Synergistic Antiproliferation of Cisplatin and Nitrated [6,6,6]Tricycle Derivative (SK2) for a Combined Treatment of Oral Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. SK2 Preparation and Inhibitors
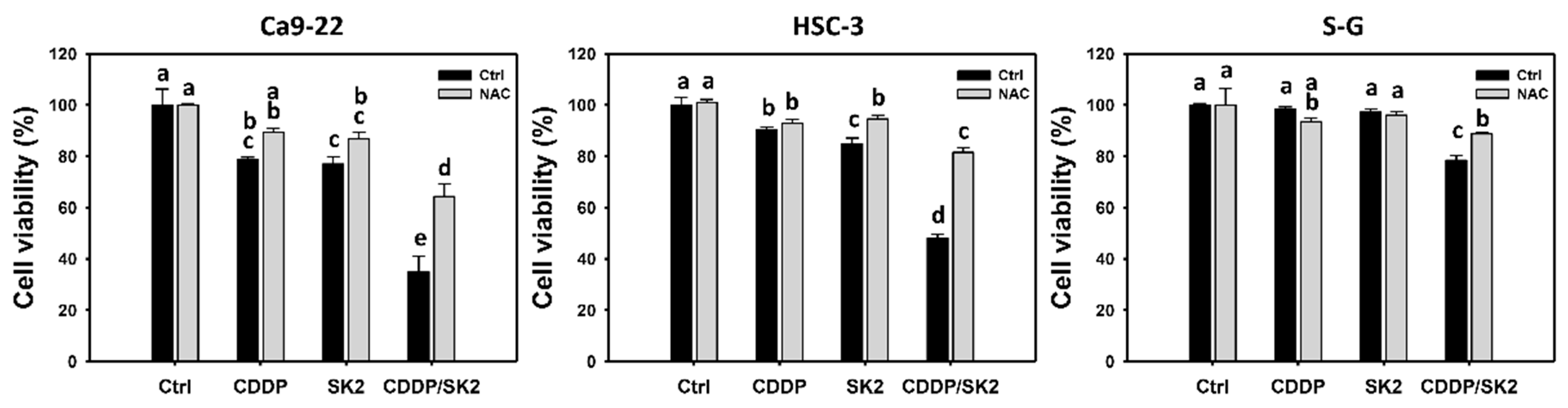
2.2. Cell Cultures and Cell Viability
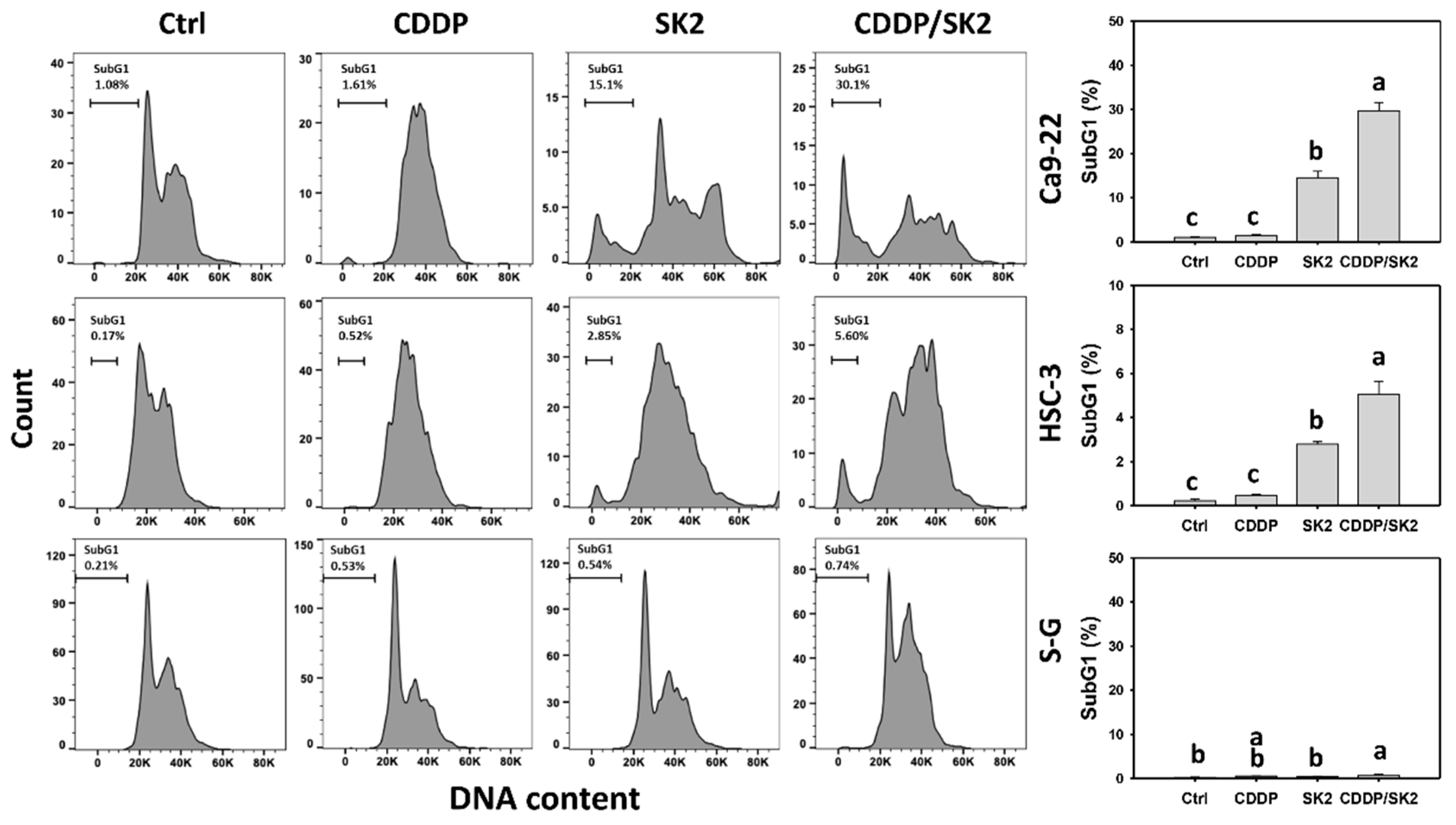
2.3. Cell Cycle Assay
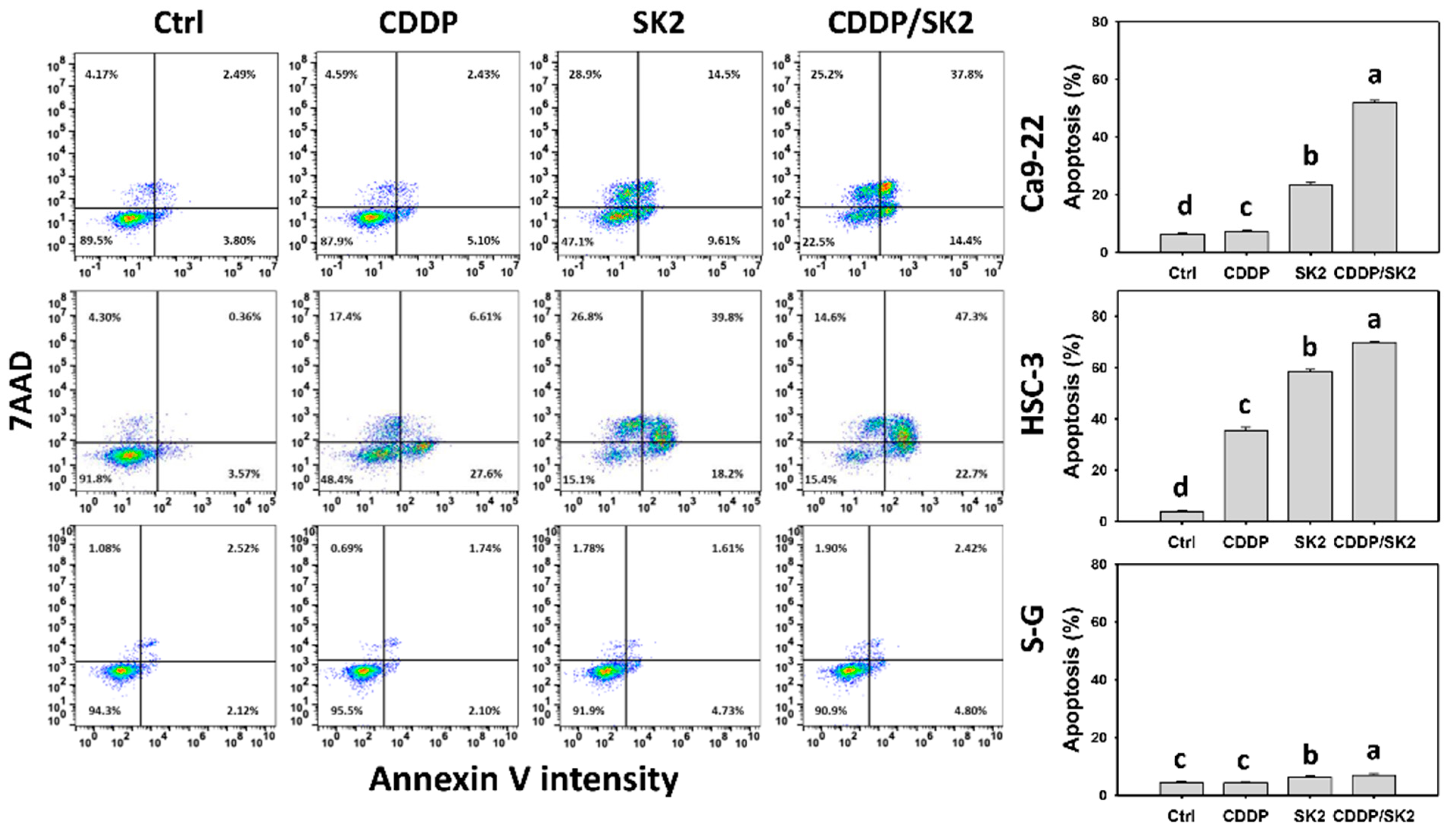
2.4. Annexin-V Apoptosis Assay
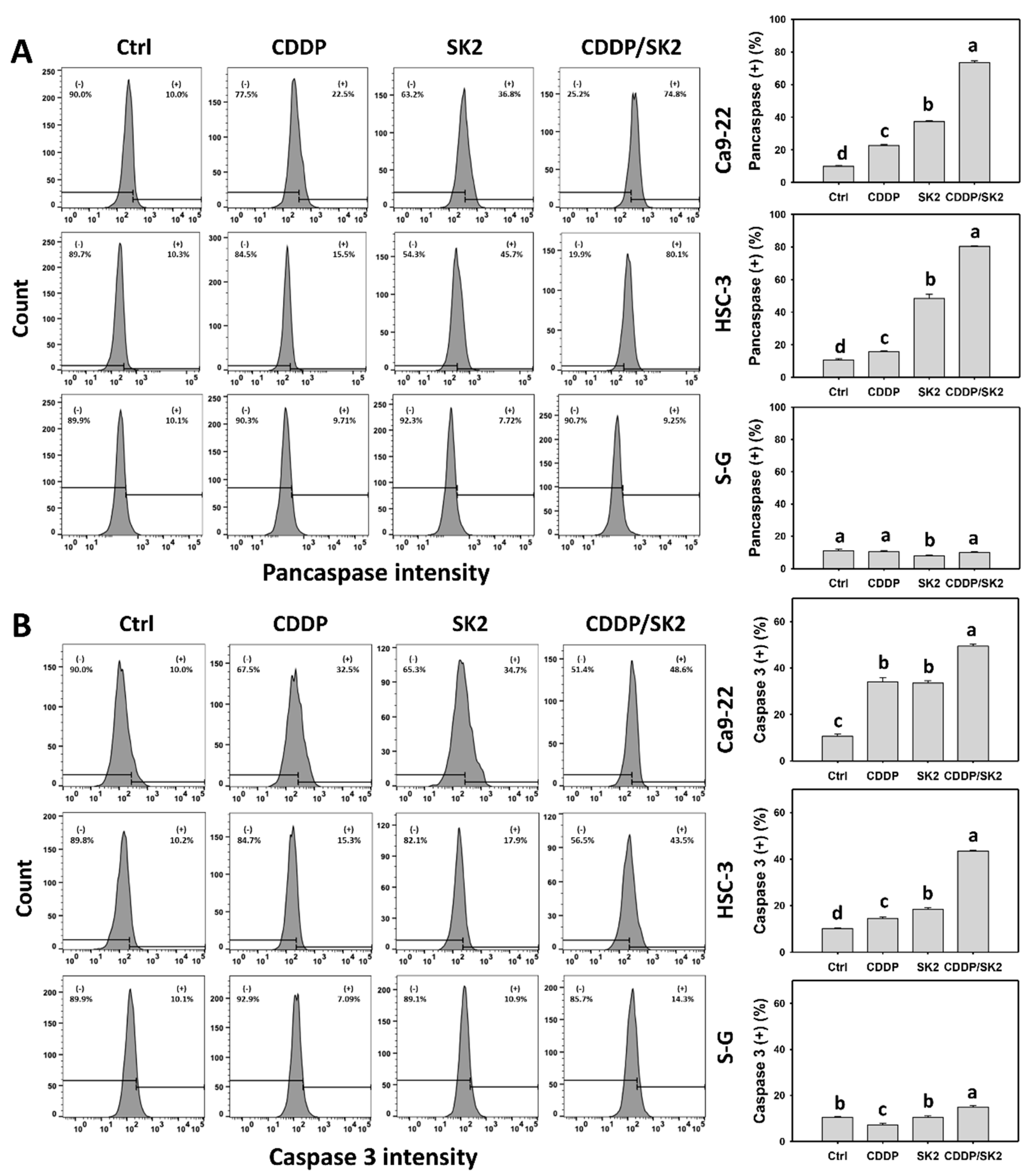
2.5. Caspase (Cas)-Apoptosis Assay
2.6. ROS, MitoSOX, and Mitochondrial Membrane Potential (MMP) Assay
2.7. γH2AX and 8-Hydroxy-2′-deoxyguanosine (8-OHdG) Assays
2.8. Statistics
3. Results
3.1. Cisplatin/SK2 (CDDP/SK2) Combined Treatment Causes Synergistic Antiproliferation in Oral Cancer Cells
3.2. Cisplatin/SK2 Causes Synergistic subG1 Accumulation in Oral Cancer Cells
3.3. Cisplatin/SK2 Causes Synergistic Annexin-V-Detected Apoptosis in Oral Cancer Cells
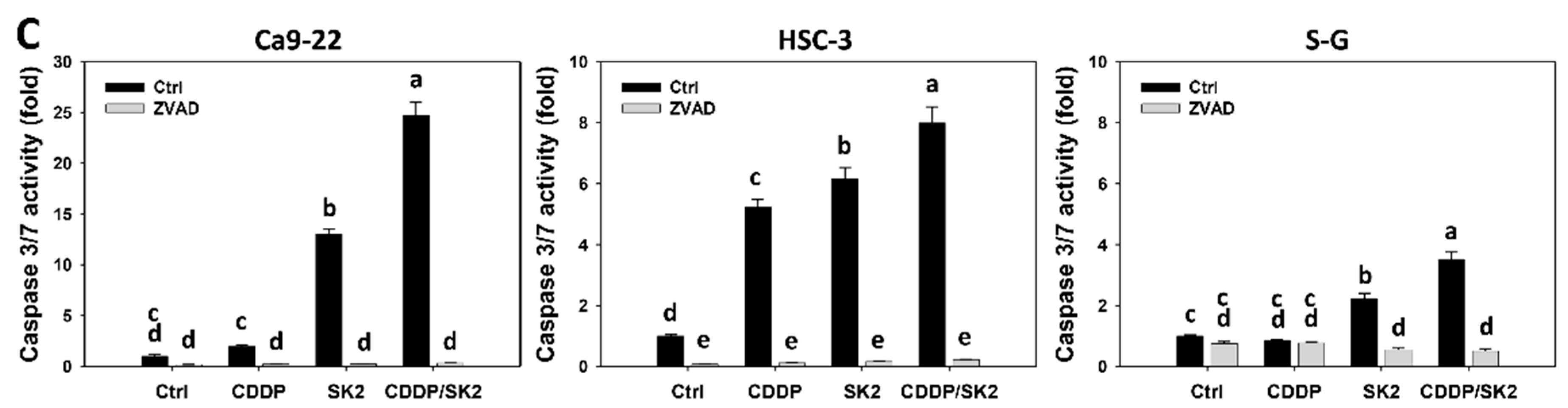
3.4. Cisplatin/SK2 Shows Synergistic Apoptosis (Caspase Activation) in Oral Cancer Cells
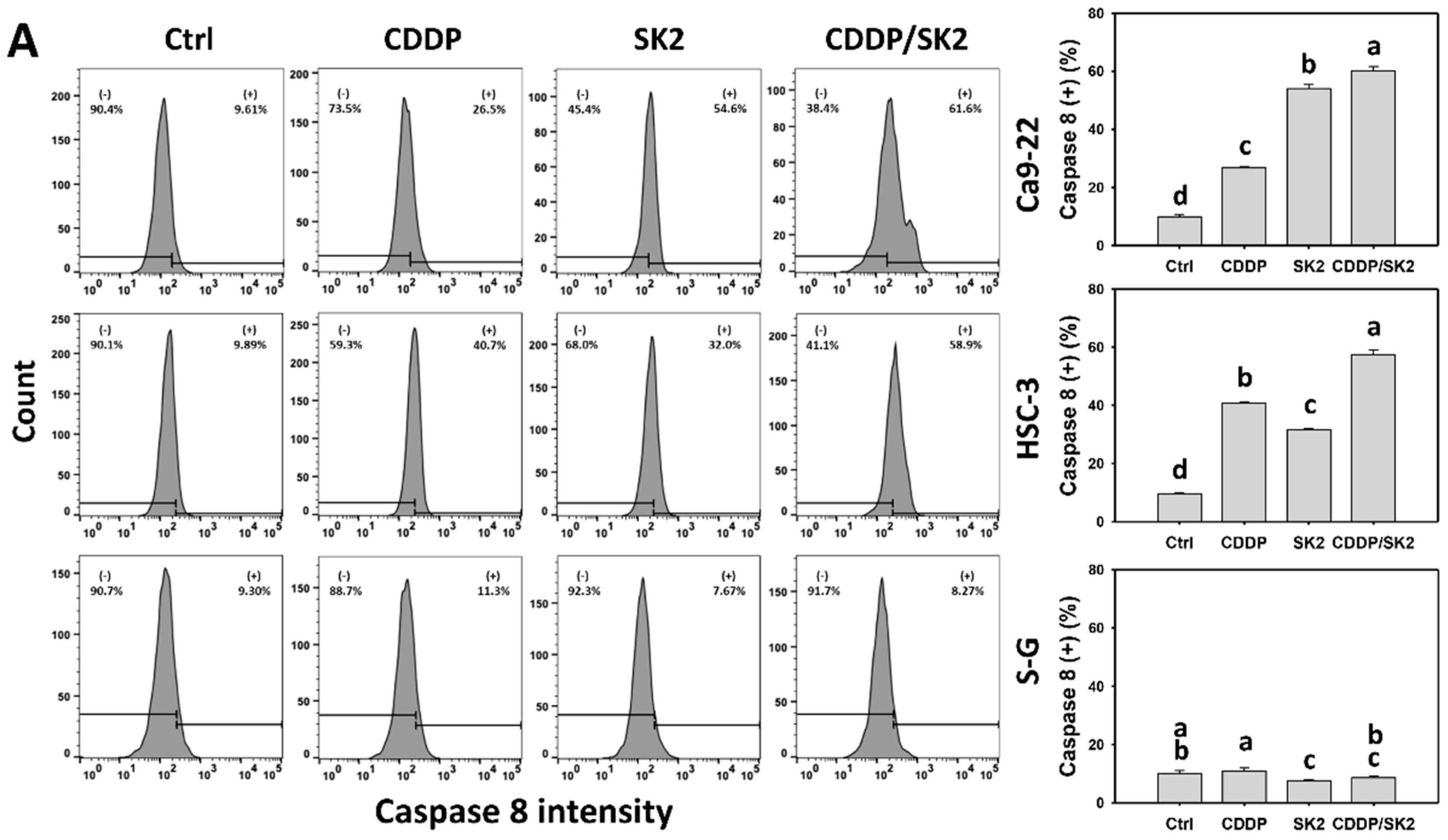
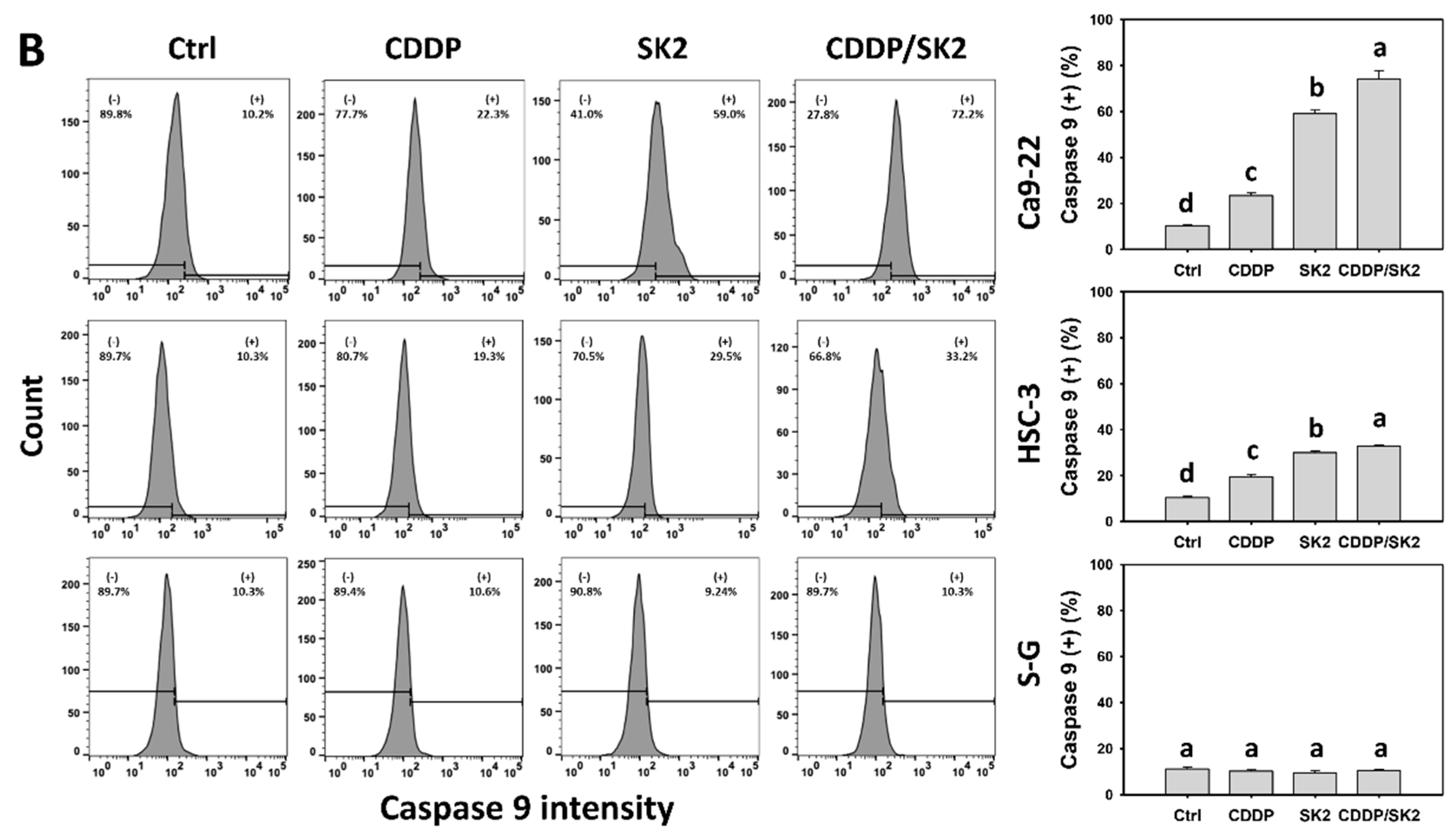
3.5. Cisplatin/SK2 Shows Synergistic Induction of Extrinsic and Intrinsic Apoptosis in Oral Cancer Cells
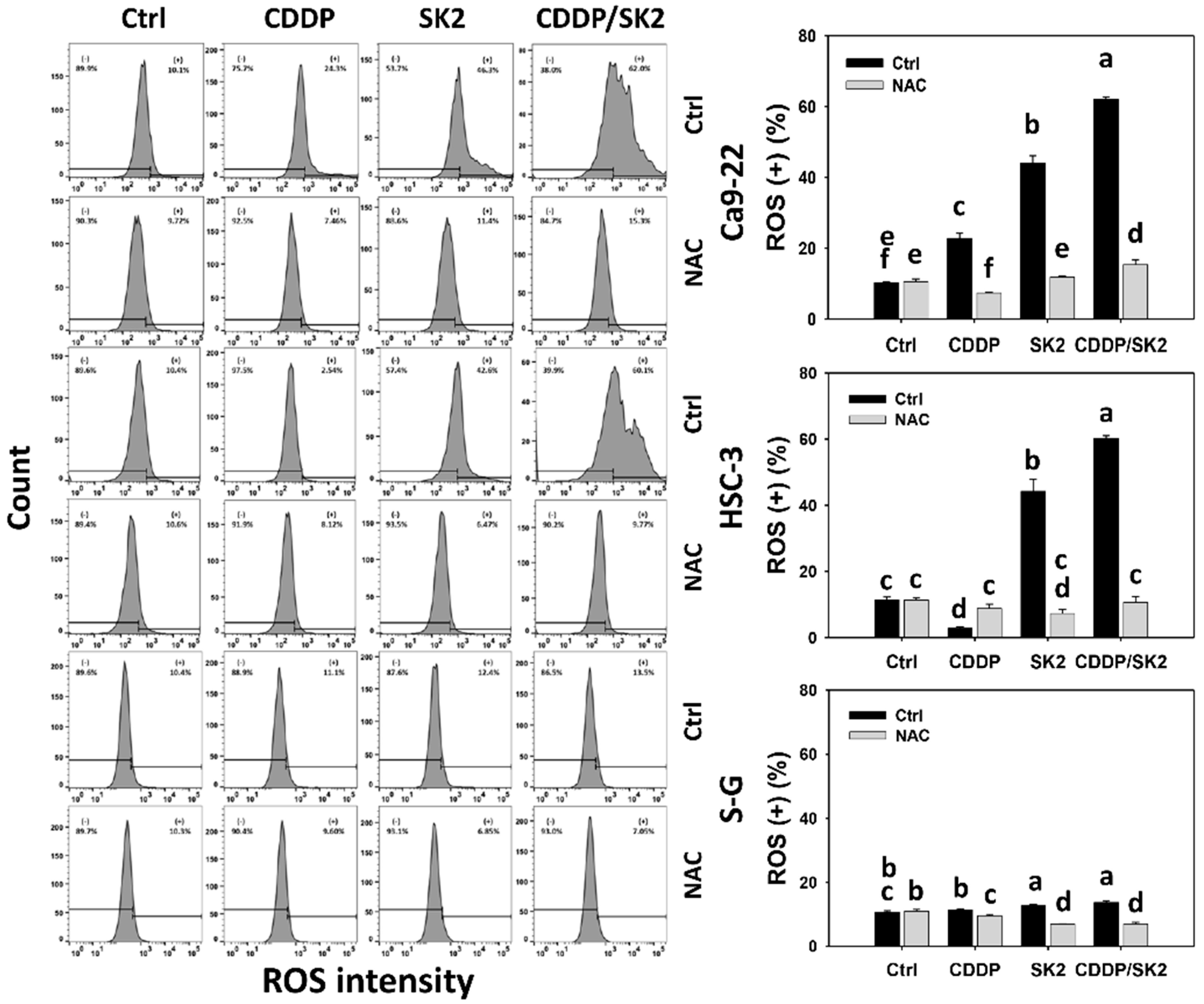
3.6. Cisplatin/SK2 Combined Treatment Shows Synergistic Induction of ROS in Oral Cancer Cells
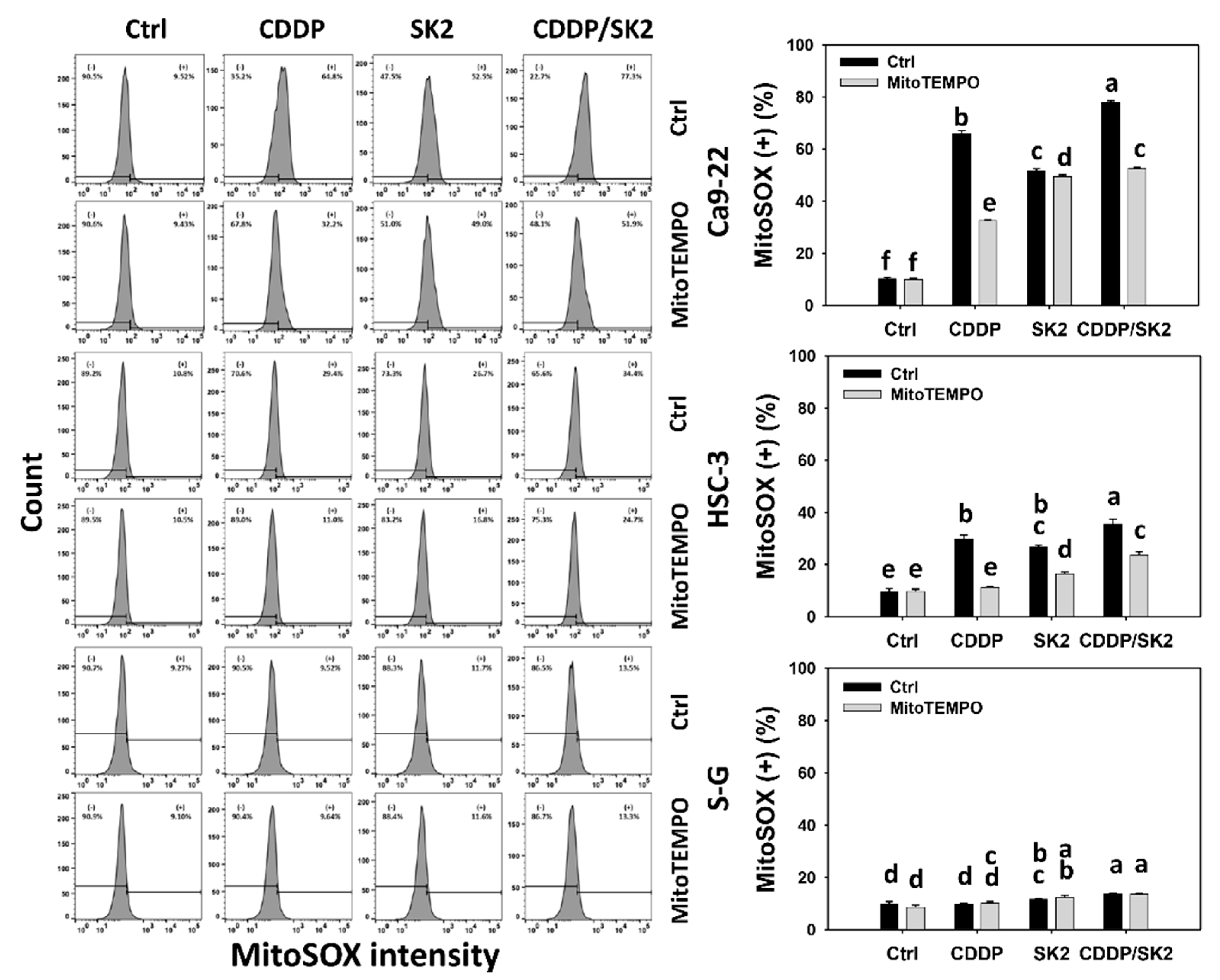
3.7. Cisplatin/SK2 Shows Synergistic Induction of MitoSOX in Oral Cancer Cells
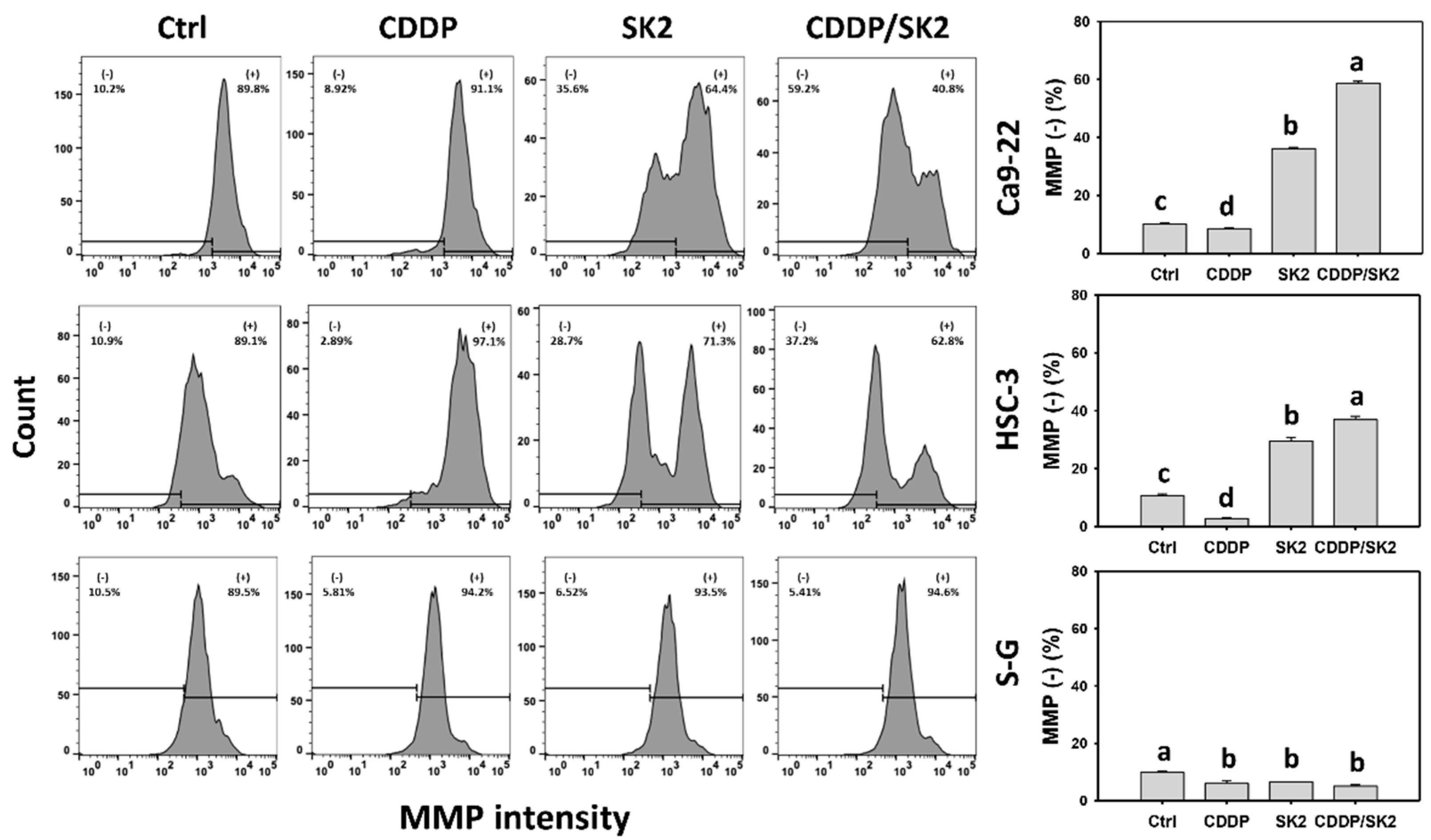
3.8. Cisplatin/SK2 Shows Synergistic MMP Destruction of Oral Cancer Cells
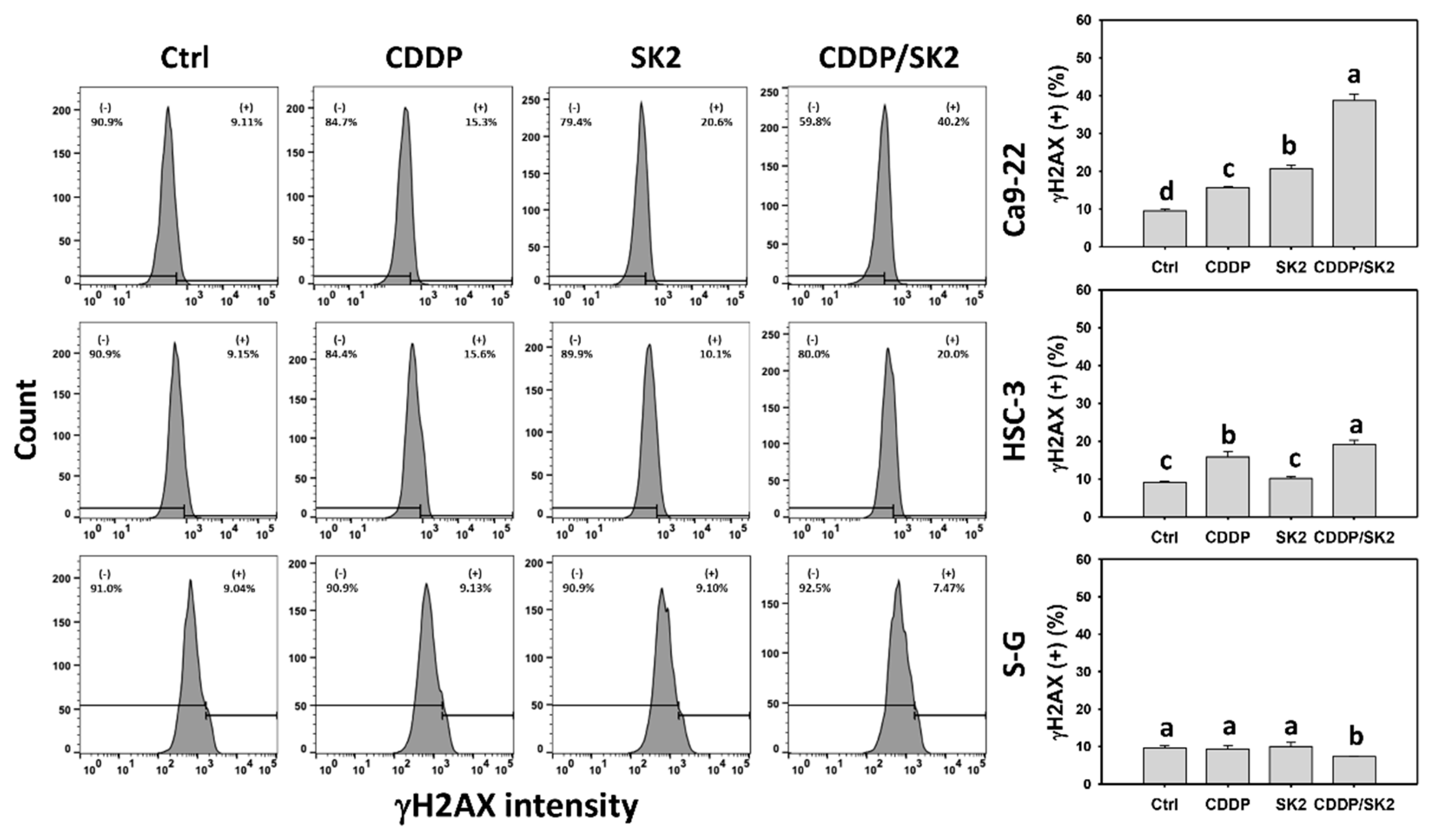
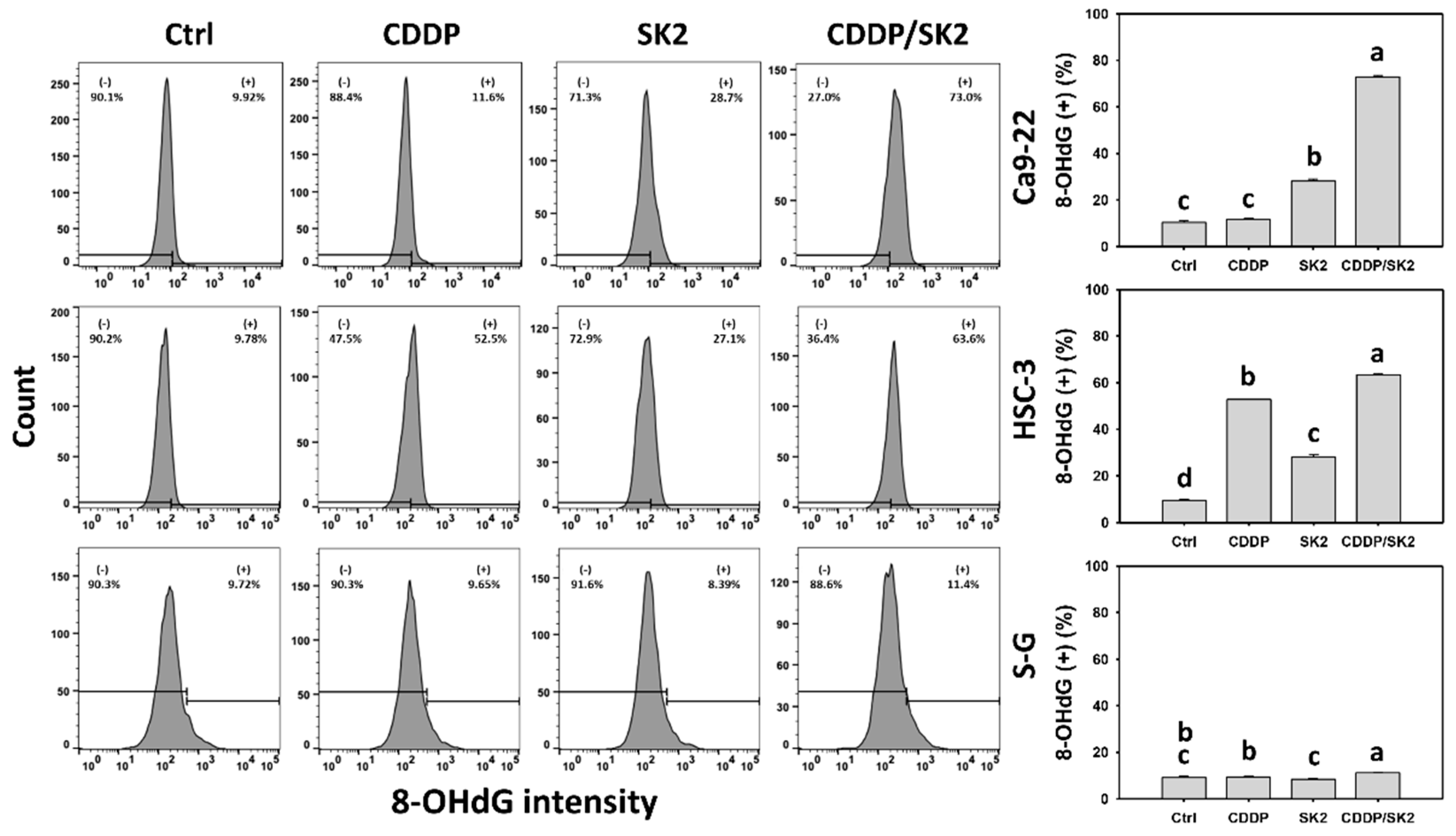
3.9. Cisplatin/SK2 Shows Synergistic Induction of γH2AX and 8-OHdG in Oral Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Health Promotion Administration, Ministry of Health and Welfare. Cancer Registry Annual Report; Health Promotion Administration, Ministry of Health and Welfare: Taiwan, China, 2016. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.E.; Grunfeld, E.A.; McGurk, M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005, 41, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Moeller, B.J.; Richardson, R.A.; Dewhirst, M.W. Hypoxia and radiotherapy: Opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007, 26, 241–248. [Google Scholar] [CrossRef]
- Riesco-Martinez, M.; Parra, K.; Saluja, R.; Francia, G.; Emmenegger, U. Resistance to metronomic chemotherapy and ways to overcome it. Cancer Lett. 2017, 400, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Sheng Sow, H.; Mattarollo, S.R. Combining low-dose or metronomic chemotherapy with anticancer vaccines: A therapeutic opportunity for lymphomas. Oncoimmunology 2013, 2, e27058. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-W.K.; Chang, V.H.S. Animal models and in vivo investigations for drug repurposing in lung cancer. In Drug Repurposing in Cancer Therapy; To, K.K.W., Cho, W.C.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 273–293. [Google Scholar]
- Pan, Y.; Shao, D.; Zhao, Y.; Zhang, F.; Zheng, X.; Tan, Y.; He, K.; Li, J.; Chen, L. Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK- HIF-1alpha. Int. J. Biol. Sci. 2017, 13, 794–803. [Google Scholar] [CrossRef]
- Perry, J.M.; Tao, F.; Roy, A.; Lin, T.; He, X.C.; Chen, S.; Lu, X.; Nemechek, J.; Ruan, L.; Yu, X.; et al. Overcoming Wnt-beta-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat. Cell Biol. 2020, 22, 689–700. [Google Scholar] [CrossRef]
- Lin, A. Radiation therapy for oral cavity and oropharyngeal cancers. Dent. Clin. North Am. 2018, 62, 99–109. [Google Scholar] [CrossRef]
- Hartner, L. Chemotherapy for oral cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, L.; Huang, B.; Li, X.; Yang, L.; Hu, X.; Jiang, Y.; Shao, Z.; Wang, Z. Efficacy and mechanism of the combination of PARP and CDK4/6 inhibitors in the treatment of triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 122. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.T.; Zhang, L.Y.; Shan, F.Y.; Shen, M.H.; Ruan, S.M. Jiedu Sangen decoction inhibits chemoresistance to 5-fluorouracil of colorectal cancer cells by suppressing glycolysis via PI3K/AKT/HIF-1alpha signaling pathway. Chin. J. Nat. Med. 2021, 19, 143–152. [Google Scholar] [PubMed]
- Petrenko, M.; Guttler, A.; Funtan, A.; Kessler, J.; Emmerich, D.; Paschke, R.; Vordermark, D.; Bache, M. Combined 3-O-acetylbetulin treatment and carbonic anhydrase IX inhibition results in additive effects on human breast cancer cells. Chem. Biol. Interact. 2021, 333, 109326. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Li, Y.; Ren, X.; Zhou, Y.; Hu, W.; Zhou, C.; Jing, Q.; Yang, C.; Wang, L.; et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis. 2021, 12, 705. [Google Scholar] [CrossRef]
- Yu, Y.; Gaillard, S.; Phillip, J.M.; Huang, T.C.; Pinto, S.M.; Tessarollo, N.G.; Zhang, Z.; Pandey, A.; Wirtz, D.; Ayhan, A.; et al. Inhibition of spleen tyrosine kinase potentiates paclitaxel-induced cytotoxicity in ovarian cancer cells by stabilizing microtubules. Cancer Cell 2015, 28, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Ho, J.N.; Byun, S.S.; Lee, S.; Oh, J.J.; Hong, S.K.; Lee, S.E.; Yeon, J.S. Synergistic antitumor effect of triptolide and cisplatin in cisplatin resistant human bladder cancer cells. J. Urol. 2015, 193, 1016–1022. [Google Scholar] [CrossRef]
- Lee, J.G.; Wu, R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1alpha in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia 2015, 17, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, T.; Chen, T.; Yang, T.; Ren, H.; Chen, M. Combination treatment of FTY720 and cisplatin exhibits enhanced antitumour effects on cisplatin-resistant non-small lung cancer cells. Oncol. Rep. 2018, 39, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.C.; Chang, M.Y.; Shiau, J.P.; Farooqi, A.A.; Huang, Y.H.; Tang, J.Y.; Chang, H.W. Antiproliferation- and apoptosis-inducible effects of a novel nitrated [6,6,6]tricycle derivative (SK2) on oral cancer cells. Molecules 2022, 27, 1576. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Wu, C.F.; Lee, M.G.; El-Shazly, M.; Lai, K.H.; Ke, S.C.; Su, C.W.; Shih, S.P.; Sung, P.J.; Hong, M.C.; Wen, Z.H.; et al. Isoaaptamine induces T-47D cells apoptosis and autophagy via oxidative stress. Mar. Drugs 2018, 16, 18. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yen, C.Y.; Wang, H.R.; Yang, H.P.; Tang, J.Y.; Huang, H.W.; Hsu, S.H.; Chang, H.W. Tenuifolide B from Cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damage. Toxins 2016, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Kasten, F.H.; Pineda, L.F.; Schneider, P.E.; Rawls, H.R.; Foster, T.A. Biocompatibility testing of an experimental fluoride releasing resin using human gingival epithelial cells in vitro. Vitr. Cell Dev. Biol. 1989, 25, 57–62. [Google Scholar] [CrossRef]
- Kasten, F.H.; Soileau, K.; Meffert, R.M. Quantitative evaluation of human gingival epithelial cell attachment to implant surfaces in vitro. Int. J. Periodontics Restor. Dent. 1990, 10, 68–79. [Google Scholar]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Shih, Y.L.; Lee, M.H.; Au, M.K.; Chen, Y.L.; Lu, H.F.; Chung, J.G. Bufalin induces apoptosis of human osteosarcoma U-2 OS cells through endoplasmic reticulum stress, caspase- and mitochondria-dependent signaling pathways. Molecules 2017, 22, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.C.; Wang, Y.Y.; Lin, L.C.; Chang, M.Y.; Yuan, S.F.; Tang, J.Y.; Chang, H.W. Combined treatment of sulfonyl chromen-4-ones (CHW09) and ultraviolet-C (UVC) enhances proliferation inhibition, apoptosis, oxidative stress, and DNA damage against oral cancer cells. Int. J. Mol. Sci. 2020, 21, 6443. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Chen, P.H.; Tang, J.Y.; Yen, C.Y.; Su, Y.C.; Huang, M.Y.; Chang, H.W. Manoalide shows mutual interaction between cellular and mitochondrial reactive species with apoptosis in oral cancer cells. Oxid. Med. Cell Longev. 2021, 2021, 6667355. [Google Scholar] [CrossRef]
- Monteiro, L.B.; Davanzo, G.G.; de Aguiar, C.F.; Moraes-Vieira, P.M.M. Using flow cytometry for mitochondrial assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef]
- Tang, J.Y.; Li, L.J.; Ou-Yang, F.; Wang, C.L.; Shu, C.W.; Wu, K.H.; Wang, H.R.; Yen, C.H.; Cheng, Y.B.; Chang, H.W. Ethyl acetate extract of Nepenthes ventricosa x maxima exerts preferential killing to oral cancer cells. DNA Cell Biol. 2019, 38, 763–772. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hao, L.J.; Hung, C.P.; Chen, J.W.; Leu, S.F.; Huang, B.M. Apoptotic effect of cisplatin and cordycepin on OC3 human oral cancer cells. Chin. J. Integr. Med. 2014, 20, 624–632. [Google Scholar] [CrossRef]
- Tang, J.Y.; Shu, C.W.; Wang, C.L.; Wang, S.C.; Chang, M.Y.; Lin, L.C.; Chang, H.W. Sulfonyl chromen-4-ones (CHW09) shows an additive effect to inhibit cell growth of X-ray irradiated oral cancer cells, involving apoptosis and ROS generation. Int. J. Radiat. Biol. 2019, 95, 1226–1235. [Google Scholar] [CrossRef]
- Aiguade, J.; Hao, J.; Forsyth, C.J. Synthesis of a 2,9-dioxabicyclo[3.3.1]nonane via double intramolecular Hetero-Michael addition: Entry to the F−G ring system of the azaspiracids. Org. Lett. 2001, 3, 979–982. [Google Scholar] [CrossRef]
- El Amrani, M.; Lai, D.; Debbab, A.; Aly, A.H.; Siems, K.; Seidel, C.; Schnekenburger, M.; Gaigneaux, A.; Diederich, M.; Feger, D. Protein kinase and HDAC inhibitors from the endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2014, 77, 49–56. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, J.Y.; Yang, J.; Lin, T.; Zeng, L.; Xu, J.F.; Hou, J.L.; Zhang, X. Targeting the cell membrane by charge-reversal amphiphilic pillar[5]arene for the selective killing of cancer cells. ACS Appl. Mater. Interfaces 2019, 11, 38497–38502. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.F.; Inague, A.; Arini, G.S.; Terra, L.F.; Wailemann, R.A.M.; Pimentel, A.C.; Yoshinaga, M.Y.; Silva, R.R.; Severino, D.; de Almeida, D.R.Q.; et al. Distinct photo-oxidation-induced cell death pathways lead to selective killing of human breast cancer cells. Cell Death Dis. 2020, 11, 1070. [Google Scholar] [CrossRef]
- Zhou, Y.; Gan, F.; Zhang, Y.; He, X.; Shen, C.; Qiu, H.; Liu, P. Selective killing of cancer cells by nonplanar aromatic hydrocarbon-induced DNA damage. Adv. Sci. 2019, 6, 1901341. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin selectively kills breast cancer cells showing G2/M arrest, apoptosis, and oxidative DNA damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, H.; Manda, T.; Matsumoto, S.; Mukumoto, S.; Nishigaki, F.; Kawamura, I.; Shimomura, K. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. III. Antitumor activities on experimental tumors in mice. J. Antibiot. 1994, 47, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Pluchino, L.A.; Choudhary, S.; Wang, H.C. Reactive oxygen species-mediated synergistic and preferential induction of cell death and reduction of clonogenic resistance in breast cancer cells by combined cisplatin and FK228. Cancer Lett. 2016, 381, 124–132. [Google Scholar] [CrossRef]
- Dewangan, J.; Tandon, D.; Srivastava, S.; Verma, A.K.; Yapuri, A.; Rath, S.K. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis 2017, 22, 1246–1259. [Google Scholar] [CrossRef]
- Nurdin, L.; Spasyuk, D.M.; Fairburn, L.; Piers, W.E.; Maron, L. Oxygen-oxygen bond cleavage and formation in Co(II)-mediated stoichiometric O2 reduction via the potential intermediacy of a Co(IV) oxyl radical. J. Am. Chem. Soc. 2018, 140, 16094–16105. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Y.; Koper, M.T.; Calle-Vallejo, F. Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis. J. Am. Chem. Soc. 2014, 136, 15694–15701. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, Z.W.; Ho, F.M.; Chan, W.H. Berberine impairs embryonic development in vitro and in vivo through oxidative stress-mediated apoptotic processes. Environ. Toxicol. 2018, 33, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Chen, C.Y.; Omar, H.A.; Huang, K.Y.; Tsao, C.C.; Chiu, C.C.; Chen, Y.L.; Chen, P.H.; Teng, Y.N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.C.; El-Shazly, M.; Juan, Y.S.; Chang, C.Y.; Su, J.H.; Chen, Y.C.; Shih, S.P.; Chen, H.M.; Wu, Y.C.; Lu, M.C. Cracking the cytotoxicity code: Apoptotic induction of 10-acetylirciformonin B is mediated through ROS generation and mitochondrial dysfunction. Mar. Drugs 2014, 12, 3072–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-C.; Yen, C.-Y.; Shiau, J.-P.; Chang, M.-Y.; Hou, M.-F.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Synergistic Antiproliferation of Cisplatin and Nitrated [6,6,6]Tricycle Derivative (SK2) for a Combined Treatment of Oral Cancer Cells. Antioxidants 2022, 11, 926. https://doi.org/10.3390/antiox11050926
Wang S-C, Yen C-Y, Shiau J-P, Chang M-Y, Hou M-F, Jeng J-H, Tang J-Y, Chang H-W. Synergistic Antiproliferation of Cisplatin and Nitrated [6,6,6]Tricycle Derivative (SK2) for a Combined Treatment of Oral Cancer Cells. Antioxidants. 2022; 11(5):926. https://doi.org/10.3390/antiox11050926
Chicago/Turabian StyleWang, Sheng-Chieh, Ching-Yu Yen, Jun-Ping Shiau, Meng-Yang Chang, Ming-Feng Hou, Jiiang-Huei Jeng, Jen-Yang Tang, and Hsueh-Wei Chang. 2022. "Synergistic Antiproliferation of Cisplatin and Nitrated [6,6,6]Tricycle Derivative (SK2) for a Combined Treatment of Oral Cancer Cells" Antioxidants 11, no. 5: 926. https://doi.org/10.3390/antiox11050926
APA StyleWang, S.-C., Yen, C.-Y., Shiau, J.-P., Chang, M.-Y., Hou, M.-F., Jeng, J.-H., Tang, J.-Y., & Chang, H.-W. (2022). Synergistic Antiproliferation of Cisplatin and Nitrated [6,6,6]Tricycle Derivative (SK2) for a Combined Treatment of Oral Cancer Cells. Antioxidants, 11(5), 926. https://doi.org/10.3390/antiox11050926






