Polyphenolic Compounds from Lespedeza bicolor Protect Neuronal Cells from Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation
2.3. General Experimental Procedures
2.4. Analytical and Semi-Preparative HPLC
2.5. HR-ESI-MS
2.6. Antiradical Activity
2.7. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. Quantum-Chemical Modeling
2.9. Cell Line and Culture Conditions
2.10. Cell Viability Assay (MTT Method)
2.11. In Vitro Model of PQ-, Rotenone-, and 6-OHDA-Induced Neurotoxicity
2.12. Reactive Oxygen Species (ROS) Level Evaluation in PQ-Treated Cells
2.13. Mitochondrial Membrane Potential (MMP) Detection
2.14. Statistical Analysis
3. Results
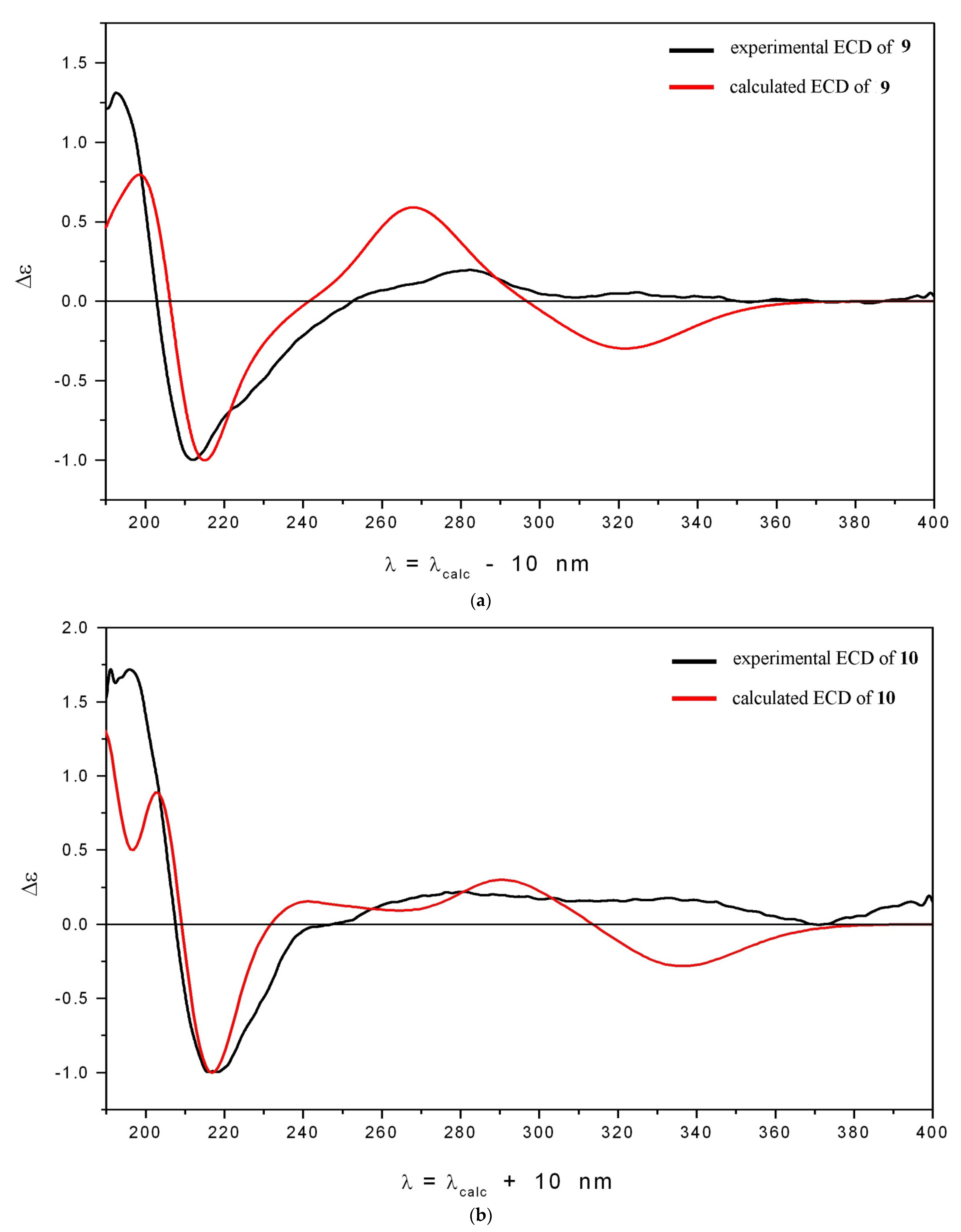
3.1. Isolation and Structure Elucidation of Compounds 10 and 11
3.2. Antioxidant Activity of Polyphenolic Compounds
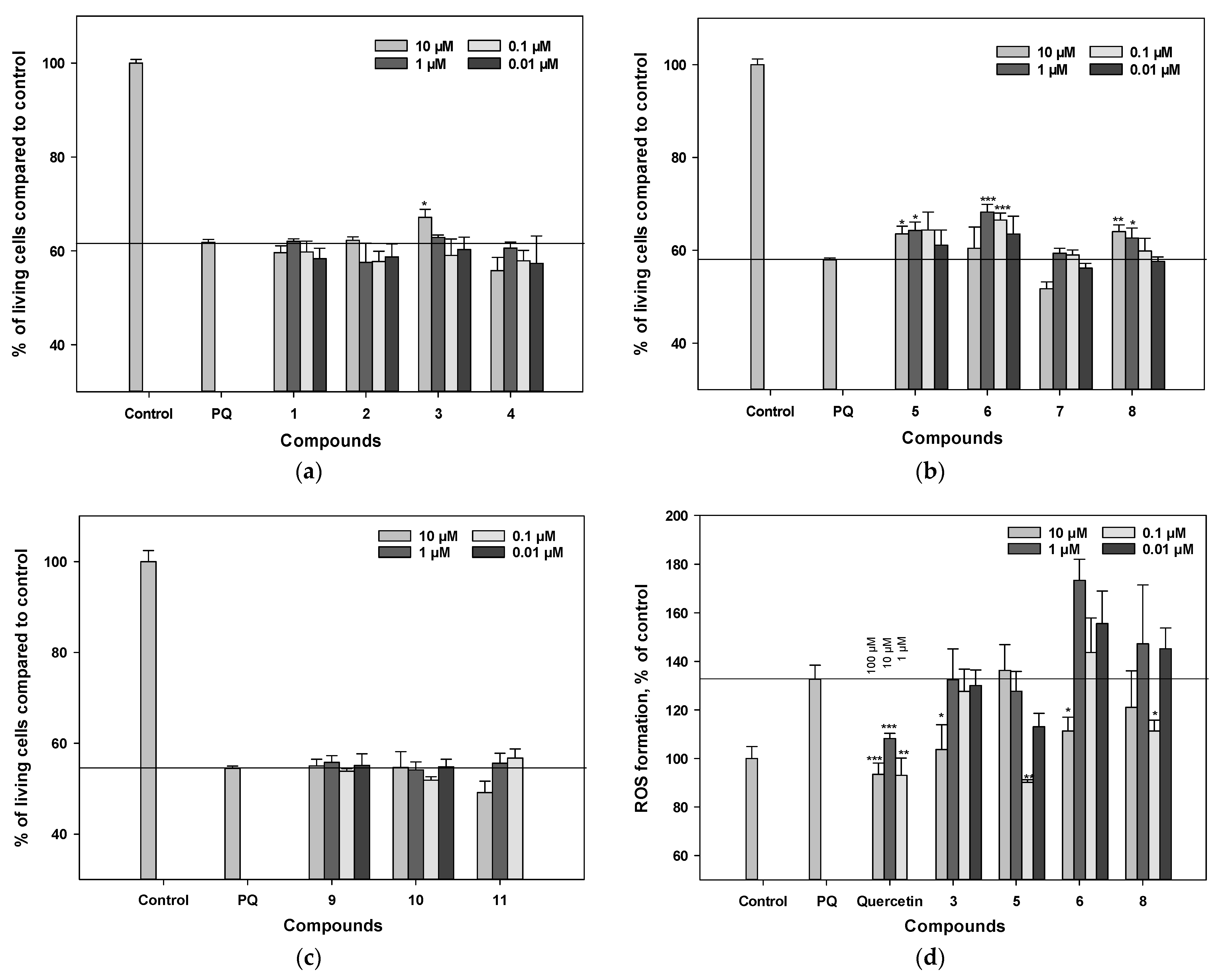
3.3. Cytotoxicity of Polyphenolic Compounds from L. bicolor against Neuro-2a Cells
3.4. Influence on Viability and ROS Level in PQ-Treated Neuro-2a Cells
3.5. Mitochondrial Membrane Potential (MMP) Detection
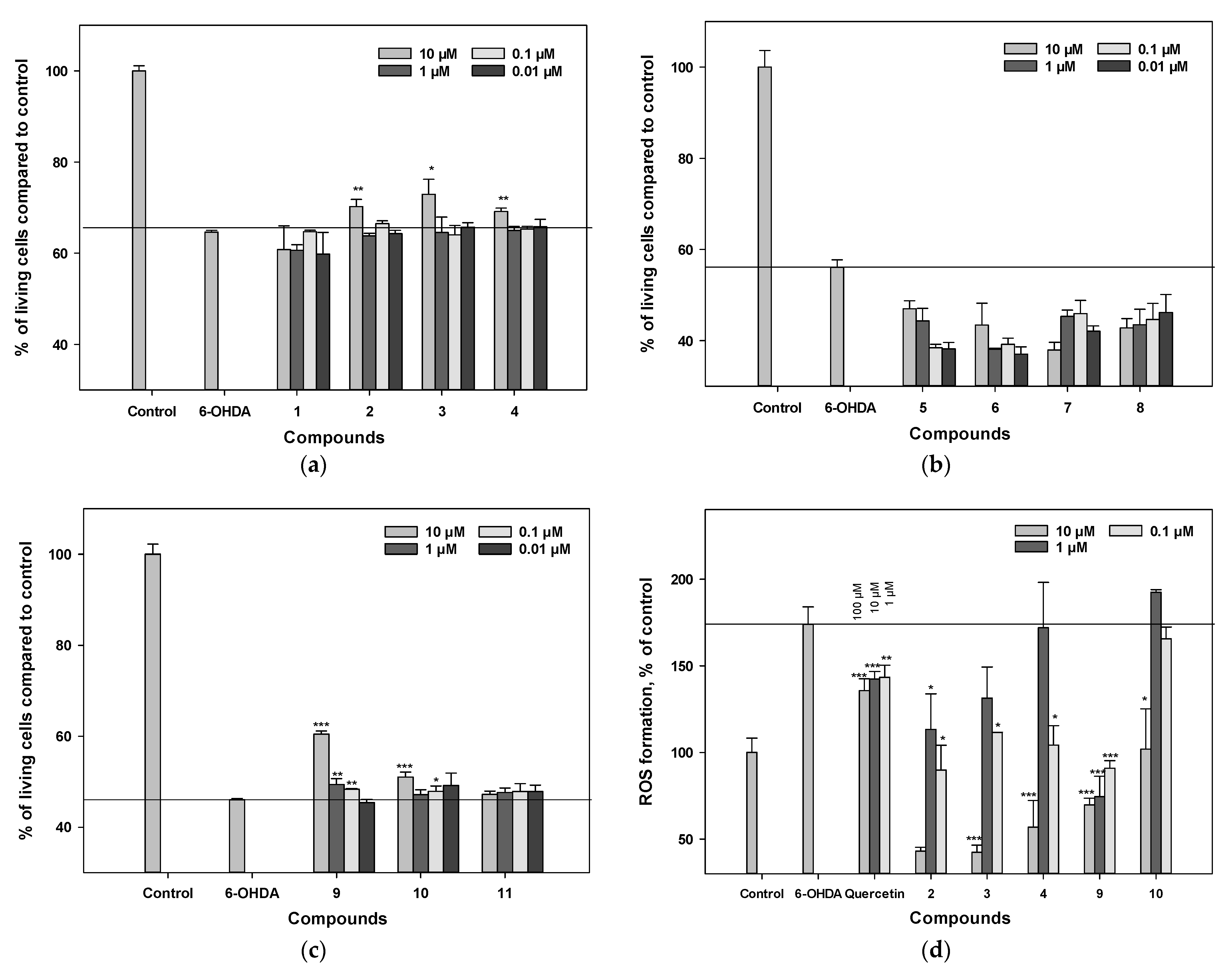
3.6. Influence on Viability and ROS Levels in 6-OHDA-Treated Neuro-2a Cells
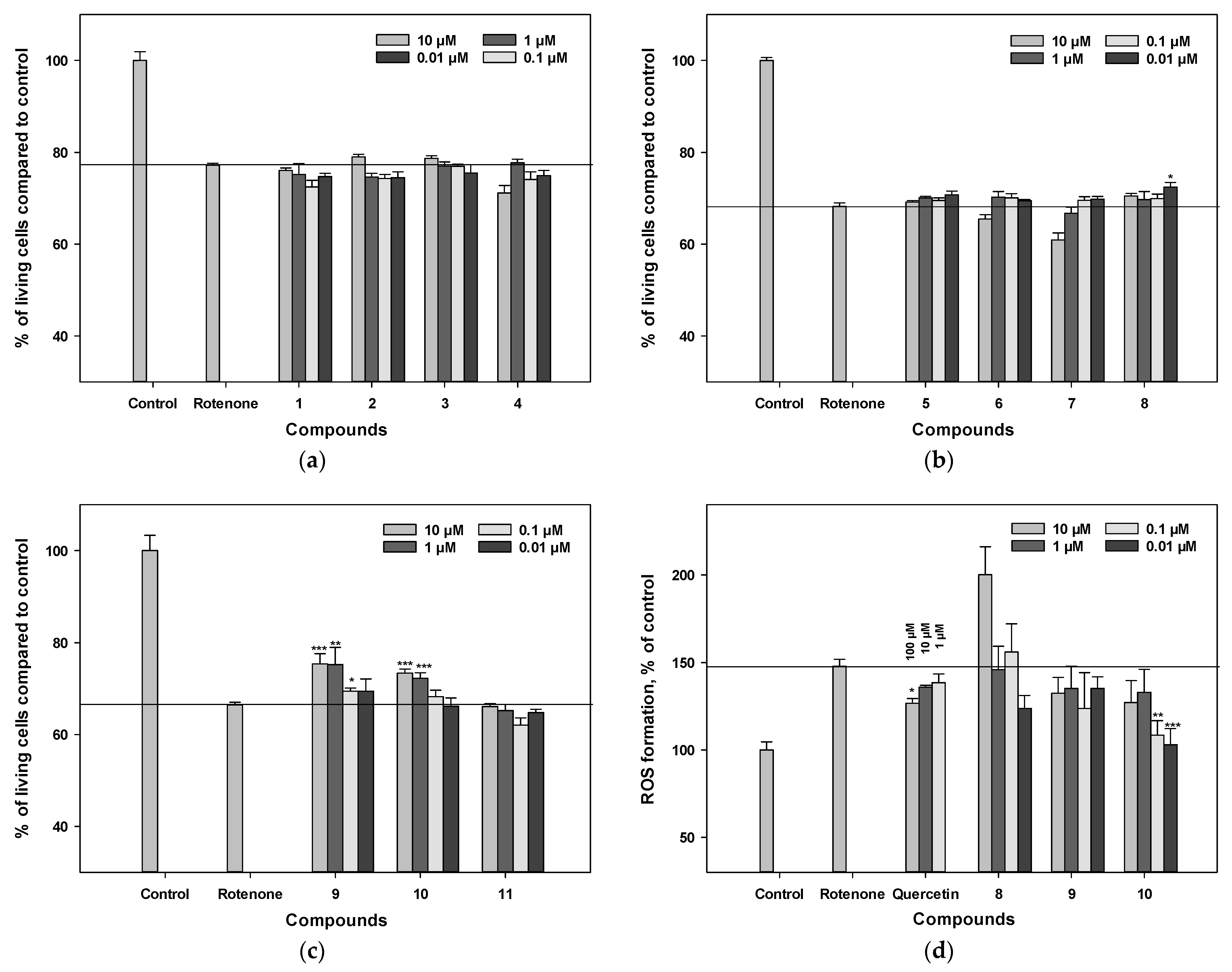
3.7. Influence on Viability and ROS Levels in Rotenone-Treated Neuro-2a Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Borrageiro, G.; Haylett, W.; Seedat, S.; Kuivaniemi, H.; Bardien, S. A review of genome-wide transcriptomics studies in Parkinson’s disease. Eur. J. Neurosci. 2018, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRX 2005, 2, 484–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastias-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the paraquat-induced sporadic Parkinson’s disease-like model. Mol. Neurobiol. 2019, 56, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.; Chingizova, E.; Pislyagin, E.; Likhatskaya, G.; Sabutski, Y.; Pelageev, D.; Polonik, S.; Aminin, D. Neuroprotective effect of 1,4-naphthoquinones in an in vitro model of paraquat and 6-OHDA-induced neurotoxicity. Int. J. Mol. Sci. 2021, 22, 9933. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Zhang, L.; Rasul, A.; Anwar, H.; Sohail, M.U.; Razzaq, A.; Aziz, N.; Shabbir, A.; Ali, M.; Sun, T. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer’s and Parkinson’s diseases: An update of recent data. Molecules 2018, 23, 814. [Google Scholar] [CrossRef] [Green Version]
- Selvam, C.; Jordan, B.C.; Prakash, S.; Mutisya, D.; Thilagavathi, R. Pterocarpan scaffold: A natural lead molecule with diverse pharmacological properties. Eur. J. Med. Chem. 2017, 128, 219–236. [Google Scholar] [CrossRef]
- Xia, W.; Luo, P.; Hua, P.; Ding, P.; Li, C.; Xu, J.; Zhou, H.; Gu, Q. Discovery of a new pterocarpan-type antineuroinflammatory compound from Sophora tonkinensis through suppression of the TLR4/NFκB/MAPK signaling pathway with PU 1 as a potential target. ACS Chem. Neurosci. 2018, 10, 295–303. [Google Scholar] [CrossRef]
- Tsai, R.T.; Tsai, C.W.; Liu, S.P.; Gao, J.X.; Kuo, Y.H.; Chao, P.M.; Hung, H.S.; Shyu, W.C.; Lin, S.Z.; Fu, R.H. Maackiain ameliorates 6-hydroxydopamine and SNCA pathologies by modulating the PINK1/Parkin pathway in models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y cell line. Int. J. Mol. Sci. 2020, 21, 4455. [Google Scholar] [CrossRef]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.J.; Kim, W.J.; Kang, M.G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, J.H.; Lee, C.; Jin, Q.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Inhibitory constituents of Sophora tonkinensis on nitric oxide production in RAW264.7 macrophages. Bioorg. Med. Chem. Lett. 2015, 25, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kang, M.; Pyo, S.; Chae, H.S.; Ryu, K.H.; Kim, J.; Chin, Y.W. SKI3301, a purified herbal extract from Sophora tonkinensis, inhibited airway inflammation and bronchospasm in allergic asthma animal models in vivo. J. Ethnopharmacol. 2017, 206, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Singh, S. Updates on immunity and inflammation in Parkinson disease pathology. J. Neurosci. Res. 2018, 96, 379–390. [Google Scholar] [CrossRef]
- Liu, M.H.; Tsuang, F.Y.; Sheu, S.Y.; Sun, J.S.; Shih, C.M. The protective effects of coumestrol against amyloid-beta peptide-and lipopolysaccharide-induced toxicity on mice astrocytes. Neurol. Res. 2011, 33, 663–672. [Google Scholar] [CrossRef]
- Castro, C.C.; Pagnussat, A.S.; Orlandi, L.; Worm, P.; Moura, N.; Etgen, A.M.; Netto, C.A. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012, 1474, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Anastacio, J.B.R.; Sanches, E.F.; Nicola, F.; Odorcyk, F.; Fabres, R.B.; Netto, C.A. Phytoestrogen coumestrol attenuates brain mitochondrial dysfunction and longterm cognitive deficits following neonatal hypoxia–ischemia. Int. J. Dev. Neurosci. 2019, 79, 86–95. [Google Scholar] [CrossRef]
- Moreira, A.C.; Silva, A.M.; Branco, A.F.; Baldeiras, I.; Pereira, G.C.; Seiça, R.; Santos, M.S.; Sardao, V.A. Phytoestrogen coumestrol improves mitochondrial activity and decreases oxidative stress in the brain of ovariectomized Wistar-Han rats. J. Funct. Foods. 2017, 34, 329–339. [Google Scholar] [CrossRef]
- Mori-Hongo, M.; Yamaguchi, H.; Warashina, T.; Miyase, T. Melanin synthesis inhibitors from Lespedeza cyrtobotrya. J. Nat. Prod. 2009, 72, 63–71. [Google Scholar] [CrossRef]
- Mori-Hongo, M.; Takimoto, H.; Katagiri, T.; Kimura, M.; Ikeda, Y.; Miyase, T. Melanin synthesis inhibitors from Lespedeza floribunda. J. Nat. Prod. 2009, 72, 194–203. [Google Scholar] [CrossRef]
- Miyase, T.; Sano, M.; Nakai, H.; Muraoka, M.; Nakazawa, M.; Suzuki, M.; Yoshino, K.; Nishihara, Y.; Tanai, J. Antioxidants from Lespedeza homoloba (I). Phytochemistry 1999, 52, 303–310. [Google Scholar] [CrossRef]
- Miyase, T.; Sano, M.; Yoshino, K.; Nonaka, K. Antioxidants from Lespedeza homoloba (II). Phytochemistry 1999, 52, 311–319. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Tarbeeva, D.V.; Fedoreyev, S.A.; Busenbender, T.; Kaune, M.; Veselova, M.V.; Kalinovskiy, A.I.; Hauschild, J.; Grigorchuk, V.P.; Kim, N.Y.; et al. Polyphenolic compounds from Lespedeza bicolor root bark inhibit progression of human prostate cancer cells via induction of apoptosis and cell cycle arrest. Biomolecules 2020, 10, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarbeeva, D.V.; Fedoreyev, S.A.; Veselova, M.V.; Blagodatski, A.S.; Klimenko, A.M.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Berdyshev, D.V.; Gorovoy, P.G. Cytotoxic polyphenolic compounds from Lespedeza bicolor stem bark. Fitoterapia 2019, 135, 64–72. [Google Scholar] [CrossRef]
- Lee, P.J.; Pham, C.H.; Thuy, N.T.T.; Park, H.J.; Lee, S.H.; Yoo, H.M.; Cho, N. 1-Methoxylespeflorin G11 protects HT22 cells from glutamate-induced cell death through inhibition of ROS production and apoptosis. J. Microbiol. Biotechnol. 2021, 31, 217–225. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Krylova, N.V.; Iunikhina, O.V.; Likhatskaya, G.N.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Shchelkanov, M.Y.; Fedoreyev, S.A. Biologically active polyphenolic compounds from Lespedeza bicolor. Fitoterapia 2022, 157, 105121. [Google Scholar] [CrossRef]
- Thuy, N.T.T.; Lee, J.E.; Yoo, H.M.; Cho, N. Antiproliferative pterocarpans and coumestans from Lespedeza bicolor. J. Nat. Prod. 2019, 82, 3025–3032. [Google Scholar] [CrossRef]
- Woo, H.S.; Kim, D.W.; Curtis-Long, M.J.; Lee, B.W.; Lee, J.H.; Kim, J.Y.; Kang, J.E.; Park, K.H. Potent inhibition of bacterial neuraminidase activity by pterocarpans isolated from the roots of Lespedeza bicolor. Bioorg. Med. Chem. Lett. 2011, 21, 6100–6103. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin(II)-quercetin complex: Synthesis, spectral characterization and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pislyagin, E.; Kozlovskiy, S.; Menchinskaya, E.; Chingizova, E.; Likhatskaya, G.; Gorpenchenko, T.; Sabutski, Y.; Polonik, S.; Aminin, D. Synthetic 1,4-naphthoquinones inhibit P2X7 receptors in murine neuroblastoma cells. Bioorg. Med. Chem. 2021, 31, 115975. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical perspective: Models of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-L.; Chao, C.-C.; Lee, Y.-C.; Lu, M.-K.; Cheng, J.-J.; Yang, Y.-C.; Huang, N.-K. Paraquat induces cell death through impairing mitochondrial membrane permeability. Mol. Neurobiol. 2015, 53, 2169–2188. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, Z.; Cao, F.; Wang, L.; Liao, Y.; Liu, X.; Pan, R.; Chang, Q. Brain pharmacokinetics and the pharmacological effects on striatal neurotransmitter levels of Pueraria lobata isoflavonoids in rat. Front. Pharmacol. 2017, 8, 599. [Google Scholar] [CrossRef] [Green Version]
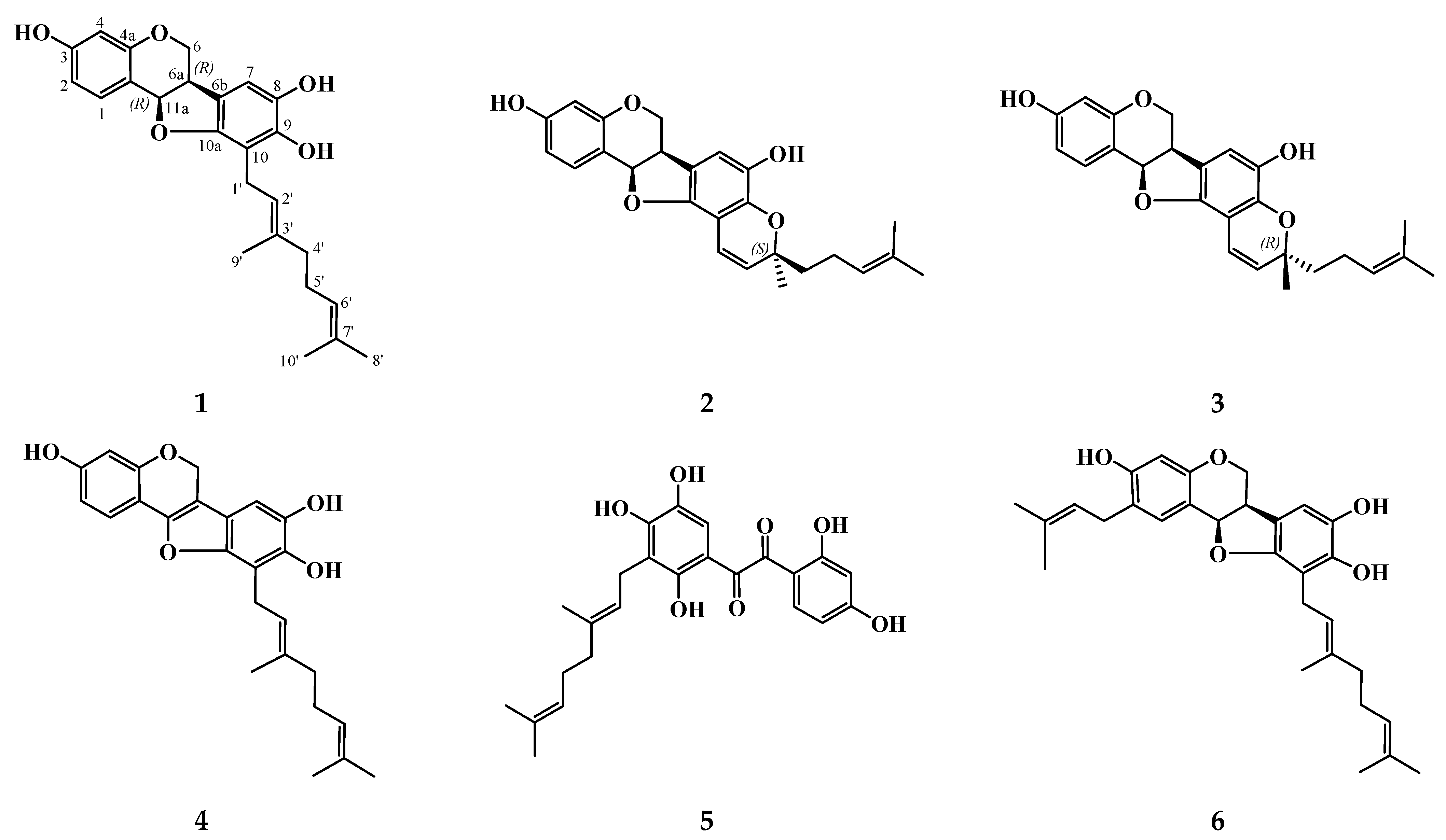
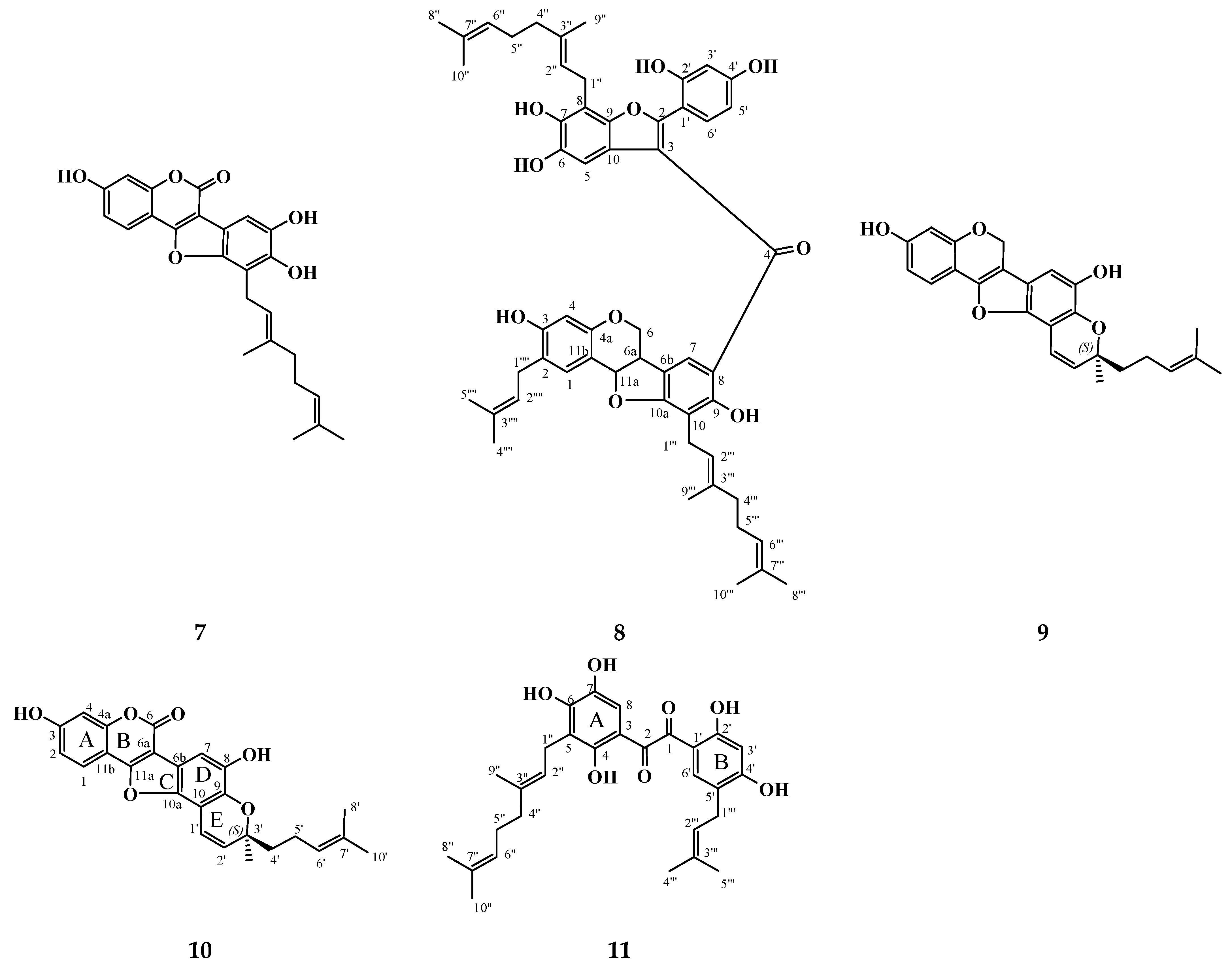

| Position | 13C | 1H | HMBC | COSY | ROESY |
|---|---|---|---|---|---|
| 1 | 122.7 | 7.85, d, J = 8.5, 1H | C-3, 4, 4a | H-2 | H-2 |
| 2 | 113.6 | 6.95, d, J = 8.5, 1H | C-3, 4, 11b | H-1 | H-1 |
| 3 | 159.2 | ||||
| 4 | 104.0 | 7.13, s, 1H | C-2, 3, 4a, 11b | ||
| 4a | 154.8 | ||||
| 6 | 159.0 | ||||
| 6a | 103.8 | ||||
| 6b | 116.0 | ||||
| 7 | 105.4 | 7.44, s, 1H | C-6a, 6b, 8, 9, 10, 10a | OH-8 | |
| 8 | 143.1 | ||||
| 9 | 138.7 | ||||
| 10 | 106.4 | ||||
| 10a | 145.2 | ||||
| 11a | 160.1 | ||||
| 11b | 106.3 | ||||
| 1′ | 115.9 | 6.89, d, J = 9.9, 1H | C-8, 9, 10, 10a, 3′, 9′ | H-2′ | H-2′ |
| 2′ | 130.6 | 5.78, d, J = 9.9, 1H | C-9, 10, 3′, 4′, 9′ | H-1′ | H-1′, 4′, 5′, 9′ |
| 3′ | 80.6 | ||||
| 4′ | 40.9 | 1.78, m, 1H | C-2′, 3′, 5′, 6′, 9′ | H-5′ | H-2′, 5′, 9′ |
| 1.84, m, 1H | C-2′, 3′, 5′, 6′, 9′ | H-5′ | H-2′, 5′, 9′ | ||
| 5′ | 22.8 | 2.13, m, 1H | C-4′, 6′, 7′ | H-4′, 6′ | H-2′, 4′, 9′ |
| 2.15, m, 1H | C-4′, 6′, 7′ | H-4′, 6′ | H-2′, 4′, 9′ | ||
| 6′ | 123.6 | 5.10, t, J = 6.9, 1H | C-4′, 6′, 8′, 10′ | H-5′, 8′, 10′ | H-5′, 8′ |
| 7′ | 132.2 | ||||
| 8′ | 25.6 | 1.67, s, 3H | C-4′ (weak), 6′, 7′, 10′ | H-6′ | H-6′ |
| 9′ | 26.3 | 1.50, s, 3H | C-2′, 3′, 4′, 5′ (weak) | H-2′, 4′, 5′ | |
| 10′ | 17.6 | 1.57, s, 3H | C-4′ (weak), 6′, 7′, 8′ | H-6′ | |
| OH-3 | 6.54, bs, 1H | ||||
| OH-8 | 5.50, bs, 1H | C-7 | H-7 |
| Position | 13C | 1H | HMBC | COSY | ROESY |
|---|---|---|---|---|---|
| 1 | 194.7 | ||||
| 2 | 194.6 | ||||
| 3 | 109.1 | ||||
| 4 | 158.9 | ||||
| 5 | 115.3 | ||||
| 6 | 152.5 | ||||
| 7 | 136.8 | ||||
| 8 | 113.7 | 6.84, s, 1H | C-2, 4, 6, 7 | OH-7 | |
| 1′ | 111.1 | ||||
| 2′ | 165.2 | ||||
| 3′ | 104.3 | 6.44, s, 1H | C-1′, 2′, 4′, 5′ | ||
| 4′ | 163.7 | ||||
| 5′ | 119.9 | ||||
| 6′ | 133.9 | 7.18, s, 1H | C-1, 1′, 2′, 5′, 1‴ | H-1‴ | |
| 1″ | 22.0 | 3.51, d, J = 7.3, 2H | C-4, 5, 6, 2″, 3″ | H-2″, 9″ | H-2″, 9″, OH-6 |
| 2″ | 120.5 | 5.33, t, J = 7.3, 1H | C-1″ | H-1″, 9″ | H-1″, 4″, OH-6 |
| 3″ | 139.7 | ||||
| 4″ | 39.7 | 2.09, m, 2H | C-2″, 3″, 5″, 6″ | H-2″, 6″ | |
| 5″ | 26.4 | 2.13, m, 2H | C-3″, 6″, 7″ | H-6″ | H-6″ |
| 6″ | 123.8 | 5.07, m, 1H | H-5″, 8″, 10″ | H-4″, 5″ | |
| 7″ | 132.1 | ||||
| 8″ | 25.7 | 1.68, s, 3H | C-6″, 7″, 10″ | H-6″ | |
| 9″ | 16.3 | 1.84, s, 3H | C-2″, 3″, 4″ | H-1″, 2″ | H-1″ |
| 10″ | 17.7 | 1.60, s, 3H | C-6″, 7″, 8″ | H-6″ | |
| 1‴ | 29.1 | 3.23, d, J = 7.2, 2H | C-4′, 5′, 6′, 2‴, 3‴ | H-2‴, 4‴, 5‴ | H-2‴, 4‴, 5‴, 6′, OH-4′ |
| 2‴ | 120.8 | 5.20, t, J = 7.2, 2H | H-1‴, 4‴, 5‴ | H-1‴, 5‴ | |
| 3‴ | 136.4 | ||||
| 4‴ | 25.7 | 1.73, s, 3H | C-2‴, 3‴, 5‴ | H-1‴, 2‴ | H-1‴ |
| 5‴ | 17.9 | 1.71, s, 3H | C-2‴, 3‴, 4‴ | H-1‴, 2‴ | H-1‴, 2‴ |
| OH-4 | 11.92, s, 1H | C-3, 4, 5 | |||
| OH-6 | 6.68, s, 1H | C-5, 6, 7 | H-2″, 1″, OH-7 | ||
| OH-7 | 5.05, bs, 1H | H-8, OH-6 | |||
| OH-2′ | 11.59, s, 1H | C1′, 2′, 3′, 4′ | |||
| OH-4′ | 6.11, s, 1H | C-3′, 4′, 5′ | H-1‴ |
| Compound | DPPH-Scavenging Effect | FRAP |
|---|---|---|
| SC50 µM, 30 min | mol (Fe2+)/mol (polyphenolic compound X) | |
| Quercetin | 8.9 ± 0.2 | 5.11 ± 0.31 |
| Ascorbic acid | 30.5 ± 2.1 | 3.43 ± 0.33 |
| 1 | 24.3 ± 3.7 * | 1.78 ± 0.16 *** |
| 2 | 25.0 ± 2.2 ** | 1.31 ± 0.02 ** |
| 3 | 23.7 ± 2.1 ** | 1.60 ± 0.08 ** |
| 4 | 18.0 ± 0.2 *** | 2.78 ± 0.18 ** |
| 5 | 26.1 ± 3.0 * | 0.86 ± 0.04 * |
| 6 | 21.8 ± 2.0 * | 1.43 ± 0.06 ** |
| 7 | 25.7 ± 3.4 * | 1.83 ± 0.16 * |
| 8 | 26.7 ± 3.8 * | 1.95 ± 0.19 * |
| 9 | 13.5 ± 1.2 ** | 0.75 ± 0.05 ** |
| 10 | 22.7 ± 3.5 * | 1.75 ± 0.20 * |
| 11 | 19.4 ± 2.2 ** | 1.45 ± 0.08 ** |
| Compound | EC50, µM |
|---|---|
| 1 | 72.0 |
| 2 | 75.0 |
| 3 | 44.0 |
| 4 | 76.0 |
| 5 | 100.0 |
| 6 | 40.6 |
| 7 | >100 |
| 8 | >100 |
| 9 | 75.0 |
| 10 | 44.0 |
| 11 | 87.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarbeeva, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Berdyshev, D.V.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Mishchenko, N.P.; Aminin, D.L.; Fedoreyev, S.A. Polyphenolic Compounds from Lespedeza bicolor Protect Neuronal Cells from Oxidative Stress. Antioxidants 2022, 11, 709. https://doi.org/10.3390/antiox11040709
Tarbeeva DV, Pislyagin EA, Menchinskaya ES, Berdyshev DV, Kalinovskiy AI, Grigorchuk VP, Mishchenko NP, Aminin DL, Fedoreyev SA. Polyphenolic Compounds from Lespedeza bicolor Protect Neuronal Cells from Oxidative Stress. Antioxidants. 2022; 11(4):709. https://doi.org/10.3390/antiox11040709
Chicago/Turabian StyleTarbeeva, Darya V., Evgeny A. Pislyagin, Ekaterina S. Menchinskaya, Dmitrii V. Berdyshev, Anatoliy I. Kalinovskiy, Valeria P. Grigorchuk, Natalia P. Mishchenko, Dmitry L. Aminin, and Sergey A. Fedoreyev. 2022. "Polyphenolic Compounds from Lespedeza bicolor Protect Neuronal Cells from Oxidative Stress" Antioxidants 11, no. 4: 709. https://doi.org/10.3390/antiox11040709
APA StyleTarbeeva, D. V., Pislyagin, E. A., Menchinskaya, E. S., Berdyshev, D. V., Kalinovskiy, A. I., Grigorchuk, V. P., Mishchenko, N. P., Aminin, D. L., & Fedoreyev, S. A. (2022). Polyphenolic Compounds from Lespedeza bicolor Protect Neuronal Cells from Oxidative Stress. Antioxidants, 11(4), 709. https://doi.org/10.3390/antiox11040709






