Dietary Iron Restriction Improves Muscle Function, Dyslipidemia, and Decreased Muscle Oxidative Stress in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Treatments
2.2. Induction of Diabetes and Experimental Design
2.3. Muscle Extraction and Tissue Preparation
2.4. Hemoglobin Measurement
2.5. Force Development and Fatigue Induction
2.6. Lipid Profile
2.7. Determination of Lipid Peroxidation
2.8. ROS Measurements
2.9. Measurement of Glutathione
2.10. Statistical Analysis
3. Results
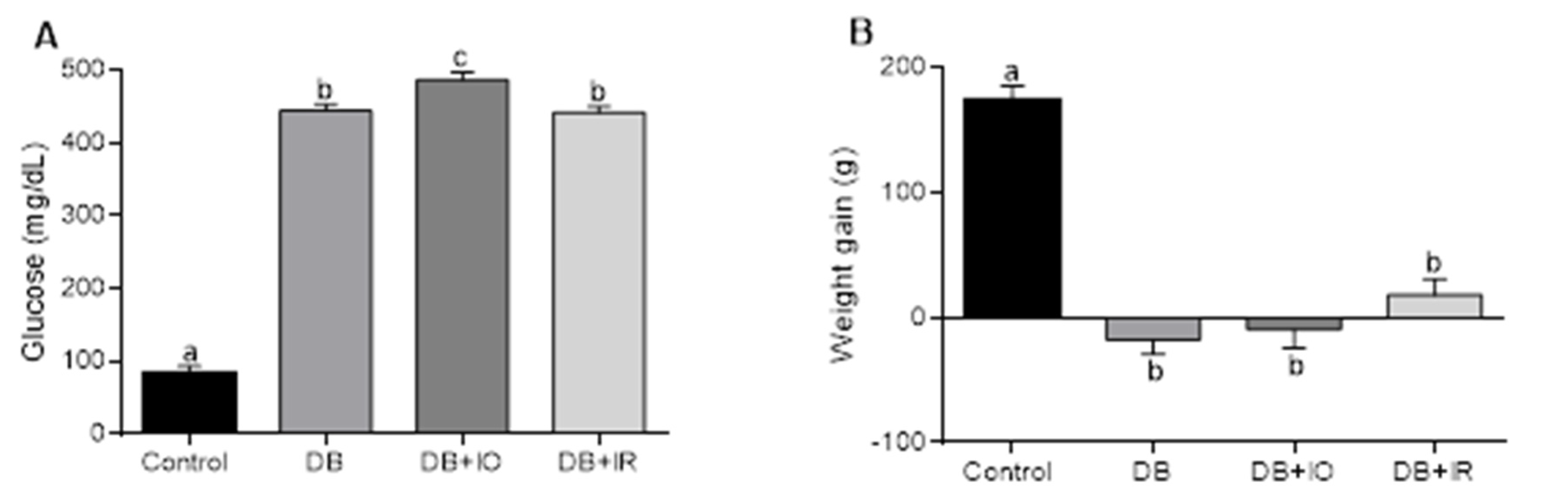
3.1. Effect of Dietary Iron on Blood Glucose and Body Weight in STZ-Diabetic Induced Rats
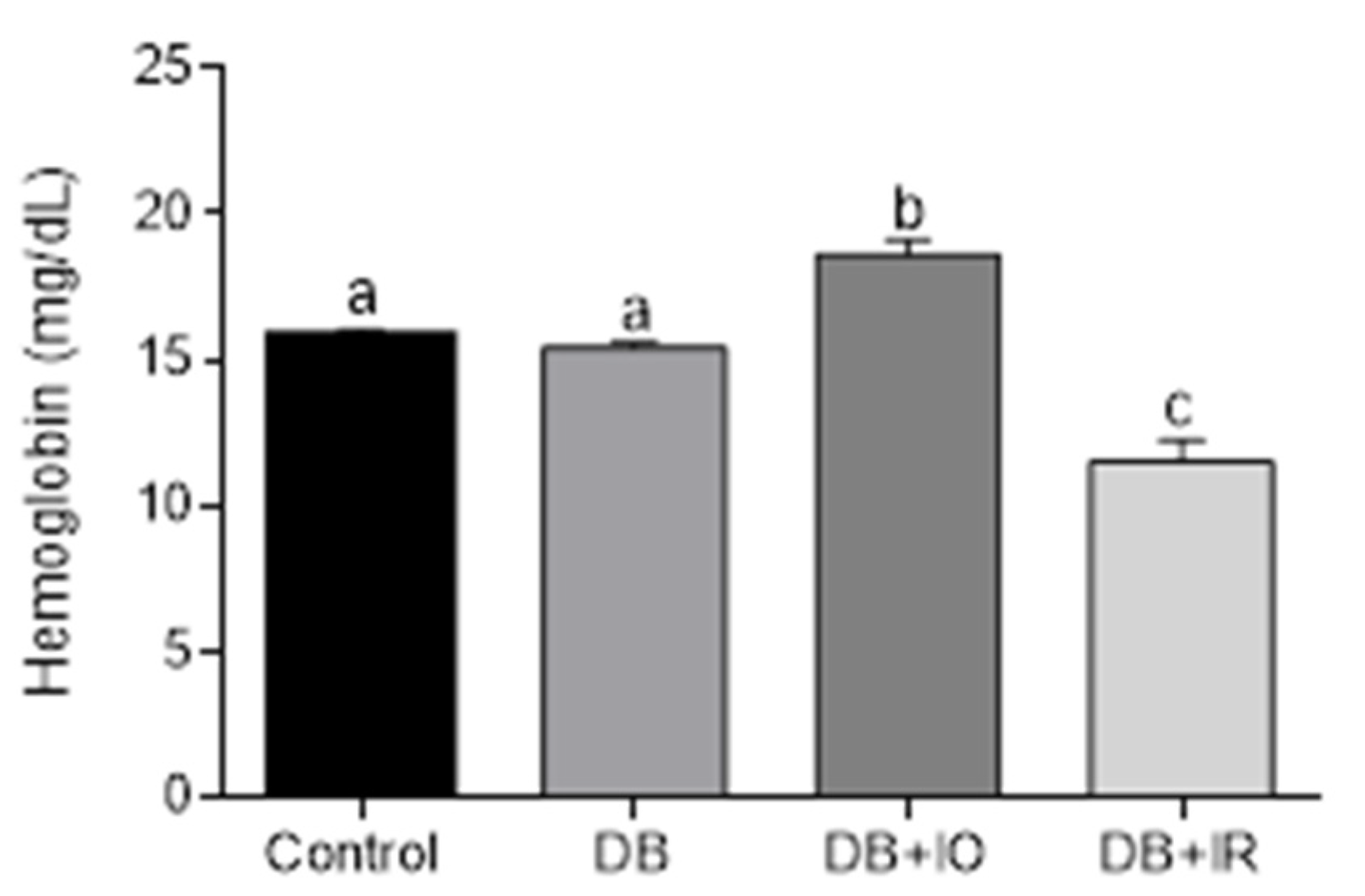
3.2. Effects of Dietary Iron on Hemoglobin
3.3. Effects of Dietary Iron on Muscle Strength Development and Fatigue
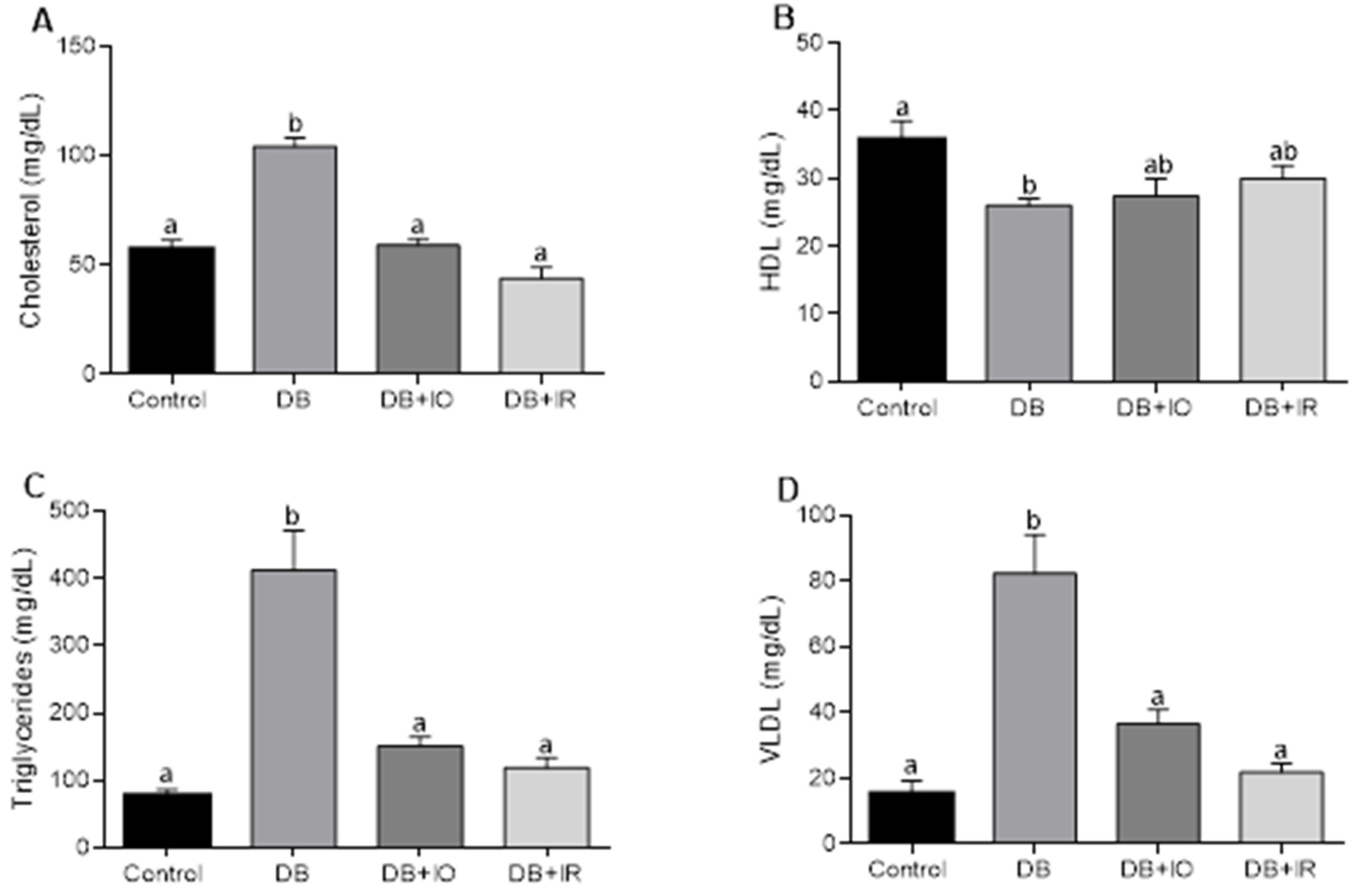
3.4. Effects of Iron on Serum Lipid Profile
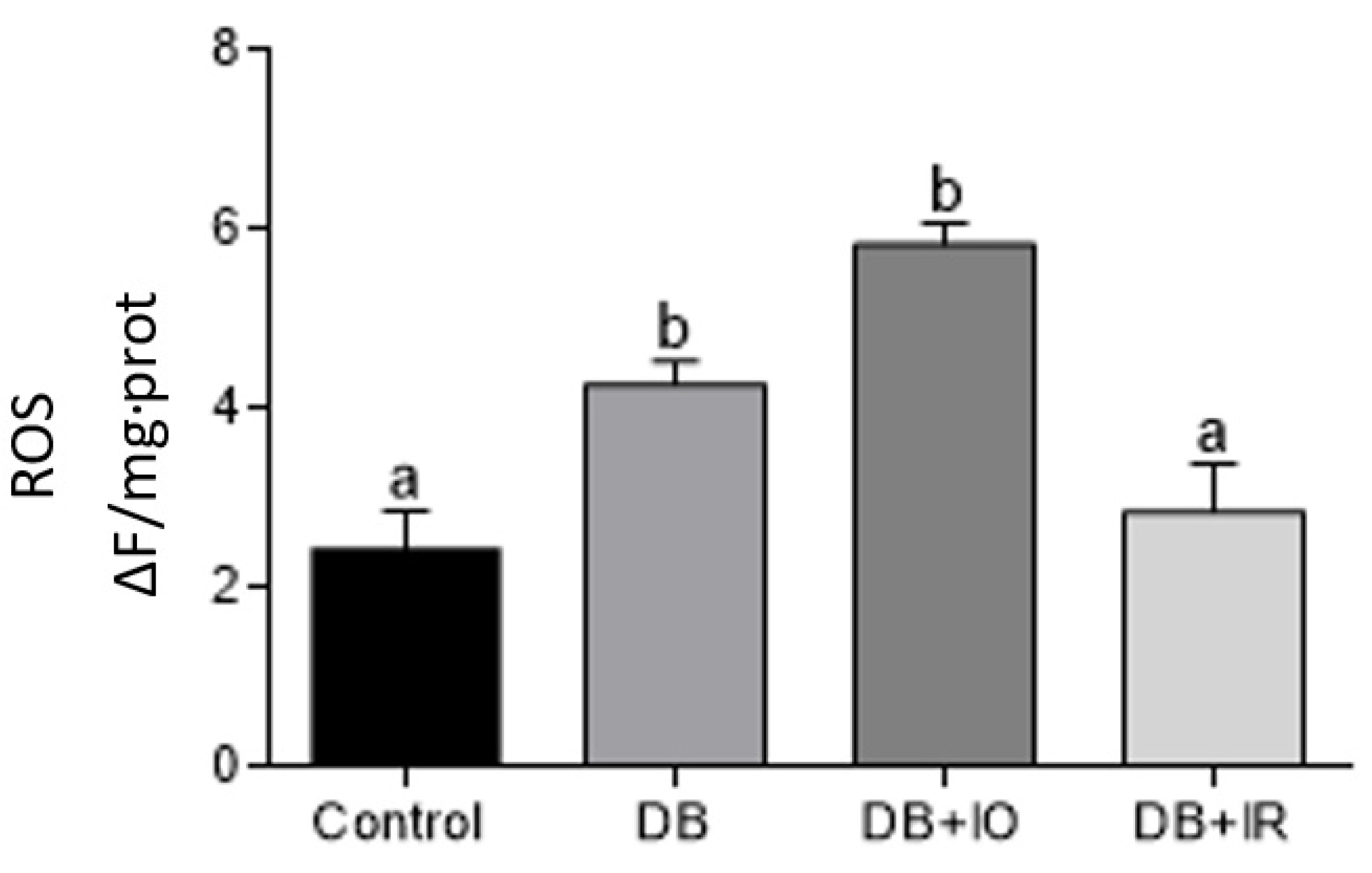
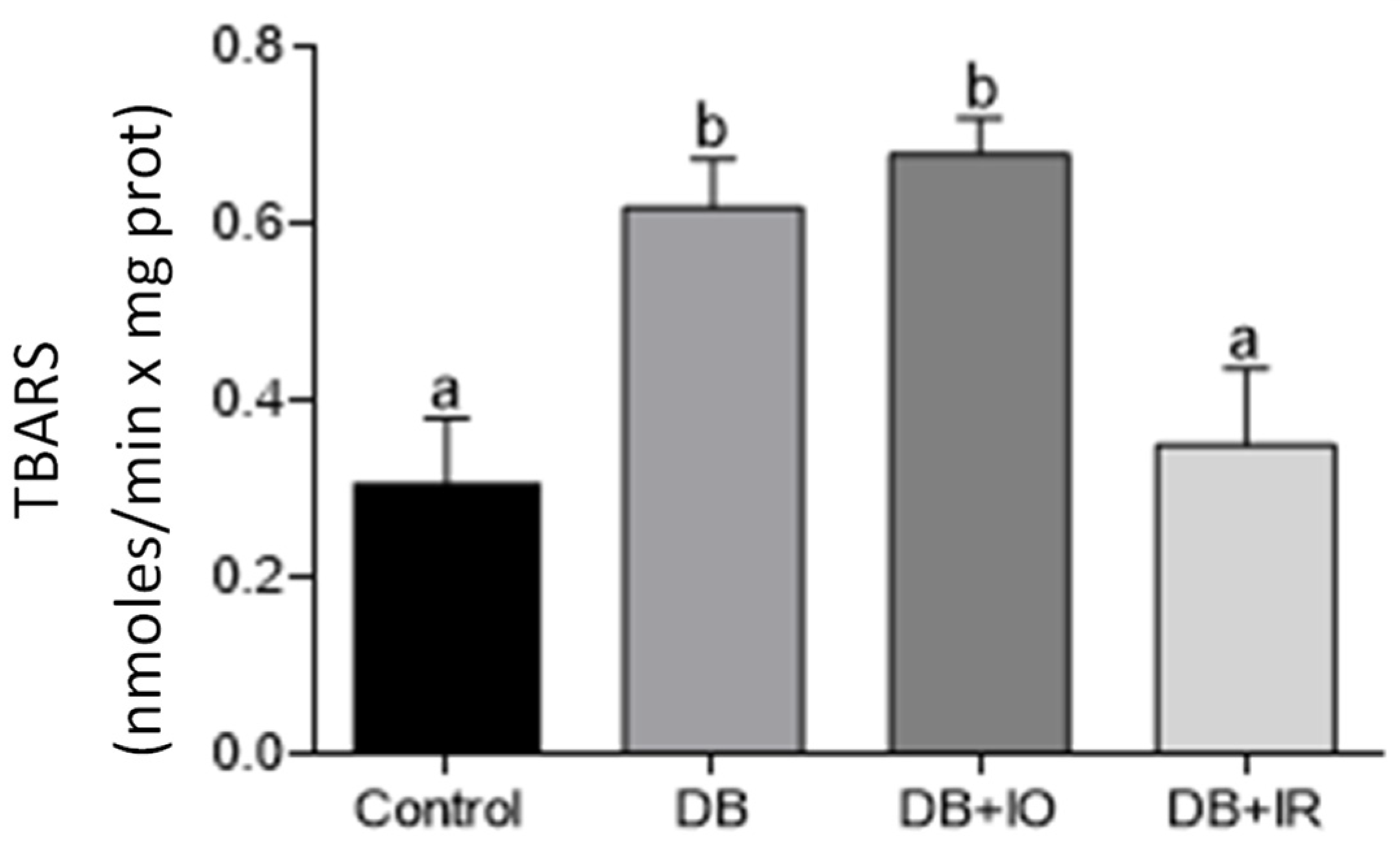
3.5. Effect of Iron on Oxidative Stress Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, J.; Souza, E.; Echazú Böschemeier, A.G.; Costa, C.; Bezerra, H.S. Diagnosis of diabetes mellitus and living with a chronic condition: Participatory study. BMC Public Health 2018, 18, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elisabetta, B.; Laura, L. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid. Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar]
- Fakhruddin, S.; Alanazi, W.; Jackson, K. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Kalra, S.; Sahay, R. Diabetes fatigue syndrome. Diabetes Ther. 2018, 9, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Leigh, C.; Gerald, S. Hyperglycemia-induced diaphragm weakness is mediated by oxidative stress. Crit. Care 2014, 18, 88. [Google Scholar]
- Devulapally, Y.; Singh, N.D.; Bai, P.K. A comparative study of skeletal muscle fatigue in diabetic and non-diabetic human beings. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1529–1532. [Google Scholar] [CrossRef]
- Jia, A.; Zeng, W.; Yu, L.; Zheng, H.; Lu, Z.; Song, Y. Very low-density lipoprotein cholesterol is associated with extent and severity of coronary artery disease in patients with type 2 diabetes mellitus. SAGE Open Med. 2019, 7, 2050312119871786. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P. Diabetes dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Warraich, H.J.; Rana, J.S. Dyslipidemia in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 27–32. [Google Scholar] [CrossRef]
- Andrich, D.; Ou, Y.; Melbouci, L. Altered lipid metabolism impairs skeletal muscle force in young rats submitted to a short-term high-fat diet. Front. Physiol. 2018, 26, 1327. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, C.E.; Van Rompay, M.I.; Schultz, N.S. Relationship between muscle strength and dyslipidemia, serum 25(OH) D, and weight status among diverse schoolchildren: A cross-sectional analysis. BMC Pediatr. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the hemodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef] [PubMed]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, S.; Fonseca, V.A.; Alam, M.G.; Shah, S.V. The role of iron in diabetes and its complications. Diabetes Care 2007, 30, 1926–1933. [Google Scholar] [CrossRef] [Green Version]
- Márquez-Ibarra, A.; Huerta, M.; Villalpando-Hernández, S.; Ríos-Silva, M.; Díaz-Reval, M.I.; Cruzblanca, H.; Mancilla, E.; Trujillo, X. The effects of dietary iron and capsaicin on hemoglobin, blood glucose, insulin tolerance, cholesterol, and triglycerides, in healthy and diabetic wistar rats. PLoS ONE 2016, 11, e0152625. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Balasubramaniam, P.; Malathi, A. Comparative study of hemoglobin estimated by Drabkin’s and Sahli’s methods. J. Postgrad. Med. 1992, 38, 8–9. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar]
- Huerta-Cervantes, M.; Peña-Montes, D.J.; Montoya-Pérez, R.; Trujillo, X.; Huerta, M.; López-Vázquez, M.Á.; Olvera-Cortés, M.E.; Saavedra-Molina, A. Gestational diabetes triggers oxidative stress in hippocampus and cerebral cortex and cognitive behavior modifications in rat offspring: Age- and sex-dependent effects. Nutrients 2020, 12, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ávila, O.; Gallegos-Corona, M.A.; Sánchez-Briones, L.A. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J. Bioenerg. Biomembr. 2015, 47, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, R.C.; Jones, D.; Gabrielsen, S.; Huang, J.; Simcox, J.A.; Luo, B.; Soesanto, Y.; Rienhoff, H.; Abel, E.D.; McClain, D.A. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep−/−) mouse. Am. J. Physiol. Endocrinol. 2010, 298, 1236–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Lee, K.; Cui, R.; Kim, D.J.; Choi, S.; Lee, W.J.; Kang, Y.; Kim, T.H.; Moon, H.U.; Jeon, J.Y.; Han, S.J.; et al. Iron overload plays an important role in er stress-induced insulin resistance in human skeletal muscle cells. Diabetes 2018, 67, 1912. [Google Scholar] [CrossRef]
- Makker, J.; Hanif, A.; Bajantri, B.; Chilimuri, S. Dysmetabolic hyperferritinemia: All iron overload is not hemochromatosis. Case Rep. Gastroenterol. 2015, 9, 7–14. [Google Scholar] [CrossRef]
- Halon-Golabek, M.; Borkowska, A.; Herman-Antosiewicz, A.; Antosiewicz, J. Iron metabolism of the skeletal muscle and neurodegeneration. Front. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef] [Green Version]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Takemura, S.; Kodai, S.; Shinkawa, H.; Tsukioka, T.; Ichikawa, H.; Naito, Y.; Yoshikawa, T.; Okada, S. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am. J. Physiol. Endocrinol. 2010, 298, E1140–E1149. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; da Costa Guerra, J.F.; Sampaio, A.F.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L. Iron dextran increases hepatic oxidative stress and alters expression of genes related to lipid metabolism contributing to hyperlipidaemia in murine model. Biomed. Res. Int. 2015, 2015, 272617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, S.; Thibault, L.; Delvin, E.; Yotov, W.; Bendayan, M.; Levy, E. Dietary iron overload and induced lipid peroxidation are associated with impaired plasma lipid transport and hepatic sterol metabolism in rats. Hepatology 1999, 29, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.M.; Chua, A.C.; Carter, K.W.; Delima, R.D.; Johnstone, D.; Herbison, C.E.; Firth, M.J.; O’Leary, R.; Milward, E.A.; Olynyk, J.K.; et al. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology 2010, 52, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, U.; Latham, P.S.; Oates, P.S. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J. Gastroenterol. 2012, 18, 4651–4658. [Google Scholar] [CrossRef]
- Tiwari, S.; Siddiqi, S.A. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 2012, 5, 1079–1086. [Google Scholar] [CrossRef] [Green Version]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The role of iron and iron overload in chronic liver disease. Med. Sci Mon. 2016, 22, 2144–2151. [Google Scholar] [CrossRef] [Green Version]
- Prenner, S.B.; Mulvey, C.K.; Ferguson, J.F.; Rickels, M.R.; Bhatt, A.B.; Reilly, M.P. Very low-density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis 2014, 236, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Kostapanos, M.S.; Elisaf, M.S. High density lipoproteins and type 2 diabetes: Emerging concepts in their relationship. World J. Exp. Med. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Wong, N.K.P.; Nicholls, S.J.; Tan, J.T.M.; Bursill, C.A. The role of high-density lipoproteins in diabetes and its vascular complications. Int. J. Mol. Sci. 2018, 19, 1680. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Vargas, M.A.; Saavedra-Molina, A.; Gómez-Barroso, M.; Peña-Montes, D.; Cortés-Rojo, C.; Miguel, H.; Trujillo, X.; Montoya-Pérez, R. Dietary Iron Restriction Improves Muscle Function, Dyslipidemia, and Decreased Muscle Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Antioxidants 2022, 11, 731. https://doi.org/10.3390/antiox11040731
Vargas-Vargas MA, Saavedra-Molina A, Gómez-Barroso M, Peña-Montes D, Cortés-Rojo C, Miguel H, Trujillo X, Montoya-Pérez R. Dietary Iron Restriction Improves Muscle Function, Dyslipidemia, and Decreased Muscle Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Antioxidants. 2022; 11(4):731. https://doi.org/10.3390/antiox11040731
Chicago/Turabian StyleVargas-Vargas, Manuel Alejandro, Alfredo Saavedra-Molina, Mariana Gómez-Barroso, Donovan Peña-Montes, Christian Cortés-Rojo, Huerta Miguel, Xochitl Trujillo, and Rocío Montoya-Pérez. 2022. "Dietary Iron Restriction Improves Muscle Function, Dyslipidemia, and Decreased Muscle Oxidative Stress in Streptozotocin-Induced Diabetic Rats" Antioxidants 11, no. 4: 731. https://doi.org/10.3390/antiox11040731
APA StyleVargas-Vargas, M. A., Saavedra-Molina, A., Gómez-Barroso, M., Peña-Montes, D., Cortés-Rojo, C., Miguel, H., Trujillo, X., & Montoya-Pérez, R. (2022). Dietary Iron Restriction Improves Muscle Function, Dyslipidemia, and Decreased Muscle Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Antioxidants, 11(4), 731. https://doi.org/10.3390/antiox11040731







