Clinical and Preclinical Studies of Fermented Foods and Their Effects on Alzheimer’s Disease
Abstract
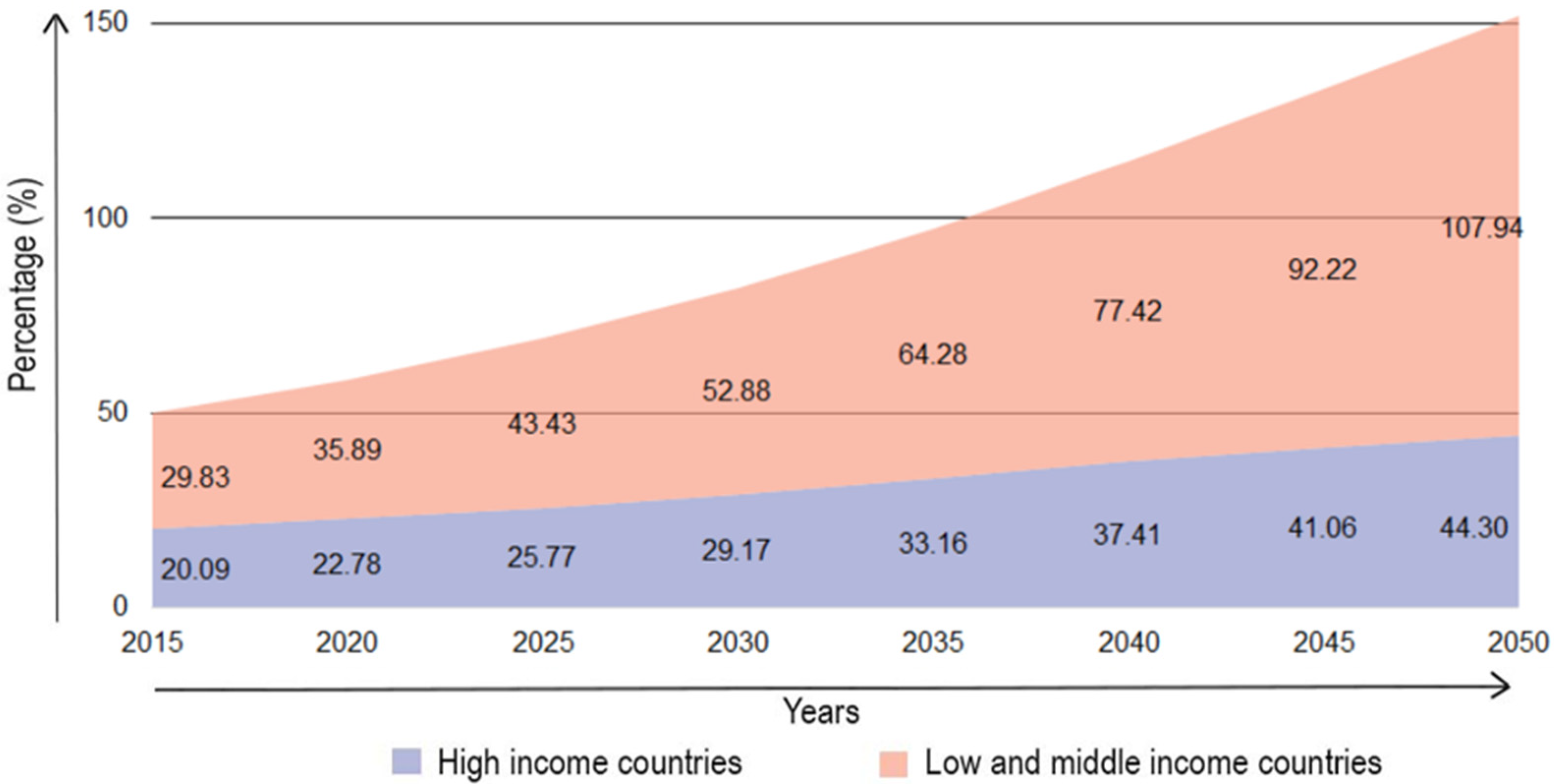
1. Introduction
2. Alzheimer’s Disease and Neuroinflammation
3. Fermented Food Products
4. Clinical Studies
5. Preclinical In Vivo Studies
5.1. Fermented Dairy Products
5.2. Kefir
5.3. Fermented Legumes and Cereal-Based Products
5.4. Fermented Plant Root Products
5.5. Fermented Fruit and Vegetable Products
5.6. Other Fermented Plant Products
5.7. Fungi
6. Preclinical In Vitro Studies
7. Gut–Brain Axis
8. Gut Microbiota and Development of Alzheimer’s Disease
9. Potential Mechanism of Fermented Foods on Gut–Brain Axis
9.1. Chemical Constituents Modulation
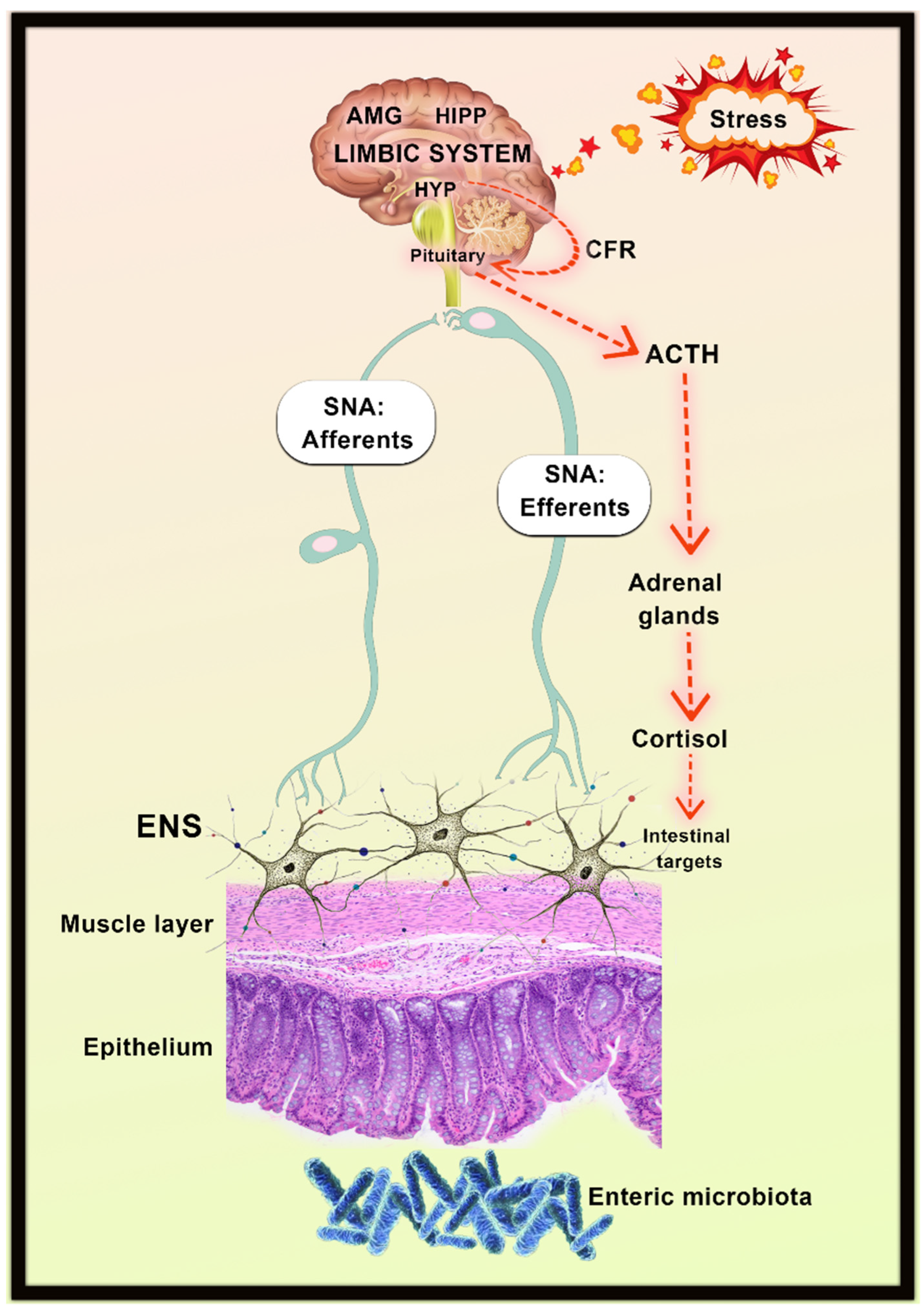
9.2. HPA Axis Inhibition
9.3. Neurochemical Modulation
10. Microbiota Modulation as a Therapeutic Target in Alzheimer’s Disease
11. Commercialized Fermented Products
12. Future Prospects and Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO|Dementia. Available online: http://www.who.int/mediacentre/factsheets/fs362/en/ (accessed on 22 February 2018).
- International, A.D.; Guerchet, M.; Prince, M.; Prina, M. Numbers of People with Dementia Worldwide: An Update to the Estimates in the World Alzheimer Report 2015. 2020. Available online: https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide/ (accessed on 1 April 2022).
- Nichols, E.; Vos, T. Estimating the Global Mortality from Alzheimer’s Disease and Other Dementias: A New Method and Results from the Global Burden of Disease Study 2019. Alzheimer’s Dement. 2020, 16, e042236. [Google Scholar] [CrossRef]
- Chen, G.; Xu, T.; Yan, Y.; Zhou, Y.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharm. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A Molecular Approach in Drug Development for Alzheimer’s Disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Adewumi, G.A. Health-Promoting Fermented Foods. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 399–418. [Google Scholar] [CrossRef]
- Li, K.J.; Brouwer-Brolsma, E.M.; Burton-Pimentel, K.J.; Vergères, G.; Feskens, E.J.M. A Systematic Review to Identify Biomarkers of Intake for Fermented Food Products. Genes Nutr. 2021, 16, 5. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Tsaltas, D. Chapter 10—Fermented Foods and Beverages. In Innovations in Traditional Foods; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 257–291. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Tamang, J.P. Religious Ethnic Foods. J. Ethn. Foods 2015, 2, 45–46. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Parveen Rani, R. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Int. 2014, 2014, e250424. [Google Scholar] [CrossRef]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of Probiotics on Cognition, and Biomarkers of Inflammation and Oxidative Stress in Adults with Alzheimer’s Disease or Mild Cognitive Impairment—A Meta-Analysis of Randomized Controlled Trials. Aging 2020, 12, 4010–4039. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, C.; Roman, P.; Rueda-Ruzafa, L.; Rodriguez-Arrastia, M.; Cardona, D. Effects of Probiotics Supplementation on Dementia and Cognitive Impairment: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110189. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Scholey, A.; Firth, J.; D’Cunha, N.M.; Lane, M.; Hockey, M.; Ashton, M.M.; Cryan, J.F.; O’Neil, A.; Naumovski, N.; et al. Prebiotics, Probiotics, Fermented Foods and Cognitive Outcomes: A Meta-Analysis of Randomized Controlled Trials. Neurosci. Biobehav. Rev. 2020, 118, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbi, F.; Mirghafourvand, M.; Shamekh, A.; Mahmoodpoor, A.; Sanaie, S. Effects of Probiotic Supplementation on Cognitive Function in Elderly: A Systematic Review and Meta-Analysis. Aging Ment. Health 2021, 25, 1–9. [Google Scholar] [CrossRef]
- Bekris, L.M.; Yu, C.-E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Piaceri, I.; Nacmias, B.; Sorbi, S. Genetics of Familial and Sporadic Alzheimer’s Disease. Front. Biosci. (Elite Ed.) 2013, 5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Guzmán-Martínez, L.; Cerda-Troncoso, C.; Farías, G.A.; Maccioni, R.B. Neuroinflammation in the Pathogenesis of Alzheimer’s Disease. A Rational Framework for the Search of Novel Therapeutic Approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Bronzuoli, M.R.; Iacomino, A.; Steardo, L.; Scuderi, C. Targeting Neuroinflammation in Alzheimer’s Disease. J. Inflamm. Res. 2016, 9, 199–208. [Google Scholar] [CrossRef]
- Anal, A.K. Quality Ingredients and Safety Concerns for Traditional Fermented Foods and Beverages from Asia: A Review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Prakash, J. Chapter 14—Safety of Fermented Cereals and Legumes. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martín-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 283–310. [Google Scholar] [CrossRef]
- Corona, O.; Randazzo, W.; Miceli, A.; Guarcello, R.; Francesca, N.; Erten, H.; Moschetti, G.; Settanni, L. Characterization of Kefir-like Beverages Produced from Vegetable Juices. LWT Food Sci. Technol. 2016, 66, 572–581. [Google Scholar] [CrossRef]
- Waché, Y.; Dijon, A. Flavours. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2013; pp. 175–193. [Google Scholar] [CrossRef]
- Marullo, P.; Dubourdieu, D. 11—Yeast Selection for Wine Flavour Modulation. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2010; pp. 293–345. [Google Scholar] [CrossRef]
- Walther, B.; Schmid, A. Chapter 7—Effect of Fermentation on Vitamin Content in Food. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 131–157. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Fermented Foods: Fermented Vegetables and Other Products. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 668–674. [Google Scholar] [CrossRef]
- Ilango, S.; Antony, U. Probiotic Microorganisms from Non-Dairy Traditional Fermented Foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Lavefve, L.; Marasini, D.; Carbonero, F. Chapter Three—Microbial Ecology of Fermented Vegetables and Non-Alcoholic Drinks and Current Knowledge on Their Impact on Human Health. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 87, pp. 147–185. [Google Scholar] [CrossRef]
- Ray, R.C.; Joshi, V.K. Fermented Foods: Past, Present and Future. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Terefe, N.S. Emerging Trends and Opportunities in Food Fermentation. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Kiers, J.L.; Van Laeken, A.E.A.; Rombouts, F.M.; Nout, M.J.R. In Vitro Digestibility of Bacillus Fermented Soya Bean. Int. J. Food Microbiol. 2000, 60, 163–169. [Google Scholar] [CrossRef]
- Chen, P.; Dong, J.; Yin, H.; Bao, X.; Chen, L.; He, Y.; Chen, R.; Wan, X.; Zhao, Y.; Hou, X. Genome Comparison and Evolutionary Analysis of Different Industrial Lager Yeasts (Saccharomyces pastorianus). J. Inst. Brew. 2016, 122, 42–47. [Google Scholar] [CrossRef]
- Sicard, D.; Legras, J.-L. Bread, Beer and Wine: Yeast Domestication in the Saccharomyces sensu stricto Complex. Comptes Rendus Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nout, M.J.R.; Aidoo, K.E. Asian Fungal Fermented Food. In Industrial Applications; Osiewacz, H.D., Ed.; The Mycota; Springer: Berlin/Heidelberg, Germany, 2002; pp. 23–47. [Google Scholar] [CrossRef]
- Joshi, V.K.; Parmar, M.; Rana, N.S. Pectin Esterase Production from Apple Pomace in Solid-State and Submerged Fermentations. Food Technol. Biotechnol. 2006, 44, 253–256. [Google Scholar]
- Hasan, M.N.; Sultan, M.Z.; Mar-E-Um, M. Significance of Fermented Food in Nutrition and Food Science. J. Sci. Res. 2014, 6, 373–386. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Röytiö, H. 1—Probiotic Fermented Foods and Health Promotion. In Advances in Fermented Foods and Beverages; Holzapfel, W., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–22. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.Y.; Hur, S.J. Effects of Different Starter Cultures on the Biogenic Amine Concentrations, Mutagenicity, Oxidative Stress, and Neuroprotective Activity of Fermented Sausages and Their Relationships. J. Funct. Foods 2019, 52, 424–429. [Google Scholar] [CrossRef]
- Kang, S.J.; Seo, J.Y.; Cho, K.M.; Lee, C.K.; Kim, J.H.; Kim, J.S. Antioxidant and Neuroprotective Effects of Doenjang Prepared with Rhizopus, Pichia, and Bacillus. Prev. Nutr. Food Sci. 2016, 21, 221–226. [Google Scholar] [CrossRef]
- Hwang, J.H.; Wu, S.J.; Wu, P.L.; Shih, Y.Y.; Chan, Y.C. Neuroprotective Effect of Tempeh against Lipopolysaccharide-Induced Damage in BV-2 Microglial Cells. Nutr. Neurosci. 2019, 22, 840–849. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Araújo, T.H.; Schneedorf, J.M.; Ferreira, C.d.S.; Moraes, G.d.O.I.; Coimbra, R.S.; Rodrigues, M.R. A Novel Beer Fermented by Kefir Enhances Anti-Inflammatory and Anti-Ulcerogenic Activities Found Isolated in Its Constituents. J. Funct. Foods 2016, 21, 58–69. [Google Scholar] [CrossRef]
- Kawahara, M.; Nemoto, M.; Nakata, T.; Kondo, S.; Takahashi, H.; Kimura, B.; Kuda, T. Anti-Inflammatory Properties of Fermented Soy Milk with Lactococcus lactis Subsp. lactis S-SU2 in Murine Macrophage RAW264.7 Cells and DSS-Induced IBD Model Mice. Int. Immunopharmacol. 2015, 26, 295–303. [Google Scholar] [CrossRef]
- Maeno, M.; Yamamoto, N.; Takano, T. Identification of an Antihypertensive Peptide from Casein Hydrolysate Produced by a Proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1996, 79, 1316–1321. [Google Scholar] [CrossRef]
- Li, C.; Kwok, L.Y.; Mi, Z.; Bala, J.; Xue, J.; Yang, J.; Ma, Y.; Zhang, H.; Chen, Y. Characterization of the Angiotensin-Converting Enzyme Inhibitory Activity of Fermented Milks Produced with Lactobacillus casei. J. Dairy Sci. 2017, 100, 9495–9507. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Q.; Ji, Z.; Shu, G.; Chen, H. Production and Fermentation Characteristics of Angiotensin-I-Converting Enzyme Inhibitory Peptides of Goat Milk Fermented by a Novel Wild Lactobacillus plantarum 69. LWT Food Sci. Technol. 2018, 91, 532–540. [Google Scholar] [CrossRef]
- Solanki, D.; Hati, S. Considering the Potential of Lactobacillus rhamnosus for Producing Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides in Fermented Camel Milk (Indian Breed). Food Biosci. 2018, 23, 16–22. [Google Scholar] [CrossRef]
- Nejati, F.; Rizzello, C.G.; Di Cagno, R.; Sheikh-Zeinoddin, M.; Diviccaro, A.; Minervini, F.; Gobbetti, M. Manufacture of a Functional Fermented Milk Enriched of Angiotensin-I Converting Enzyme (ACE)-Inhibitory Peptides and γ-Amino Butyric Acid (GABA). LWT Food Sci. Technol. 2013, 51, 183–189. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Torino, M.I.; Martín, V.; Arroyo, R.; Garcia-Mora, P.; Estrella Pedrola, I.; Vidal-Valverde, C.; Rodriguez, J.M.; Frias, J. Multifunctional Properties of Soy Milk Fermented by Enterococcus faecium Strains Isolated from Raw Soy Milk. J. Agric. Food Chem. 2012, 60, 10235–10244. [Google Scholar] [CrossRef]
- Liu, C.F.; Tung, Y.T.; Wu, C.L.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Antihypertensive Effects of Lactobacillus-Fermented Milk Orally Administered to Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2011, 59, 4537–4543. [Google Scholar] [CrossRef]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood Pressure Lowering Effect of a Novel Fermented Milk Containg GABA in Mild Hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef]
- Pouliot-Mathieu, K.; Gardner-Fortier, C.; Lemieux, S.; St-Gelais, D.; Champagne, C.P.; Vuillemard, J.C. Effect of Cheese Containing Gamma-Aminobutyric Acid-Producing Lactic Acid Bacteria on Blood Pressure in Men. PharmaNutrition 2013, 1, 141–148. [Google Scholar] [CrossRef]
- Suwanmanon, K.; Hsieh, P.C. Effect of γ-Aminobutyric Acid and Nattokinase-Enriched Fermented Beans on the Blood Pressure of Spontaneously Hypertensive and Normotensive Wistar-Kyoto Rats. J. Food Drug Anal. 2014, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- GRUNEWALD, K.K. Serum Cholesterol Levels in Rats Fed Skim Milk Fermented by Lactobacillus acidophilus. J. Food Sci. 1982, 47, 2078–2079. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, M.J.; Yang, H.J.; Park, S. Isoflavonoids and Peptides from Meju, Long-Term Fermented Soybeans, Increase Insulin Sensitivity and Exert Insulinotropic Effects In Vitro. Nutrition 2011, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D. Diversity of Bacteriocinogenic Lactic Acid Bacteria Isolated from Boza, a Cereal-Based Fermented Beverage from Bulgaria. Food Control 2010, 21, 1011–1021. [Google Scholar] [CrossRef]
- Aslim, B.; Yuksekdag, Z.N.; Sarikaya, E.; Beyatli, Y. Determination of the Bacteriocin-like Substances Produced by Some Lactic Acid Bacteria Isolated from Turkish Dairy Products. LWT Food Sci. Technol. 2005, 38, 691–694. [Google Scholar] [CrossRef]
- Iranmanesh, M.; Ezzatpanah, H.; Mojgani, N. Antibacterial Activity and Cholesterol Assimilation of Lactic Acid Bacteria Isolated from Traditional Iranian Dairy Products. LWT Food Sci. Technol. 2014, 58, 355–359. [Google Scholar] [CrossRef]
- Luo, F.; Feng, S.; Sun, Q.; Xiang, W.; Zhao, J.; Zhang, J.; Yang, Z. Screening for Bacteriocin-Producing Lactic Acid Bacteria from Kurut, a Traditional Naturally-Fermented Yak Milk from Qinghai-Tibet Plateau. Food Control 2011, 22, 50–53. [Google Scholar] [CrossRef]
- Bhanja, T.; Kumari, A.; Banerjee, R. Enrichment of Phenolics and Free Radical Scavenging Property of Wheat Koji Prepared with Two Filamentous Fungi. Bioresour. Technol. 2009, 100, 2861–2866. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, S.J.; Shyu, Y.T. Antioxidant Properties of Certain Cereals as Affected by Food-Grade Bacteria Fermentation. J. Biosci. Bioeng. 2014, 117, 449–456. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Kuhad, R.C. Upgrading the Antioxidant Potential of Cereals by Their Fungal Fermentation under Solid-State Cultivation Conditions. Lett. Appl. Microbiol. 2014, 59, 493–499. [Google Scholar] [CrossRef]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D. 21.Comparative Study of the Effects of Solid-State Fermentation with. J. Agric. Food Chem. 2012, 60, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the Antioxidant Capacity of Soy Whey by Fermentation with Lactobacillus plantarum B1–6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.H.; Seo, W.T.; Yun, H.D.; Hong, S.Y.; Kim, M.K.; Cho, K.M. The Production of Surfactin during the Fermentation of Cheonggukjang by Potential Probiotic Bacillus subtilis CSY191 and the Resultant Growth Suppression of MCF-7 Human Breast Cancer Cells. Food Chem. 2012, 131, 1347–1354. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In Vitro Investigation of Anticancer, Antihypertensive, Antidiabetic, and Antioxidant Activities of Camel Milk Fermented with Camel Milk Probiotic: A Comparative Study with Fermented Bovine Milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef]
- Ebringer, L.; Ferenčík, M.; Krajčovič, J. Beneficial Health Effects of Milk and Fermented Dairy Products—Review. Folia Microbiol. 2008, 53, 378–394. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and Phytic Acid Interactions: Is It a Real Problem for Human Nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Tasdemir, S.S.; Sanlier, N. An Insight into the Anticancer Effects of Fermented Foods: A Review. J. Funct. Foods 2020, 75, 104281. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra e Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; Pereira, T.M.C.; et al. Oxidative Stress and Dementia in Alzheimer’s Patients: Effects of Synbiotic Supplementation. Oxidative Med. Cell. Longev. 2020, 2020, e2638703. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Kim, D.S.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented Milk of Lactobacillus helveticus IDCC3801 Improves Cognitive Functioning during Cognitive Fatigue Tests in Healthy Older Adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Ohsawa, K.; Nakamura, F.; Uchida, N.; Mizuno, S.; Yokogoshi, H. Lactobacillus helveticus-Fermented Milk Containing Lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) Improves Cognitive Function in Healthy Middle-Aged Adults: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Food. Sci. Nutr. 2018, 69, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Park, S.; Paik, J.-W.; Chae, S.-W.; Kim, D.-H.; Jeong, D.-G.; Ha, E.; Kim, M.; Hong, G.; Park, S.-H.; et al. Efficacy and Safety of Lactobacillus plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Mursjid, F.; Priandini, D.; Setyawan, H.; Ismael, R.I.; Bandelow, S.; Rahardjo, T.B. Borobudur Revisited: Soy Consumption May Be Associated with Better Recall in Younger, but Not in Older, Rural Indonesian Elderly. Brain Res. 2011, 1379, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Handajani, Y.S.; Turana, Y.; Yogiara, Y.; Widjaja, N.T.; Sani, T.P.; Christianto, G.A.M.; Suwanto, A. Tempeh Consumption and Cognitive Improvement in Mild Cognitive Impairment. DEM 2020, 49, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary Patterns and Risk of Dementia in an Elderly Japanese Population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Marotta, F.; Dominguez, L.J. Oxidative Stress in Patients with Alzheimer’s Disease: Effect of Extracts of Fermented Papaya Powder. Mediat. Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Bhatia, V.; Sharma, S. Role of Mitochondrial Dysfunction, Oxidative Stress and Autophagy in Progression of Alzheimer’s Disease. J. Neurol. Sci. 2021, 421, 117253. [Google Scholar] [CrossRef]
- Agrawal, I.; Jha, S. Mitochondrial Dysfunction and Alzheimer’s Disease: Role of Microglia. Front. Aging Neurosci. 2020, 12, 252. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharm. 2019, 176, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Sadjimim, T.; Yesufu, A.; Kreager, P.; Rahardjo, T.B. High Tofu Intake Is Associated with Worse Memory in Elderly Indonesian Men and Women. Dement. Geriatr. Cogn. Disord. 2008, 26, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of Reactive Oxygen Species in the Progression of Alzheimer’s Disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive Effects of a Fermented Dairy Product against Alzheimer’s Disease and Identification of a Novel Oleamide with Enhanced Microglial Phagocytosis and Anti-Inflammatory Activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef]
- Liu, T.-H.; Chiou, J.; Tsai, T.-Y. Effects of Lactobacillus plantarum TWK10-Fermented Soymilk on Deoxycorticosterone Acetate-Salt-Induced Hypertension and Associated Dementia in Rats. Nutrients 2016, 8, 260. [Google Scholar] [CrossRef]
- Ohsawa, K.; Uchida, N.; Ohki, K.; Nakamura, Y.; Yokogoshi, H. Lactobacillus helveticus-Fermented Milk Improves Learning and Memory in Mice. Nutr. Neurosci. 2015, 18, 232–240. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel Lactopeptides in Fermented Dairy Products Improve Memory Function and Cognitive Decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef]
- Ano, Y.; Yoshino, Y.; Kutsukake, T.; Ohya, R.; Fukuda, T.; Uchida, K.; Takashima, A.; Nakayama, H. Tryptophan-Related Dipeptides in Fermented Dairy Products Suppress Microglial Activation and Prevent Cognitive Decline. Aging 2019, 11, 2949–2967. [Google Scholar] [CrossRef]
- Liu, J.; Yu, C.; Li, R.; Liu, K.; Jin, G.; Ge, R.; Tang, F.; Cui, S. High-Altitude Tibetan Fermented Milk Ameliorated Cognitive Dysfunction by Modified Gut Microbiota in Alzheimer’s Disease Transgenic Mice. Food Funct. 2020, 11, 5308–5319. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Takaichi, Y.; Washinuma, T.; Uchida, K.; Takashima, A.; Nakayama, H. β-Lactolin, a Whey-Derived Lacto-Tetrapeptide, Prevents Alzheimer’s Disease Pathologies and Cognitive Decline. J. Alzheimer’s Dis. 2020, 73, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Kim, J.E.; Kwak, M.H.; Koh, E.K.; Song, S.H.; Sung, J.E.; Kim, D.S.; Hong, J.T.; Hwang, D.Y. Neuroprotective Effects of Fermented Soybean Products (Cheonggukjang) Manufactured by Mixed Culture of Bacillus subtilis MC31 and Lactobacillus sakei 383 on Trimethyltin-Induced Cognitive Defects Mice. Nutr. Neurosci. 2016, 19, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Kwon, D.Y.; Kim, H.J.; Kim, M.J.; Jung, D.Y.; Kang, H.J.; Kim, D.S.; Kang, S.; Moon, N.R.; Shin, B.K.; et al. Fermenting Soybeans with Bacillus Licheniformis Potentiates Their Capacity to Improve Cognitive Function and Glucose Homeostaisis in Diabetic Rats with Experimental Alzheimer’s Type Dementia. Eur. J. Nutr. 2015, 54, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Kuo, T.-F.; Wu, C.-L.; Wang, J.-J.; Pan, T.-M. Red Mold Rice Promotes Neuroprotective SAPPalpha Secretion Instead of Alzheimer’s Risk Factors and Amyloid Beta Expression in Hyperlipidemic Abeta40-Infused Rats. J. Agric. Food Chem. 2010, 58, 2230–2238. [Google Scholar] [CrossRef]
- Kanouchi, H.; Kakimoto, T.; Nakano, H.; Suzuki, M.; Nakai, Y.; Shiozaki, K.; Akikoka, K.; Otomaru, K.; Nagano, M.; Matsumoto, M. The Brewed Rice Vinegar Kurozu Increases HSPA1A Expression and Ameliorates Cognitive Dysfunction in Aged P8 Mice. PLoS ONE 2016, 11, e0150796. [Google Scholar] [CrossRef]
- Hamad, A.; Mani, V.; Ramasamy, K.; Lim, S.M.; Majeed, A. Memory Enhancement in Rats by Soybean and Tempeh Extracts Is Associated with Improved Cholinergic and Reduced Neuroinflammatory Activities (Peningkatan Daya Ingatan Dalam Tikus Oleh Ekstrak Soya Dan Tempeh Dikaitkan Dengan Peningkatan Aktiviti Kolinergik Dan Pengurangan Aktiviti Keradangan). Sains Malays. 2016, 45, 1299–1310. [Google Scholar]
- Chan, Y.-C.; Lee, I.-T.; Wang, M.-F.; Yeh, W.-C.; Liang, B.-C. Tempeh Attenuates Cognitive Deficit, Antioxidant Imbalance, and Amyloid β of Senescence-Accelerated Mice by Modulating Nrf2 Expression via MAPK Pathway. J. Funct. Foods 2018, 50, 112–119. [Google Scholar] [CrossRef]
- Ayuningtyas, A.; Murbawani, E.A.; Nuryanto, N. The Effect of Tempeh Intake on Spatial Memory in Prediabetic Rats. Nutr. Food Sci. 2018, 49, 592–599. [Google Scholar] [CrossRef]
- Yoo, D.-H.; Kim, D.-H. Lactobacillus pentosus Var. Plantarum C29 Increases the Protective Effect of Soybean against Scopolamine-Induced Memory Impairment in Mice. Int. J. Food Sci. Nutr. 2015, 66, 912–918. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hwang, Y.-H.; Kim, D.-H. Lactobacillus plantarum C29-Fermented Soybean (DW2009) Alleviates Memory Impairment in 5XFAD Transgenic Mice by Regulating Microglia Activation and Gut Microbiota Composition. Mol. Nutr. Food Res. 2018, 62, 1800359. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Pathak, S.; Kumar, V.; Panda, B.P. Attenuation of Neurobehavioral and Neurochemical Abnormalities in Animal Model of Cognitive Deficits of Alzheimer’s Disease by Fermented Soybean Nanonutraceutical. Inflammopharmacology 2018, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Chung, Y.-S.; Kwak, C.S.; Kwon, Y.H. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.L.; Malta, S.M.; Guerra Silva, H.C.; Borges, L.D.F.; Rocha, L.O.; da Silva, J.R.; Rodrigues, T.S.; Venturini, G.; Padilha, K.; da Costa Pereira, A.; et al. Kefir Metabolites in a Fly Model for Alzheimer’s Disease. Sci. Rep. 2021, 11, 11262. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, N.S.; Kandil, E.A.; Ghoneum, M.H. Probiotics Fermentation Technology, a Novel Kefir Product, Ameliorates Cognitive Impairment in Streptozotocin-Induced Sporadic Alzheimer’s Disease in Mice. Oxidative Med. Cell. Longev. 2021, 2021, e5525306. [Google Scholar] [CrossRef]
- Ali, O.; Ali, M.; Anwar, M. The Approaching Therapy Targeting Alzheimer’s Disease Using Kefir Grain and Mesenchymal Stem Cells. Int. J. Appl. Pharm. Biol. Res. 2016, 1, 1–21. [Google Scholar]
- Anwar, M.; Ali, O.S.; Rashed, L.A.; Badawi, A.M.; Eltablawy, N.A. The Neuro Engraftment and Neuroregenerative Effects of Hydrogen Sulphide Donor, Intracerebral MSCs, Ginko Biloba and Kefir in Attenuating Neuropathological Hallmarks of Lipopolysaccharide Induced Alzheimer’s Disease Rat Models. Int. J. Aging Res. 2019, 2, 38. [Google Scholar] [CrossRef]
- He, X.; Zou, Y.; Yoon, W.-B.; Park, S.-J.; Park, D.-S.; Ahn, J. Effects of Probiotic Fermentation on the Enhancement of Biological and Pharmacological Activities of Codonopsis Lanceolata Extracted by High Pressure Treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef]
- Weon, J.B.; Yun, B.-R.; Lee, J.; Eom, M.R.; Ko, H.-J.; Lee, H.Y.; Park, D.-S.; Chung, H.-C.; Chung, J.Y.; Ma, C.J. Cognitive-Enhancing Effect of Steamed and Fermented Codonopsis Lanceolata: A Behavioral and Biochemical Study. Evid. Based Complement. Altern. Med. 2014, 2014, 319436. [Google Scholar] [CrossRef]
- Hermawati, E.; Sari, D.C.R.; Partadiredja, G. The Effects of Black Garlic Ethanol Extract on the Spatial Memory and Estimated Total Number of Pyramidal Cells of the Hippocampus of Monosodium Glutamate-Exposed Adolescent Male Wistar Rats. Anat. Sci. Int. 2015, 90, 275–286. [Google Scholar] [CrossRef]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by β-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef]
- Wichai, T.; Pannangrong, W.; Welbat, J.; Chaichun, A.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of Aged Garlic Extract on Spatial Memory and Oxidative Damage in the Brain of Amyloid-β Induced Rats. Songklanakarin J. Sci. Technol. 2019, 41, 311–318. [Google Scholar]
- Lee, Y.; Oh, S. Administration of Red Ginseng Ameliorates Memory Decline in Aged Mice. J. Ginseng Res. 2015, 39, 250–256. [Google Scholar] [CrossRef]
- Tasi, Y.-C.; Chin, T.-Y.; Chen, Y.-J.; Huang, C.-C.; Lee, S.-L.; Wu, T.-Y. Potential Natural Products for Alzheimer’s Disease: Targeted Search Using the Internal Ribosome Entry Site of Tau and Amyloid-β Precursor Protein. Int. J. Mol. Sci. 2015, 16, 8789–8810. [Google Scholar] [CrossRef]
- Han, S.-H.; Kim, S.-J.; Yun, Y.W.; Nam, S.Y.; Lee, H.-J.; Lee, B.-J. Protective Effects of Cultured and Fermented Ginseng Extracts against Scopolamine-Induced Memory Loss in a Mouse Model. Lab. Anim. Res. 2018, 34, 37–43. [Google Scholar] [CrossRef]
- Nagao, M.; Yamano, S.; Imagawa, N.; Igami, K.; Miyazaki, T.; Ito, H.; Watanabe, T.; Kubota, K.; Katsurabayashi, S.; Iwasaki, K. Effect of Lactobacillus paracasei A221-Fermented Ginseng on Impaired Spatial Memory in a Rat Model with Cerebral Ischemia and β-Amyloid Injection. Tradit. Kampo Med. 2019, 6, 96–104. [Google Scholar] [CrossRef]
- Kim, C.-J.; Ryu, H.-Y.; Lee, S.; Lee, H.-J.; Chun, Y.-S.; Kim, J.-K.; Yu, C.-Y.; Ghimire, B.K.; Lee, J.-G. Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax Ginseng C.A. Meyer in Mice. Molecules 2021, 26, 3001. [Google Scholar] [CrossRef]
- Imao, K.; Kameyama, T.; Ukai, M. PS-501, Fermented Papaya Preparation, Improves Scopolamine-Induced Amnesia in Mice. Res. Commun. Pharmacol. Toxicol. 2001, 6, 197–204. [Google Scholar]
- Yoshino, F.; Lee, M.; Kobayashi, K.; Hayashi, Y.; Aruoma, O. Assessment of the Effect of Fermented Papaya Preparation on Oxidative Damage in Spontaneously Hypertensive Rat Brain Using Electron Spin Resonance (ESR) Imaging and L-Band ESR Spectroscopy. J. Funct. Foods 2009, 1, 375–380. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, J.E.; Lee, S.; Cho, E.J.; Kim, H.Y. Effects of the Fermented Zizyphus Jujuba in the Amyloid Β25-35-Induced Alzheimer’s Disease Mouse Model. Nutr. Res. Pract. 2021, 15, 173–186. [Google Scholar] [CrossRef]
- Jung, I.-H.; Jung, M.-A.; Kim, E.-J.; Han, M.J.; Kim, D.-H. Lactobacillus pentosus Var. Plantarum C29 Protects Scopolamine-Induced Memory Deficit in Mice. J. Appl. Microbiol. 2012, 113, 1498–1506. [Google Scholar] [CrossRef]
- Woo, M.; Noh, J.S.; Cho, E.J.; Song, Y.O. Bioactive Compounds of Kimchi Inhibit Apoptosis by Attenuating Endoplasmic Reticulum Stress in the Brain of Amyloid β-Injected Mice. J. Agric. Food Chem. 2018, 66, 4883–4890. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Kim, M.J.; Song, Y.O. Bioactive Compounds in Kimchi Improve the Cognitive and Memory Functions Impaired by Amyloid Beta. Nutrients 2018, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Soe, K.H.; Lee, T.H.; Kim, I.S.; Lee, Y.M.; Lim, B.O. Cognitive Improving Effects by Highbush Blueberry (Vaccinium Crymbosum L.) Vinegar on Scopolamine-Induced Amnesia Mice Model. J. Agric. Food Chem. 2018, 66, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-S.; Han, Y.H.; Hong, S.-J.; Hwang, S.-M.; Kwon, Y.-S.; Lee, H.J.; Kim, S.-S.; Kim, M.-J.; Chun, W. Bark Constituents from Mushroom-Detoxified Rhus Verniciflua Suppress Kainic Acid-Induced Neuronal Cell Death in Mouse Hippocampus. Korean J. Physiol. Pharm. 2010, 14, 279–283. [Google Scholar] [CrossRef]
- Mathiyazahan, D.B.; Justin Thenmozhi, A.; Manivasagam, T. Protective Effect of Black Tea Extract against Aluminium Chloride-Induced Alzheimer’s Disease in Rats: A Behavioural, Biochemical and Molecular Approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Cai, S.; Yang, H.; Wen, B.; Zhu, K.; Zheng, X.; Huang, J.; Wang, Y.; Liu, Z.; Tu, P. Inhibition by Microbial Metabolites of Chinese Dark Tea of Age-Related Neurodegenerative Disorders in Senescence-Accelerated Mouse Prone 8 (SAMP8) Mice. Food Funct. 2018, 9, 5455–5462. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yang, H.S.; Jo, J.H.; Lee, S.C.; Park, T.Y.; Choi, B.S.; Seo, K.S.; Huh, C.K. Anti-Amnesic Effect of Fermented Ganoderma lucidum Water Extracts by Lactic Acid Bacteria on Scopolamine-Induced Memory Impairment in Rats. Prev. Nutr. Food Sci. 2015, 20, 126–132. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, L.; Han, M.; Jin, M.; Wu, J.; Tan, L.; Chen, Z.; Zhang, X. The Prophylactic and Therapeutic Effects of Fermented Cordyceps Sinensis Powder, Cs-C-Q80, on Subcortical Ischemic Vascular Dementia in Mice. Evid.-Based Complement. Altern. Med. 2018, 2018, e4362715. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Lee, C.-L. Cordyceps Cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-Induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation 2021, 7, 39. [Google Scholar] [CrossRef]
- Ano, Y.; Nakayama, H. Preventive Effects of Dairy Products on Dementia and the Underlying Mechanisms. Int. J. Mol. Sci. 2018, 19, 1927. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.; Sherzai, A.; Adeghate, J.; Sherzai, D. Gut Dysbiosis, Insulin Resistance and Alzheimer’s Disease: Review of a Novel Approach to Neurodegeneration. Front. Biosci. 2021, 13, 17–29. [Google Scholar] [CrossRef]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s Disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef]
- García-Ayllón, M.-S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the Role of Acetylcholinesterase in Alzheimer’s Disease: Cross-Talk with P-Tau and β-Amyloid. Front. Mol. Neurosci. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Eu, W.Z.; Chen, Y.-J.; Chen, W.-T.; Wu, K.-Y.; Tsai, C.-Y.; Cheng, S.-J.; Carter, R.N.; Huang, G.-J. The Effect of Nerve Growth Factor on Supporting Spatial Memory Depends upon Hippocampal Cholinergic Innervation. Transl. Psychiatry 2021, 11, 162. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Ginsberg, S.D.; Mahady, L.; Perez, S.E.; Massa, S.M.; Longo, F.M.; Ikonomovic, M.D. Nerve Growth Factor Pathobiology During the Progression of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 533. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kuo, T.-F.; Wang, J.-J.; Pan, T.-M. Red Mold Rice Ameliorates Impairment of Memory and Learning Ability in Intracerebroventricular Amyloid Beta-Infused Rat by Repressing Amyloid Beta Accumulation. J. Neurosci. Res. 2007, 85, 3171–3182. [Google Scholar] [CrossRef]
- Lee, B.-H.; Ho, B.-Y.; Wang, C.-T.; Pan, T.-M. Red Mold Rice Promoted Antioxidase Activity against Oxidative Injury and Improved the Memory Ability of Zinc-Deficient Rats. J. Agric. Food Chem. 2009, 57, 10600–10607. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wang, J.-J.; Pan, T.-M. Red Mold Rice Extract Represses Amyloid Beta Peptide-Induced Neurotoxicity via Potent Synergism of Anti-Inflammatory and Antioxidative Effect. Appl. Microbiol. Biotechnol. 2008, 79, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Du, Y.; Zhang, Y.; Huang, Z.; Fu, M.; Li, J.; Pang, Y.; Lei, P.; Wang, Y.T.; Song, W.; et al. MKP-1 Reduces Aβ Generation and Alleviates Cognitive Impairments in Alzheimer’s Disease Models. Signal Transduct. Target. Ther. 2019, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, H.; Raina, A.K.; Perry, G.; Smith, M.A. The Role of Mitogen-Activated Protein Kinase Pathways in Alzheimer’s Disease. Neurosignals 2002, 11, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Kim, M.-Y.; Kim, J.-H.; Cho, J.Y. Codonopsis Lanceolata: A Review of Its Therapeutic Potentials. Phytother. Res. 2016, 30, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Farooqui, T.; Koh, H.-L.; Farooqui, A.A.; Ling, E.-A. Protective Effects of Ginseng on Neurological Disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Hayashi, Y.; Marotta, F.; Mantello, P.; Rachmilewitz, E.; Montagnier, L. Applications and Bioefficacy of the Functional Food Supplement Fermented Papaya Preparation. Toxicology 2010, 278, 6–16. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wu, C.-S.; Wang, M. The Jujube (Ziziphus jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus Ostreatus Alleviate Cognitive Impairment in a Rat Model of Alzheimer’s Disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef]
- Deng, L.-L.; Yuan, D.; Zhou, Z.-Y.; Wan, J.-Z.; Zhang, C.-C.; Liu, C.-Q.; Dun, Y.-Y.; Zhao, H.-X.; Zhao, B.; Yang, Y.-J.; et al. Saponins from Panax Japonicus Attenuate Age-Related Neuroinflammation via Regulation of the Mitogen-Activated Protein Kinase and Nuclear Factor Kappa B Signaling Pathways. Neural Regen. Res. 2017, 12, 1877–1884. [Google Scholar] [CrossRef]
- Park, J.-M.; Shin, J.-H.; Lee, D.-W.; Song, J.-C.; Suh, H.-J.; Chang, U.-J.; Kim, J.-M. Identification of the Lactic Acid Bacteria in Kimchi According to Initial and Over-Ripened Fermentation Using PCR and 16S RRNA Gene Sequence Analysis. Food Sci. Biotechnol. 2010, 19, 541–546. [Google Scholar] [CrossRef]
- Shiao, M.-S. Natural Products of the Medicinal Fungus Ganoderma lucidum: Occurrence, Biological Activities, and Pharmacological Functions. Chem. Rec. 2003, 3, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qu, Z.; Zeng, Y.; Lin, Y.; Li, Y.; Chung, P.; Wong, R.; Hägg, U. Neuroprotective Effect of Preadministration with Ganoderma lucidum Spore on Rat Hippocampus. Exp. Toxicol. Pathol. 2012, 64, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal Uses of the Mushroom Cordyceps Militaris: Current State and Prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.-B.; Chang, J.; Zhu, Y.; Sun, X. Chemical Constituents of Cordyceps Cicadae. Nat. Prod. Commun. 2015, 10, 2145–2146. [Google Scholar] [CrossRef]
- Kumar, M.R.; Yeap, S.K.; Lee, H.C.; Mohamad, N.E.; Nazirul Mubin Aziz, M.; Khalid, M.; Masarudin, M.J.; Leow, A.T.C.; Abdullah, J.O.; Alitheen, N.B. Selected Kefir Water from Malaysia Attenuates Hydrogen Peroxide-Induced Oxidative Stress by Upregulating Endogenous Antioxidant Levels in SH-SY5Y Neuroblastoma Cells. Antioxidants 2021, 10, 940. [Google Scholar] [CrossRef]
- Lee, B.-H.; Hsu, W.-H.; Hou, C.-Y.; Chien, H.-Y.; Wu, S.-C. The Protection of Lactic Acid Bacteria Fermented-Mango Peel against Neuronal Damage Induced by Amyloid-Beta. Molecules 2021, 26, 3503. [Google Scholar] [CrossRef]
- Lee, N.-K.; Lim, S.-M.; Cheon, M.-J.; Paik, H.-D. Physicochemical Analysis of Yogurt Produced by Leuconostoc Mesenteroides H40 and Its Effects on Oxidative Stress in Neuronal Cells. Food Sci. Anim. Resour. 2021, 41, 261–273. [Google Scholar] [CrossRef]
- Cheon, M.-J.; Lim, S.-M.; Lee, N.-K.; Paik, H.-D. Probiotic Properties and Neuroprotective Effects of Lactobacillus buchneri KU200793 Isolated from Korean Fermented Foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, J.; Lee, J.-H.; Akanda, M.R.; Cho, J.-H.; Kim, S.-K.; Choi, Y.-J.; Park, B.-Y. Neuroprotective Effects of Cornus officinalis on Stress-Induced Hippocampal Deficits in Rats and H2O2-Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Antioxidants 2020, 9, 27. [Google Scholar] [CrossRef]
- Zhang, S.; Duangjan, C.; Tencomnao, T.; Liu, J.; Lin, J.; Wink, M. Neuroprotective Effects of Oolong Tea Extracts against Glutamate-Induced Toxicity in Cultured Neuronal Cells and β-Amyloid-Induced Toxicity in Caenorhabditis elegans. Food Funct. 2020, 11, 8179–8192. [Google Scholar] [CrossRef]
- Rho, T.; Choi, M.S.; Jung, M.; Kil, H.W.; Hong, Y.D.; Yoon, K.D. Identification of Fermented Tea (Camellia Sinensis) Polyphenols and Their Inhibitory Activities against Amyloid-Beta Aggregation. Phytochemistry 2019, 160, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of Gamma-Aminobutyric Acid (GABA) by Lactobacillus buchneri Isolated from Kimchi and Its Neuroprotective Effect on Neuronal Cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar] [PubMed]
- Yim, N.-H.; Gu, M.J.; Park, H.R.; Hwang, Y.-H.; Ma, J.Y. Enhancement of Neuroprotective Activity of Sagunja-Tang by Fermentation with Lactobacillus Strains. BMC Complement. Altern. Med. 2018, 18, 312. [Google Scholar] [CrossRef] [PubMed]
- Musa, N.H.; Mani, V.; Lim, S.M.; Vidyadaran, S.; Abdul Majeed, A.B.; Ramasamy, K. Lactobacilli-Fermented Cow’s Milk Attenuated Lipopolysaccharide-Induced Neuroinflammation and Memory Impairment in Vitro and in Vivo. J. Dairy Res. 2017, 84, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Eun, C.-S.; Lim, J.-S.; Lee, J.; Lee, S.-P.; Yang, S.-A. The Protective Effect of Fermented Curcuma longa L. on Memory Dysfunction in Oxidative Stress-Induced C6 Gliomal Cells, Proinflammatory-Activated BV2 Microglial Cells, and Scopolamine-Induced Amnesia Model in Mice. BMC Complement. Altern. Med. 2017, 17, 367. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, B.-K.; Lee, Y.-C. Effects of Corni Fructus on Ovalbumin-Induced Airway Inflammation and Airway Hyper-Responsiveness in a Mouse Model of Allergic Asthma. J. Inflamm. 2012, 9, 9. [Google Scholar] [CrossRef]
- Lee, N.-H.; Seo, C.-S.; Lee, H.; Jung, D.-Y.; Lee, J.-K.; Lee, J.-A.; Song, K.Y.; Shin, H.; Lee, M.-Y.; Seo, Y.B.; et al. Hepatoprotective and Antioxidative Activities of Cornus officinalis against Acetaminophen-Induced Hepatotoxicity in Mice. Evid.-Based Complement. Altern. Med. 2011, 2012, e804924. [Google Scholar] [CrossRef]
- Lee, M.; McGeer, E.G.; McGeer, P.L. Mechanisms of GABA Release from Human Astrocytes. Glia 2011, 59, 1600–1611. [Google Scholar] [CrossRef]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA Exerts Protective and Regenerative Effects on Islet Beta Cells and Reverses Diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef]
- Ercolini, D.; Fogliano, V. Food Design To Feed the Human Gut Microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Regulation of the Stress Response by the Gut Microbiota: Implications for Psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Microbiota and Neuroimmune Signalling—Metchnikoff to Microglia. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 494–496. [Google Scholar] [CrossRef]
- Sherwin, E.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. May the Force Be With You: The Light and Dark Sides of the Microbiota-Gut-Brain Axis in Neuropsychiatry. CNS Drugs 2016, 30, 1019–1041. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Patho-Physiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Li, H.; Li, X.-N.; Yuan, C.-H.; Zhao, H. Gut-Brain Axis: Possible Role of Gut Microbiota in Perioperative Neurocognitive Disorders. Front. Aging Neurosci. 2021, 13, 745774. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The Role of Microbial Amyloid in Neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef] [PubMed]
- Cherny, I.; Rockah, L.; Levy-Nissenbaum, O.; Gophna, U.; Ron, E.Z.; Gazit, E. The Formation of Escherichia Coli Curli Amyloid Fibrils Is Mediated by Prion-like Peptide Repeats. J. Mol. Biol. 2005, 352, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, J.; Du, Y.; Wu, W.; Nie, W.; Zhang, D.; Luo, Y.; Lu, H.; Lei, M.; Xiao, S.; et al. Effects of Gut Microbiota and Probiotics on Alzheimer’s Disease. Transl. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, J.H.; Newman, T.N.; Oppong, G.O.; Rapsinski, G.J.; Yen, J.-H.; Biesecker, S.G.; Wilson, R.P.; Butler, B.P.; Winter, M.G.; Tsolis, R.M.; et al. Microbial Amyloids Induce Interleukin 17A (IL-17A) and IL-22 Responses via Toll-Like Receptor 2 Activation in the Intestinal Mucosa. Infect. Immun. 2012, 80, 4398–4408. [Google Scholar] [CrossRef]
- Hill, J.M.; Lukiw, W.J. Microbial-Generated Amyloids and Alzheimer’s Disease (AD). Front. Aging Neurosci. 2015, 7, 9. [Google Scholar] [CrossRef]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides Fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7, 1544. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating Endotoxin and Systemic Immune Activation in Sporadic Amyotrophic Lateral Sclerosis (SALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Jin, L.-W.; De Carli, C.; Phinney, B.; Sharp, F.R. Gram-Negative Bacterial Molecules Associate with Alzheimer Disease Pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Hauss-Wegrzyniak, B.; Vraniak, P.D.; Wenk, G.L. LPS-Induced Neuroinflammatory Effects Do Not Recover with Time. Neuroreport 2000, 11, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.S.; Kranjac, D.; Alonzo, C.A.; Haase, J.H.; Cedillos, R.O.; McLinden, K.A.; Boehm, G.W.; Chumley, M.J. Prolonged Elevation in Hippocampal Aβ and Cognitive Deficits Following Repeated Endotoxin Exposure in the Mouse. Behav. Brain Res. 2012, 229, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Can a Bacterial Endotoxin Be a Key Factor in the Kinetics of Amyloid Fibril Formation? J. Alzheimer’s Dis. 2014, 39, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Diamond, M.I. Prion-like Mechanisms in Neurodegenerative Diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Emery, D.C.; Shoemark, D.K.; Batstone, T.E.; Waterfall, C.M.; Coghill, J.A.; Cerajewska, T.L.; Davies, M.; West, N.X.; Allen, S.J. 16S RRNA Next Generation Sequencing Analysis Shows Bacteria in Alzheimer’s Post-Mortem Brain. Front. Aging Neurosci. 2017, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the Presence of Periodontopathic Virulence Factors in Short-Term Postmortem Alzheimer’s Disease Brain Tissue. J. Alzheimer’s Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Carter, C. Alzheimer’s Disease: APP, Gamma Secretase, APOE, CLU, CR1, PICALM, ABCA7, BIN1, CD2AP, CD33, EPHA1, and MS4A2, and Their Relationships with Herpes Simplex, C. Pneumoniae, Other Suspect Pathogens, and the Immune System. Int. J. Alzheimer’s Dis. 2011, 2011, e501862. [Google Scholar] [CrossRef]
- Hammond, C.J.; Hallock, L.R.; Howanski, R.J.; Appelt, D.M.; Little, C.S.; Balin, B.J. Immunohistological Detection of Chlamydia Pneumoniae in the Alzheimer’s Disease Brain. BMC Neurosci. 2010, 11, 121. [Google Scholar] [CrossRef]
- Miklossy, J. Bacterial Amyloid and DNA Are Important Constituents of Senile Plaques: Further Evidence of the Spirochetal and Biofilm Nature of Senile Plaques. J. Alzheimer’s Dis. 2016, 53, 1459–1473. [Google Scholar] [CrossRef]
- Kountouras, J.; Boziki, M.; Gavalas, E.; Zavos, C.; Deretzi, G.; Grigoriadis, N.; Tsolaki, M.; Chatzopoulos, D.; Katsinelos, P.; Tzilves, D.; et al. Increased Cerebrospinal Fluid Helicobacter Pylori Antibody in Alzheimer’s Disease. Int. J. Neurosci. 2009, 119, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Kotronis, G.; Thomann, R.; Polyzos, S.A.; Boziki, M.; Gialamprinou, D.; Deretzi, G.; Katsinelos, P.; Kountouras, J. Review: Impact of Helicobacter Pylori on Alzheimer’s Disease: What Do We Know so Far? Helicobacter 2018, 23, e12454. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Neufeld, K.-A.M. Gut–Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R. Handbook of Fermented Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular Analysis of Commensal Host-Microbial Relationships in the Intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.-L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of Caffeic and Ferulic Acid Equivalents in Plasma after Coffee Consumption: Small Intestine and Colon Are Key Sites for Coffee Metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef]
- Park, E.-K.; Shin, J.; Bae, E.-A.; Lee, Y.-C.; Kim, D.-H. Intestinal Bacteria Activate Estrogenic Effect of Main Constituents Puerarin and Daidzin of Pueraria thunbergiana. Biol. Pharm. Bull. 2006, 29, 2432–2435. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Sudo, T.; Nishida, R.; Kawahara, A.; Saisho, K.; Mimori, K.; Yamada, A.; Mizoguchi, A.; Kadoya, K.; Matono, S.; Mori, N.; et al. Clinical Impact of Tumor-Infiltrating Lymphocytes in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2017, 24, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of Gut Leakiness by a Probiotic Treatment Leads to Attenuated HPA Response to an Acute Psychological Stress in Rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Jury, J.; MacQueen, G.; Sherman, P.M.; Perdue, M.H. Probiotic Treatment of Rat Pups Normalises Corticosterone Release and Ameliorates Colonic Dysfunction Induced by Maternal Separation. Gut 2007, 56, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, M.; Wu, X.; Kong, X.; Liu, Y.; Xu, X. Lactobacillus rhamnosus Zz-1 Exerts Preventive Effects on Chronic Unpredictable Mild Stress-Induced Depression in Mice via Regulating the Intestinal Microenvironment. Food Funct. 2022. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-Gut-Brain Axis and the Central Nervous System. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Yunes, R.A.; Poluektova, E.U.; Vasileva, E.V.; Odorskaya, M.V.; Marsova, M.V.; Kovalev, G.I.; Danilenko, V.N. A Multi-Strain Potential Probiotic Formulation of GABA-Producing Lactobacillus plantarum 90sk and Bifidobacterium Adolescentis 150 with Antidepressant Effects. Probiotics Antimicrob. Proteins 2019, 12, 973–979. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic Gastrointestinal Inflammation Induces Anxiety-like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterology 2010, 139, 2102–2112. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharm. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front. Psychiatry 2021, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B. Inflammaging Decreases Adaptive and Innate Immune Responses in Mice and Humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Divyashri, G.; Krishna, G.; Prapulla, S.G. Probiotic Attributes, Antioxidant, Anti-Inflammatory and Neuromodulatory Effects of Enterococcus Faecium CFR 3003: In Vitro and in Vivo Evidence. J. Med. Microbiol. 2015, 64, 1527–1540. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-Z. Therapeutic Potential of Bifidobacterium Breve Strain A1 for Preventing Cognitive Impairment in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and Bifidobacteria Ameliorate Memory and Learning Deficits and Oxidative Stress in β-Amyloid (1-42) Injected Rats. Appl. Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal Microbiota Transplantation Alleviated Alzheimer’s Disease-like Pathogenesis in APP/PS1 Transgenic Mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, J.H.; Shin, J.; Kim, J.S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive Function Improvement after Fecal Microbiota Transplantation in Alzheimer’s Dementia Patient: A Case Report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The Role of Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef]
- Szablewski, L. Human Gut Microbiota in Health and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pardo, P.; Kliest, T.; Dodiya, H.B.; Broersen, L.M.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. The Gut-Brain Axis in Parkinson’s Disease: Possibilities for Food-Based Therapies. Eur. J. Pharm. 2017, 817, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional Patterns Associated with the Maintenance of Neurocognitive Functions and the Risk of Dementia and Alzheimer’s Disease: A Focus on Human Studies. Pharm. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of Gut Microbiota and Nutrients in Amyloid Formation and Pathogenesis of Alzheimer Disease. Nutr. Rev. 2016, 74, 624–634. [Google Scholar] [CrossRef]
- Fermented Food and Ingredients Market Size USD 875.21 Bn by 2027|CAGR of 6.0%. Available online: https://secure.livechatinc.com/ (accessed on 28 August 2021).
- Hansen, E.B. STARTER CULTURES|Uses in the Food Industry. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 529–534. [Google Scholar] [CrossRef]
- Hayes, M.; García-Vaquero, M. Bioactive Compounds from Fermented Food Products. In Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Food Engineering Series; Springer International Publishing: Cham, Switzerland, 2016; pp. 293–310. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic Phenolic Antioxidants: Metabolism, Hazards and Mechanism of Action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Das, G.; Paramithiotis, S.; Sundaram Sivamaruthi, B.; Wijaya, C.H.; Suharta, S.; Sanlier, N.; Shin, H.-S.; Patra, J.K. Traditional Fermented Foods with Anti-Aging Effect: A Concentric Review. Food Res. Int. 2020, 134, 109269. [Google Scholar] [CrossRef]

| Health-Promoting Activity | Health-Promoting Compounds | Fermented Food Products | Fermenting Microorganism(s) | Reference |
|---|---|---|---|---|
| Neuroprotection | Antioxidant enzymes, GABA, genistein, anthocyanin | Sausage | Enterococcus thailandicus, Enterococcus faecalis | [41] |
| Soybean | Bacillus amyloliquefaciens, Bacillus amyloliquefaciens, Rhizopus oryzae, Pichia farinose | [42] | ||
| Tempeh | Rhizopus spp., Lactobacillus spp. | [43] | ||
| Anti-inflammatory | Polyphenol | Kefir | LAB | [44] |
| Soy milk | Lactococcus lactis subsp. lactis S-SU2 | [45] | ||
| Anti-hypertensive | ACE inhibitory peptides, GABA | Milk | Lactobacillus helveticus strain CP790, Saccharomyces cerevisiae | [46] |
| Milk | Lactobacillus casei strain IMAU20411 | [47] | ||
| Goat milk | Lactobacillus plantarum strain 69 | [48] | ||
| Camel milk | Lactobacillus rhamnosus strain MTCC 5945 (NS4) | [49] | ||
| Milk | Lactococcus lactis strain DIBCA2 | [50] | ||
| Milk | Lactobacillus plantarum strain PU11 | [50] | ||
| Soy milk | Enterococcus faecium | [51] | ||
| Skim milk | Lactobacillus plantarum | [52] | ||
| Milk | Lactobacillus casei strain Shirota, Lactobacillus lactis strain YIT 2027 | [53] | ||
| Cheese | Lactobacillus lactis | [54] | ||
| Beans | Bacillus subtilis strain B060 | [55] | ||
| Anti-cholesterol | Hydroxy-methylglutaric acid, Orotic acid (inhibitor of cholesterol synthesis) | Milk | Lactobacillus acidophilus | [56] |
| Anti-diabetic | Isoflavonoids, peptides | Soybeans (Meju) | Bacillus spp., Aspergillus spp. | [57] |
| Cereal (Boza) | LAB | [58] | ||
| Anti-microbial | Bacteriocins, | Kefir Yoghurt | LAB | [59] |
| Bacteriocin-like inhibitory substances (BLIS), | Ewe milk Yoghurt Buttermilk | LAB | [60] | |
| Carboxylic acids | Yak milk (Kurut) | LAB | [61] | |
| Cheese (Jben) | LAB | |||
| Wort | Lactobacillus plantarum strain FST1.7, Lactobacillus brevis strain R2D | |||
| Anti-oxidative | Phenolic, flavonoid compounds | Wheat koji | Aspergillus oryzae, Aspergillus awamori strain nakazawa | [62] |
| Cereal | Bacillus subtilis, Lactobacillus plantarum | [63] | ||
| Wheat | Aspergillus oryzae, Rhizopus oryzae | [64] | ||
| Wheat | Aspergillus oryzae, Aspergillus niger | [65] | ||
| Soy whey | Lactobacillus plantarum strain B1-6 | [66] | ||
| Anti-cancer | Peptides, Surfactin-like compounds | Soybeans (Cheonggukjang) | Bacillus subtilis strain CSY 191 | [67] |
| Camel milk | Lactobacillus lactis, Lactobacillus acidophilus | [68] | ||
| Alleviation of lactose intolerant | Lactase | Milk | Lactobacillus acidophilus | [69] |
| Anti-nutritive | Phytase | Bread | Yeast species | [70] |
| Fermented Food Products | Fermenting Microorganism(s) | Experimental Subjects | Assessments | Effects | Reference |
|---|---|---|---|---|---|
| Kefir fermented in milk | Acetobacter aceti, Acetobacter sp., Lactobacillus delbrueckii delbrueckii, Lactobacillus fermentum, Lactobacillus fructivorans, Enterococcus faecium, Leuconostoc spp., Lactobacillus kefiranofaciens, Candida famata, and Candida krusei | AD patients (n = 13) | Cognitive assessment, determination of cytokines, ROS, advanced oxidation protein products, MMP, p53, and cleaved PARP levels, cell cycle, cell viability, and apoptosis analyses | Marked improvement in memory, executive/language functions, and visual-spatial/abstraction abilities, decreased oxidative stress and inflammation, increased NO bioavailability, and improved serum protein oxidation, mitochondrial dysfunction, apoptosis, and DNA damage/repair. | [73] |
| Milk | Lactobacillus helveticus IDCC3801 | Older people (60–75 years old) | Cognitive tests, PSS, BDNF, GDS-SF, and WBV | Improved cognitive function. | [74] |
| Milk | Lactobacillus helveticus CM4 | Healthy adults (middle age) | RBANS test | Improved attention and delayed memory. | [75] |
| Soybean (DW2009) | Lactobacillus plantarum C29 | Patients with mild cognitive impairment (n = 100) (55–85 years old) | Neurocognitive function tests, BDNF levels, and fecal microbiota analysis | Enhanced cognitive function, increased BDNF levels, and lactobacilli in the gut microbiota. | [76] |
| Probiotic milk | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum | AD patients (n = 60) (60–95 years old) | MMSE score and biomarkers test | Enhanced cognitive function and a significant decrease in MDA, hs-CRP, insulin metabolism markers, triglyceride, and VLDL. | [77] |
| Tofu, tempeh and other genistein-rich foods | Not reported as it is an observational study | Older people (n = 115) (52–98 years old) | Medical examination, cognitive and socioeconomic assessments | Improved memory and cognitive function in middle-aged people but not in older people. | [78] |
| Tempeh | Rhizopus oligosporus, Enterobacteriaceae and lactic acid bacteria | Older people with mild cognitive impairment (n = 90) (≥60 years old) | Cognitive tests and blood uric acid level | Improved global cognitive function | [79] |
| Soybean and soybean products | Not reported as it is a population study | Japanese subjects without dementia (60–79 years old) | Neuropsychological tests, dietary surveys, and health examinations | Reduced risk of dementia | [80] |
| Papaya | Yeast | Patients with initial or mild AD (n = 40) (mean age 78.2 ± 1.1 years) | Urinary 8-OHdG test | Significant decrease in the 8-OHdG levels | [81] |
| Fermented Food Products | Fermenting Microorganism(s) | Experimental Subjects | Assessments | Effects | Reference |
|---|---|---|---|---|---|
| Dairy | |||||
| Camembert cheese | Penicillium candidum | C57BL/6J mice, CD-1 mice, and B6SJL-Tg mice (6–8 weeks old) | Aβ1–42 deposition analysis, anti-inflammatory and phagocytosis assays | Reduction of Aβ and inflammation increased BDNF and GDNF | [89] |
| Soymilk | Lactobacillus plantarum strain TWK10 | Wistar rats (n = 30) (8 weeks old, 260–300 g) | Learning and memory, antihypertensive, biochemical and histological analysis | Significant decrease in blood pressure improved the learning ability and reduced the occurrence of dementia | [90] |
| Calpis sour milk whey | Lactobacillus helveticus | ddY mice (n = 255) (7 weeks old) | SABT and NORT | Significantly improved cognitive impairment and object recognition memory | [91] |
| Lactopeptides | Digested with enzyme from Aspergillus melleus and Bacillus stearothermophilus | C57BL/6J mice (7 and 22 months old) and Crl: CD1 (ICR) mice (6 weeks old) | SABT, NORT, and monoamine oxidase inhibitory and monoamine analyses | Improved memory function, inhibited monoamine oxidase-B activity, and enhanced dopamine levels in brain tissue | [92] |
| Tryptophan-related dipeptides | Digested by enzymes from Aspergillus melleus | C57BL/6J mice (newborn <7 day, 7 weeks, and 68-weeks old) and ICR mice (6 weeks old) | Electrophysiology, SABT, and NORT | Suppressed microglial inflammatory response, increased Aβ phagocytosis, improved cognitive and memory impairment | [93] |
| Tibetan fermented milk | Not reported | B6C3 mice (n = 12) (2 months old, 19.86 ± 3.37 g) APPswe/PS1dE9-transgenic mice (n = 36) (2 months old, 20.03 ± 3.52 g) | MWMT, NORT, immunohistochemistry, 16S rRNA sequencing, and taxonomic analysis of gut microbiota | Improved cognitive impairment, reduced Aβ deposition in the cerebral cortex and hippocampus, increased intestinal microbial diversity | [94] |
| β-lactolin, a whey-derived lacto-tetrapeptide | Not reported | B6SJL-Tg mice (2.5 months old) and B6; C3-Tg mice (3 months old) | Cytokine, synaptophysin, Aβ, and tau by ELISA, immunohistochemistry, dopamine analysis, NORT, and OFT | Ameliorated synaptophysin, dopamine, Aβ, BDNF, inflammatory cytokines, and IGF-1 levels, and improved impaired long-term object memory and behavioral abnormality | [95] |
| Legumes and Cereal | |||||
| Cheonggukjang | Bacillus subtilis MC31 and Lactobacillus sakei 383 | ICR mice (n = 80) (6 weeks old) | PAT, NORT, AChE, MDA, SOD, and NGF detection, and histological analysis | Improved short- and long-term memory, NGF signaling pathway, NGF concentration, Bax/Bcl-2 levels, AChE and SOD activity | [96] |
| Cheonggukjang and soybeanss | Bacillus licheniformis SCD 111067P | Sprague Dawley rats (n = 80) (223 ± 16 g) | PAT, MWMT, and immunohistochemistry | Significantly reduced Aβ accumulation, ameliorated insulin signaling, improved cognitive functions, and glucose regulations | [97] |
| Red mould rice | Monascus purpureus NTU 568 | Wistar rats (n = 49) (280–320 g) | PAT, MWMT, detection of TBARS, ROS, ApoE, β-secretase, sAPPα, and brain cholesterol levels in the hippocampusand cortex | Improved memory deficits, brain cholesterol level, oxidative stress and lipid peroxidation, decreased Aβ formation and deposition, and suppressed ApoE expression | [98] |
| Kurozu and Kurozu Moromi | Not reported | R1 mice (n =16) (10 weeks old) and P8 mice (n = 27) (12 weeks old) | MWMT, antioxidant assays, and detection of HSPA1A mRNA expression | Suppressed Aβ accumulation and cognitive dysfunction and enhanced HSPA1A mRNA expression | [99] |
| Soybean and Tempeh | Rhizophus sp. | Sprague Dawley rats (n = 96) (180 ± 20 g) (3–4 months old) | Radial arm maze, elevated plus maze, ACh and AChE assays, and IL-10 and IL-1β measurements | Tempeh showed significant improvement in memory, ACh and AChE activities, and a decrease in inflammation | [100] |
| Tempeh | Rhizopusoligosporus (BCR C 31750) | SMAP8 mice (n = 32) (6 months old) and SAMR1 mice (n = 18) (6 months old) | Cognitive evaluation, redox status analysis, and RT-PCR and western blot analyses of Nrf2, p-JNK, and p-p38 expressions | Stronger cognition, reduced Aβ, carbonyl protein, and MDA levels, enhanced Nrf2, catalase, and SOD activities | [101] |
| Tempeh | Not reported | Wistar rats (n = 15) (180–280 g) (2.5–3 months old) | MWMT | Improved spatial memory impairment | [102] |
| Defatted soybean powder | Lactobacillus pentosus var. plantarum C29 | ICR mice (24–28 g) (6 weeks old) | PAT, Y-maze and MWMT, and detection of AChE and BDNF activity | Improved memory impairment, increased BDNF activity, and inhibited AChE activity | [103] |
| Soybean | Lactobacillus plantarum C29 | B6SJL-Tg mice (4 months old) | Y-maze task, PAT, NORT, MWMT, pyrosequencing, and in vivo intestinal permeability assay | Improved cognitive function, significantly reduced Aβ, β/γ-secretases, NF-κB activation, and caspase-3 expression, and enhanced BDNF expression | [104] |
| Nanonutraceuticals of soybean | Bacillus subtilis | Wistar albino rats (180–200 g) | MWMT, PAT, and assays for AChE, MDA, protein carbonyl, and oxidative markers | Ameliorated learning and memory, AChE and antioxidant status, reduced MDA, protein carbonyl, and Aβ deposition | [105] |
| Doenjang | Aspergillus oryzae and Bacillus licheniformis | C57BL/6J mice (n = 47) (4 weeks old) | Brain tissue histopathology, MDA and protein carbonylation measurement, immunoblotting, and qPCR analyses | Enhanced neurotrophic factor mRNA levels, alleviated neuronal loss, reduced neuroinflammation- and oxidative stress-related mRNA levels and oxidative metabolites contents | [106] |
| Kefir | |||||
| Kefir and kefir fractions fermented in cow milk | Lactobacillus kefiranofaciens, Lactobacillus kefiri, Acetobacter fabarum, Lactococcus lactis, and Rickettsiales | Drosophila melanogaster | Total amyloid quantification, survival assay, rapid iterative negative geotaxis assay, and histopathological analysis | Improved climbing ability, vacuolar lesions, survival rate, and neurodegeneration index. | [107] |
| Probiotics Fermentation Technology (PFT) kefir grain product | Lactobacillus kefiri P-IF, Lactobacillus kefiri P-B1, Kazachstania turicensis, Kazachstania unispora, and Kluyveromyces marxianus | Albino mice (25–30 g) | NORT, MWMT, evaluation of Aβ1-42, ACh, MDA, Nrf2, NF-κB, TNF-α, and Caspase-3 levels | Attenuated neuronal degeneration improved cognition, restored ACh levels, reduced apoptosis, oxidative damage, and proinflammatory cytokine expression. | [108] |
| Kefir fermented in organic powdered milk | Not reported | Albino rats (n = 60) (150–200 g) | T-maze test, biochemical analysis, detection of cholesterol, TNF-α and IL-10 levels | Attenuated cognitive impairment, Aβ and tau pathology, lipid profile, oxidative stress, and Bax expression | [109] |
| Kefir fermented in milk | Not reported | Albino rats (n = 72) (200–250 g) | MWMT, estimation of brain tissue expression of MAPK, Tau protein, ACAT, CBS, Aβ42, MDA, and GSH, and histopathology | Improved memory, decreased MAPK, Tau, ACAT, CBS, Aβ42, MDA, and oxidative stress levels, and increased GSH levels | [110] |
| Plant Root | |||||
| Codonopsis lanceolata extract | Bifidobacterium longum and Lactobacillus rhamnosus | ICR mice (n = 40) (27.7 ± 2.4 g) (5 weeks old) | PAT | Improved memory deficit | [111] |
| Codonopsis lanceolata | Bifidobacterium longum (KACC 20587), Lactobacillus acidophilus (KACC 12419), and Leuconostoc mesenteroides (KACC 12312) | ICR mice (n = 35) (25–30 g) (3 weeks old) | MWMT, PAT, AChE, BDNF, and CREB level | Increased cognition, BDNF, and CREB expressions, and inhibited AChE activity. | [112] |
| Black garlic | No fermenting microorganism is involved | Wistar rats (n = 25) (3–4 weeks) | MWMT, and estimation of the total number of hippocampal pyramidal cells | Ameliorated memory deficits and estimated a higher total number of hippocampal pyramidal cells | [113] |
| Aged garlic | No fermenting microorganism is involved | Wistar rats (n = 48) (180–220 g) | NORT, immunohistochemistry, and western blotting analysis | Significant increase in short-term memory and decrease in inflammatory responses | [114] |
| Aged garlic | No fermenting microorganism is involved | Wistar rats (n = 48) (180–220 g) (8 weeks old) | MWMT, histological analysis, neurons quantification, and biochemical analysis | Improved learning and short-term memory impairment, reversed neuronal loss, and increased GSH and SOD activities | [115] |
| Red ginseng | Not reported | C57BL/6 mice (28–30 g) (21 months old) | Y-maze task, NORT, MWMT, and immunoblot analysis | Attenuated iNOS, TNF-α, IL-1β, and COX-2 expressions, restored GSH levels and increased Nrf2 and HO-1. | [116] |
| Radix notoginseng | Lactobacillus spp. | ApoE−/− mice (n = 16) (10 weeks old) | MWMT | Ameliorated spatial memory | [117] |
| Wild ginseng root extract (HLJG0701) | Lactic acid bacteria | ICR mice (n = 48) (8 weeks old) | AChE, ACh and BDNF expressions, MWMT, and Y-maze test | Significant reduction in AChE activity, increased ACh and BDNF levels, improved memory | [118] |
| Ginseng | Lactobacillus paracasei A221 | Wistar rats (300–350 g) (10 weeks old) | MWMT, immunofluorescence, and western blotting | Improved memory, caspase-3, and Iba-1 levels, and loss of hippocampal neurons | [119] |
| Wild ginseng root extract (HLJG0701-β) | Pediococcus pentosaceus | Male C57BL mice (18.37–23.92 g) (9 weeks old) and female C57BL mice (18.40–20.97 g) (9 weeks old) | MWMT, Y-maze task, measurement of AChE, ACh, MDA, and catalase levels | Ameliorated the long-term memory impairment, ACh, and catalase levels, and reduced AChE and MDA levels | [120] |
| Fruits and Vegetables | |||||
| Papaya | Yeast | Mice | SABT and PAT | Improved short- and long-term memory | [121] |
| Papaya | Yeast | SHR rat (350–450 g) | Electronspin resonance imaging analysis | Up-regulated the redox defense activity | [122] |
| Zizyphus jujuba | Saccharomyces cerevisiae | ICR mice (n = 28) (5 weeks old) | T-maze test, NORT, MWMT, and measurement of ALT, AST, MDA, and NO levels | Ameliorated cognitive function and suppressed the elevations of NO and MDA | [123] |
| Kimchi | No fermenting microorganism is involved | ICR mice (28–30 g) | PAT, Y-maze test, MWMT, and immunoblotting | Ameliorated memory impairment and increased BDNF and p-CREB expressions | [124] |
| Kimchi | No fermenting microorganism is involved | ICR mice (5 weeks old) | Measurement of ROS, TBARS, AD-related markers, endoplasmic reticulum stress markers, and apoptosis-related molecules | Reduced APP, p-Tau, BACE, endoplasmic reticulum stress markers, and pro-apoptotic molecules, and enhanced cIAP and Bcl-2 expressions | [125] |
| Kimchi | No fermenting microorganism is involved | ICR mice (5 weeks old) | MWMT, NORT, T-maze test, measurement of ROS, peroxynitrite, TBARS, and GSH levels, and western blot analysis | Improved cognitive deficits and GSH level, and reduced TBARS, peroxynitrite, and ROS levels | [126] |
| Highbush blueberry | Saccharomyces cerevisiae KCCM 34709 and Acetobacter sp. KCCM 40085 | ICR mice (6 weeks old) | Y-maze test, PAT, detection of ACh, AChE, SOD, catalase, and MDA levels, and immunohistochemistry | Significantly ameliorated cognitive functions, inhibited AChE activity, and facilitated ACh activity | [127] |
| Other Plant Products | |||||
| Rhus verniciflua | Mushroom-mediated fermentation. No fermenting microorganism is involved | ICR mice (23–25 g) | Immunohistochemistry and In situ labeling of DNA fragmentation | Significantly attenuated pyramidal neuronal cell death and microglia activation | [128] |
| Black tea | Fully-fermented tea produced through oxidation. No fermenting microorganism is involved | Albino Wistar rats (n = 36) (200–225 g) (10–12 weeks old) | PAT, MWMT, estimation of AChE, TBARS, SOD, GPx, and GSH levels, and western blot analysis | Improved memory deficits, inhibited AChE activity, reduced oxidative stress and Aβ1–42 related and apoptotic markers | [129] |
| Chinese dark tea | Not reported | SAMR1 mice (n = 8) and SAMP8 mice (n = 32) (25–30 g) (4 months old) | Measurement of oxidative stress- and Aβ42, H&E staining, Nissl dyeing, myelin staining, and Roche apoptotic staining | Attenuated Aβ metabolic pathway, downregulated 4-HNE formation, enhanced endogenous antioxidant capacity, and protected neurons by reducing oxidative stress | [130] |
| Fungi | |||||
| Ganoderma lucidum | Bifidobacterium bifidum and Lactobacillus sakei LI033 | Sprague Dawley rats (n = 42) (200–250 g) (6 weeks old) | MWMT, PAT, rotarod test, vertical pole test, and measurement of AChE activity | Improved memory and lowered AChE activities in the brain | [131] |
| Cordyceps sinensis (Berk) Sacc. | Not reported | ICR mice (22–25 g) (8–10 weeks old) | NORT, MWMT, histopathology, immunohistochemistry, and western blot analysis | Improved learning and memory deficit, and significantly decreased MBP, TNF-α, and IL-1β expressions | [132] |
| Cordyceps cicadae NTTU 868 | Fermented with potato dextrose broth powder and yeast extract | Sprague Dawley rats (n = 48) (6–8 weeks old) | MWMT and measurements of TNF-α, IL-1β, IL-6, and Aβ40-related proteins levels | Improved memory deficit, suppressed Aβ40, BACE, and pro-inflammatory cytokine expression, and increased MAGT1 expression | [133] |
| Fermented Food Products | Fermenting Microorganism(s) | Experimental Subjects | Assessments | Effects | Reference |
|---|---|---|---|---|---|
| Kefir | Not reported | SH-SY5Y cells | Measurement of TPC, TFC, FRAP, and DPPH levels, MTT, AO/PI, Annexin V-FITC, SEM, TEM, and qPCR analysis for SOD, catalase, and Tp73 expressions | Increased TPC, TFC, FRAP, and DPPH activities, a significantly lower percentage of necrotic cells, greater protection to cytoplasmic and cytoskeleton inclusion of SH-SY5Y cells, upregulation of SOD and catalase activities, and downregulation of Tp73 | [159] |
| Mango peel extracts | Lactobacillus acidophilus (BCRC14079) | Neuron-2A cells | MitoSOX-red stain, cell cycle, and immunocytochemistry | Upregulated BDNF expressions, attenuated oxidative stress, Aβ accumulation, and the elevation of subG1 | [160] |
| Kimchi | Leuconostoc mesenteroides H40 * | SH-SY5Y cells | MTT assay and qPCR analysis of BDNF, Bax, and Bcl-2 expression | Increased cell viability and BDNF expression, and reduced Bax/Bcl-2 ratio | [161] |
| Kimchi | Lactobacillus buchneri KU200793 * | SH-SY5Y cells | MTT assay and qPCR analysis of BDNF, Bax, and Bcl-2 expression | Significantly increased BDNF expression and decreased Bax/Bcl-2 ratio | [162] |
| Cornus officinalis | Lactobacillus rhamnosus, Enterococcus faecium, and Lactobacillus acidophilus | SH-SY5Y cells | MTT assay, detection of ROS and LDH release, qPCR, and western blot analysis for Bax/Bcl-2 and MAPK expressions | Significantly inhibited ROS and LDH release, enhanced catalase, SOD, and BDNF expressions, and regulated the Bax/Bcl-2 ratio and MAPK phosphorylation. | [163] |
| Oolong tea | Semi-fermented Chinese tea produced through oxidation. No fermenting microorganism is involved | Neuro-2A and HT22 cells | MTT assay, measurement of ROS, and qRT-PCR analysis for SODs, GPx, and GSTs | Decreased ROS accumulation, increased SODs, GPx, GSTs, GAP-43, and Ten-4 expressions | [164] |
| Camellia sinensis | Fermented Camellia sinensis is produced via heating and enzymatic fermentation of leaves. No fermenting microorganism is involved | SH-SY5Y cells | ThT fluorescence-based assay, TEM, and CCK-8 assay | Significantly reduced Aβ aggregation and stronger protection against Aβ-induced toxicity | [165] |
| Tempeh | Rhizopus and Lactobacillus | BV2 cells | MTT assay, detection of ROS, and western immunoblot analysis for nitric oxide synthase, CREB, and BDNF expressions | Decreased ROS, CREB, and nitric oxide synthase levels, and upregulated BDNF expression | [43] |
| Kimchi | Lactobacillus buchneri * | PC12 cells | MTT assay | Increased cell viability and showed complete neuroprotection by retaining 100% cell viability | [166] |
| Sagunja-tang | Lactobacillus rhamnosus KFRI127, Lactobacillus zeae KFR129, Lactobacillus rhamnosus KFRI144, Lactobacillus acidophilus KFRI150, Lactobacillus fermentum KFRI162, Lactobacillus plantarum KFRI166, Lactobacillus acidophilus KFRI217, and Lactobacillus helveticus KFRI341 | SH-SY5Y cells | CCK-8 assay, measurement of ROS, and MMPs assay | High protection against cell death and reduced ROS and mitochondrial membrane potential disruption | [167] |
| Cow’s milk | Lactobacillus fermentum LAB9 or Lactobacillus casei LABPC | BV2 cells | MTT assay, Griess reagent, and CD40 immunophenotyping | Decreased in NO level without affecting cell viability and no effect in CD40 expression | [168] |
| Curcuma longa L. | Lactobacillus plantarum K154 containing 2% (w/v) yeast extract | BV2 and C6 cells | MTT, NO, PGE2, and TNF-α assays | Prevented the cell death and inhibited PGE2 and NO production | [169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.R.; Azizi, N.F.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Leow, A.T.C.; Mortadza, S.A.S.; Alitheen, N.B. Clinical and Preclinical Studies of Fermented Foods and Their Effects on Alzheimer’s Disease. Antioxidants 2022, 11, 883. https://doi.org/10.3390/antiox11050883
Kumar MR, Azizi NF, Yeap SK, Abdullah JO, Khalid M, Omar AR, Osman MA, Leow ATC, Mortadza SAS, Alitheen NB. Clinical and Preclinical Studies of Fermented Foods and Their Effects on Alzheimer’s Disease. Antioxidants. 2022; 11(5):883. https://doi.org/10.3390/antiox11050883
Chicago/Turabian StyleKumar, Muganti Rajah, Nor Farahin Azizi, Swee Keong Yeap, Janna Ong Abdullah, Melati Khalid, Abdul Rahman Omar, Mohd. Azuraidi Osman, Adam Thean Chor Leow, Sharifah Alawieyah Syed Mortadza, and Noorjahan Banu Alitheen. 2022. "Clinical and Preclinical Studies of Fermented Foods and Their Effects on Alzheimer’s Disease" Antioxidants 11, no. 5: 883. https://doi.org/10.3390/antiox11050883
APA StyleKumar, M. R., Azizi, N. F., Yeap, S. K., Abdullah, J. O., Khalid, M., Omar, A. R., Osman, M. A., Leow, A. T. C., Mortadza, S. A. S., & Alitheen, N. B. (2022). Clinical and Preclinical Studies of Fermented Foods and Their Effects on Alzheimer’s Disease. Antioxidants, 11(5), 883. https://doi.org/10.3390/antiox11050883









