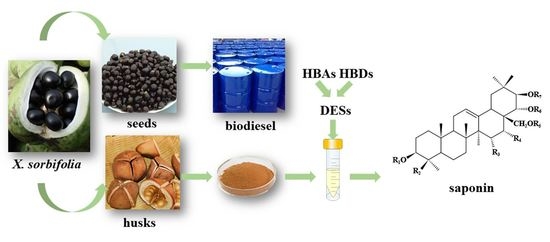
Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras sorbifolia Bunge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Preparations of DESs
2.3. Extraction of Total Saponins and Total Flavonoids
2.4. Microstructure of Plant Material
2.5. Optimal DESs Extraction Conditions for Total Saponins
2.6. Determination of Total Saponins and Total Flavonoids Content
2.6.1. Determination of Total Saponins Content
2.6.2. Determination of Total Flavonoids Content
2.7. Qualitative Analysis of Recovered Saponins Composition by HPLC-ESI-MS
2.8. Recovery of Bioactive Saponins from TPMBr-La Extracts
2.8.1. Screening of Macroporous Resins
2.8.2. Study on Adsorption Kinetics of D101 Macroporous Resin
2.9. Determination of Antioxidant Activity
2.9.1. DPPH Radical Scavenging Assay
2.9.2. ABTS Radical Scavenging Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Design and Screening of Saponin−Oriented DESs
3.2. Preliminary Extraction Mechanism for the Efficiency and Specificity of Saponins Extraction
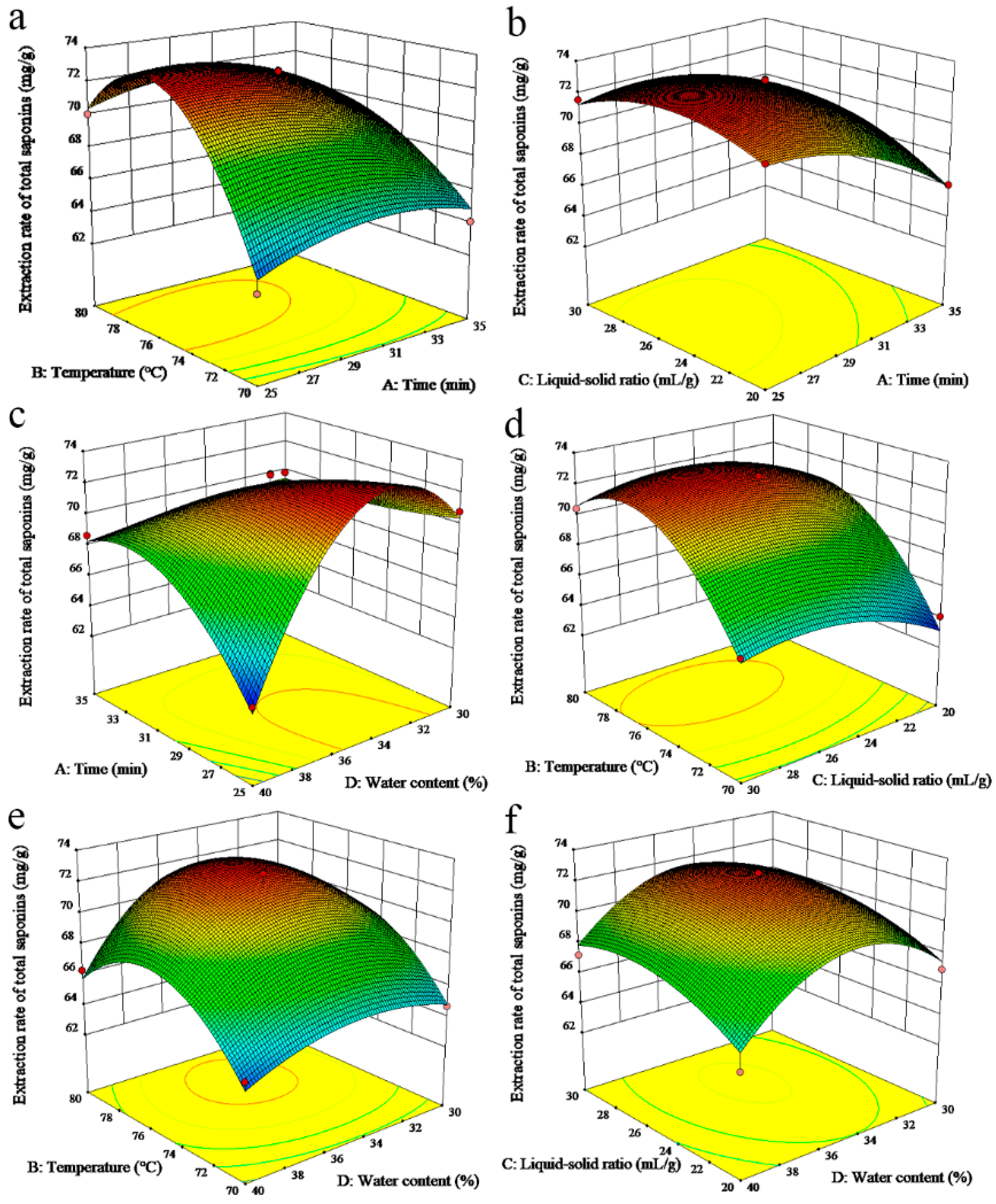
3.3. Box-Behnken Design (BBD) Optimization
3.4. Recovery of Total Saponins and Kinetic Analysis of Adsorption Process
3.4.1. Screening of Macroporous Resins
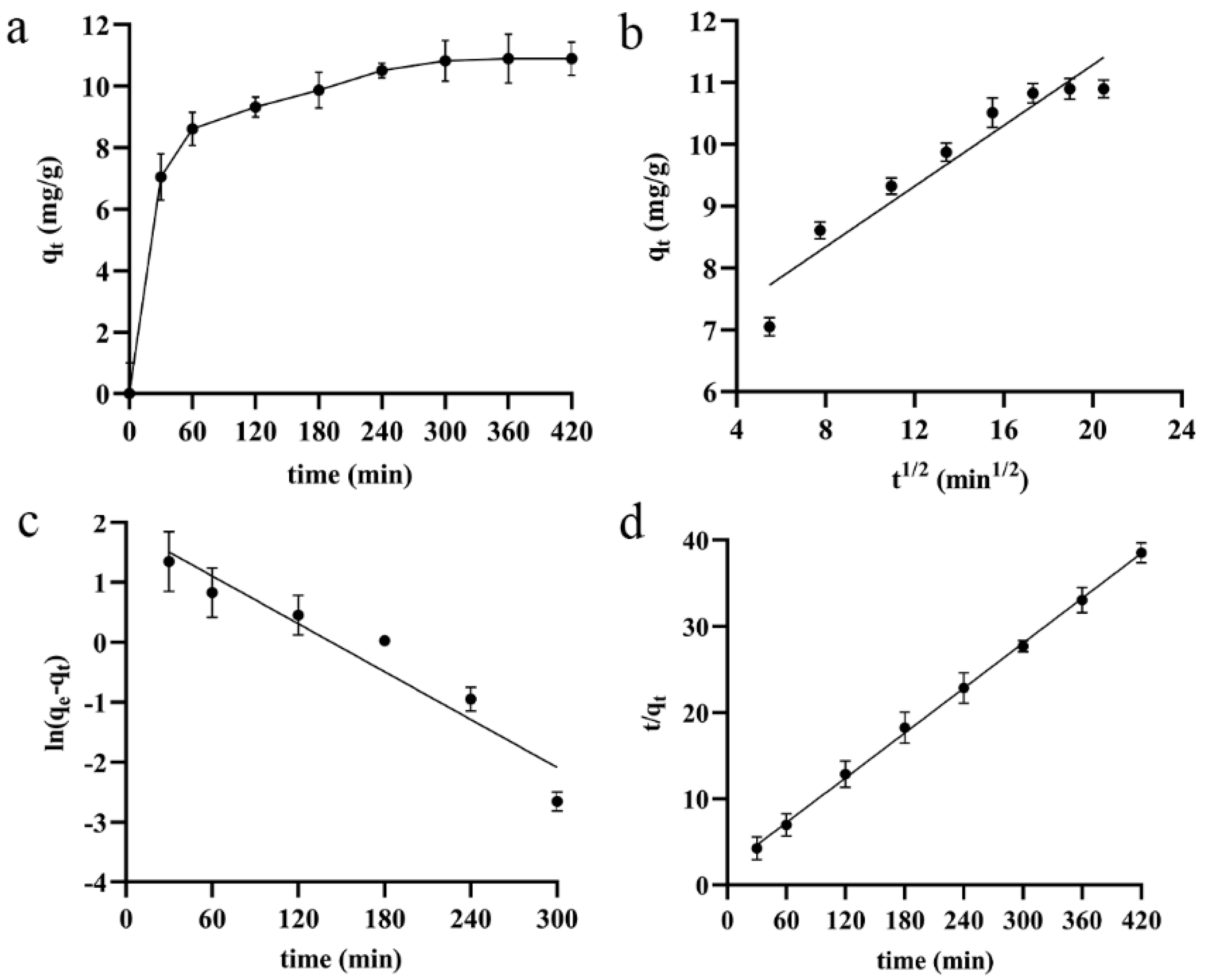
3.4.2. Kinetic Analysis of the Adsorption Process
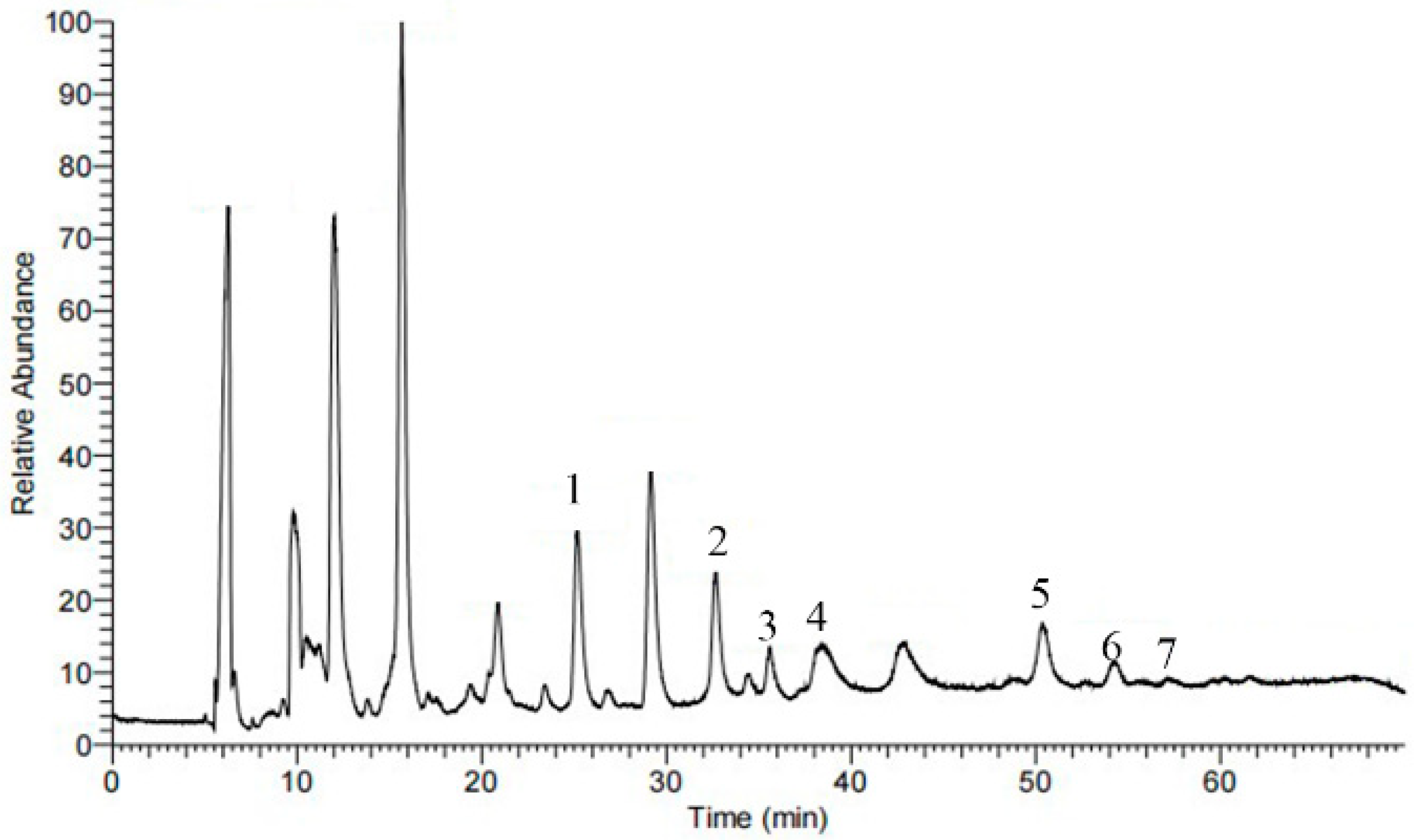
3.5. Identification of Barrigenol-Like Saponins in the TPMBr-La Extracts
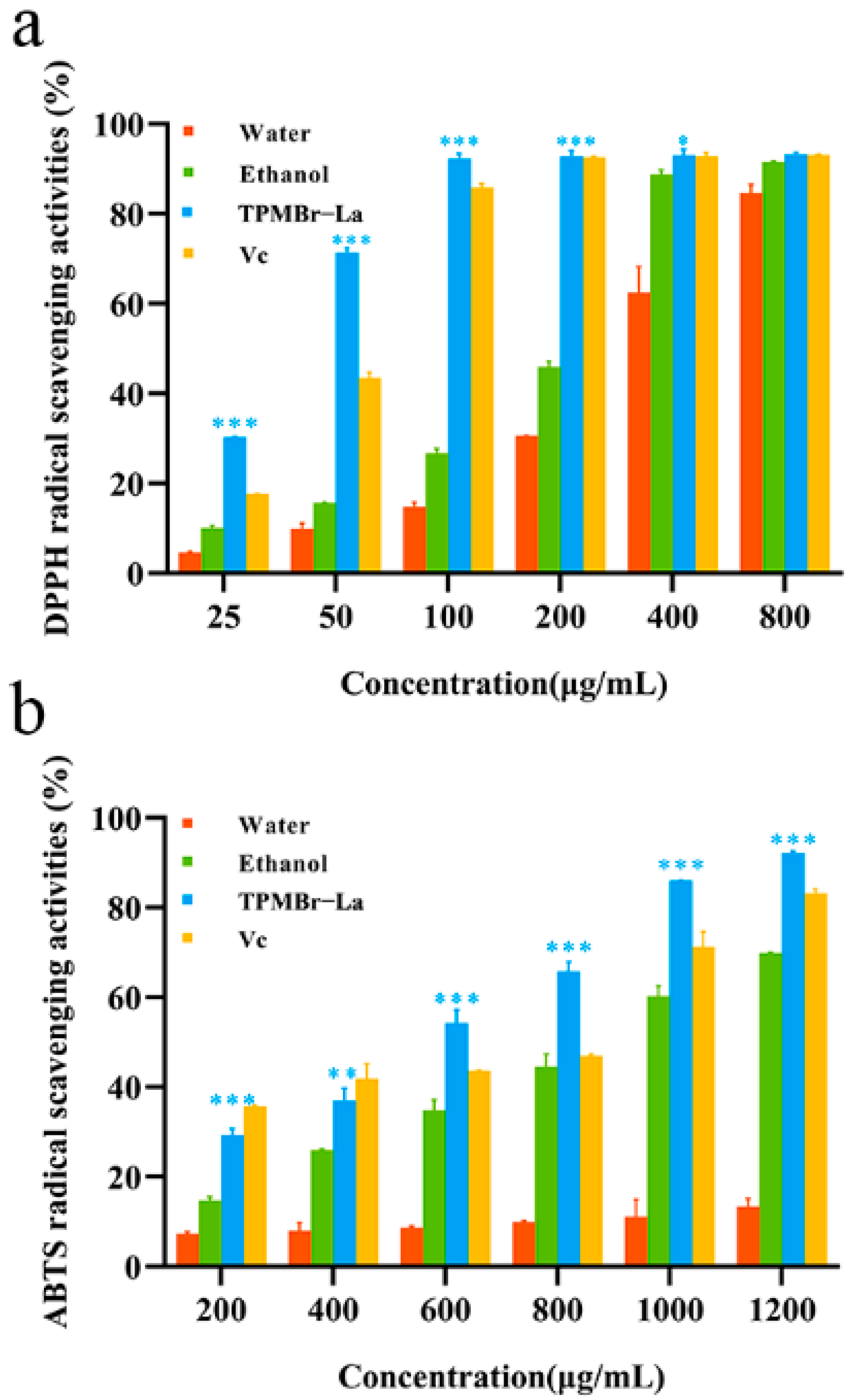
3.6. Evaluation of the Antioxidant Activity of the TPMBr-La Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Z.Y.; Qi, J.H.; Yin, L.M. Biodiesel production from Xanthoceras sorbifolia in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2013, 24, 57–65. [Google Scholar] [CrossRef]
- Jin, H.; Zou, J.X.; Li, L.L.; Bai, X.L.; Zhu, T.; Li, J.B.; Xu, B.C.; Wang, Z. Physiological responses of yellow-horn seedlings to high temperatures under drought condition. Plant Biotechnol. Rep. 2020, 14, 111–120. [Google Scholar] [CrossRef]
- Li, X.F.; Hou, S.L.; Su, M.; Yang, M.F.; Shen, S.H.; Jiang, G.M.; Qi, D.M.; Chen, S.Y.; Liu, G.S. Major Energy Plants and Their Potential for Bioenergy Development in China. Environ. Manag. 2010, 46, 579–589. [Google Scholar] [CrossRef]
- Hu, D.; Cui, Y.J.; Zhang, J. Nervonic acid amends motor disorder in a mouse model of Parkinson’s disease. Transl. Neurosci. 2021, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, X. Advances in studies on chemical constituents and pharmacological effects of Xanthoceras sorbifolia. Northwestern Pharm. J. 2004, 5, 235–237. [Google Scholar]
- Chai, X.; Zhang, J.W.; Li, S.H.; Cheng, Q.S.; Qin, M.M.; Yang, C.Y.; Gao, J.L.; Huang, H.B. Xanthoceraside induces cell apoptosis through downregulation of the PI3K/Akt/Bcl-2/Bax signaling pathway in cell lines of human bladder cancer. Indian J. Pathol. Microbiol. 2021, 64, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Lei, Z.L.; Cao, J. Traditional uses, phytochemistry, pharmacology and +current uses of underutilized Xanthoceras sorbifolium bunge: A review. J. Ethnopharmacol. 2022, 283, 114747. [Google Scholar] [CrossRef]
- Wang, H.D. Research Progress on chemical constituents and comprehensive utilization of Xanthoceras sorbifolia Bunge. Chin. Wild Plant Resour. 1998, 17, 13–16. [Google Scholar]
- Liu, X.X.; Yang, X.A.; Qv, C.; Wu, Z.; Yang, B.Z.; Wang, L.H.; Zou, L.B. Effects of Extracts from the Pericarp of Xanthoceras sorbifolia Bunge on Learning and Memory Obstacle. Tradit. Chin. Drug Res. Clin. Pharmacol. 2007, 18, 23–25. [Google Scholar] [CrossRef]
- Wu, W.J.; Li, B.S. Extraction of saponins from Xanthoceras sorbifolia husks by foam separation. J. Zhejiang Agric. Sci. 2010, 4, 816–819. [Google Scholar]
- Zhang, X.T.; Hao, Y.N.; Wang, X.M.; Chen, Z.J. Adsorption of iron(III), cobalt(II), and nickel(II) on activated carbon derived from Xanthoceras Sorbifolia Bunge hull: Mechanisms, kinetics and influencing parameters. Water Sci. Technol. 2017, 75, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Xiang, Z.; Cui, H.; Xiao, H.; Kang, T.G.; Dou, D.-Q.; Kuang, H.X. Two new oleanane-type saponins from the husks of Xanthoceras sorbifolia Bunge. Nat. Prod. Res. 2013, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Ding, K.; Guo, S.; Xie, F.; Li, Q.; Bi, K. Metabolomics analysis of Xanthoceras sorbifolia husks protection of rats against Alzheimer’s disease using liquid chromatography mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1126, 121739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.N.; Ma, C.L.; Qi, Z.; Ma, C.M. Simultaneous quantification of ten constituents of Xanthoceras sorbifolia Bunge using UHPLC-MS methods and evaluation of their radical scavenging, DNA scission protective, and alpha-glucosidase inhibitory activities. Chin. J. Nat. Med. 2015, 13, 873–880. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, Y.R. Natural barrigenol-like triterpenoids: A comprehensive review of their contributions to medicinal chemistry. Phytochemistry 2019, 161, 41–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Han, S.S.; Nan, Y. Progress in research and development of Xanthoceras Sorbifolia. China J. Chin. Mater. Med. 2021, 46, 4334–4343. [Google Scholar] [CrossRef]
- Wang, L.H.; Chen, W. Optimization of Extraction Technology of Xanthoceraside from Xanthoceras sorbifolia Bunge Husks by Orthogonal Test. Food Sci. 2009, 30, 58–60. [Google Scholar]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Ruesgas-Ramon, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef] [Green Version]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry: A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.Z.; Wang, L.T.; Kang, Y.F.; Meng, Y.; Jiao, J.; Fu, Y.J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Abranches, D.O.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS in the design of deep eutectic solvents for the extraction of antioxidants from Rosemary. ACS Sustain. Chem. Eng. 2020, 8, 12132–12141. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Zarur Coelho, M.A.; Marrucho, I.M. Extraction of saponins from sisal (Agave sisalana) and jua (Ziziphus joazeiro) with cholinium-based ionic liquids and deep eutectic solvents. Eur. Food Res. Technol. 2013, 237, 965–975. [Google Scholar] [CrossRef]
- Yang, G.Y.; Song, J.N.; Chang, Y.Q.; Wang, L.; Zheng, Y.G.; Zhang, D.; Guo, L. Natural Deep Eutectic Solvents for the Extraction of Bioactive Steroidal Saponins from Dioscoreae Nipponicae Rhizoma. Molecules 2021, 26, 2079. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, W.H.; Zhang, Z.H.; Liu, E.H.; Guo, L. Evaluation of natural deep eutectic solvents for the extraction of bioactive flavone C-glycosides from Flos Trollii. Microchem. J. 2019, 145, 180–186. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, H.; Wu, C.E. Screening of Antioxidant and Antitumor Activities of Major Ingredients from Defatted Camellia oleifera Seeds. Food Sci. Biotechnol. 2014, 23, 873–880. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Z.; Xu, C.; Liang, J.; Wei, B.; Wu, D.; Zhu, S. Study on poly (methyl methacrylate)/carbon nanotube composites. Mater. Sci. Eng. A 1999, 271, 395–400. [Google Scholar] [CrossRef]
- Shen, D.; Labreche, F.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Zhu, J. Ultrasound-assisted adsorption/desorption of jujube peel flavonoids using macroporous resins. Food Chem. 2022, 368, 130800. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J. Chromatogr. A 2016, 1443, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Yang, Y.; Ren, H.X.; Sun, S.Y.; Mu, L.; Chen, X.; Wang, Y.Z.; Zhang, Y.; Wang, L.; Sun, C.H. Identification of multiple components in deep eutectic solvent extract of Acanthopanax senticosus root by ultra-high-performance liquid chromatography with quadrupole orbitrap mass spectrometry. Phytochem. Lett. 2020, 35, 175–185. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; He, Y.; Xu, Y.; Li, L.; Luo, Z. UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: Composition, extraction mechanism and in vitro biological activities. Food Chem. 2022, 370, 131042. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zheng, Y.L.; Yang, S.K.; Zhang, J.R.; Cheng, X.Y.; Ghiladi, R.; Ma, Z.; Wang, J.; Deng, W.W. One-pot method based on deep eutectic solvent for extraction and conversion of polydatin to resveratrol from Polygonum cuspidatum. Food Chem. 2021, 343, 28498. [Google Scholar] [CrossRef]
- Saeed, T.; Naeem, A.; Din, I.U.; Farooq, M.; Khan, I.W.; Hamayun, M.; Malik, T. Synthesis of chitosan composite of metal-organic framework for the adsorption of dyes; kinetic and thermodynamic approach. J. Hazard. Mater. 2022, 427, 127902. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Su, D.; Li, X.Z.; Liu, D.; Xi, R.G.; Gao, H.Y.; Wang, X.B. Barrigenol triterpenes from the husks of Xanthoceras sorbifolia Bunge and their antitumor activities. RSC Adv. 2016, 6, 27434–27446. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Yang, J.; Meng, D.L.; Li, N. Two new triterpenoid saponins from the carpophore of Xanthoceras sorbifolia Bunge. J. Asian Nat. Prod. Res. 2008, 10, 260–264. [Google Scholar] [CrossRef]
- Chan, P.K.; Zhao, M.; Che, C.T.; Mak, E. Cytotoxic acylated triterpene saponins from the husks of Xanthoceras sorbifolia. J. Nat. Prod. 2008, 71, 1247–1250. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.G.; Fu, Y.J.; Yang, Y.C.; Li, S.M.; Li, Z.N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Ling, J.H.; Liu, L.L.; Wang, Y.X.; Li, Z.Y.; Liu, R.; Li, Q.; Wang, Y.; Yang, B.Z.; Chen, X.H.; Bi, K.S. Characterization and quantification of the triterpenoids in different parts of Xanthoceras sorbifolia by HPLC-ESI-MS. J. Pharm. Biomed. 2011, 55, 259–264. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xiao, H.F.; Shi, H.X.; Song, Z.X.; Tang, Z.S. Antioxidant activity of fruit Shells of Xanthoceras sorbifolia and screening of active components inhibiting proliferation of HepG2 cell lines. Mod. Chin. Med. 2017, 19, 1572–1574. [Google Scholar] [CrossRef]
- Mrsan, A.; Nadpal, J.; Stupar, A.; Pojic, M.; Mandic, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
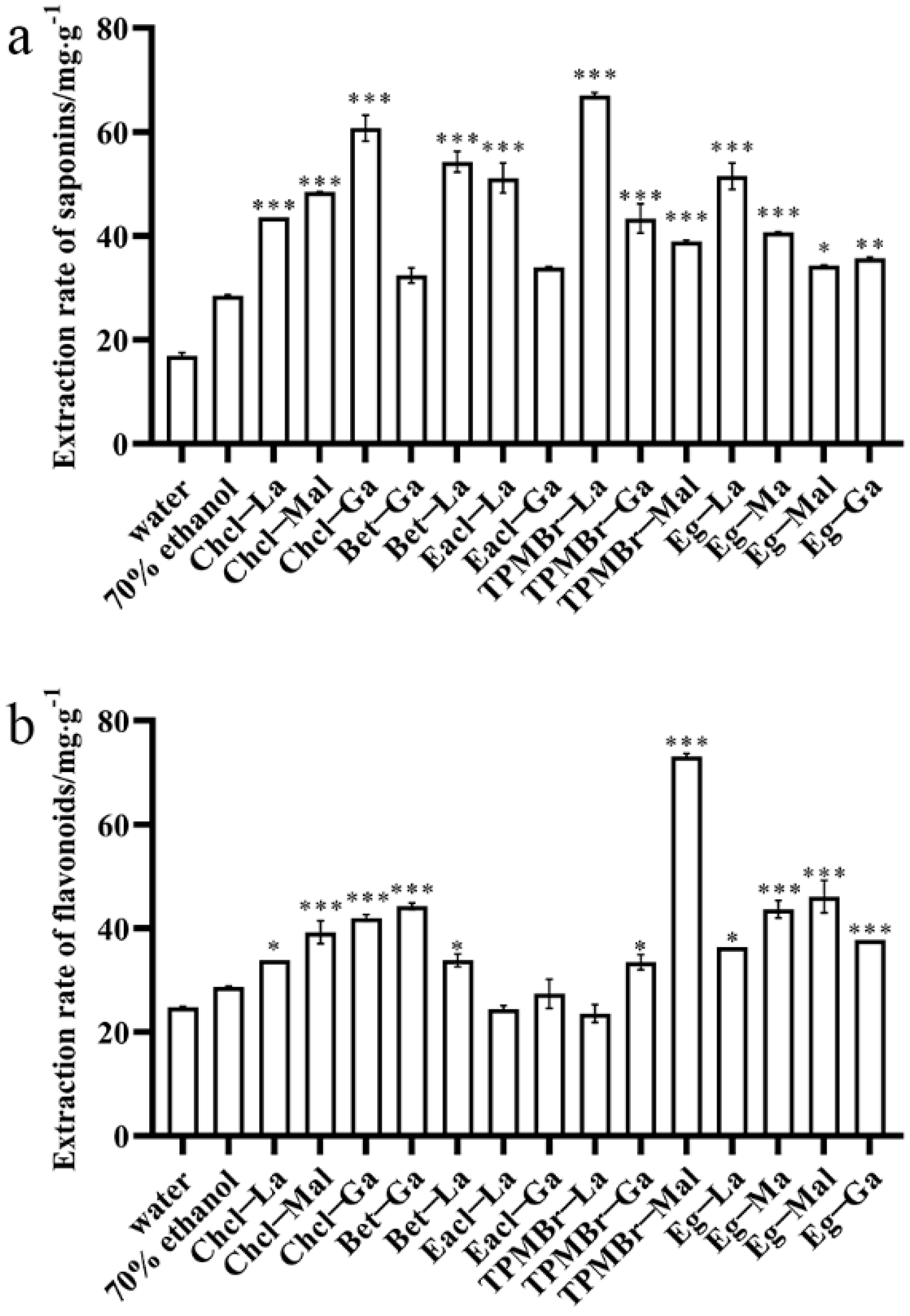



| No. | Solvent Code | Hydrogen Bond Acceptors (HBAs) | Hydrogen Bond Donors (HBDs) | Molar Ratio |
|---|---|---|---|---|
| 1 | Chcl-La | Choline chloride | Lactic acid | 1:2 |
| 2 | Chcl-Mal | Choline chloride | Malonic acid | 1:2 |
| 3 | Chcl-Ga | Choline chloride | Glycolic acid | 1:2 |
| 4 | Bet-Ga | Betaine | Glycolic acid | 1:2 |
| 5 | Bet-La | Betaine | Lactic acid | 1:2 |
| 6 | Eacl-La | Ethylamine hydrochloride | Lactic acid | 1:2 |
| 7 | Eacl-Ga | Ethylamine hydrochloride | Glycolic acid | 1:2 |
| 8 | TPMBr-La | Tetrapropylammonium bromide | Lactic acid | 1:2 |
| 9 | TPMBr-Ga | Tetrapropylammonium bromide | Glycolic acid | 1:2 |
| 10 | TPMBr-Mal | Tetrapropylammonium bromide | Malonic acid | 1:2 |
| 11 | Eg-La | Ethylene glycol | Lactic acid | 1:2 |
| 12 | Eg-Ma | Ethylene glycol | Malic acid | 1:2 |
| 13 | Eg-Mal | Ethylene glycol | Malonic acid | 1:2 |
| 14 | Eg-Ga | Ethylene glycol | Glycolic acid | 1:2 |
| Independent Factor | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A Extraction time (min) | 25 | 30 | 35 |
| B Extraction temperature (°C) | 70 | 75 | 80 |
| C Liquid-solid ratio (mL/g) | 20 | 25 | 30 |
| D water content (%) | 25 | 30 | 35 |
| Variables | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 282.84 | 18 | 15.71 | 12.547 | 0.0003 |
| A-Extraction time | 13.25 | 1 | 13.25 | 10.57 | 0.0100 |
| B-Extraction temperature | 67.98 | 1 | 67.98 | 54.23 | <0.0001 |
| C-Liquid-solid ratio | 8.23 | 1 | 8.23 | 6.57 | 0.0305 |
| D-Water content | 3.11 | 1 | 3.11 | 2.48 | 0.1495 |
| AB | 0.34 | 1 | 0.34 | 0.27 | 0.6169 |
| Model | 282.84 | 18 | 15.71 | 12.547 | 0.0003 |
| A-Extraction time | 13.25 | 1 | 13.25 | 10.57 | 0.0100 |
| B-Extraction temperature | 67.98 | 1 | 67.98 | 54.23 | <0.0001 |
| C-Liquid-solid ratio | 8.23 | 1 | 8.23 | 6.57 | 0.0305 |
| D-Water content | 3.11 | 1 | 3.11 | 2.48 | 0.1495 |
| AB | 0.34 | 1 | 0.34 | 0.27 | 0.6169 |
| AC | 2.72 | 1 | 2.72 | 2.17 | 0.1746 |
| AD | 11.73 | 1 | 11.73 | 9.36 | 0.0136 |
| BC | 0.35 | 1 | 0.35 | 0.28 | 0.6110 |
| BD | 0.013 | 1 | 0.013 | 0.011 | 0.9204 |
| CD | 0.17 | 1 | 0.17 | 0.13 | 0.7227 |
| A2 | 11.08 | 1 | 11.08 | 8.84 | 0.0156 |
| B2 | 87.33 | 1 | 87.33 | 69.67 | <0.0001 |
| C2 | 14.02 | 1 | 14.02 | 11.19 | 0.0086 |
| D2 | 72.21 | 1 | 72.21 | 57.60 | <0.0001 |
| Residual | 11.28 | 9 | 1.25 | ||
| Lack of Fit | 3.24 | 6 | 0.54 | 0.20 | 0.9541 |
| R2 | 0.9616 | ||||
| Adj R2 | 0.8849 |
| Kinetic Model | Equations | qe exp. | qe calc. | K | R2 |
|---|---|---|---|---|---|
| Intra-particle diffusion | qe = 0.2454t1/2 + 6.3797 | 10.90 | 11.0358 | 0.2454 | 0.9168 |
| pseudo-first-order | ln (qe−qt) = −0.0133t + 1.9051 | 10.90 | 6.7201 | 0.0133 | 0.9211 |
| pseudo-second-order | = 0.0867t + 2.0116 | 10.90 | 11.5340 | 0.0037 | 0.9991 |
| Samples | IC50 (μg/mL) | |
|---|---|---|
| DPPH | ABTS | |
| Water | 321.79 ± 6.90 | >1200 |
| Ethanol | 215.35 ± 5.43 | 855.25 ± 1.66 |
| TPMBr-La | 36.54 ± 0.46 | 541.13 ± 0.03 |
| Vc | 59.43 ± 0.53 | 620.14 ± 3.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wu, G.; Wang, L.; Cao, F.; Jiang, Y.; Zhao, L. Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras sorbifolia Bunge. Antioxidants 2022, 11, 736. https://doi.org/10.3390/antiox11040736
Cao J, Wu G, Wang L, Cao F, Jiang Y, Zhao L. Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras sorbifolia Bunge. Antioxidants. 2022; 11(4):736. https://doi.org/10.3390/antiox11040736
Chicago/Turabian StyleCao, Jinteng, Guangwei Wu, Lei Wang, Fuliang Cao, Yan Jiang, and Linguo Zhao. 2022. "Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras sorbifolia Bunge" Antioxidants 11, no. 4: 736. https://doi.org/10.3390/antiox11040736
APA StyleCao, J., Wu, G., Wang, L., Cao, F., Jiang, Y., & Zhao, L. (2022). Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras sorbifolia Bunge. Antioxidants, 11(4), 736. https://doi.org/10.3390/antiox11040736