Angiotensin II Promotes Skeletal Muscle Angiogenesis Induced by Volume-Dependent Aerobic Exercise Training: Effects on miRNAs-27a/b and Oxidant–Antioxidant Balance
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
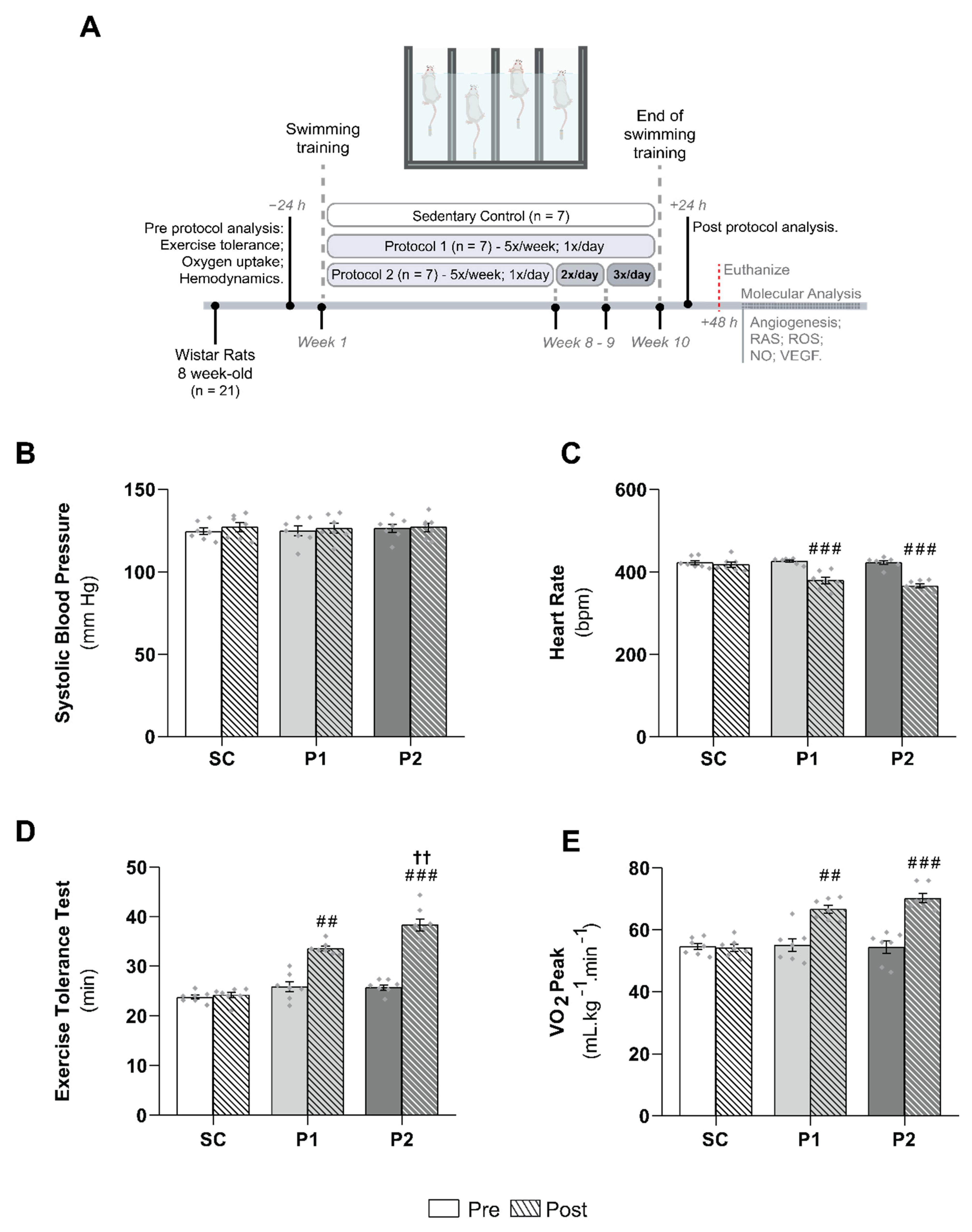
2.2. Exercise Training Protocol
2.3. Cardiovascular Measurements
2.4. Exercise Tolerance Test
2.5. Oxygen Uptake Measurements
2.6. Preparation of Biological Samples
2.7. Capillary-to-Fiber Ratio
2.8. Measurement of Plasmatic Nitric Oxide Concentration
2.9. Measurement of Plasmatic Nitrotyrosine
2.10. ACE1 and ACE2 Activity Assay
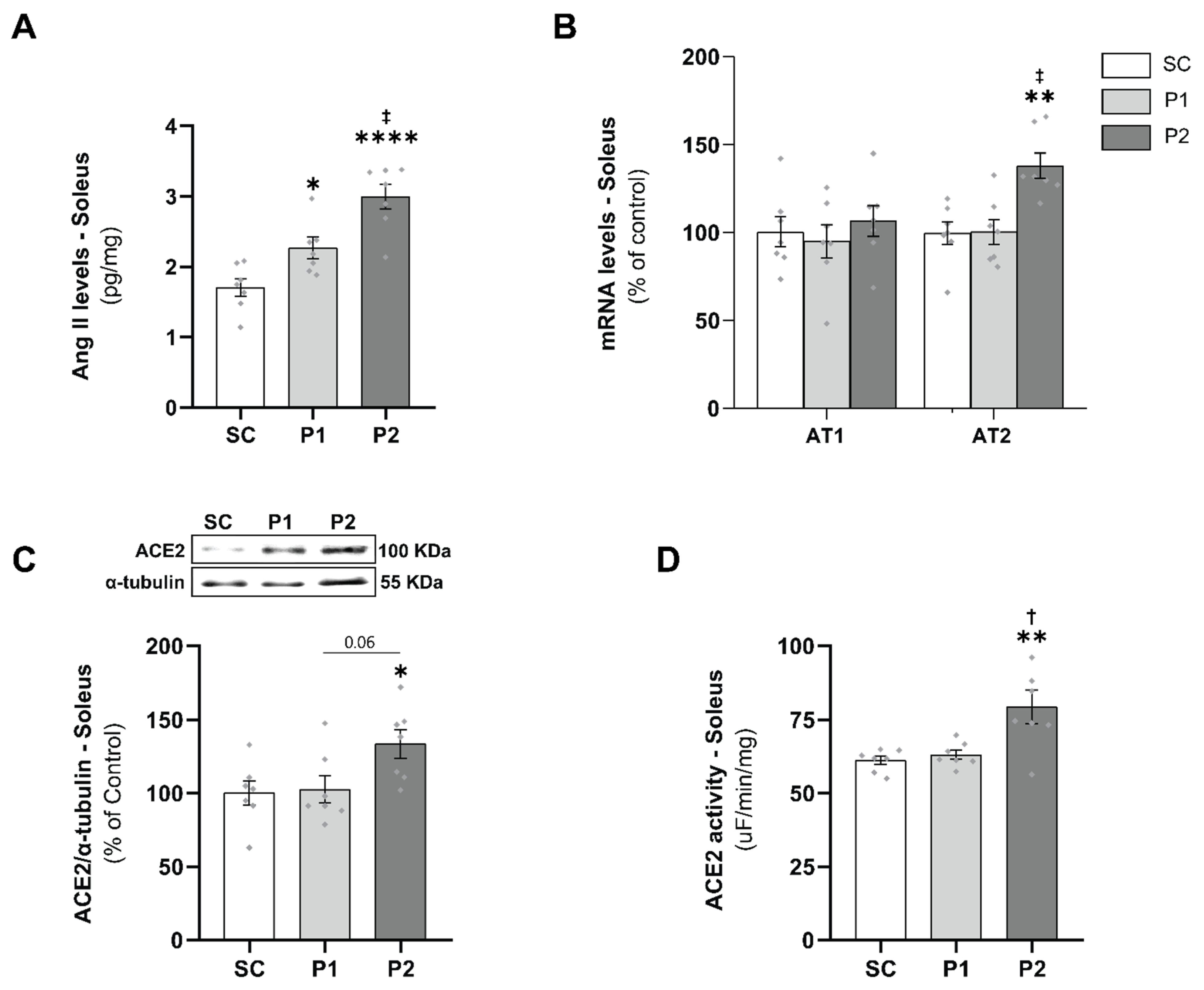
2.11. Measurement of Angiotensin II Levels
2.12. NADPH Oxidase Activity
2.13. RNA Isolation and mRNA Quantification Using Real-Time PCR
2.14. miRNA Quantitation Using Real-Time PCR
2.15. Quantification of Protein Expression
2.16. Statistical Analysis
3. Results
3.1. Hemodynamics Parameters and Aerobic Training Markers
3.2. miRNAs-27a and -27b Expression and ACE1 Target Gene Levels
3.3. Skeletal Muscle RAS Analysis
3.4. Exercise Training-Induced VEGF Levels and Capillary-to-Fiber Ratio
3.5. Aerobic Exercise Training Induces Antioxidant Defense
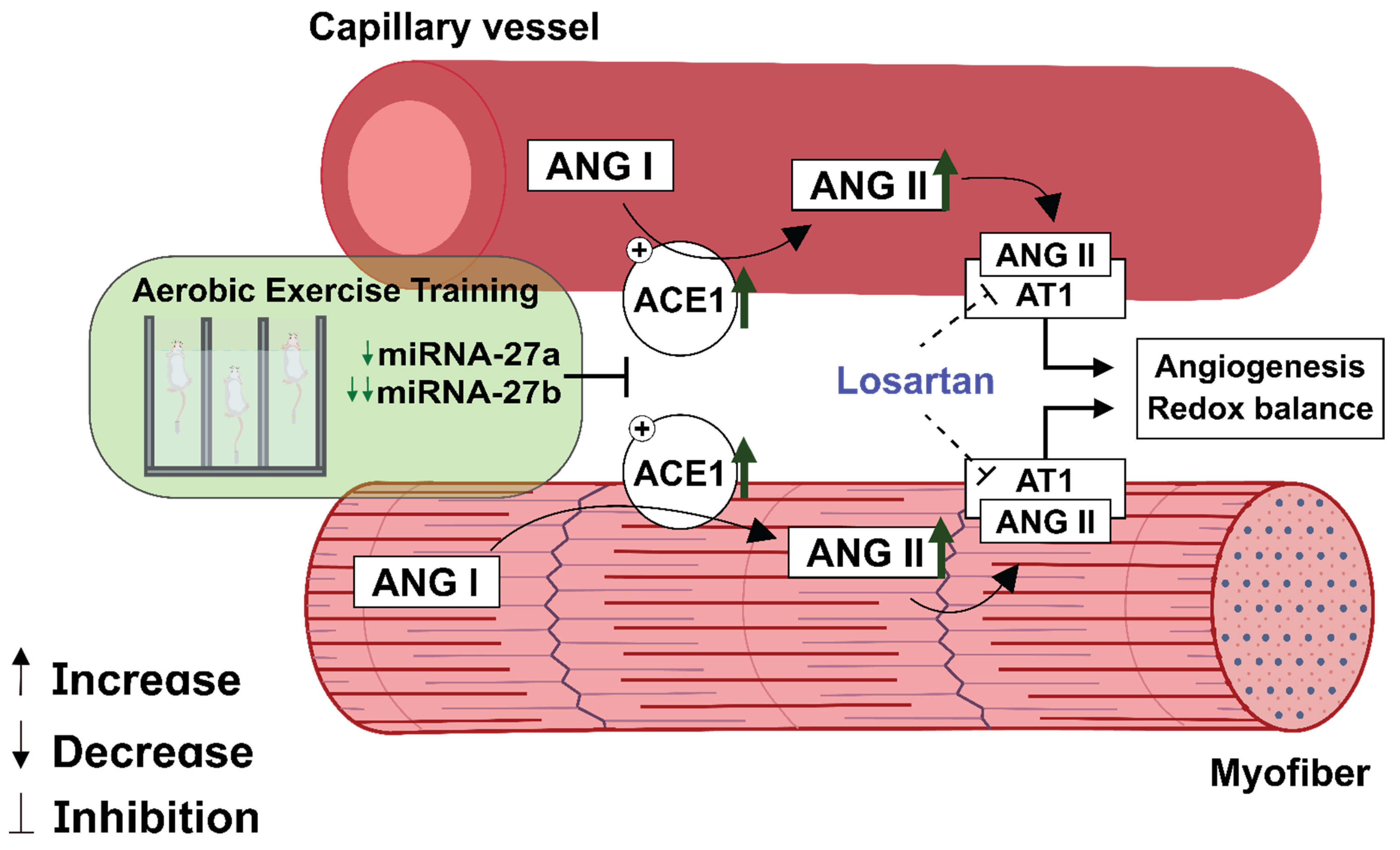
3.6. Losartan Treatment Prevents Aerobic Exercise Training-Induced Skeletal Muscle Angiogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, A.J.; Jude, B.; Lanner, J.T. Intramuscular mechanisms of overtraining. Redox Biol. 2020, 35, 101480. [Google Scholar] [CrossRef] [PubMed]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Ruegsegger, G.N.; Toedebusch, R.G.; Yan, Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 129–151. [Google Scholar] [PubMed]
- Duscha, B.D.; E Kraus, W.; Jones, W.S.; Robbins, J.L.; Piner, L.W.; Huffman, K.M.; Allen, J.D.; Annex, B.H. Skeletal muscle capillary density is related to anaerobic threshold and claudication in peripheral artery disease. Vasc. Med. 2020, 25, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Hashimoto, N.Y.; Magalhães, F.C.; Fernandes, F.B.; Casarini, D.; Carmona, A.; Krieger, J.; Phillips, M.I.; Oliveira, E.M. Aerobic Exercise Training-Induced Left Ventricular Hypertrophy Involves Regulatory MicroRNAs, Decreased Angiotensin-Converting Enzyme-Angiotensin II, and Synergistic Regulation of Angiotensin-Converting Enzyme 2-Angiotensin (1-7). Hypertension 2011, 58, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Treviño-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129.e14. [Google Scholar] [CrossRef] [PubMed]
- Roks, A.J.; Rodgers, K.; Walther, T. Effects of the renin angiotensin system on vasculogenesis-related progenitor cells. Curr. Opin. Pharmacol. 2011, 11, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Woods, D.R. Skeletal muscle RAS and exercise performance. Int. J. Biochem. Cell Biol. 2003, 35, 855–866. [Google Scholar] [CrossRef]
- Saijonmaa, O.; Nyman, T.; Kosonen, R.; Fyhrquist, F. Upregulation of angiotensin-converting enzyme by vascular endothelial growth factor. Am. J. Physiol. Circ. Physiol. 2001, 280, H885–H891. [Google Scholar] [CrossRef]
- Amaral, S.; Papanek, P.E.; Greene, A.S. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am. J. Physiol. Circ. Physiol. 2001, 281, H1163–H1169. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.L.; Linderman, J.R.; Morse, M.M.; Greene, A.S. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation 2001, 8, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.; Puntschart, A.; Kaijser, L.; Jansson, E.; Sundberg, C.J. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am. J. Physiol. Circ. Physiol. 1999, 276, H679–H685. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Wagner, H.; Mudaliar, S.R.D.; Saucedo, E.; Henry, R.; Wagner, P.D. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am. J. Physiol. Circ. Physiol. 2000, 279, H772–H778. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Wagner, H.; Mudaliar, S.R.D.; Henry, R.; Noyszewski, E.A.; Wagner, P.D. Human VEGF gene expression in skeletal muscle: Effect of acute normoxic and hypoxic exercise. Am. J. Physiol. Content 1999, 277, H2247–H2252. [Google Scholar] [CrossRef] [PubMed]
- Breen, E.; Johnson, E.C.; Wagner, H.; Tseng, H.M.; Sung, L.A.; Wagner, P.D. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J. Appl. Physiol. 1996, 81, 355–361. [Google Scholar] [CrossRef]
- Gavin, T.P.; Spector, D.A.; Wagner, H.; Breen, E.; Wagner, P.D. Effect of captopril on skeletal muscle angiogenic growth factor responses to exercise. J. Appl. Physiol. 2000, 88, 1690–1697. [Google Scholar] [CrossRef][Green Version]
- Gavin, T.P.; Spector, D.A.; Wagner, H.; Breen, E.; Wagner, P.D. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J. Appl. Physiol. 2000, 88, 1192–1198. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox. Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- DA Silva, N.D.; Fernandes, T.; Soci, U.P.R.; Monteiro, A.W.A.; Phillips, M.I.; DE Oliveira, E.M. Swimming Training in Rats Increases Cardiac MicroRNA-126 Expression and Angiogenesis. Med. Sci. Sports Exerc. 2012, 44, 1453–1462. [Google Scholar] [CrossRef]
- Soci, U.P.R.; Fernandes, T.; Hashimoto, N.Y.; Mota, G.F.; Amadeu, M.A.; Rosa, K.T.; Irigoyen, M.C.; Phillips, M.I.; Oliveira, E.M. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol. Genom. 2011, 43, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Soci, U.P.R.; Fernandes, T.; Barauna, V.G.; Hashimoto, N.Y.; Mota, G.D.F.A.; Rosa, K.T.; Irigoyen, M.C.; Philips, M.I.; De Oliveira, E.M. Epigenetic control of exercise training-induced cardiac hypertrophy by miR-208. Clin. Sci. 2016, 130, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Casaes, L.; Soci, U.; Silveira, A.; Gomes, J.; Barretti, D.; Roque, F.; Oliveira, E. Exercise Training Restores the Cardiac Microrna-16 Levels Preventing Microvascular Rarefaction in Obese Zucker Rats. Obes. Facts 2018, 11, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.L.P.; Fernandes, T.; Soci, U.P.R.; Silveira, A.C.; Barretti, D.L.M.; Negrão, C.E.; Oliveira, E.M. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxidative Med. Cell. Longev. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Veliceasa, D.; Biyashev, D.; Qin, G.; Misener, S.; Mackie, A.R.; Kishore, R.; Volpert, O.V. Therapeutic manipulation of angiogenesis with miR-27b. Vasc. Cell 2015, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.M.; Sasaki, M.S.; Cerêncio, M.; Baraúna, V.G.; Krieger, J.E. Local renin-angiotensin system regulates left ventricular hypertrophy induced by swimming training independent of circulating renin: A pharmacological study. J. Renin. Angiotensin. Aldosterone Syst. 2009, 10, 15–23. [Google Scholar] [CrossRef]
- Jamieson, M.J.; Gonzales, G.M.; Jackson, T.I.; Koerth, S.M.; Romano, W.F.; Tan, D.-X.; Castillon, F.; Skinner, M.H.; Grossmann, M.; Shepherd, A.M. Evaluation of the IITC tail cuff blood pressure recorder in the rat against intraarterial pressure according to criteria for human devices. Am. J. Hypertens. 1997, 10, 209–216. [Google Scholar] [CrossRef][Green Version]
- Pelozin, B.R.; Soci, U.P.R.; Gomes, J.L.P.; Oliveira, E.M.; Fernandes, T. mTOR signaling-related microRNAs as cardiac hypertrophy modulators in high-volume endurance training. J. Appl. Physiol. 2021, 132, 126–139. [Google Scholar] [CrossRef]
- Brooks, G.A.; White, T.P. Determination of metabolic and heart rate responses of rats to treadmill exercise. J. Appl. Physiol. 1978, 45, 1009–1015. [Google Scholar] [CrossRef]
- Fernandes, T.; Nakamuta, J.S.; Magalhães, F.C.; Roque, F.R.; Lavini-Ramos, C.; Schettert, I.T.; Coelho, V.; Krieger, J.E.; Oliveira, E.M. Exercise training restores the endothelial progenitor cells number and function in hypertension. J. Hypertens. 2012, 30, 2133–2143. [Google Scholar] [CrossRef]
- Barauna, V.G.; Magalhaes, F.C.; Krieger, J.E.; Oliveira, E.M. AT1 receptor participates in the cardiac hypertrophy induced by resistance training in rats. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R381–R387. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laurindo, F.R.; De Souza, H.P.; Pedro, M.D.A.; Janiszewski, M. Redox aspects of vascular response to injury. Methods Enzymol. 2002, 352, 432–454. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Fernandes, T.; Couto, G.K.; Ferreira-Filho, J.C.A.; Salemi, V.; Fernandes, F.B.; Casarini, D.; Brum, P.C.; Rossoni, L.V.; Oliveira, E.; et al. Effects of Exercise Training on Circulating and Skeletal Muscle Renin-Angiotensin System in Chronic Heart Failure Rats. PLoS ONE 2014, 9, e98012. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021. [Google Scholar]
- Chinsomboon, J.; Ruas, J.; Gupta, R.K.; Thom, R.; Shoag, J.; Rowe, G.C.; Sawada, N.; Raghuram, S.; Arany, Z. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. USA 2009, 106, 21401–21406. [Google Scholar] [CrossRef] [PubMed]
- Hudlicka, O.; Brown, M.; Egginton, S. Angiogenesis in skeletal and cardiac muscle. Physiol. Rev. 1992, 72, 369–417. [Google Scholar] [CrossRef] [PubMed]
- Prior, B.M.; Yang, H.T.; Terjung, R.L. What makes vessels grow with exercise training? J. Appl. Physiol. 2004, 97, 1119–1128. [Google Scholar] [CrossRef]
- Karakilic, A.; Yuksel, O.; Kizildag, S.; Hosgorler, F.; Topcugil, B.; Ilgin, R.; Gumus, H.; Guvendi, G.; Koc, B.; Kandis, S.; et al. Regular aerobic exercise increased VEGF levels in both soleus and gastrocnemius muscles correlated with hippocampal learning and VEGF levels. Acta Neurobiol. Exp. 2021, 81, 1–9. [Google Scholar] [CrossRef]
- Gustafsson, T.; Knutsson, A.; Puntschart, A.; Kaijser, L.; Nordqvist, S.A.C.; Sundberg, C.; Jansson, E. Increased expression of vascular endothelial growth factor in human skeletal muscle in response to short-term one-legged exercise training. Pflügers Arch. Eur. J. Physiol. 2002, 444, 752–759. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef]
- Levy, B.; Ambrosio, G.; Pries, A.; Struijker-Boudier, H. Microcirculation in Hypertension. Circulation 2001, 104, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, H.; Uzan, L.; Mourad, J.-J.; Safar, M.; Levy, B.I.; Tibiriçà, E. Increased Skin Capillary Density in Treated Essential Hypertensive Patients. Am. J. Hypertens. 2006, 19, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Baraúna, V.G.; Negrão, C.E.; Phillips, M.I.; Oliveira, E.M. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am. J. Physiol. Circ. Physiol. 2015, 309, H543–H552. [Google Scholar] [CrossRef] [PubMed]
- Clauss, S.; Wakili, R.; Hildebrand, B.; Kaab, S.; Hoster, E.; Klier, I.; Martens, E.; Hanley, A.; Hanssen, H.; Halle, M.; et al. MicroRNAs as Biomarkers for Acute Atrial Remodeling in Marathon Runners (The miRathon Study—A Sub-Study of the Munich Marathon Study). PLoS ONE 2016, 11, e0148599. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.E.; Lo, C.; Estephan, L.E.; Tai, Y.-Y.; Tang, Y.; Zhao, J.; Sugahara, M.; Gorcsan, J.; Brown, M.G.; Lieberman, D.E.; et al. Specific circulating microRNAs display dose-dependent responses to variable intensity and duration of endurance exercise. Am. J. Physiol. Circ. Physiol. 2018, 315, H273–H283. [Google Scholar] [CrossRef] [PubMed]
- Soplinska, A.; Zareba, L.; Wicik, Z.; Eyileten, C.; Jakubik, D.; Siller-Matula, J.M.; De Rosa, S.; Malek, L.A.; Postula, M. MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise—A Comprehensive Review. Diagnostics 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.; de Gonzalo-Calvo, D.; Toro, R.; Fernandes, T.; Theisen, D.; Wang, D.-Z.; Devaux, Y.; Network, O.B.O.T.C. Non-coding RNAs and exercise: Pathophysiological role and clinical application in the cardiovascular system. Clin. Sci. 2018, 132, 925–942. [Google Scholar] [CrossRef]
- Sun, L.; Li, W.; Lei, F.; Li, X. The regulatory role of micro RNA s in angiogenesis-related diseases. J. Cell. Mol. Med. 2018, 22, 4568–4587. [Google Scholar] [CrossRef]
- Caporali, A.; Emanueli, C. MicroRNA regulation in angiogenesis. Vasc. Pharmacol. 2011, 55, 79–86. [Google Scholar] [CrossRef]
- Wang, J.; Tao, J.; Chen, D.-D.; Cai, J.-J.; Irani, K.; Wang, Q.; Yuan, H.; Chen, A.F. MicroRNA miR-27b Rescues Bone Marrow-Derived Angiogenic Cell Function and Accelerates Wound Healing in Type 2 Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2014, 34, 99–109. [Google Scholar] [CrossRef]
- Ding, L.; Ni, J.; Yang, F.; Huang, L.; Deng, H.; Wu, Y.; Ding, X.; Tang, J. Promising therapeutic role of miR-27b in tumor. Tumor Biol. 2017, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lv, C.; Zhang, B.; Zhou, Q.; Cao, Z. MicroRNA-27b functions as a new inhibitor of ovarian cancer-mediated vasculogenic mimicry through suppression of VE-cadherin expression. RNA 2017, 23, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Goyal, D.; Leitzke, A.; Gheorghe, C.P.; Longo, L.D. Brain Renin-Angiotensin System: Fetal Epigenetic Programming by Maternal Protein Restriction During Pregnancy. Reprod. Sci. 2010, 17, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.C.; Hamdy, R.C.; Chua, B.H. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim. Biophys. Acta 1998, 1401, 187–194. [Google Scholar] [CrossRef]
- Prasannarong, M.; Santos, F.R.; Henriksen, E.J. ANG-(1-7) reduces ANG II-induced insulin resistance by enhancing Akt phosphorylation via a Mas receptor-dependent mechanism in rat skeletal muscle. Biochem. Biophys. Res. Commun. 2012, 426, 369–373. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Li, P.; Chappell, M.C.; Ferrario, C.M.; Brosnihan, K.B. Angiotensin-(1-7) Augments Bradykinin-Induced Vasodilation by Competing with ACE and Releasing Nitric Oxide. Hypertension 1997, 29, 394–398. [Google Scholar] [CrossRef]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef]
- Nunes-Silva, A.; Rocha, G.C.; Magalhaes, D.M.; Vaz, L.N.; De Faria, M.H.S.; E Silva, A.C.S. Physical Exercise and ACE2-Angiotensin-(1-7)-Mas Receptor Axis of the Renin Angiotensin System. Protein Pept. Lett. 2017, 24, 809–816. [Google Scholar] [CrossRef]
- Evangelista, F.S. Physical Exercise and the Renin Angiotensin System: Prospects in the COVID-19. Front. Physiol. 2020, 11, 561403. [Google Scholar] [CrossRef]
- Magalhães, D.M.; Nunes-Silva, A.; Rocha, G.C.; Vaz, L.N.; de Faria, M.H.S.; Vieira, E.L.M.; Rocha, N.P.; e Silva, A.C.S. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin-angiotensin system. Heliyon 2020, 6, e03208. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Farrow, M.; Biglands, J.; Fernandes, R.J.; Abraldes, J.A.; Castro, F.A.D.S.; de Souza, H.L.; Arriel, R.A.; Meireles, A.; Marocolo, M.; et al. Commentaries on Viewpoint: The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J. Appl. Physiol. 2020, 129, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.S.; Jae, S.Y. Exercise as medicine for COVID-19: An ACE in the hole? Med Hypotheses 2020, 142, 109835. [Google Scholar] [CrossRef] [PubMed]
- Libera, L.D.; Ravara, B.; Angelini, A.; Rossini, K.; Sandri, M.; Thiene, G.; Ambrosio, G.B.; Vescovo, G. Beneficial Effects on Skeletal Muscle of the Angiotensin II Type 1 Receptor Blocker Irbesartan in Experimental Heart Failure. Circulation 2001, 103, 2195–2200. [Google Scholar] [CrossRef]
- Russell, S.; Eley, H.; Tisdale, M. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell. Signal. 2007, 19, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.F.; Bechara, L.R.G.; Bacurau, A.V.N.; Jannig, P.R.; Voltarelli, V.A.; Dourado, P.M.; Vasconcelos, A.R.; Scavone, C.; Ferreira, J.C.B.; Brum, P.C. Exercise training decreases NADPH oxidase activity and restores skeletal muscle mass in heart failure rats. J. Appl. Physiol. 2017, 122, 817–827. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; A Freeman, B.; Griendling, K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.F.; Pelozin, B.R.A.; da Silva Junior, N.D.; Soci, U.P.R.; do Carmo, E.C.; da Mota, G.d.F.A.; Cachofeiro, V.; Lahera, V.; Oliveira, E.M.; Fernandes, T. Angiotensin II Promotes Skeletal Muscle Angiogenesis Induced by Volume-Dependent Aerobic Exercise Training: Effects on miRNAs-27a/b and Oxidant–Antioxidant Balance. Antioxidants 2022, 11, 651. https://doi.org/10.3390/antiox11040651
Rodrigues LF, Pelozin BRA, da Silva Junior ND, Soci UPR, do Carmo EC, da Mota GdFA, Cachofeiro V, Lahera V, Oliveira EM, Fernandes T. Angiotensin II Promotes Skeletal Muscle Angiogenesis Induced by Volume-Dependent Aerobic Exercise Training: Effects on miRNAs-27a/b and Oxidant–Antioxidant Balance. Antioxidants. 2022; 11(4):651. https://doi.org/10.3390/antiox11040651
Chicago/Turabian StyleRodrigues, Luis Felipe, Bruno Rocha Avila Pelozin, Natan Daniel da Silva Junior, Ursula Paula Renó Soci, Everton Crivoi do Carmo, Glória de Fatima Alves da Mota, Victoria Cachofeiro, Vicente Lahera, Edilamar Menezes Oliveira, and Tiago Fernandes. 2022. "Angiotensin II Promotes Skeletal Muscle Angiogenesis Induced by Volume-Dependent Aerobic Exercise Training: Effects on miRNAs-27a/b and Oxidant–Antioxidant Balance" Antioxidants 11, no. 4: 651. https://doi.org/10.3390/antiox11040651
APA StyleRodrigues, L. F., Pelozin, B. R. A., da Silva Junior, N. D., Soci, U. P. R., do Carmo, E. C., da Mota, G. d. F. A., Cachofeiro, V., Lahera, V., Oliveira, E. M., & Fernandes, T. (2022). Angiotensin II Promotes Skeletal Muscle Angiogenesis Induced by Volume-Dependent Aerobic Exercise Training: Effects on miRNAs-27a/b and Oxidant–Antioxidant Balance. Antioxidants, 11(4), 651. https://doi.org/10.3390/antiox11040651








