytaminogenesis, fatty acid synthesis, and OXPHOS, which is summarized in Figure 4.
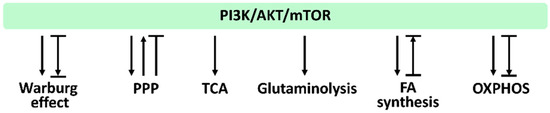
Figure 4.
PI3K/AKT/mTOR and its associated metabolisms. Arrows indicate activation; T indicates inhibition; T with an arrow indicates PI3K/AKT/mTOR inhibition leading to metabolic activation. Abbreviations: PPP, pentose phosphate pathway; TCA, tricarboxylic acid cycle; FA, fatty acid; OXPHOS, oxidative phosphorylation. These effects were summarized from the reports mentioned in Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5, Section 3.6. Different studies reported differential regulations to these metabolisms by modulating PI3K/AKT/mTOR signaling. Various reports show different PI3K/AKT/mTOR responses for the same metabolism.
3.1. Relationship between the Warburg Effect and PI3K/AKT/mTOR
The PI3K/AKT/mTOR axis is a tightly connected pathway starting at PI3K and subsequently activates AKT and mTOR. Some studies report parts of such pathways but do not exclude participation in others.
Several studies examined the function of PI3K/AKT/mTOR in the Warburg effect by upregulation or downregulation strategies. Overexpressing the activated AKT increases glucose uptake by activating the glucose transporter GLUT1 [61] and enhances the Warburg effect [61]. In contrast, inhibiting AKT/mTOR/GLUT1 signaling by berberine can suppress the Warburg effect for antiproliferation of breast and liver cancer cells [62]. Accordingly, modulating the PI3K/AKT/mTOR axis regulates the Warburg effect (Figure 4).
3.2. Relationship between PPP and PI3K/AKT/mTOR
Several studies examined the function of PI3K/AKT/mTOR in PPP by modulating their protein or enzyme levels and activities. Glucose-6-phosphate dehydrogenase (G6PD), a priming enzyme of PPP, is stabilized by PI3K/AKT activation to promote PPP [63]. Suppressing the expression of the pleckstrin homology like domain family A member 3 (PHLDA3), an intrinsic AKT inhibitor, can improve the PI3K activation and switch glycolysis to PPP [63].
Transketolase (TKT), one of the PPP enzymes, is highly expressed in colorectal cancer, giving a poor prognosis [64]. TKT is also upregulated in colorectal cancer cell lines, promoting proliferation and metastasis. TKT overexpression induces AKT activation [64]. Accordingly, PPP may activate AKT. However, PPP may have a different response to AKT. PPP can inactivate AKT to induce antiproliferation of neuroblastoma cells [65]. Therefore, PPP and PI3K/AKT/mTOR offer reciprocal regulation (Figure 4).
3.3. Relationship between the TCA Cycle and PI3K/AKT/mTOR
The PDH complex catalyzes the reaction converting pyruvate to acetyl-CoA. Then, acetyl-CoA joints oxoacetate to enter the TCA cycle and becomes citrate. PI3K activation can regulate the Warburg effect, partly inhibit pyruvate kinase 2 (PKM2), a rate-limited enzyme of glycolysis, and finally dissociates the connection between glycolysis and the TCA cycle [63]. PDH activity is suppressed by pyruvate dehydrogenase kinase (PDK1) [18]. AKT can activate PDK1 by phosphorylation to enhance its PDH inhibition for pausing the TCA cycle and switching to the LDH response in the Warburg effect [18]. Accordingly, PI3K/AKT/mTOR shows a close relationship to TCA cycle regulation (Figure 4).
Moreover, the metabolites at the pausing TCA cycle also change to other pathways such as lipid synthesis [66]. mTOR involved in glutaminolysis contributing to TCA cycle regulation is described later [67]. Therefore, PI3K/AKT/mTOR signaling functions as a TCA modulator.
3.4. Relationship between Glutaminolysis and PI3K/AKT/mTOR
Glutaminolysis is connected to the TCA cycle at the entry of α-ketoglutarate, fueling the TCA cycle [66]. Mitochondrial pyruvate carrier (MPC) can transport pyruvate from the cytoplasm to mitochondria. Inhibiting MPC activates glutamate dehydrogenase (GDH), which converts glutamate to α-ketoglutarate, and generates acetyl-CoA from glutamine [66]. Accordingly, MPC inhibition changes the paths to replenish TCA intermediates [66]. Since PI3K/AKT/mTOR regulates the TCA cycle, it also controls glutaminolysis.
Cancer cells highly express PI3K-AKT-mTOR and improve glutaminolysis [68]. NAD(P)H: quinone oxidoreductase 1 (NQO1) is an antioxidant signaling protein. In NQO1-defective liver cancer cells, both glycolysis and glutaminolysis-associated gene expressions are suppressed by AKT [69]. In contrast, NQO1 overexpression induces PI3K/AKT activation to improve liver cancer cell proliferation. Therefore, PI3K/AKT/mTOR function as modulators for controlling glutaminolysis (Figure 4).
3.5. Relationship between Fatty Acid Metabolism and PI3K/AKT/mTOR
Several studies examined the function of PI3K/AKT/mTOR in the gene expressions for fatty acid metabolism. ATP citrate lyase (ACLY) catalyzes the conversion of TCA cycle-derived citrate to acetyl-CoA in the cytoplasm. AKT can activate ACLY by phosphorylation [70] to control fatty acid synthesis [18], providing de novo lipid synthesis. PI3K/AKT/mTOR is the upstream regulator for the melanoma antigen ganglioside GD3. GD3 can activate SREBP1 and, in turn, regulates ACC1 expression [71].
Upregulation of human epidermal growth factor receptor 2 (HER2) in breast cancer cells enhances the expression of fatty acid synthesis genes such as ACC1 and FASN, which are suppressed by PI3K and mTOR inhibitors, indicating that PI3K/AKT/mTOR can regulate fatty acid synthesis [72]. AKT/mTOR is overexpressed in liver cancer cells and induces upregulation of lipogenesis [73]. In ACC2 knockdown mice, fatty acid synthesis-associated genes, including ACC1, FASN, and ATP citrate lyase (ACL), are downregulated [74]. Therefore, PI3K/Akt/mTOR plays a vital role in regulating fatty acid metabolism (Figure 4).
3.6. Relationship between OXPHOS and PI3K/AKT/mTOR
AKT activation enhances OXPHOS in both normal and cancer cells. After a nephrotoxic injury in renal proximal tubular cells, AKT is activated [75]. ETC activity and ATP generation rate are the critical indicators for OXPHOS, which is proportional to oxygen consumption rate (OCR). PI3K/AKT/mTOR pathway inhibition suppresses OCR in head and neck cancer cells [76]. Therefore, PI3K/AKT/mTOR plays a vital role in regulating OXPHOS metabolism (Figure 4).
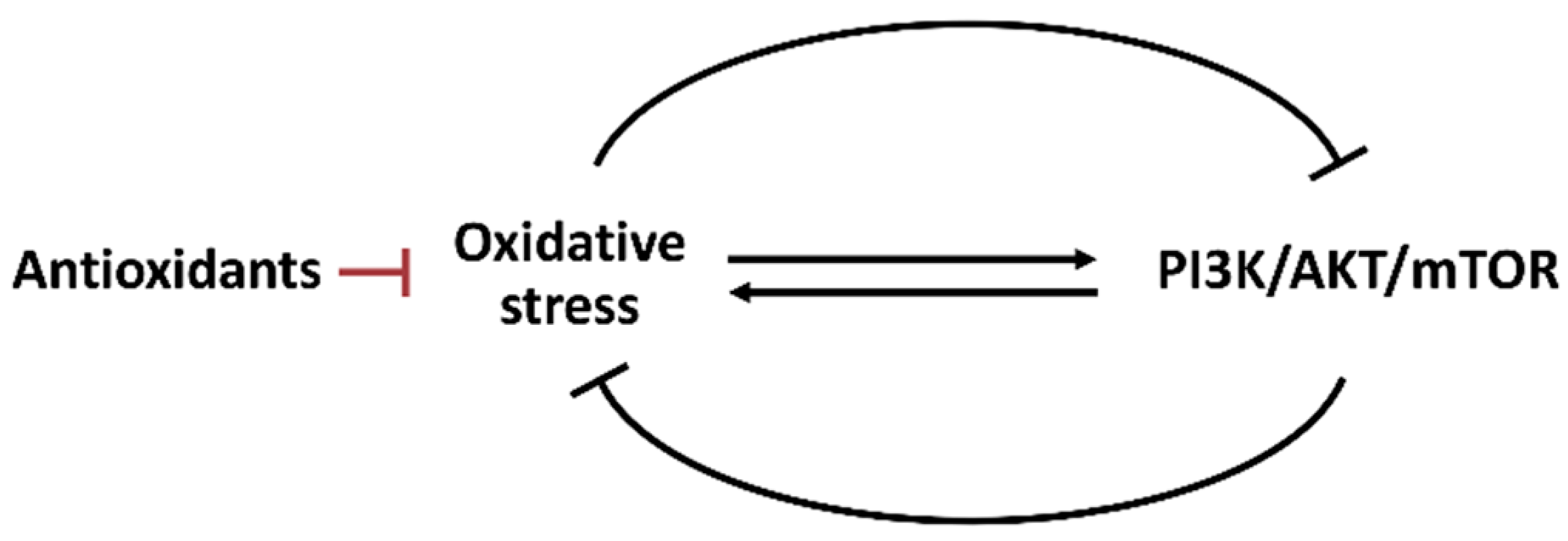
4. Interaction between Oxidative Stress and PI3K/AKT/mTOR
Oxidative stress and oncogene signal transduction are essential in regulating metabolism [77]. Oxidative stress and PI3K/AKT/mTOR pathways show the reciprocal modulation of several cell stress responses such as apoptosis [78], autophagy [79], senescence [79,80], and ER stress [81]. Oxidative stress may regulate the PI3K/AKT/mTOR activity (Figure 5). Hydrogen peroxide inactivates protein tyrosine phosphatase 1B (PTP1B) to inhibit PI3K. Hydrogen peroxide also inactivates protein phosphatase 2A (PP2A) to inhibit AKT [18].
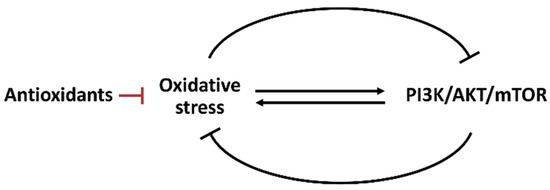
Figure 5.
Interaction between oxidative stress and PI3K/AKT/mTOR. Oxidative stress and PI3K/AKT/mTOR can reciprocally induce or suppress each other. The antioxidant system also regulates oxidative stress.
Under moderate levels of ROS, AKT activation happens [79] through PI3K signaling [82]. However, ROS may dephosphorylate and inactivate PI3K/AKT of gastric cancer cells following the treatment of the thioredoxin reductase-1 inhibitor chaetocin [78].
In contrast, PI3K/AKT/mTOR may regulate oxidative stress (Figure 5). Phosphatase and tensin homologous (PTEN) proteins suppress PI3K signaling [79]. Hence, in PTEN-deficient prostate cancer cells, AKT is hyperactivated to highly induce ROS generation attributed to OXPHOS induction [83]. Growth factor activates AKT to enhance ROS generation, leading to uncontrolled cancer cell proliferation [84]. Notably, PI3K/AKT/mTOR may regulate oxidative stress differently. PI3K/AKT is activated in dental pulp cells at hypoxia to suppress oxidative stress [85].
Moreover, redox homeostasis is balanced between oxidative stress and the antioxidant system. AKT activation improves oxidative stress adaptation by activating NRF2-associated antioxidant signaling [18]. Hence, oxidative stress and PI3K/AKT/mTOR show the complex interaction in redox homeostasis. Due to reciprocal regulation, drug treatments that directly affect one may indirectly influence the other. Accordingly, oxidative stress and PI3K/AKT/mTOR exhibit multiple functions regulating their respective metabolisms.
5. The Roles of Oxidative Stress and PI3K/AKT/mTOR in Fucoidan
Several bioactive compounds have been identified in algae [86,87,88,89,90]. Fucoidan is a brown algae-derived polysaccharide capable of generating abundant sulfated fucoses [91]. Fucoidan has been isolated from several brown algae, including Alaria esculenta, Ascophyllum nodosum, Cladosiphon okamuranus, Colpomenia sinuosa, Fucus vesiculosus, Fucus evanescens, Ecklonia cava, Hizikia fusiforme, Laminaria hyperborea, Laminaria japonicia, Macrocystis pyrifera, Saccharina japonica, Sargassum confusum, Sargassum coreanum, Sargassum filipendula, Sargassum horneri, Sargassum mcclurei, Sargassum natans, Sargassum polycystum, and Sargassum siliquastrum [92,93,94,95,96,97,98,99,100,101,102,103].
Fucoidan is a safe food supplement authorized by the United States Food and Drug Administration (FDA) [25]. Several bioactivities of fucoidan have been reported in suppressing inflammation, coagulant, microbial infection, and oxidation [104]. Moreover, some studies focus on the chemopreventive effects of fucoidan [98,105,106,107,108,109]. Since most of these bioactivities, such as inflammation [110], chemoprevention [111], and anticancer [112] effects, primarily rely on the modulation of oxidative stress, we discuss the development of oxidative stress for fucoidan research in Section 5.1.
Moreover, the anticancer effects of fucoidan were emphasized in several reviews [92,113,114,115]. Mounting evidence shows that fucoidan exhibits antiproliferation effects against several types of cancers, such as cholangiocarcinoma [116], breast [117,118], pancreas [119], lung [120,121], and cervical [122] cancer cells. Since these anticancer effects are associated with modulating PI3K/AKT/mTOR [123,124,125,126,127,128], PI3K/AKT/mTOR signaling of fucoidan-related studies is also summarized in Section 5.2.
5.1. Oxidative Stress Studies of Fucoidan
Exogenous antioxidants are capable of biphasic functions for modulating oxidative stress, i.e., decreasing and increasing oxidative stress at normal and lethal concentrations [129]. Fucoidan from Undaria pinnatifida [130] and Sargassum filipendula [131] show biochemical antioxidant effects by examining 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging ability. Several studies reported that fucoidan exhibits chemopreventive effects on hazardous chemicals, radiation, and toxins, while others reported antiproliferative effects against cancer cells.
Given the chemopreventive effects, fucoidan exhibits protective effects against acetaminophen-triggered hepatotoxicity by improving the expression and translocation of antioxidant NRF2, upregulating GSH, superoxide dismutase (SOD), and catalase (CAT), and suppressing ROS and lipid peroxidation (oxidative stress) of hepatocytes [105]. Similarly, fucoidan increases viability and decreases apoptosis, DNA damage, and ROS generation in UVB-irradiated human keratinocytes, accompanied by NRF2 activation [107]. Fucoidan also inhibits tumor necrosis factor (TNF)-α/interferon (IFN)-γ triggered inflammation and ROS generation of keratinocytes by turning on NRF2 signaling [98].
Moreover, fucoidan shows in vivo chemopreventative effects. Fucoidan increases survival and decreases oxidative stress and heart-beating induced by hydrogen peroxide in zebrafish embryos [132]. Besides NRF2 activation, the GSH level increases in fucoidan and high-fat diet-fed mice, accompanied by decreasing protein and lipid peroxidation [106].
Given the antiproliferation effects against cancer cells, fucoidan shows antiproliferation, oxidative stress, and apoptosis-inducing results in several kinds of cancer cells, such as breast [133], liver [134], lung [135], and colon [136]. Since redox homeostasis is the outcome of balancing oxidative stress and the antioxidant system, it is possible that the antioxidant system is down-regulated and subsequently induces oxidative stress. Therefore, fucoidan can modulate oxidative stress to protect normal cells or kill cancer cells (Table 1).

Table 1.
Relationship between fucoidan-modulated oxidative stress and PI3K/AKT/mTOR in metabolic regulations *.
5.2. PI3K/AKT/mTOR Studies of Fucoidan
Fucoidan decreases phosphor-PI3K/AKT to inactivate PI3K/AKT and induce apoptosis in acute promyelocytic leukemia [123], lung [124], prostate [125], liver [126], bladder [127], and colon [128] cancer cells. Fucoidan also shows preferential killing of ovarian cancer cells but not normal ovarian epithelial cells by downregulating cancer cell PI3K/AKT signaling [137]. In a 7,12-dimethylbenz[a]anthracene (DMBA)-induced animal tumor model, fucoidan suppresses breast cancer cell-xenografted tumor growth by inhibiting PI3K/AKT/GSK3β signaling [138]. Moreover, fucoidan shows anti-migration and anti-invasion in lung [139] and colon [128] cancer cells by downregulating PI3K/AKT/mTOR signaling. Therefore, fucoidan induces several PI3K/AKT/mTOR-mediated responses (Table 1).
6. The Roles of Fucoidan-Modulated Oxidative Stress and PI3K/AKT/mTOR in Metabolic Regulations
The relationship between fucoidan-modulated oxidative stress and PI3K/AKT/mTOR in metabolic regulations is summarized in Table 1. As mentioned in Section 2 and Section 3, oxidative stress and PI3K/AKT/mTOR show the modulating effects on these mechanisms. Fucoidan shows the regulation of oxidative stress and PI3K/AKT/mTOR. However, the impact of fucoidan-modulated oxidative stress and PI3K/AKT/mTOR on these mechanisms remain unclear. We next discuss the potential role of oxidative stress and PI3K/AKT/mTOR in the metabolic regulation of fucoidan.
6.1. The Roles of Fucoidan-Induced Oxidative Stress in Metabolic Regulations Need Further Investigation
Fucoidan shows antiproliferation, apoptosis, and oxidative stress-related responses on several cancer cells. However, the impact of fucoidan-induced oxidative stress on regulating metabolism was not thoroughly investigated (Table 1). Fucoidan shows antiproliferation effects on liver cancer cells by triggering oxidative stress generation and apoptosis, accompanied by GSH depletion [134]. Fucoidan also sensitizes breast cancer cells to anticancer drugs such as cisplatin, tamoxifen, or paclitaxel by downregulating GSH levels [140]. Therefore, fucoidan provides oxidative stress-dependent antiproliferation to cancer cells (Table 1). However, the role of oxidative stress in regulating the metabolism of fucoidan treatment lacks detailed investigation.
6.2. The Roles of Fucoidan-Inactivated PI3K/AKT/mTOR in Metabolic Regulations Need Further Investigation
Fucoidan also shows PI3K/AKT/mTOR inactivation-related responses on several cancer cells. However, the impact of PI3K/AKT/mTOR signaling on regulating the metabolism of fucoidan treatment was not thoroughly investigated (Table 1).
Several fatty acid metabolism studies affected by fucoidan were reported (Table 1); however, the roles of oxidative stress and PI3K/AKT/mTOR in regulating fatty acid metabolism remain unclear. Fucoidan inhibits proliferation by inhibiting fatty acid synthesis (ACC) in hepatoma HLF cells [141]. Moreover, fucoidan suppresses HMG-CoA reductase (HMGCR) and improves lecithin-cholesterol acyltransferase (LCAT) expressions, and reduces cholesterol synthesis [142]. Fucoidan also suppresses SREBP1c expression and reduces fatty acid synthesis. It enhances the peroxisome proliferator-activated receptor α (PPARα), PPARγ, and lipoprotein lipase (LPL) expression to drive the β-oxidation reaction of fatty acids [142]. Similarly, fucoidan activates lipolysis enzymes, e.g., hormone-sensitive lipase (HSL), to decrease lipid storage in adipocytes [143].
Except for the fatty acid metabolism, the remaining metabolisms such as Warburg Effect, PPP, TCA cycle, glutaminolysis, and OXPHOS were not connected to fucoidan studies. Moreover, fucoidan can modulate oxidative stress and PI3K/AKT/mTOR signaling. Nevertheless, the contributions of fucoidans to regulating the Warburg Effect, PPP, TCA cycle, glutaminolysis, fatty acid metabolism, and OXPHOS remain unstudied. This holds particularly for a detailed examination of the effects that oxidative stress and PI3K/AKT/mTOR may provide in regulating the Warburg Effect, PPP, TCA cycle, glutaminolysis, fatty acid metabolism, and OXPHOS of fucoidan (Table 1).
7. Conclusions
Metabolism is at the center of cancer cell proliferation. Oxidative stress and PI3K/AKT/mTOR signaling play crucial roles in controlling metabolism for carcinogenesis. The relationships between oxidative stress and PI3K/AKT/mTOR signaling and individual metabolism were summarized. These signaling pathways exhibit a diverse regulation of different metabolisms of cancer cells. As mentioned above, oxidative stress and PI3K/AKT/mTOR signaling are well organized and connected to several metabolisms in cancer cells. They may reroute when some of them are suppressed. The relationships between the Warburg effect, PPP, fatty acid metabolism, TCA cycle, glutaminolysis, and OXPHOS were demonstrated here.
Accordingly, natural products or other chemical agents exhibiting oxidative stress and PI3K/AKT/mTOR modulating functions may potentially regulate cancer cell development. This review chose the brown algae-derived fucoidan to discuss its impact on oxidative stress and PI3K/AKT/mTOR and their modulating effects on metabolisms. Although fucoidan impacts oxidative stress and PI3K/AKT/mTOR signaling, their possible regulating metabolisms remain unclear.
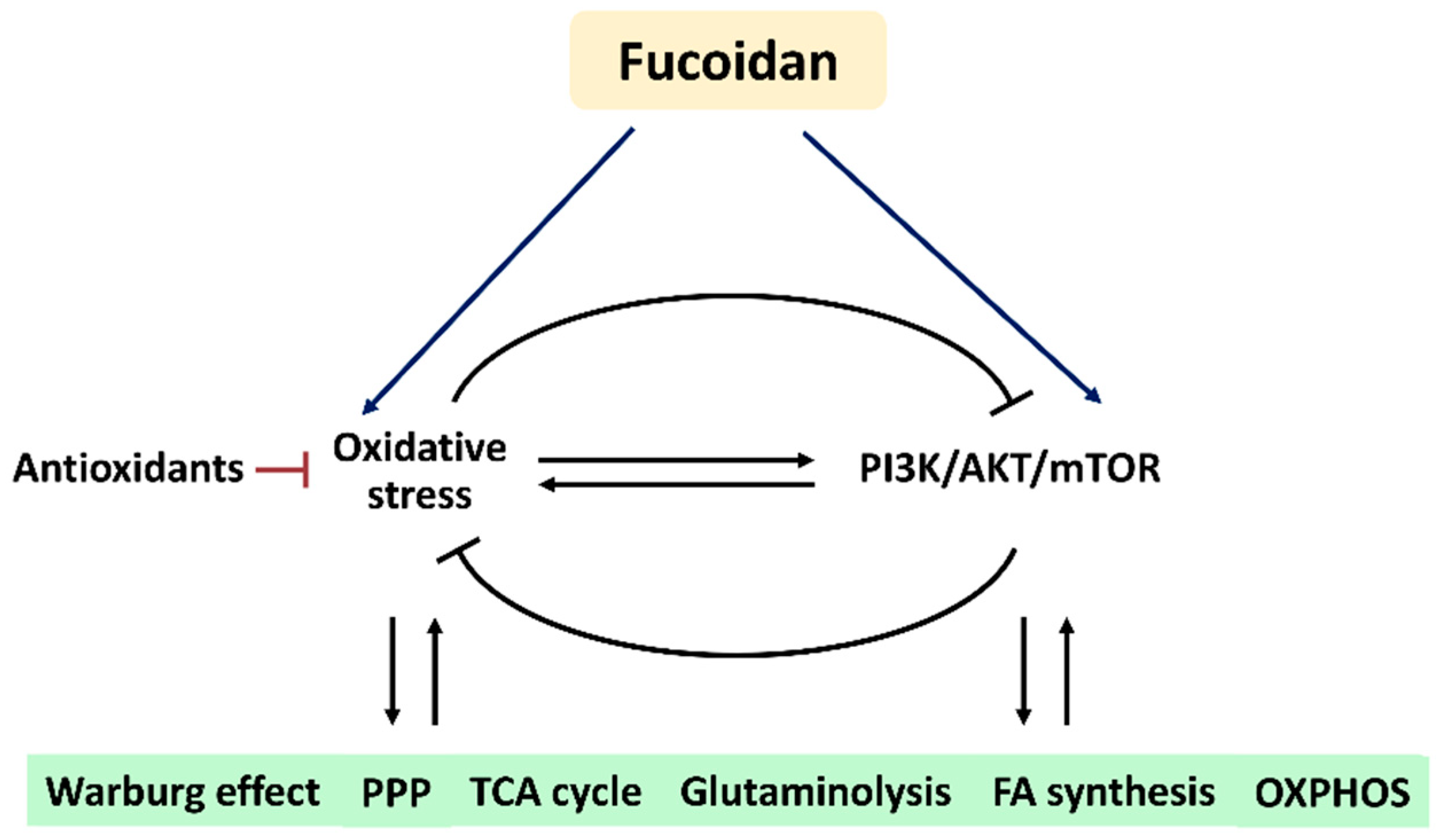
Based on these findings, we hypothesize that fucoidan regulates oxidative stress and PI3K/AKT/mTOR signaling to modulate their associated metabolic regulations (Figure 6). Understanding this connection and mechanism may provide a novel strategy to investigate the roles of oxidative stress and PI3K/AKT/mTOR signaling in metabolism-based cancer therapy using fucoidan in the future.
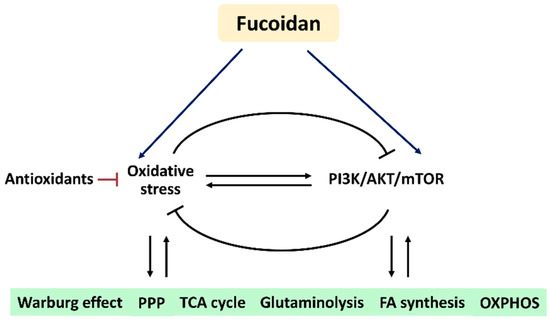
Figure 6.
Hypothesis. Fucoidan may modulate oxidative stress and PI3K/AKT/mTOR in metabolic regulations. Oxidative stress and PI3K/AKT/mTOR can reciprocally induce or suppress each other. The antioxidant system also regulates oxidative stress. Fucoidan can modulate oxidative stress and PI3K/AKT/mTOR, but their impacts on fucoidan-modulated metabolisms are rarely investigated. Accordingly, fucoidan may trigger oxidative stress and PI3K/AKT/mTOR to control several metabolic functions. Abbreviations: PPP, pentose phosphate pathway; TCA, tricarboxylic acid cycle; FA, fatty acid; OXPHOS, oxidative phosphorylation.
Notably, these metabolisms crosstalk with each other, and they receive integrating effects from oxidative stress and PI3K/AKT/mTOR signaling. Using the inhibitors or activators of these metabolisms may provide a deep understanding of the metabolism functions of drug-modulating changes on oxidative stress and PI3K/AKT/mTOR signaling. Moreover, the combined treatments with some of these metabolic modulators may also improve the anticancer therapeutic effects. In addition to fucoidan, other anticancer agents with the modulating ability of oxidative stress and PI3K/AKT/mTOR signaling may use the same strategy to enhance their antiproliferation effects on cancers.
Therefore, the contribution of this review is to shed light on the existing knowledge of individual and interacting effects of oxidative stress and PI3K-AKT-mTOR and provide an effective strategy for applying these metabolism-related regulations in cancer therapy.
Author Contributions
Conceptualization, J.-P.S., Y.-T.C., C.-Y.Y. and H.-W.C.; methodology, Y.-B.C., J.-Y.T. and M.-F.H.; supervision, C.-Y.Y. and H.-W.C.; writing—original draft, J.-P.S., Y.-T.C. and H.-W.C.; writing—review and editing, C.-Y.Y. and H.-W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by funds from the Ministry of Science and Technology (MOST 108-2314-B-037-021-MY3; MOST 110-2314-B-037-074-MY3), the National Sun Yat-sen University KMU Joint Research Project (#NSYSUKMU 111-P20), and the Kaohsiung Medical University (KMU-DK(A)111008).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. Links between metabolism and cancer. Genes Dev. 2012, 26, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Takahashi, E.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Ogura, S.-I. Inverse correlation between heme synthesis and the Warburg effect in cancer cells. BioRxiv 2019, 753079. [Google Scholar] [CrossRef]
- Ghanbari Movahed, Z.; Rastegari-Pouyani, M.; Mohammadi, M.H.; Mansouri, K. Cancer cells change their glucose metabolism to overcome increased ROS: One step from cancer cell to cancer stem cell? Biomed. Pharmacother. 2019, 112, 108690. [Google Scholar] [CrossRef]
- Lien, E.C.; Lyssiotis, C.A.; Cantley, L.C. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. Recent Results Cancer Res. 2016, 207, 39–72. [Google Scholar]
- Huang, S. mTOR signaling in metabolism and cancer. Cells 2020, 9, 2278. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Y.; Yu, S.; Li, X.; Wang, A.; Wang, S.; Chen, W.; Lu, Y. Critical role of mTOR in regulating aerobic glycolysis in carcinogenesis (Review). Int. J. Oncol. 2021, 58, 9–19. [Google Scholar] [CrossRef]
- Chung, Y.K.; Or, R.C.-H.; Lu, C.H.; Ouyang, W.T.; Yang, S.Y.; Chang, C.C. Sulforaphane down-regulates SKP2 to stabilize p27(KIP1) for inducing antiproliferation in human colon adenocarcinoma cells. J. Biosci. Bioeng. 2015, 119, 35–42. [Google Scholar] [CrossRef]
- Yang, K.H.; Tang, J.Y.; Chen, Y.N.; Chuang, Y.T.; Tsai, I.H.; Chiu, C.C.; Li, L.J.; Chien, T.M.; Cheng, Y.B.; Chang, F.R.; et al. Nepenthes extract induces selective killing, necrosis, and apoptosis in oral cancer cells. J. Pers. Med. 2021, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Huang, H.W.; Wang, H.R.; Chan, Y.C.; Haung, J.W.; Shu, C.W.; Wu, Y.C.; Chang, H.W. 4beta-Hydroxywithanolide E selectively induces oxidative DNA damage for selective killing of oral cancer cells. Environ. Toxicol. 2018, 33, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Huang, J.W.; Chang, F.R.; Huang, K.J.; Huang, H.M.; Huang, H.W.; Chou, C.K.; Wu, Y.C.; Chang, H.W. Golden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 2013, 8, e64739. [Google Scholar]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Lab. Investig. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/mTOR in oral cancer: Mechanisms and advances in clinical trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Kang, B.W.; Chau, I. Molecular target: Pan-AKT in gastric cancer. ESMO Open 2020, 5, e000728. [Google Scholar] [CrossRef]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef]
- Ringel, M.D.; Hayre, N.; Saito, J.; Saunier, B.; Schuppert, F.; Burch, H.; Bernet, V.; Burman, K.D.; Kohn, L.D.; Saji, M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001, 61, 6105–6111. [Google Scholar]
- Ghoneum, A.; Abdulfattah, A.Y.; Said, N. Targeting the PI3K/AKT/mTOR/NFkappaB axis in ovarian cancer. J. Cell. Immunol. 2020, 2, 68–73. [Google Scholar]
- Mroweh, M.; Roth, G.; Decaens, T.; Marche, P.N.; Lerat, H.; Macek Jilkova, Z. Targeting Akt in hepatocellular carcinoma and its tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 1794. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Hossain, F.; Andreana, P.R. Developments in carbohydrate-based cancer therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef]
- de Padua, M.C.; Delodi, G.; Vucetic, M.; Durivault, J.; Vial, V.; Bayer, P.; Noleto, G.R.; Mazure, N.M.; Zdralevic, M.; Pouyssegur, J. Disrupting glucose-6-phosphate isomerase fully suppresses the “Warburg effect” and activates OXPHOS with minimal impact on tumor growth except in hypoxia. Oncotarget 2017, 8, 87623–87637. [Google Scholar] [CrossRef]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef]
- Pan, J.G.; Mak, T.W. Metabolic targeting as an anticancer strategy: Dawn of a new era? Sci. STKE 2007, 2007, pe14. [Google Scholar] [CrossRef][Green Version]
- Zdralevic, M.; Vucetic, M.; Daher, B.; Marchiq, I.; Parks, S.K.; Pouyssegur, J. Disrupting the ‘Warburg effect’ re-routes cancer cells to OXPHOS offering a vulnerability point via ‘ferroptosis’-induced cell death. Adv. Biol. Regul. 2018, 68, 55–63. [Google Scholar] [CrossRef]
- El Sayed, S.M.; Mahmoud, A.A.; El Sawy, S.A.; Abdelaal, E.A.; Fouad, A.M.; Yousif, R.S.; Hashim, M.S.; Hemdan, S.B.; Kadry, Z.M.; Abdelmoaty, M.A.; et al. Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med. Hypotheses 2013, 81, 866–870. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Cho, E.S.; Cha, Y.H.; Kim, H.S.; Kim, N.H.; Yook, J.I. The pentose phosphate pathway as a potential target for cancer therapy. Biomol. Ther. 2018, 26, 29–38. [Google Scholar] [CrossRef]
- Movahed, Z.G.; Yarani, R.; Mohammadi, P.; Mansouri, K. Sustained oxidative stress instigates differentiation of cancer stem cells into tumor endothelial cells: Pentose phosphate pathway, reactive oxygen species and autophagy crosstalk. Biomed. Pharmacother. 2021, 139, 111643. [Google Scholar] [CrossRef]
- Liu, C.L.; Hsu, Y.C.; Lee, J.J.; Chen, M.J.; Lin, C.H.; Huang, S.Y.; Cheng, S.P. Targeting the pentose phosphate pathway increases reactive oxygen species and induces apoptosis in thyroid cancer cells. Mol. Cell. Endocrinol. 2020, 499, 110595. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Y.; Liu, Z.; Yang, J.; Tang, H.; Wang, Y. Itaconic acid exerts anti-inflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. Sci. Rep. 2021, 11, 18173. [Google Scholar] [CrossRef]
- Noster, J.; Persicke, M.; Chao, T.C.; Krone, L.; Heppner, B.; Hensel, M.; Hansmeier, N. Impact of ROS-induced damage of TCA cycle enzymes on metabolism and virulence of Salmonella enterica serovar Typhimurium. Front. Microbiol. 2019, 10, 762. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Sokolov, A.P.; Kondrashova, M.N. Nonezymatic formation of succinate in mitochondria under oxidative stress. Free Radic. Biol. Med. 2006, 41, 56–64. [Google Scholar] [CrossRef]
- Mates, J.M.; Di Paola, F.J.; Campos-Sandoval, J.A.; Mazurek, S.; Marquez, J. Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer. Semin. Cell Dev. Biol. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Tang, K.; Yu, Y.; Zhu, L.; Xu, P.; Chen, J.; Ma, J.; Zhang, H.; Fang, H.; Sun, W.; Zhou, L.; et al. Hypoxia-reprogrammed tricarboxylic acid cycle promotes the growth of human breast tumorigenic cells. Oncogene 2019, 38, 6970–6984. [Google Scholar] [CrossRef]
- Ciccarone, F.; Di Leo, L.; Lazzarino, G.; Maulucci, G.; Di Giacinto, F.; Tavazzi, B.; Ciriolo, M.R. Aconitase 2 inhibits the proliferation of MCF-7 cells promoting mitochondrial oxidative metabolism and ROS/FoxO1-mediated autophagic response. Br. J. Cancer 2020, 122, 182–193. [Google Scholar] [CrossRef]
- Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979, 254, 2669–2676. [Google Scholar] [CrossRef]
- Lu, W.; Pelicano, H.; Huang, P. Cancer metabolism: Is glutamine sweeter than glucose? Cancer Cell 2010, 18, 199–200. [Google Scholar] [CrossRef]
- Jin, L.; Alesi, G.; Kang, S. Glutaminolysis as a target for cancer therapy. Oncogene 2016, 35, 3619–3625. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Xu, L.; Xu, R.; Saw, P.E.; Wu, J.; Cheng, S.X.; Xu, X. Nanoparticle-mediated inhibition of mitochondrial glutaminolysis to amplify oxidative stress for combination cancer therapy. Nano Lett. 2021, 21, 7569–7578. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Rosca, M.G.; Vazquez, E.J.; Chen, Q.; Kerner, J.; Kern, T.S.; Hoppel, C.L. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 2012, 61, 2074–2083. [Google Scholar] [CrossRef]
- Ito, H.; Nakamae, I.; Kato, J.Y.; Yoneda-Kato, N. Stabilization of fatty acid synthesis enzyme acetyl-CoA carboxylase 1 suppresses acute myeloid leukemia development. J. Clin. Investig. 2021, 131, e141529. [Google Scholar] [CrossRef]
- Adeshakin, A.O.; Liu, W.; Adeshakin, F.O.; Afolabi, L.O.; Zhang, M.; Zhang, G.; Wang, L.; Li, Z.; Lin, L.; Yan, D.; et al. Regulation of lipid accumulation-induced ROS in myeloid-derived suppressor cells via targeting fatty-acid transport protein 2 enhanced anti-PD-L1 tumor immunotherapy. BioRxiv 2020. [Google Scholar] [CrossRef]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The impact of oxidative stress on adipose tissue energy balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Hao, W.; Chang, C.P.; Tsao, C.C.; Xu, J. Oligomycin-induced bioenergetic adaptation in cancer cells with heterogeneous bioenergetic organization. J. Biol. Chem. 2010, 285, 12647–12654. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.; Jang, H.; Woo, S.M.; Park, J.B.; Lee, S.H.; Kang, J.H.; Kim, H.Y.; Song, J.; Kim, S.Y. Targeting oxidative phosphorylation reverses drug resistance in cancer cells by blocking autophagy recycling. Cells 2020, 9, 2013. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Ying, M.; Jin, C.; Li, J.; Hu, X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target. Ther. 2021, 6, 242. [Google Scholar] [CrossRef]
- Hosios, A.M.; Manning, B.D. Cancer signaling drives cancer metabolism: AKT and the Warburg effect. Cancer Res. 2021, 81, 4896–4898. [Google Scholar] [CrossRef]
- Guo, X.H.; Jiang, S.S.; Zhang, L.L.; Hu, J.; Edelbek, D.; Feng, Y.Q.; Yang, Z.X.; Hu, P.C.; Zhong, H.; Yang, G.H.; et al. Berberine exerts its antineoplastic effects by reversing the Warburg effect via downregulation of the Akt/mTOR/GLUT1 signaling pathway. Oncol. Rep. 2021, 46, 253. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, Y.; Zhang, X.; Yu, Y.; Wu, S.; Jiao, J.; Tran, L.; Zhang, W.; Liu, R.; Zhang, L.; et al. TRIM21 and PHLDA3 negatively regulate the crosstalk between the PI3K/AKT pathway and PPP metabolism. Nat. Commun. 2020, 11, 1880. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Yong, H.; Xu, J.; Qu, P.; Qiao, S.; Hou, P.; Li, Z.; Chu, S.; Zheng, J.; et al. Transketolase promotes colorectal cancer metastasis through regulating AKT phosphorylation. Cell Death Dis. 2022, 13, 99. [Google Scholar] [CrossRef]
- Qi, L.; Toyoda, H.; Shankar, V.; Sakurai, N.; Amano, K.; Kihira, K.; Iwasa, T.; Deguchi, T.; Hori, H.; Azuma, E.; et al. Heterogeneity of neuroblastoma cell lines in insulin-like growth factor 1 receptor/Akt pathway-mediated cell proliferative responses. Cancer Sci. 2013, 104, 1162–1171. [Google Scholar] [CrossRef]
- Gotting, I.; Jendrossek, V.; Matschke, J. A new twist in protein kinase B/Akt signaling: Role of altered cancer cell metabolism in Akt-mediated therapy resistance. Int. J. Mol. Sci. 2020, 21, 8563. [Google Scholar] [CrossRef]
- de la Cruz Lopez, K.G.; Toledo Guzman, M.E.; Sanchez, E.O.; Garcia Carranca, A. mTORC1 as a regulator of mitochondrial functions and a therapeutic target in cancer. Front. Oncol. 2019, 9, 1373. [Google Scholar] [CrossRef]
- Favela-Hernández, J.; Balderas, R.; Guerrero, G. Glutamate metabolism regulates immune escape of glioma. Madr. J. Immunol. 2018, 3, 58–64. [Google Scholar] [CrossRef]
- Dimri, M.; Humphries, A.; Laknaur, A.; Elattar, S.; Lee, T.J.; Sharma, A.; Kolhe, R.; Satyanarayana, A. NAD(P)H quinone dehydrogenase 1 ablation inhibits activation of the phosphoinositide 3-kinase/Akt serine/threonine kinase and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways and blocks metabolic adaptation in hepatocellular carcinoma. Hepatology 2020, 71, 549–568. [Google Scholar]
- Berwick, D.C.; Hers, I.; Heesom, K.J.; Moule, S.K.; Tavare, J.M. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J. Biol. Chem. 2002, 277, 33895–33900. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Furukawa, K.; Hamamura, K.; Furukawa, K. Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: Induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 2011, 71, 4989–4997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, M.Y.; Park, S.W.; Moon, J.S.; Koh, Y.K.; Ahn, Y.H.; Park, B.W.; Kim, K.S. Up-regulation of acetyl-CoA carboxylase alpha and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J. Biol. Chem. 2007, 282, 26122–26131. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.F.; Wang, C.; Ho, C.; Ladu, S.; Lee, S.A.; Mattu, S.; Destefanis, G.; Delogu, S.; Zimmermann, A.; Ericsson, J.; et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011, 140, 1071–1083. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA carboxylases and diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef]
- Shaik, Z.P.; Fifer, E.K.; Nowak, G. Akt activation improves oxidative phosphorylation in renal proximal tubular cells following nephrotoxicant injury. Am. J. Physiol. Renal Physiol. 2008, 294, F423–F432. [Google Scholar] [CrossRef]
- Cerniglia, G.J.; Dey, S.; Gallagher-Colombo, S.M.; Daurio, N.A.; Tuttle, S.; Busch, T.M.; Lin, A.; Sun, R.; Esipova, T.V.; Vinogradov, S.A.; et al. The PI3K/Akt pathway regulates oxygen metabolism via pyruvate dehydrogenase (PDH)-E1alpha phosphorylation. Mol. Cancer Ther. 2015, 14, 1928–1938. [Google Scholar] [CrossRef]
- Choe, J.H.; Mazambani, S.; Kim, T.H.; Kim, J.W. Oxidative stress and the intersection of oncogenic signaling and metabolism in squamous cell carcinomas. Cells 2021, 10, 606. [Google Scholar] [CrossRef]
- Wen, C.; Wang, H.; Wu, X.; He, L.; Zhou, Q.; Wang, F.; Chen, S.; Huang, L.; Chen, J.; Wang, H.; et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 2019, 10, 809. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Nogueira, V.; Park, Y.; Chen, C.C.; Xu, P.Z.; Chen, M.L.; Tonic, I.; Unterman, T.; Hay, N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, F.; Liu, X.; Liu, K.; Zhou, X.; Zhong, C. Cr(VI) induces cytotoxicity in vitro through activation of ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction via the PI3K/Akt signaling pathway. Toxicol Vitr. 2017, 41, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Patra, K.C.; Hay, N. Selective eradication of cancer displaying hyperactive Akt by exploiting the metabolic consequences of Akt activation. Elife 2018, 7, e32213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, X.; Luo, Z.; He, J.; Haider, F.; Song, C.; Peng, L.; Chen, T.; Wu, B. Hypoxia-activated PI3K/Akt inhibits oxidative stress via the regulation of reactive oxygen species in human dental pulp cells. Oxid. Med. Cell. Longev. 2019, 2019, 6595189. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.F.; Chou, H.N.; Sung, P.J. Porphyra-334 isolated from the marine algae Bangia atropurpurea: Conformational performance for energy conversion. Mar. Drugs 2014, 12, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; Garcia-Blairsy Reina, G.; Herrero, M.; Senorans, F.J.; Ibanez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenco-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of bioactive properties in brown algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Al Monla, R.; Dassouki, Z.; Sari-Chmayssem, N.; Mawlawi, H.; Gali-Muhtasib, H. Fucoidan and alginate from the brown algae Colpomenia sinuosa and their combination with vitamin C trigger apoptosis in colon cancer. Molecules 2022, 27, 358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Kang, M.C.; Jeon, Y.J. Fucoidan from acid-processed Hizikia fusiforme attenuates oxidative damage and regulate apoptosis. Int. J. Biol. Macromol. 2020, 160, 390–397. [Google Scholar] [CrossRef]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef]
- Dorschmann, P.; Apitz, S.; Hellige, I.; Neupane, S.; Alban, S.; Kopplin, G.; Ptak, S.; Frette, X.; Roider, J.; Zille, M.; et al. Evaluation of the effects of fucoidans from Fucus species and Laminaria hyperborea against oxidative stress and iron-dependent cell death. Mar. Drugs 2021, 19, 557. [Google Scholar] [CrossRef]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Han, E.J.; Oh, G.W.; Jung, W.K.; Ahn, G. Fucoidan isolated from Sargassum confusum suppresses inflammatory responses and oxidative stress in TNF-alpha/IFN-gamma- stimulated HaCaT keratinocytes by activating Nrf2/HO-1 signaling pathway. Mar. Drugs 2022, 20, 117. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Lee, K.; Cheong, S.H.; Han, Y.S.; Park, S.R.; et al. Human keratinocyte UVB-protective effects of a low molecular weight fucoidan from Sargassum horneri purified by step gradient ethanol precipitation. Antioxidants 2020, 9, 340. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.S.; Vaas, A.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, W.W.; Lee, D.S.; et al. Characterization and cytoprotective properties of Sargassum natans fucoidan against urban aerosol-induced keratinocyte damage. Int. J. Biol. Macromol. 2020, 159, 773–781. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020, 163, 26–35. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.J.; Ahn, G. Fucoidan fractionated from Sargassum coreanum via step-gradient ethanol precipitation indicate promising UVB-protective effects in human keratinocytes. Antioxidants 2021, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Mori, N. In vitro and in vivo anti-primary effusion lymphoma activities of fucoidan extracted from Cladosiphon okamuranus Tokida. Oncol. Rep. 2017, 38, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Wei, J.G.; Tu, M.J.; Gu, J.G.; Zhang, W. Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum fusiforme fucoidan alleviates high-fat diet-induced obesity and insulin resistance associated with the improvement of hepatic oxidative stress and gut microbiota profile. J. Agric. Food Chem. 2020, 68, 10626–10638. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Aleissa, M.S.; Alkahtani, S.; Abd Eldaim, M.A.; Ahmed, A.M.; Bungau, S.G.; Almutairi, B.; Bin-Jumah, M.; AlKahtane, A.A.; Alyousif, M.S.; Abdel-Daim, M.M. Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B1. Oxid. Med. Cell. Longev. 2020, 2020, 9316751. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant potential of sulfated polysaccharides from Padina boryana; protective effect against oxidative stress in in vitro and in vivo zebrafish model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Munoz, A.; Costa, M. Nutritionally mediated oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2013, 2013, 610950. [Google Scholar] [CrossRef]
- John, A.S.; Ankem, M.K.; Damodaran, C. Oxidative stress: A promising target for chemoprevention. Curr. Pharmacol. Rep. 2016, 2, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects—Involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef] [PubMed]
- van Weelden, G.; Bobinski, M.; Okla, K.; van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef]
- Jin, J.O.; Chauhan, P.S.; Arukha, A.P.; Chavda, V.; Dubey, A.; Yadav, D. The therapeutic potential of the anticancer activity of fucoidan: Current advances and hurdles. Mar. Drugs 2021, 19, 265. [Google Scholar] [CrossRef]
- Chantree, P.; Na-Bangchang, K.; Martviset, P. Anticancer activity of fucoidan via apoptosis and cell cycle arrest on cholangiocarcinoma cell. Asian Pac. J. Cancer Prev. 2021, 22, 209–217. [Google Scholar] [CrossRef]
- Malhao, F.; Ramos, A.A.; Macedo, A.C.; Rocha, E. Cytotoxicity of seaweed compounds, alone or combined to reference drugs, against breast cell lines cultured in 2D and 3D. Toxics 2021, 9, 24. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, M.; Wang, Q.; Liang, H.; Yang, J.; Pei, Z.; Qin, K. Inhibition of fucoidan on breast cancer cells and potential enhancement of their sensitivity to chemotherapy by regulating autophagy. Phytother. Res. 2021, 35, 6904–6917. [Google Scholar] [CrossRef]
- Etman, S.M.; Mehanna, R.A.; Bary, A.A.; Elnaggar, Y.S.R.; Abdallah, O.Y. Undaria pinnatifida fucoidan nanoparticles loaded with quinacrine attenuate growth and metastasis of pancreatic cancer. Int. J. Biol. Macromol. 2021, 170, 284–297. [Google Scholar] [CrossRef]
- Chen, X.; Sun, L.; Wei, X.; Lu, H.; Tan, Y.; Sun, Z.; Jiang, J. Antitumor effect and molecular mechanism of fucoidan in NSCLC. BMC Complement. Med. Ther. 2021, 21, 25. [Google Scholar] [CrossRef]
- Hsiao, H.H.; Wu, T.C.; Tsai, Y.H.; Kuo, C.H.; Huang, R.H.; Hong, Y.H.; Huang, C.Y. Effect of oversulfation on the composition, structure, and in vitro anti-lung cancer activity of fucoidans extracted from Sargassum aquifolium. Mar. Drugs 2021, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Pawar, J.S.; Ghosh, I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021, 181, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Karpiniec, S.S.; Dickinson, J.L. Fucoidan suppresses the growth of human acute promyelocytic leukemia cells in vitro and in vivo. J. Cell. Physiol. 2016, 231, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef]
- Boo, H.J.; Hong, J.Y.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, E.J.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S.; Kwon, J.M.; et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs 2013, 11, 2982–2999. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Jing, X.; Ding, X.; Yu, Y.; Zhao, Q. Fucoidan induces apoptosis and inhibits proliferation of hepatocellular carcinoma via the p38 MAPK/ERK and PI3K/Akt signal pathways. Cancer Manag. Res. 2020, 12, 1713–1723. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, D.S.; Jeong, J.W.; Hong, S.H.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Kim, G.Y.; et al. Fucoidan induces ROS-dependent apoptosis in 5637 human bladder cancer cells by downregulating telomerase activity via inactivation of the PI3K/Akt signaling pathway. Drug Dev. Res. 2017, 78, 37–48. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Laeliocattleya, R.A.; Suloi, A.; Gayatri, P.; Putri, N.; Anggraeni, Y. Fucoidan Content from Brown Seaweed (Sargassum filipendula) and Its Potential as Radical Scavenger. J. Phys. Conf. Ser. 2020, 1430, 012023. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS ONE 2011, 6, e27441. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, T.Y.; Lu, M.K.; Leng, P.J.; Tsao, S.M.; Wu, Y.C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, 44990. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Peng, X.; Li, J.; Zhu, C. The natural product fucoidan inhibits proliferation and induces apoptosis of human ovarian cancer cells: Focus on the PI3K/Akt signaling pathway. Cancer Manag. Res. 2020, 12, 6195–6207. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Xue, C.; Liang, H.; Ge, Y.; He, X.; Zhang, L.; Bian, K.; Zhang, L. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3beta pathway in vivo and in vitro. Biomed. Pharmacother. 2017, 94, 898–908. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Hayakawa, M.; Koga, H.; Torimura, T. Effects of fucoidan on proliferation, AMP-activated protein kinase, and downstream metabolism- and cell cycle-associated molecules in poorly differentiated human hepatoma HLF cells. Int. J. Oncol. 2015, 46, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, Q.; Yang, Y.; Hu, Y.; Song, Y.; He, Y.; Liu, S.; Wu, L. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int. J. Biol. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Jung, U.; Roh, C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).