Abstract
The developing brain is highly sensitive to environmental disturbances, and adverse exposures can act through oxidative stress. Given that oxidative stress susceptibility is determined partly by genetics, multiple studies have employed genetic scores to explore the role of oxidative stress in human disease. However, traditional approaches to genetic score construction face a range of challenges, including a lack of interpretability, bias towards the disease outcome, and often overfitting to the study they were derived on. Here, we develop an alternative strategy by first generating a genetic pathway function score for oxidative stress (gPFSox) based on the transcriptional activity levels of the oxidative stress response pathway in brain and other tissue types. Then, in the Barwon Infant Study (BIS), a population-based birth cohort (n = 1074), we show that a high gPFSox, indicating reduced ability to counter oxidative stress, is linked to higher autism spectrum disorder risk and higher parent-reported autistic traits at age 4 years, with AOR values (per 2 additional pro-oxidant alleles) of 2.10 (95% CI (1.12, 4.11); p = 0.024) and 1.42 (95% CI (1.02, 2.01); p = 0.041), respectively. Past work in BIS has reported higher prenatal phthalate exposure at 36 weeks of gestation associated with offspring autism spectrum disorder. In this study, we examine combined effects and show a consistent pattern of increased neurodevelopmental problems for individuals with both a high gPFSox and high prenatal phthalate exposure across a range of outcomes, including high gPFSox and high DEHP levels against autism spectrum disorder (attributable proportion due to interaction 0.89; 95% CI (0.62, 1.16); p < 0.0001). The results highlight the utility of this novel functional genetic score and add to the growing evidence implicating gestational phthalate exposure in adverse neurodevelopment.
1. Introduction
Disorders of child neurodevelopment have increased over recent decades. Since the 2000s, for instance, the prevalence of autism spectrum disorder (ASD) has risen from 6.7 in 1000 [1] to 17.2 in 1000 in the United States [2], with a global estimate of 1–2.5% [3]. Reported prevalence rates of developmental delay in childhood have also risen [4,5]. The increases may partly reflect heightened awareness, improved detection, and changes in diagnostic criteria [6]. However, given the sensitivity of the developing brain to environmental disturbances [7], there is growing concern around the role of prenatal chemical exposures, including exposure to manufactured plastic chemicals [8,9,10].
Phthalates are one chemical class of concern [11,12]. They are a ubiquitous environmental contaminant [13] and can pass through the placental barrier and affect the developing fetus [14]. We have previously reported that the majority of pregnant women in our location harbor phthalate chemicals [15]. These chemicals appear to derive from ingested food (plastic food packaging, tinned food, and high-fat milk) and inhaled (air freshener and aerosols) and absorbed sources (hair treatment and cleaning chemicals) [15]. This is consistent with previous reports [16,17,18,19,20,21], which have highlighted plastic food packaging in particular as a major phthalate source.
Epidemiological studies have overall reported a positive association between prenatal phthalate exposure and adverse neurodevelopment [22], as described in recent systematic reviews [23,24,25,26], although not all findings have been consistent. There is some evidence for a possible adverse effect on child cognitive development, with maternal urinary phthalate metabolites linked to lower cognition [23,26]. Prenatal phthalate exposure is also associated in some studies with increased ASD symptomology and diagnosis risk [27,28,29,30,31], including in our location [31]. We have suggested that disparities may, in part, be attributable to differing susceptibility to toxicants, due to genetics and concomitant oxidant exposures [31]. In the Barwon Infant Study cohort, we reported that an infant’s genetic vulnerability to oxidative stress, an imbalance between the production and detoxification of reactive oxygen species (ROS) [32], may interplay with maternal phthalate exposure to increase the risk of adverse neurodevelopment [31].
In other studies of human cohorts and preclinical animal and cell culture models, phthalates have been linked to elevated oxidative stress [33,34,35]. A consequent impact on neurodevelopment is plausible as the brain’s high oxygen requirements make it especially susceptible to the damaging effects of ROS. Excess ROS can impair neurodevelopment by suppressing neuronal proliferation and migration, synapse formation, and long-term potentiation, and by activating inflammatory and apoptotic pathways in the brain [36]. Indeed, genome-wide association studies (GWAS) and other genomic analyses of ASD routinely identify genes implicated in oxidative stress [37,38,39,40].
Given that the ability of an individual to respond effectively to oxidative stress is determined in part by genetic makeup, several studies of ROS-related disorders have used polygenic risk scores (PRSs) to quantify genetic predisposition to oxidative stress [41,42]. A PRS is computed by summing the number of single-nucleotide polymorphisms (SNPs) an individual carries that show association with the trait or disease of interest, with each SNP often weighted by its GWAS effect size [43,44].
However, standard approaches to genetic score construction, including for oxidative stress, face a range of challenges. A key limitation is that the SNPs employed in PRSs rarely map to functionally coherent gene sets, making such scores difficult to interpret mechanistically [45]. Further, some oxidative-stress-related PRSs encode a disease outcome as well as oxidative stress, creating a bias towards this outcome that strengthens associations but obscures the impact of oxidative stress, per se, on the disease. For instance, we have previously published on a genetic score for oxidative stress derived from SNPs in genes relevant to oxidant balance that were further filtered based on association with adverse neurodevelopment [31]. Moreover, these challenges are frequently compounded by overfitting due to scores being both generated and evaluated on the same study [46,47].
Here, we present an alternative approach using a genetic pathway function score for oxidative stress (gPFSox), constructed independently of our study cohort, and capturing transcriptional activity of the oxidative stress response pathway. This biologically motivated, disease-agnostic score has the potential to be more interpretable and applicable to a wider range of brain and other disorders than existing PRSs. By encoding activity at the pathway level, it can help elucidate how the mechanism of ROS production and elimination impinges on neurodevelopment.
In this study, we aim (i) to examine the association between this gPFSox score and key neurodevelopmental outcomes relating to cognition and attention-deficit hyperactivity disorder at 2 years of age and autism at ages 2 and 4 years in a large population-based cohort, and (ii) to investigate the interplay between gPFSox and prenatal phthalate exposure in adverse neurodevelopment. We discuss how the findings compare to our past use of a disease-based genetic score for the same study [31].
2. Materials and Methods
2.1. Study Cohort
The Barwon Infant Study (BIS) is a birth cohort from Victoria, Australia, consisting of 1074 mother-infant pairs and aimed at investigating early-life causes of non-communicable diseases [48]. Women were recruited between 15 and 28 weeks of completed pregnancy from 2010 to 2013 but later excluded if their child was born before 32 weeks or diagnosed with a congenital disorder or serious illness. As described elsewhere, extensive biological, clinical, and questionnaire measures were collected prenatally, at birth, and in intervals up to age 4 years [48]. The study was approved by the Barwon Health Human Research Ethics Committee (HREC 10/24) and written informed consent was obtained from the participating families.
2.2. Phthalate Measurement
Phthalate metabolite levels were measured in 842 pregnant women using a single spot urine specimen collected at 36 weeks of gestation. High-performance liquid chromatography–tandem mass spectroscopy with direct injection was performed by the Queensland Alliance for Environmental Health Science (QAEHS). QAEHS procedures are detailed elsewhere [49]. For monoethyl phthalate (MEP), monoisobutyl phthalate (MiBP), mono-n-butyl phthalate (MnBP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), repeated spot specimens taken in the third trimester had intra-class correlation coefficients over 0.4 in one or both of two previous studies [50,51]. This suggests that single spot tests can capture third-trimester exposure to phthalates with reasonable reliability.
2.3. Child Neurodevelopmental Outcomes
At age 2–3 years, the child’s caregiver completed the Child Behavior Checklist for Ages 1.5–5. The DSM-5-oriented autism spectrum problems (CBCL-ASP) and attention-deficit hyperactivity disorder (CBCL-ADHD) subscales were derived by summing responses to 12 behavioral statements (0: not true; 1: somewhat true; 2: very true) [52]. A scaled T-score of 50 represents the median in the normative sample, so a binary variable was created whereby children with a T-score above 50 were classified as having “above-median CBCL autism spectrum problems”. For CBCL-ADHD, a binary variable was created whereby for children with a T-score above 65, the threshold for “borderline or clinical ADHD symptoms” [53] was classified as having “high-CBCL attention-deficit hyperactivity problems”. At age 2–3 years, the Bayley Scales of Infant and Toddler Development 3rd edition (BAYLEY-III) cognitive scale was administered, and the raw scores were used in analyses [54]. At age 4 years, parents reported if their child had doctor-diagnosed autism spectrum disorder (ASD) or any ASD traits.
2.4. Genotyping
DNA samples were extracted from cord and 12-month whole blood using the QIAamp 96 DNA QIAcube HT kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions and stored at −80 °C. Whole-genome genotyping was performed with an Illumina Global Screening Array (Illumina, San Diego, CA, USA). Imputation was completed using the Sanger Imputation Server (Wellcome Sanger Institute, Hinxton, UK) based on the Haplotype Reference Consortium reference panel [55,56]. Infants were excluded for quality control if initial genotyping was unsuccessful at more than 5% of SNPs, and SNPs were dropped if (i) genotyping failed across more than 5% of infants, (ii) minor allele frequency was less than 0.01 or differed by more than 0.2 from the reference population, or (iii) the SNPs were not in Hardy–Weinberg equilibrium [31].
2.5. Genetic Pathway Function Score for Oxidative Stress
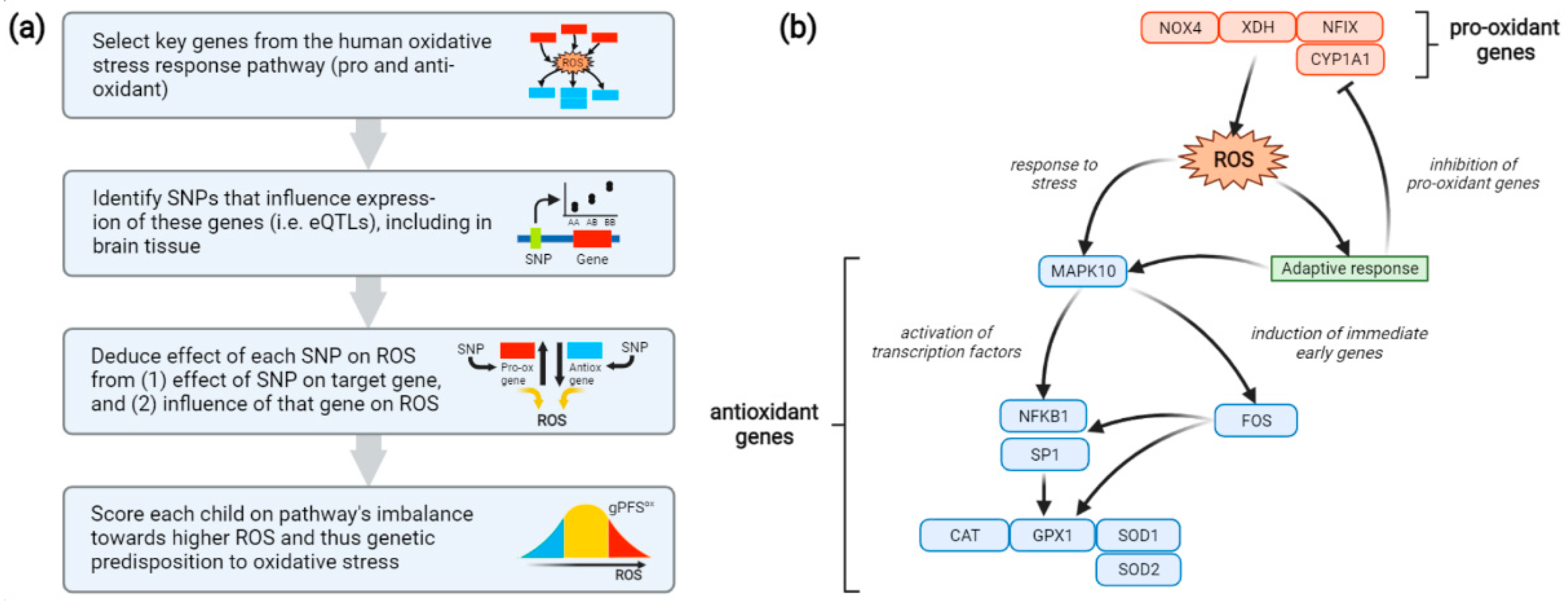
To capture each participant’s genetic predisposition to oxidative stress, a genetic pathway function score (gPFSox) was generated as follows (Figure 1a). First, a minimal pathway for the human oxidative stress response was constructed, consisting of 4 pro-oxidant and 8 antioxidant genes acting in opposing directions (Figure 1b) [57,58,59,60].
Figure 1.
(a) Method used to construct a genetic pathway function score for oxidative stress (gPFSox). (b) Oxidative-stress pathway encoded using gPFSox. Included are 4 genes involved in the production of ROS (red) and 8 genes involved in the response to ROS (blue). NOX4, NAD(P)H oxidase 4; XDH, xanthine dehydrogenase; NFIX, nuclear factor I X; CYP1A1, cytochrome P450 family 1 subfamily A member 1; MAPK10, mitogen-activated protein kinase 10; NFKB1, nuclear factor kappa B subunit 1; SP1, specificity protein 1; CAT, catalase; GPX1, glutathione peroxidase 1; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; ROS, reactive oxygen species; SNP, single-nucleotide polymorphism; eQTL, expression quantitative trait loci.
Single-nucleotide genetic polymorphisms (SNPs) linked to the activity of these genes were then identified using the Genotype-Tissue Expression (GTEx) database [61,62]. GTEx provides information on SNPs associated with tissue-specific expression of query genes by leveraging genotyping and RNA sequencing data across 54 tissue types from nearly 1000 individuals. For each oxidative stress gene, the SNP most strongly associated with its expression in any tissue type was chosen. These SNPs showed generally consistent effects across multiple tissue types, including the brain. The influence of each SNP on oxidative stress was then deduced from two factors: (1) whether the SNP up- or down-regulates expression of its target gene, and (2) whether the target gene elevates or reduces oxidative stress (Table 1). For instance, a pro-oxidant SNP might up-regulate expression of a pro-oxidant gene or down-regulate expression of an antioxidant gene. Finally, a score was created for each BIS participant reflecting the number of pro-oxidant alleles they carry for the oxidative stress response pathway and hence the pathway’s cumulative imbalance, at the transcriptional level, towards oxidative stress. Thus, if the p SNPs are ordered such that SNPs 1, …, ppro are pro-oxidant and SNPs ppro + 1, …, p are antioxidant, the score is computed as:
where gPFSiox is the score for individual i, j is the current SNP out of p = 12 total SNPs across the pathway (one for each gene), and C is the allele count for SNP j (0, 1 or 2). Thus, for each SNP, the count of pro-oxidant alleles at that locus is added; for a pro-oxidant SNP, this is simply the number of effect alleles present, while for an antioxidant SNP it becomes 2 minus the number of effect alleles. For convenience, the score is then divided by 2 to range from 0 to 12 units, the number of genes in the pathway, rather than 0 to 24 units, the number of alleles for the 12 corresponding SNPs.

Table 1.
Single-nucleotide polymorphisms (SNPs) used to create a genetic pathway function score for oxidative stress response (gPFSox). SNPs were identified using the GTEx database as expression quantitative trait loci (eQTLs) for genes within this pathway.
2.6. Statistical Analysis
Phthalate metabolite measurements were corrected for processing batch, urine dilution, and time of day for spot sample collection [31]. To estimate the biological dose delivered to the fetus, phthalate daily intakes were calculated accounting for maternal prenatal weight, average daily urine volume, fractional excretion of the compound, and compound-to-metabolite molecular weight ratio [31]. The metabolites MEHHP, MEOHP, and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) were used to calculate di-(2-ethylhexyl) phthalate (DEHP) daily intake; MEP for diethyl phthalate (DEP); MiBP for diisobutyl phthalate (DiBP); and MnBP for di-n-butyl phthalate (DnBP). DiBP and DnBP daily intakes were summed to make a DBPs daily intake measure and were not considered individually in the following analyses. DEHP, DEP, DiBP, and DnBP daily intakes were summed to make a composite phthalate daily intake measure (Σ phthalates).
Multiple linear regression was used to test for association between gPFSox and Bayley-III cognition, a continuous measure of cognitive ability. For binary outcomes, multiple logistic regression was used; these were high-CBCL attention-deficit hyperactivity problems, above-median CBCL autism spectrum problems, ASD diagnosis, and ASD traits.
Interplay with prenatal phthalate exposure was assessed by (i) evaluating the risk of adverse neurodevelopment associated with top quintile of gPFSox and top quintile of phthalate levels vs. bottom four quintiles for both and (ii) testing for additive interaction. We present the attributable proportion (AP), which measures the proportion of the disease in the doubly exposed group that is due to the interaction [63] and the relative excess risk due to interaction (RERI), a measure of interaction on the additive scale for risk ratios [64].
For (i), Ghi or Phi indicated children with values in the top quintile for gPFSox or phthalates respectively, whereas children classified as Glo or Plo were in the lower 4 quintiles for gPFSox or phthalates, respectively.
For the cognition outcome, adjustment was made for post-conceptional age at testing, sex, administering researcher, and experience of researcher in test administration. For high-CBCL attention-deficit hyperactivity problems, above-median CBCL autism spectrum problems, ASD diagnosis, and ASD traits, adjustment was made for post-conceptional age at testing and sex. A p-value threshold of 0.05 was used for statistical significance. All analyses were conducted in R 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria) and Stata/SE 16.1 (Stata Statistical Software, College Station, TX, USA).
3. Results
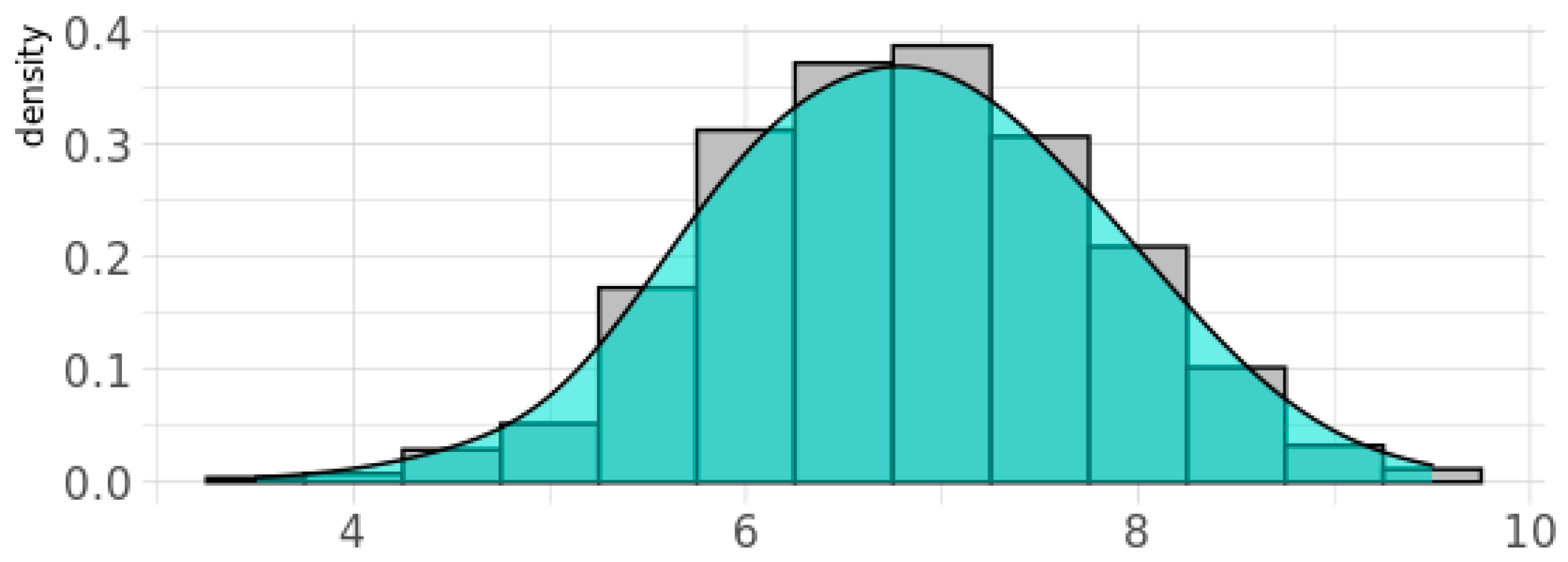
The genetic score for oxidative stress, gPFSox, was normally distributed for BIS children, with 20% of children having a value of 8 units or above (Figure 2). Children in this category were classified as Ghi (i.e., showing greater genetic vulnerability to oxidant damage due to reduced antioxidant defenses), while those below were classified as Glo. Of the 842 women with phthalate measurements in the inception cohort, the percentage with detectable levels of metabolites of DEHP was 100%, for DnBP and DEP was 99%, and for DiBP was 98%, and these levels varied by more than 1000-fold [31]. For each phthalate daily intake measure, children in the top quintile (i.e., those with the highest prenatal exposure levels) were classified as Phi and children in the bottom four quintiles as Plo. At age two years, 37% of children (55% male) had ASD symptoms classified as above average according to the CBCL-ASP scale (Table 2). At age four years, 1.4% (11/791) of children (55% male) were reported to be diagnosed with ASD, most of which were verified (9/11, 82%) in pediatric medical records, while 4.9% (39/791; 62% male) were reported to have ASD traits (Table 2). A continuous CBCL ASP scale at the age of 2 years predicted later ASD diagnosis at the age of 4 years with an area under the curve (AUC) score of 0.93 (95% CI (0.82, 1.00)) [65].
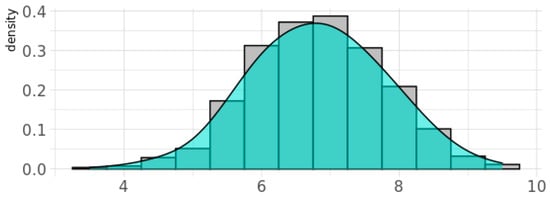
Figure 2.
Density plot for the genetic pathway function score for oxidative stress response (gPFSox) in the BIS cohort. Note: Y-axis gives the probability density function for the kernel density estimation used to fit the curve.

Table 2.
The distribution of key characteristics in the Barwon Infant Study.
3.1. Association between Phthalate Daily Intake and Neurodevelopmental Outcomes
As previously reported [31], prenatal daily intake of DBPs, DEHP, and Σ phthalates was each associated with an increased likelihood of offspring ASD diagnosis at 4 years (AOR 1.89, 95% CI (1.01, 3.53), p = 0.05; AOR 1.55, 95% CI (1.06, 2.28), p = 0.03; AOR 1.55, 95% CI (1.00, 2.40), p = 0.05; respectively) and ASD traits at 4 years (AOR 1.44, 95% CI (1.03, 2.03), p = 0.04; AOR 1.51, 95% CI (1.15, 1.98), p = 0.003; AOR 1.55, 95% CI (1.20, 2.01), p = 0.0009; respectively) [31]. No associations were found between any phthalate daily intake levels and cognition at 2–3 years [31].
3.2. Association between gPFSox and Neurodevelopmental Outcomes
Adjusting for age and sex, a higher gPFSox was associated with a greater likelihood of offspring ASD diagnosis and parent-reported ASD traits (Table 3). Each unit increase in gPFSox, indicating the presence of two additional pro-oxidant alleles for genes in the oxidative stress response pathway, was associated with an AOR of 1.42 (95% CI (1.02, 2.01), p = 0.041) for ASD traits and 2.10 (95% CI (1.12, 4.11), p = 0.024) for ASD diagnosis. The associations (for log-odds) showed no significant departure from linearity. Associations for cognition, high-CBCL attention-deficit hyperactivity problems, and above-median CBCL autism spectrum problems did not reach significance (Table 3).

Table 3.
Main effect of genetic pathway function score for oxidative stress response (gPFSox) against cognitive and ASD outcomes.
3.3. Interplay between gPFSox and Prenatal Phthalate Exposure against Neurodevelopmental Outcomes
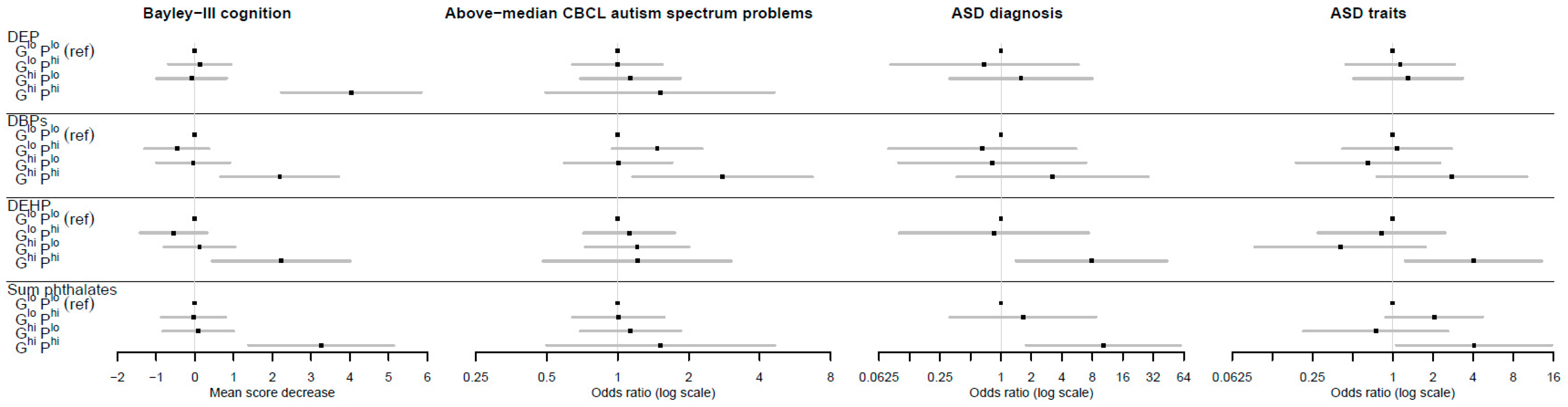
To assess combined effects, gPFSox and prenatal maternal phthalate levels were each dichotomized as high (top quintile) vs. low (bottom four quintiles). The reference category was designated as individuals with both low gPFSox and low phthalate levels, denoted by GloPlo. A clear pattern emerged (Figure 3, Table 4 and Table 5) in which individuals in the doubly exposed GhiPhi group, but not other combinations, were at significantly higher risk of adverse neurodevelopment. For instance, for DEP and Bayley’s cognition, the estimated difference in mean score was 0.00 for the reference category; −1.2, p = 0.266 for GloPhi; 0.09, p = 0.852 for GhiPlo; and −4.04, p < 0.0001 for GhiPhi. For DEHP and ASD diagnoses, the adjusted odds ratios were 1.00 for the reference category; 0.85, p = 0.884 for GloPhi; insufficient numbers for GhiPlo; and 7.84, p = 0.019 for GhiPhi. Relative excess risk due to interaction (RERI) values were not significant, but using the attributable proportion (AP) metric, there was strong evidence of interactions for multiple phthalates across cognition, ASD diagnosis, and ASD traits outcomes. For example, the AP for DEHP and ASD diagnosis was 0.89 (95% CI (0.62, 1.16); p < 0.0001), indicating that the proportion of disease in the doubly exposed group (GhiPhi) due to interaction was 89%. The results for CBCL attention-deficit hyperactivity problems showed similar patterns (see Table S1).
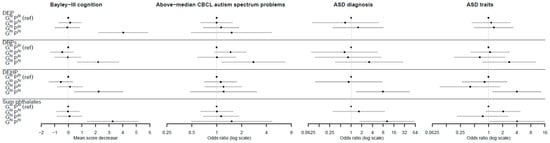
Figure 3.
The association between the genetic pathway function score for oxidative stress response (gPFSox) and prenatal phthalate level combinations and neurodevelopment. Note: Error bars are 95% confidence intervals; Ghi = top quintile of gPFSox; Glo = bottom four quintiles of gPFSox; Phi = top quintile of phthalate exposure; Plo = bottom four quintiles of phthalate exposure; DEP = diethyl phthalate; DBPs = di-n-butyl phthalate (DnBP) + diisobutyl phthalate (DiBP); DEHP = di-(2-ethyl-5-oxohexyl) phthalate; sum phthalates = sum of DEP, DBPs, and DEHP.

Table 4.
Distributions of neurodevelopmental outcomes by genetic pathway function score for oxidative stress response (gPFSox) and prenatal phthalate level combinations.

Table 5.
Interplay on the additive scale between genetic pathway function score for oxidative stress response (gPFSox) and prenatal phthalate levels against neurodevelopmental outcomes.
4. Discussion
In this study, we employed a genetic pathway function score, gPFSox, to capture genetic predisposition to oxidative stress based on transcriptional activity of the oxidative stress response pathway in multiple tissue types, including the brain. Using DNA samples sourced from cord and infant blood, we found that higher genetic scores for oxidative stress vulnerability were associated with ASD and autistic traits at 4 years of age. Unlike in our previous work, gene-environment interaction was evident, with children with both higher phthalate levels and a higher gPFSox (top quintile for both) having excess additive risk for ASD diagnosis or ASD traits at age 4 years. These findings expand our previous work with the use here of an externally derived score independent of disease status.
The human brain has high energy demands and is vulnerable to oxidative damage [66]. Although oxidative damage in the adult brain has been well studied [67], the importance of oxidative stress in adverse brain development is being increasingly recognized. These findings support past work indicating that impaired redox control appears to be an underlying mechanism for gene–environment interactions in ASD [68,69], as both ASD-associated genes [37] and environmental risk factors of ASD have been associated with oxidative stress. Specifically regarding plastic chemicals, DBP metabolites have been associated with prenatal markers of oxidative stress [70]. Similarly, oxidative stress markers have been found to mediate the effect of higher DEHP levels on preterm birth [33].
Our findings are consistent with previous genetic studies linking oxidative stress to ASD etiology [31,39]. However, unlike existing genetic scores for oxidative stress, this functional, disease-agnostic approach provides clearer evidence that elevated ROS, independent of other factors, is at play. Evidence for an association with ASD at the genetic level also suggests that oxidative stress is causally involved rather than merely a byproduct of disrupted neurodevelopment, and importantly it reduces the likelihood of confounding by environmental factors.
Our findings highlight the interplay between genetic susceptibility to oxidative stress and prenatal phthalate exposure. Individuals in the top quintile for gPFSox and top quintile for prenatal phthalate exposure, but generally not other combinations, were consistently at significantly higher risk of adverse neurodevelopment compared to those in the lower four quintiles for both. For doctor-diagnosed and parent-reported autism outcomes at 4 years, the combined top-quintile effect was evident for prenatal exposure to the high-molecular-weight phthalate DEHP and for Σ phthalates, a measure that includes DEHP, suggesting that DEHP may drive the association with ASD. This possibility is further supported by metabolomic analyses, with the association between higher prenatal DEHP exposure and increased ASD symptomology partly mediated through metabolic shifts closely linked to oxidative stress [71]. Testing for interaction, we found that the majority of the disease occurring in these doubly exposed groups was attributable to a gene–environment interaction. For example, the attributable proportion for the combined top quintiles of gPFSox and DEHP against ASD diagnosis due to interaction was more than 80 percent. These results for combined exposures are intuitively plausible given that the deleterious impact of impaired ROS processing—as reflected in a high gPFSox—would be accentuated by any additional sources of ROS, such as phthalate exposure [33]. The results also highlight the risk posed to one-fifth of the population (i.e., individuals in the top quintile of gPFSox) by phthalates and likely other common ROS-producing environmental pollutants.
A key strength of this study is the improved interpretability of the genetic score. Here, gPFSox was derived from SNPs linked to expression levels of genes in the oxidative stress response pathway—encoding the logic of pro- and antioxidant genes acting in opposing directions—rather than to a disease or other outcome. This functional approach helps shed light on how the mechanism of oxidative stress response, including in brain tissue, relates to child cognition and ASD. Furthermore, gPFSox was constructed independently of the study cohort, using genotyping and RNA sequencing data from the GTEx consortium [61], thereby improving its potential to generalize to other study populations. Given that the score is disease-agnostic, gPFSox could be evaluated against other outcomes beyond early life hypothesized to involve oxidative stress, for instance neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis [72]. Additional strengths of the study include comprehensive data and a range of neurodevelopmental outcomes from ages 2 to 4 years [31].
The study has several limitations. The number of doctor-diagnosed ASD cases was small and only 82% were verified by medical records. The autism problem score at 2 years was based on parental report, but we have previously demonstrated that this ASP score had an area under the curve of 0.93 (95% CI (0.82, 1.00)) for verified ASD diagnosis at age 4 years [65].
Here we only studied one environmental factor with possible oxidant action, phthalate plastic chemicals, but we have shown that other environmental factors such as prenatal maternal smoking or a lack of fish oil consumption (a putative antioxidant) may also be relevant [31]. Indeed, interpreted in the context of Mendelian randomization, where genetic data are used as proxies for environmental exposures, the findings for gPFSox itself suggest that wider oxidant exposures are important to consider. We plan to assess the level of agreement between the infant gPFSox and infant urinary oxidative markers such as 8-isopostane and 8-hydroxydeoxyguanosine [73,74] when these become available. Overall, our findings indicate that future studies should examine oxidant burden from both composite environmental and composite genetic factors as comprehensively as possible. This may provide additional clarity as to the possible adverse role of phthalate chemicals in neurodevelopment [24].
It is worth noting that gPFSox was constructed from a minimalist pathway of 12 genes, and several of these genes, such as SP1, exert pleiotropic effects across multiple signaling pathways. Despite this, by combining these genes in a shared pathway and encoding their cumulative influence on ROS, we found consistent effects that accord with past work on phthalates, oxidative stress, and neurodevelopment. However, future iterations could employ expanded pathways that capture a more comprehensive picture of the complex mechanisms underlying ROS balance. Moreover, while we aimed to generate a score with relevance to multiple disorders and therefore selected SNPs with the strongest overall effect on the expression of each gene, brain-region- or cell-type-specific variants of the score could be developed to study more localized pathophysiology.
5. Conclusions
A novel genetic pathway function score for oxidative stress (gPFSox), capturing transcriptional activity of the oxidative stress response pathway, has provided a genetic marker of oxidative stress that associates with adverse neurodevelopmental outcomes. Unlike our previous score for oxidative stress, gPFSox was constructed independently of our study cohort and has no a priori links to the neurodevelopmental conditions assessed here, improving its potential to generalize to other populations and to other ROS-related traits and disorders, as well as providing more robust evidence for the associations described. We also extended our previous work by using this improved functional genetic score to demonstrate that prenatal phthalate-induced adverse neurodevelopment will vary by host genetic oxidative stress vulnerability status. Future work on the causation of these neurodevelopmental disorders is likely to benefit from examining both environmental and genetic factors in the context of shared biological mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox11040659/s1, Table S1: Interplay on the additive scale between the genetic Pathway Function Score for oxidative stress response (gPFSox) and prenatal phthalate levels against borderline/clinical attention-deficit hyperactivity problems.
Author Contributions
Conceptualization, S.T. (Samuel Tanner), M.O. and A.-L.P.; methodology, S.T. (Samuel Tanner), S.T. (Sarah Thomson), M.O., C.S., T.M., R.S., E.J.S., P.V. and A.-L.P.; formal analysis, S.T. (Samuel Tanner), S.T. (Sarah Thomson), and M.O.; resources, Barwon Infant Study Investigator Group; writing—original draft preparation, S.T. (Samuel Tanner), S.T. (Sarah Thomson), K.D. and A.-L.P.; writing—review and editing, all; funding acquisition, C.S., R.S., P.D.S., F.C., D.B., T.D., P.V. and A.-L.P. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was secured from National Health and Medical Research Council of Australia (NHMRC), The Minderoo Foundation, The Shepherd Foundation, The Jack Brockhoff Foundation, the Scobie and Claire McKinnon Trust, the Shane O’Brien Memorial Asthma Foundation, the Our Women’s Our Children’s Fund Raising Committee Barwon Health, the Rotary Club of Geelong, the Ilhan Food Allergy Foundation, GMHBA, Vanguard Investments Australia Ltd., and the Percy Baxter Charitable Trust, Perpetual Trustees. In-kind support was provided by the Cotton on Foundation and CreativeForce. The study sponsors were not involved in the collection, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication. Research at Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program.
Institutional Review Board Statement
The study was approved by the Barwon Health Human Research Ethics Committee (HREC 10/24).
Informed Consent Statement
Informed consent was obtained from all participating families.
Data Availability Statement
For laboratory data access contact the corresponding author. Access to BIS data including all data used in this paper can be requested through the BIS Steering Committee by contacting annelouise.ponsonby@florey.edu.au. Requests to access cohort data are considered on scientific and ethical grounds and, if approved, provided under collaborative research agreements. Additional project information, including cohort data description and access procedure, is available at the project’s website https://www.barwoninfantstudy.org.au (last accessed 28 March 2022).
Acknowledgments
The authors thank the BIS participants for the generous contribution they have made to this project. The authors also thank current and past staff for their efforts in recruiting and maintaining the cohort and in obtaining and processing the data and biospecimens. The establishment work and infrastructure for the BIS was provided by the Murdoch Children’s Research Institute, Deakin University, and Barwon Health. The members of the BIS Investigator Group are as follows: Peter Vuillermin, Anne-Louise Ponsonby, John Carlin, Mimi LK Tang, Amy Loughman, Martin O’Hely, Toby Mansell, Lawrence Gray, Richard Saffery, Sarath Ranganathan, David Burgner, and Peter Sly. We thank Terry Dwyer and Katie Allen for their past work as foundation investigators.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, Six Sites, United States, 2000; Morbidity and Mortality Weekly Report; Surveillance summaries; Centers for Disease Control and Prevention: Washington, DC, USA, 2007; Volume 56, pp. 1–11. [Google Scholar]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014; Morbidity and Mortality Weekly Report; Surveillance summaries; Centers for Disease Control and Prevention: Washington, DC, USA, 2018; Volume 67, pp. 1–23. [Google Scholar]
- Atladottir, H.O.; Gyllenberg, D.; Langridge, A.; Sandin, S.; Hansen, S.N.; Leonard, H.; Gissler, M.; Reichenberg, A.; Schendel, D.E.; Bourke, J. The increasing prevalence of reported diagnoses of childhood psychiatric disorders: A descriptive multinational comparison. Eur. Child Adolesc. Psychiatry 2015, 24, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Muo, C.-H.; Chang, Y.-T.; Lin, C.-K. Change in prevalence status for children with developmental delay in taiwan: A nationwide population-based retrospective study. Neuropsychiatr. Dis. Treat. 2015, 11, 1541. [Google Scholar] [PubMed]
- Boyle, C.A.; Boulet, S.; Schieve, L.A.; Cohen, R.A.; Blumberg, S.J.; Yeargin-Allsopp, M.; Visser, S.; Kogan, M.D. Trends in the prevalence of developmental disabilities in us children, 1997–2008. Pediatrics 2011, 127, 1034–1042. [Google Scholar] [CrossRef]
- Matson, J.L.; Kozlowski, A.M. The increasing prevalence of autism spectrum disorders. Res. Autism Spectr. Disord. 2011, 5, 418–425. [Google Scholar] [CrossRef]
- Miguel, P.M.; Pereira, L.O.; Silveira, P.P.; Meaney, M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019, 61, 1127–1133. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Passini, E.; Righi, B.; Plessi, C.; Predieri, B.; Iughetti, L. Perinatal exposure to phthalates: From endocrine to neurodevelopment effects. Int. J. Mol. Sci. 2021, 22, 4063. [Google Scholar] [CrossRef]
- WHO. United Nations Environment Programme. State of the Science of Endocrine Disrupting Chemicals-2012; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Toxicological and Health Aspects of Bisphenol A; Report of Joint FAO/WHO Expert Meeting, 2–5 November 2010; Report of Stakeholder Meeting on Bisphenol A, 1 November 2010, Ottawa, Canada; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Edc-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Uldbjerg, C.S.; Lim, Y.H.; Gregersen, L.S.; Krause, M.; Frederiksen, H.; Andersson, A.M. Presence of parabens, phenols and phthalates in paired maternal serum, urine and amniotic fluid. Environ. Int. 2022, 158, 106987. [Google Scholar] [CrossRef]
- Sugeng, E.J.; Symeonides, C.; O’Hely, M.; Vuillermin, P.; Sly, P.D.; Vijayasarathy, S.; Thompson, K.; Pezic, A.; Mueller, J.F.; Ponsonby, A.-L. Predictors with regard to ingestion, inhalation and dermal absorption of estimated phthalate daily intakes in pregnant women: The barwon infant study. Environ. Int. 2020, 139, 105700. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, W.; Zhu, H.; Wang, C. Determination of free and total phthalates in commercial whole milk products in different packaging materials by gas chromatography-mass spectrometry. J. Dairy Sci. 2015, 98, 8278–8284. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.E.; Karr, C.J.; Seixas, N.S.; Nguyen, R.H.; Barrett, E.S.; Janssen, S.; Redmon, B.; Swan, S.H.; Sathyanarayana, S. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int. J. Environ. Res. Public Health 2014, 11, 6193–6215. [Google Scholar] [CrossRef] [PubMed]
- González-Castro, M.I.; Olea-Serrano, M.F.; Rivas-Velasco, A.M.; Medina-Rivero, E.; Ordoñez-Acevedo, L.G.; De León-Rodríguez, A. Phthalates and bisphenols migration in mexican food cans and plastic food containers. Bull. Environ. Contam. Toxicol. 2011, 86, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, F.; Wang, Y.; Ning, Y.; Yang, J.Y.; Zhou, Y.K. Phthalate levels and related factors in children aged 6–12 years. Environ. Pollut. 2017, 220, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Palmieri, R.T.; Matuszewski, J.M.; Herring, A.H.; Baird, D.D.; Hartmann, K.E.; Hoppin, J.A. Consumer product exposures associated with urinary phthalate levels in pregnant women. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 468–475. [Google Scholar] [CrossRef]
- Parlett, L.E.; Calafat, A.M.; Swan, S.H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 197–206. [Google Scholar] [CrossRef]
- Eales, J.; Bethel, A.; Galloway, T.; Hopkinson, P.; Morrissey, K.; Short, R.E.; Garside, R. Human health impacts of exposure to phthalate plasticizers: An overview of reviews. Environ. Int. 2022, 158, 106903. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, M.S.; Lim, Y.H.; Lee, N.; Hong, Y.C. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res. 2018, 167, 558–566. [Google Scholar] [CrossRef]
- Radke, E.G.; Braun, J.M.; Nachman, R.M.; Cooper, G.S. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int. 2020, 137, 105408. [Google Scholar] [CrossRef]
- Ejaredar, M.; Nyanza, E.C.; Ten Eycke, K.; Dewey, D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015, 142, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.Z.; Huang, X.; Wang, M.; Wu, J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology 2019, 73, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Miodovnik, A.; Engel, S.M.; Zhu, C.; Ye, X.; Soorya, L.V.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011, 32, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Just, A.C.; Yolton, K.; Calafat, A.M.; Sjödin, A.; Hauser, R.; Webster, G.M.; Chen, A.; Lanphear, B.P. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4-and 5-year-old children: The home study. Environ. Health Perspect. 2014, 122, 513–520. [Google Scholar] [CrossRef]
- Haggerty, D.K.; Strakovsky, R.S.; Talge, N.M.; Carignan, C.C.; Glazier-Essalmi, A.N.; Ingersoll, B.R.; Karthikraj, R.; Kannan, K.; Paneth, N.S.; Ruden, D.M. Prenatal phthalate exposures and autism spectrum disorder symptoms in low-risk children. Neurotoxicol. Teratol. 2021, 83, 106947. [Google Scholar] [CrossRef]
- Oulhote, Y.; Lanphear, B.; Braun, J.M.; Webster, G.M.; Arbuckle, T.E.; Etzel, T.; Forget-Dubois, N.; Seguin, J.R.; Bouchard, M.F.; MacFarlane, A.J.E. Gestational exposures to phthalates and folic acid, and autistic traits in canadian children. Environ. Health Perspect. 2020, 128, 027004. [Google Scholar] [CrossRef]
- Ponsonby, A.-L.; Symeonides, C.; Saffery, R.; Mueller, J.F.; O’Hely, M.; Sly, P.D.; Wardrop, N.; Pezic, A.; Mansell, T.; Collier, F. Prenatal phthalate exposure, oxidative stress-related genetic vulnerability and early life neurodevelopment: A birth cohort study. Neurotoxicology 2020, 80, 20–28. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Chen, Y.-H.; VanderWeele, T.J.; McElrath, T.F.; Meeker, J.D.; Mukherjee, B. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environ. Health Perspect. 2017, 125, 488–494. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Yan, B.; Zhu, Y.; Liu, X.; Chen, M.; Li, D.; Lee, C.-C.; Yang, X.; Ma, P. Oral exposure to dibutyl phthalate exacerbates chronic lymphocytic thyroiditis through oxidative stress in female wistar rats. Sci. Rep. 2017, 7, 15469. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Zuo, H.; Yuan, Y.; Sun, Y.; Yang, X. Primary neuronal-astrocytic co-culture platform for neurotoxicity assessment of di-(2-ethylhexyl) phthalate. J. Environ. Sci. 2014, 26, 1145–1153. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Kushima, I.; Aleksic, B.; Nakatochi, M.; Shimamura, T.; Okada, T.; Uno, Y.; Morikawa, M.; Ishizuka, K.; Shiino, T.; Kimura, H.; et al. Comparative analyses of copy-number variation in autism spectrum disorder and schizophrenia reveal etiological overlap and biological insights. Cell Rep. 2018, 24, 2838–2856. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Cai, J.J.; Ji, G.; Sham, P.C. Commonality in dysregulated expression of gene sets in cortical brains of individuals with autism, schizophrenia, and bipolar disorder. Transl. Psychiatry 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lintas, C.; Sacco, R.; Persico, A.M. Genome-wide expression studies in autism spectrum disorder, rett syndrome, and down syndrome. Neurobiol. Dis. 2012, 45, 57–68. [Google Scholar] [CrossRef]
- Bowers, K.; Li, Q.; Bressler, J.; Avramopoulos, D.; Newschaffer, C.; Fallin, M.D. Glutathione pathway gene variation and risk of autism spectrum disorders. J. Neurodev. Disord. 2011, 3, 132–143. [Google Scholar] [CrossRef]
- Heslop, C.L.; Tebbutt, S.J.; Podder, M.; Ruan, J.; Hill, J.S. Combined polymorphisms in oxidative stress genes predict coronary artery disease and oxidative stress in coronary angiography patients. Ann. Hum. Genet. 2012, 76, 435–447. [Google Scholar] [CrossRef]
- Bind, M.-A.; Coull, B.; Suh, H.; Wright, R.; Baccarelli, A.; Vokonas, P.; Schwartz, J. A novel genetic score approach using instruments to investigate interactions between pathways and environment: Application to air pollution. PLoS ONE 2014, 9, e96000. [Google Scholar]
- Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013, 9, e1003348. [Google Scholar] [CrossRef]
- Dudbridge, F. Polygenic epidemiology. Genet. Epidemiol. 2016, 40, 268–272. [Google Scholar] [CrossRef]
- Chasioti, D.; Yan, J.; Nho, K.; Saykin, A.J. Progress in polygenic composite scores in alzheimer’s and other complex diseases. Trends Genet. 2019, 35, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Patel, K.P.; Teng, A.K.; Berens, A.J.; Lachance, J. Genetic disease risks can be misestimated across global populations. Genome Biol. 2018, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, F.M.; Bustamante, C.D. Polygenic risk scores: A biased prediction? Genome Med. 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Vuillermin, P.; Saffery, R.; Allen, K.J.; Carlin, J.B.; Tang, M.L.; Ranganathan, S.; Burgner, D.; Dwyer, T.; Collier, F.; Jachno, K. Cohort profile: The barwon infant study. Int. J. Epidemiol. 2015, 44, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Gomez-Ramos, M.; Symeonides, C.; Hare, D.; Vijayasarathy, S.; Thompson, K.; Mueller, J.; Ponsonby, A.; Sly, P.; BIS Investigator Group. Harmonizing analytical chemistry and clinical epidemiology for human biomonitoring studies. A case-study of plastic product chemicals in urine. Chemosphere 2020, 238, 124631. [Google Scholar] [CrossRef]
- Adibi, J.J.; Whyatt, R.M.; Williams, P.L.; Calafat, A.M.; Camann, D.; Herrick, R.; Nelson, H.; Bhat, H.K.; Perera, F.P.; Silva, M.J. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008, 116, 467–473. [Google Scholar] [CrossRef]
- Suzuki, Y.; Niwa, M.; Yoshinaga, J.; Watanabe, C.; Mizumoto, Y.; Serizawa, S.; Shiraishi, H. Exposure assessment of phthalate esters in japanese pregnant women by using urinary metabolite analysis. Environ. Health Prev. Med. 2009, 14, 180–187. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L. Achenbach System of Empirically Based Assessment; Mental Measurements Yearbook; EBSCOhost: Ipswich, MA, USA, 2007. [Google Scholar]
- Achenbach, T.M.; Rescorla, L. Manual for ASEBA Preschool Forms and Profiles; University of Vermont, Research Centre for Children, Youth and Families: Burlington, VT, USA, 2000. [Google Scholar]
- Bayley, N. Bayley Scales of Infant and Toddler Development: Bayley-III; Harcourt Assessment; Psych. Corporation: San Antonio, TX, USA, 2006. [Google Scholar]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar]
- The Haplotype Reference Consortium. Available online: http://www.haplotype-reference-consortium.org/ (accessed on 24 March 2022).
- Slenter, D.N.; Kutmon, M.; Hanspers, K.; Riutta, A.; Windsor, J.; Nunes, N.; Mélius, J.; Cirillo, E.; Coort, S.L.; Digles, D.; et al. Wikipathways: A multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018, 46, D661–D667. [Google Scholar] [CrossRef]
- Martens, M.; Ammar, A.; Riutta, A.; Waagmeester, A.; Slenter, D.N.; Hanspers, K.; Miller, R.A.; Digles, D.; Lopes, E.N.; Ehrhart, F.; et al. Wikipathways: Connecting communities. Nucleic Acids Res. 2020, 49, D613–D621. [Google Scholar] [CrossRef]
- Morel, Y.; Barouki, R. Repression of gene expression by oxidative stress. Biochem. J. 1999, 342, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Wikipathways. Available online: https://www.wikipathways.org/index.php/WikiPathways (accessed on 14 March 2022).
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The genotype-tissue expression (gtex) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Gtex Portal. Available online: https://gtexportal.org/home/ (accessed on 26 March 2022).
- VanderWeele, T.J. Reconsidering the denominator of the attributable proportion for interaction. Eur. J. Epidemiol. 2013, 28, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; VanderWeele, T.J. Additive interaction in the presence of a mismeasured outcome. Am. J. Epidemiol. 2015, 181, 81–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pham, C.; Symeonides, C.; O’Hely, M.; Sly, P.D.; Knibbs, L.D.; Thomson, S.; Vuillermin, P.; Saffery, R.; Ponsonby, A.L. Early life environmental factors associated with autism spectrum disorder symptoms in children at age 2 years: A birth cohort study. Autism Int. J. Res. Pract. 2022; advance online publication. [Google Scholar] [CrossRef]
- Linseman, D.A. Targeting oxidative stress for neuroprotection. Antioxid. Redox Signal. 2009, 11, 421–424. [Google Scholar] [CrossRef]
- Newton, D.F.; Naiberg, M.R.; Goldstein, B.I. Oxidative stress and cognition amongst adults without dementia or stroke: Implications for mechanistic and therapeutic research in psychiatric disorders. Psychiatry Res. 2015, 227, 127–134. [Google Scholar] [CrossRef]
- Carter, C.J.; Blizard, R.A. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol a, phthalates and many others in food, cosmetics or household products. Neurochem. Int. 2016, 101, 83–109. [Google Scholar] [CrossRef]
- Mandic-Maravic, V.; Pljesa-Ercegovac, M.; Mitkovic-Voncina, M.; Savic-Radojevic, A.; Lecic-Tosevski, D.; Simic, T.; Pejovic-Milovancevic, M. Impaired redox control in autism spectrum disorders: Could it be the x in gxe? Curr. Psychiatry Rep. 2017, 19, 52. [Google Scholar] [CrossRef]
- Ferguson, K.; McElrath, T.; Chen, Y.; Mukherjee, B.; Meeker, J. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: A repeated measures analysis. Environ. Health Perspect. 2015, 123, 210–216. [Google Scholar] [CrossRef]
- Thomson, S.; Drummond, K.; O’Hely, M.; Symeonides, C.; Chandran, C.; Mansell, T.; Sly, P.; Ponsonby, A.-L.; BIS Investigator Group. Increased maternal non-oxidative energy metabolism mediates association between prenatal DEHP exposure and offspring ASD symptoms: A birth cohort study. Environ. Health Perspect. 2022; under review. [Google Scholar]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-ohdg): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Wild, P.; Sauvain, J.; Hemmendinger, M.; Canu, I.G.; Hopf, N. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol. Lett. 2020, 328, 19–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).