Artemisia gmelinii Attenuates Lung Inflammation by Suppressing the NF-κB/MAPK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Artemisia Gmelinii Ethanol Extract (AGE)
2.3. Preparation of Cigarette Smoke Extract (CSE)
2.4. Cell Culture
2.5. Animal Study
2.6. Cell Viability
2.7. Measurement of Nitric Oxide (NO) Production in MH-S Macrophages
2.8. Measurement of Cytokines and Chemokines Production in MH-S Macrophages and BALF
2.9. Western Blot Analysis of iNOS&COX-2 and MAPK/NF-ĸB in MH-S Macrophages
2.10. Immunofluorescence Assay of Nuclear Translocation of NF-κB in MH-S Macrophages
2.11. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-PCR) in MH-S Macrophages and Lung Tissues
2.12. Analysis of the Differential Cell Counts in BALF
2.13. Protein Simple Capillary Immunoassay of Nuclear Translocation of NF-κB in Lung Tissues
2.14. Histological Analysis of Lung Tissues
2.15. NETosis Assay
2.16. High-Performance Liquid Chromatography (HPLC) Analysis
2.17. Statistical Analysis
3. Results
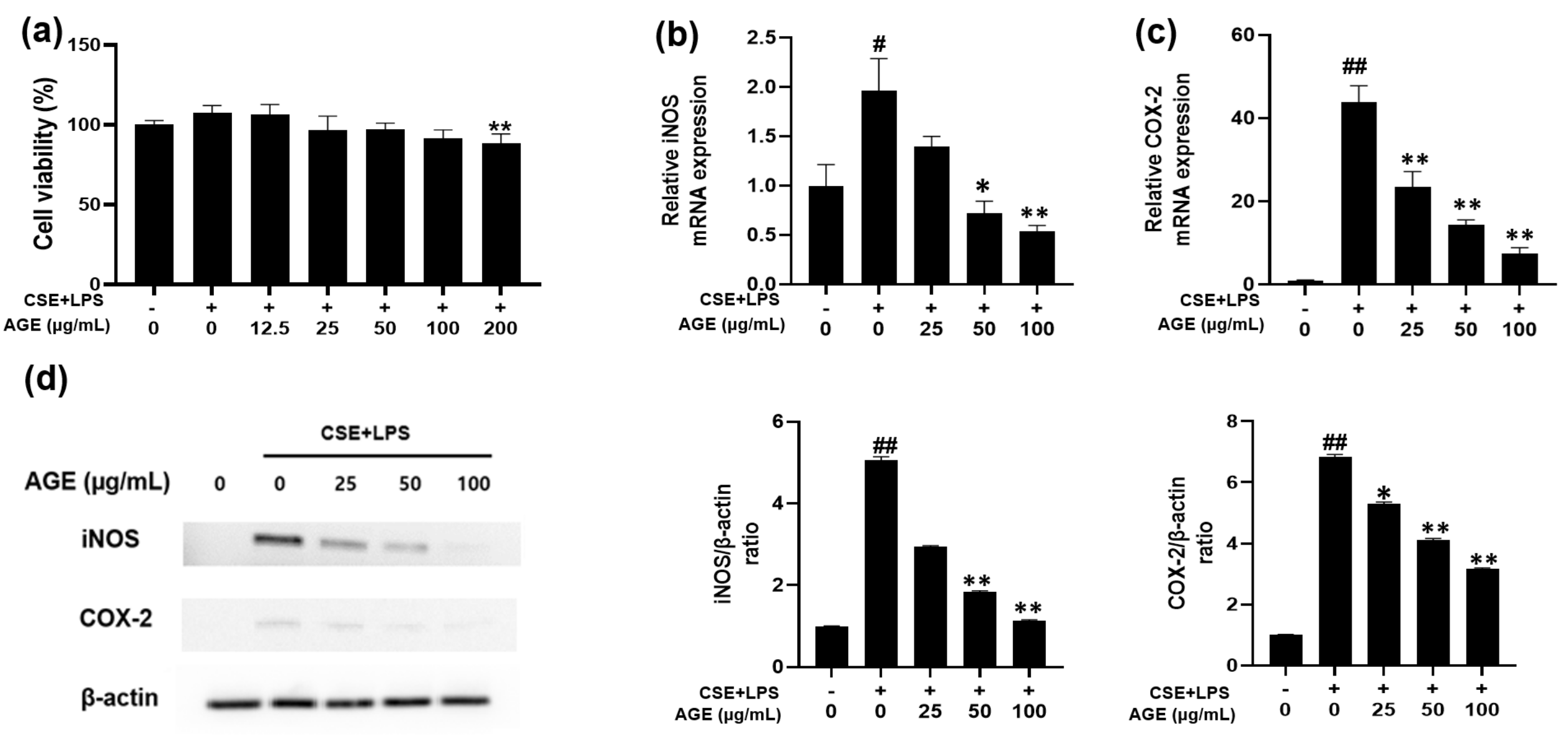
3.1. Effect of AGE on Cell Viability and iNOS and COX-2 Expression in MH-S Macrophages
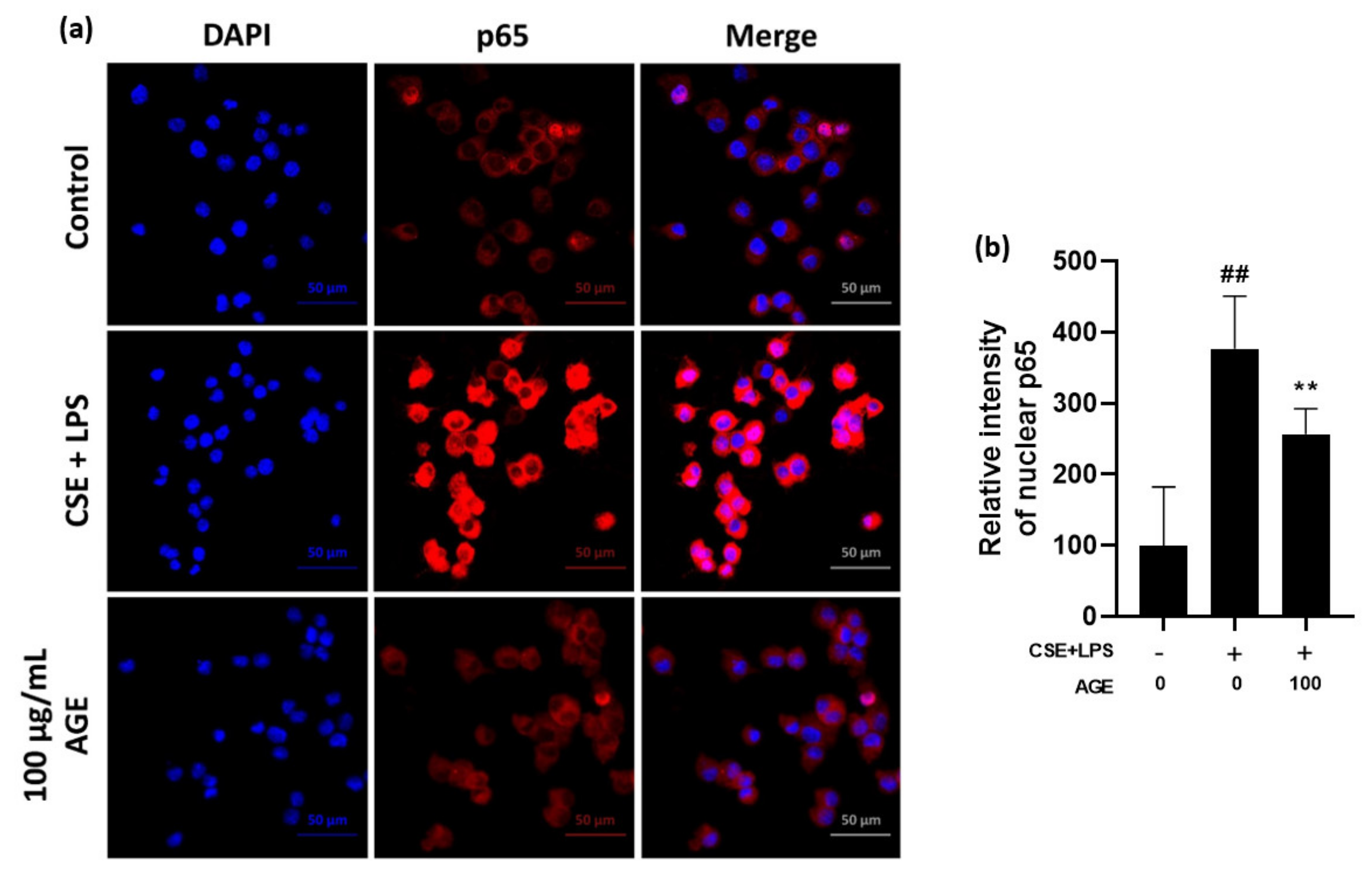
3.2. Effect of AGE on Pro-Infammatory Cytokines and Chemokines and the NF-κB Pathway in MH-S Machrophages
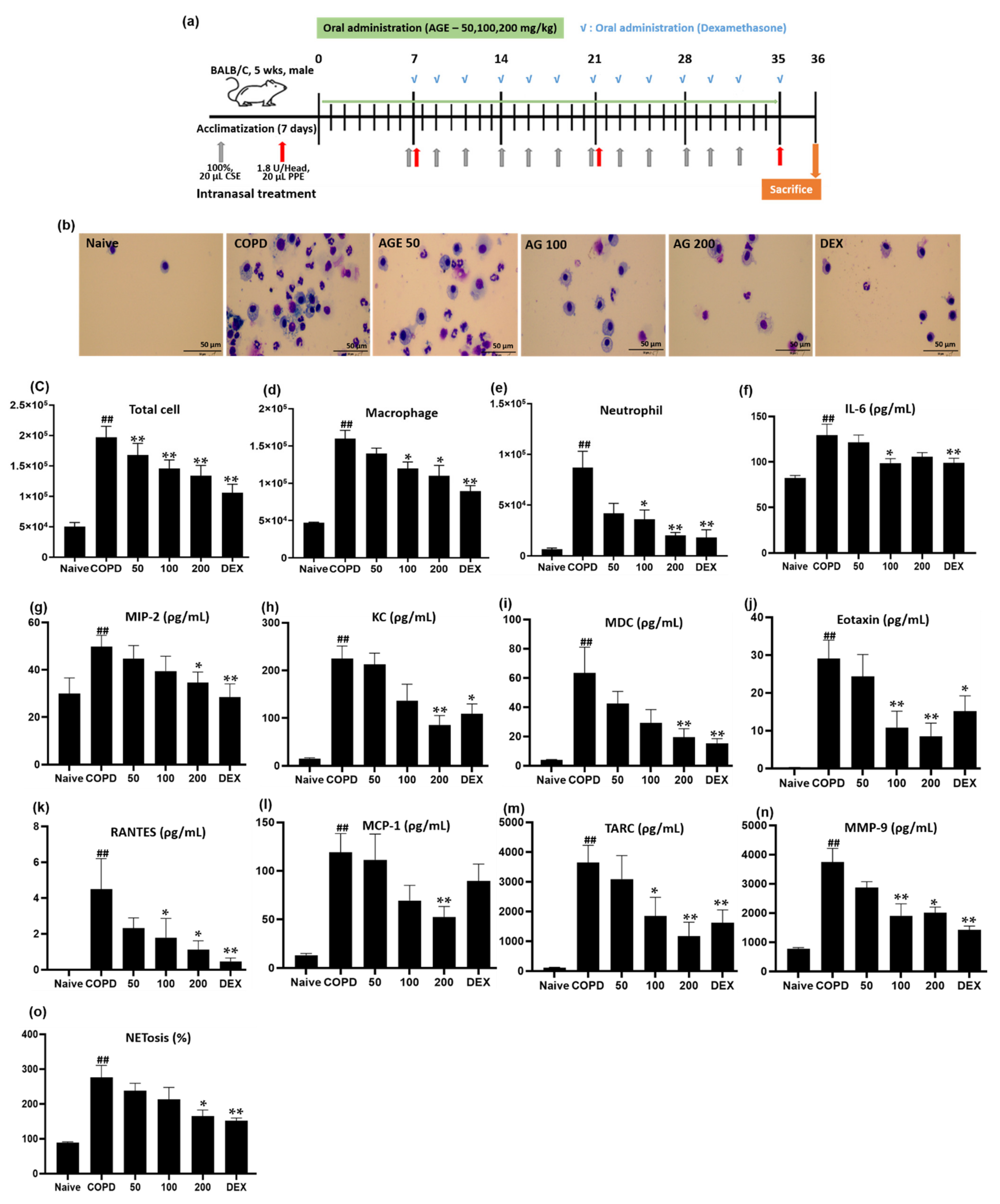
3.3. Effect of AGE on Inflammatory Cell Counts, Cytokines, Chemokines, MMP-9, and NETosis in BALF
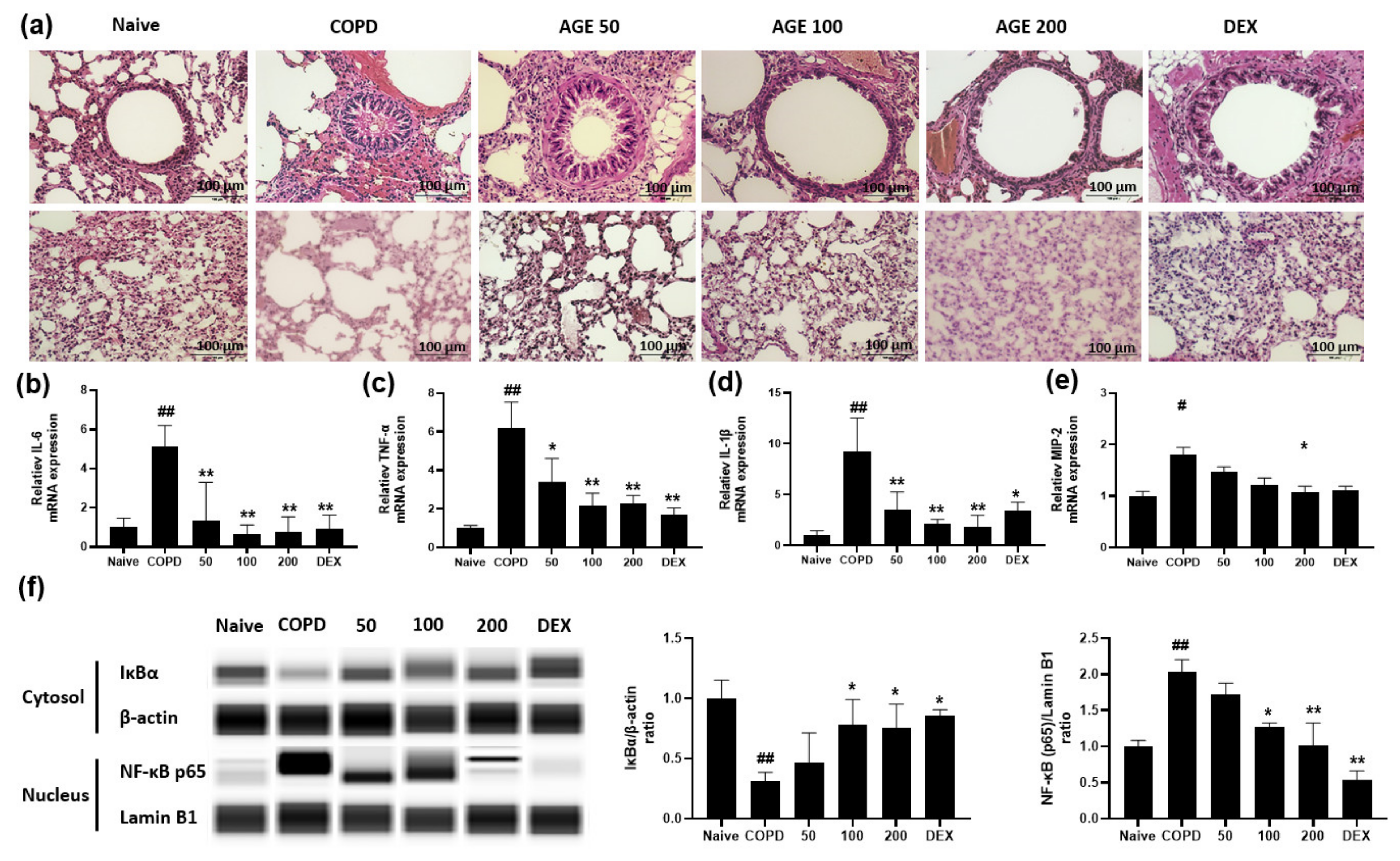
3.4. Effect of AGE on Airway Inflammation, Alveolar Enlargement, Cytokines and Chemokines and NF-κB in Lung Tissue
3.5. Active Compounds in AGE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AG | Artemisia gmelinii |
| AGE | Artemisia gmelinii ethanol extract |
| AM | Alveolar macrophage |
| BALF | Bronchoalveolar lavage fluid |
| COPD | Chronic obstructive pulmonary disease |
| COX-2 | Cyclooxygenase-2 |
| CS | Cigarette smoke |
| CSE | Cigarette smoke extract |
| ELISA | Enzyme-linked immunosorbent assay |
| HO-1 | Heme oxygenase-1 |
| HPLC | High-performance liquid chromatography |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| KC | Keratinocytes-derived chemokine |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDC | Macrophage-derived chemokine |
| MIP-2 | Macrophage inflammatory protein-2 |
| MMP-9 | Matrix metalloproteinase-9 |
| NET | Neutrophil extracellular traps |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor-E2—related factor 2 |
| PDTC | Ammonium pyrrolidine dithiocarbamate |
| PPE | Porcine pancreas elastase |
| RANTES | Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted |
| ROS | Reactive oxygen species |
| TARC | Thymus and activation-regulated chemkine |
| TNF-α | Tumor necrosis factor-α |
References
- Kim, D.S.; Kim, Y.S.; Jung, K.-S.; Chang, J.H.; Lim, C.-M.; Lee, J.H.; Uh, S.-T.; Shim, J.J.; Lew, W.J. Prevalence of chronic obstructive pulmonary disease in Korea: A population-based spirometry survey. Am. J. Respir. Crit. Care Med. 2005, 172, 842–847. [Google Scholar] [CrossRef] [PubMed]
- OCDE. Economic Consequences of Outdoor Air Pollution; Organisation for Economic Co-operation and Development: Paris, France, 2016. [Google Scholar]
- Lyu, Y.R.; Kim, J.; Yang, W.-k.; Kim, S.-h.; Park, Y.-C. Clinical Research Trends in Respiratory Diseases Related to Particulate Matter. J. Int. Korean Med. 2019, 40, 443–457. [Google Scholar] [CrossRef]
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M.; Gillespie, S.; Burney, P.; Mannino, D.M.; Menezes, A.M.; Sullivan, S.D.; Lee, T.A.; Weiss, K.B. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- Vlahos, R.; Bozinovski, S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front. Immunol. 2014, 5, 435. [Google Scholar] [CrossRef] [Green Version]
- Miravitlles, M.; Ribera, A. Understanding the impact of symptoms on the burden of COPD. Respir. Res. 2017, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). Prevention. How Tobacco Smoke Causes Disease: The biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention: Antlanta, GA, USA, 2010. [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mio, T.; Romberger, D.J.; Thompson, A.B.; Robbins, R.A.; Heires, A.; Rennard, S.I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 1997, 155, 1770–1776. [Google Scholar] [CrossRef]
- Arai, N.; Kondo, M.; Izumo, T.; Tamaoki, J.; Nagai, A. Inhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in mice. Eur. Respir. J. 2010, 35, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Voynow, J.A.; Young, L.R.; Wang, Y.; Horger, T.; Rose, M.C.; Fischer, B.M. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 1999, 276, L835–L843. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.C.; Chen, C.-H. Cigarette smoking-mediated macrophage reprogramming: Mechanistic insights and therapeutic implications. J. Nat. Sci. 2018, 4, e539. [Google Scholar]
- Zou, L.; He, E.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef]
- Olloquequi, J.; Jaime, S.; Parra, V.; Cornejo-Cordova, E.; Valdivia, G.; Agusti, A.; Silva, O.R. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir. Res. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Jung, K.-S. Management of COPD. Korean J. Med. 2009, 77, 422–428. [Google Scholar]
- Liu, J.; Gao, F.; Li, Z. Effect of yiqibushenhuoxue decoction on chronic obstructive pulmonary disease measured by St. George’s respiratory disease questionnaire scores and forced expiratory volume. J. Tradit. Chin. Med. 2014, 34, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, A.; Asai, K.; Kadotani, H.; Maruyama, N.; Kubo, H.; Okamoto, A.; Sato, K.; Yamada, K.; Ijiri, N.; Watanabe, T. Ninjin’yoeito Ameliorates Skeletal Muscle Complications in COPD Model Mice by Upregulating Peroxisome Proliferator-Activated Receptor γ Coactivator-1α Expression. Int. J. Chron. Obstruct. Pulmon Dis. 2020, 15, 3063. [Google Scholar] [CrossRef]
- Seo, E.-J.; Hong, E.-S.; Choi, M.-H.; Kim, K.-S.; Lee, S.-J. The antioxidant and skin whitening effect of Artemisia iwayomogi extracts. J. Food Sci. Technol. 2012, 44, 89–93. [Google Scholar]
- Kim, S.-H.; Choi, C.-H.; Kim, S.-Y.; Eun, J.-S.; Shin, T.-Y. Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allergy model. Exp. Biol. Med. 2005, 230, 82–88. [Google Scholar] [CrossRef]
- Vardy, J.; Chiew, K.S.; Galica, J.; Pond, G.R.; Tannock, I.F. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br. J. Cancer 2006, 94, 1011–1015. [Google Scholar] [CrossRef]
- Wong, J.; Tran, L.T.; Lynch, K.A.; Wood, L.J. Dexamethasone exacerbates cytotoxic chemotherapy induced lethargy and weight loss in female tumor free mice. Cancer Biol. Ther. 2018, 19, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, A.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Ethnopharmacological properties of Artemisia asiatica: A comprehensive review. J. Ethnopharmacol. 2018, 220, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rubins, J.B. Alveolar macrophages: Wielding the double-edged sword of inflammation. Am. J. Respir. Crit. Care Med. 2003, 167, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Rodriguez, F.; De Cabo, M.; Salgado, M.; Losada, J.; Villaron, L.; Lopez, A.; Arellano, J. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediat. Inflamm. 1999, 8, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikota, J.K.; Shen, P.; Morissette, M.C.; Fernandes, K.; Roos, A.; Chu, D.K.; Barra, N.G.; Iwakura, Y.; Kolbeck, R.; Humbles, A.A. Cigarette smoke primes the pulmonary environment to IL-1α/CXCR-2–dependent nontypeable Haemophilus influenzae–exacerbated neutrophilia in mice. J. Immunol. 2014, 193, 3134–3145. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.-T.; Liu, X.-S.; Xu, Y.-J.; Ni, W.; Chen, S.-X. Expression of nitric oxide synthase isoenzyme in lung tissue of smokers with and without chronic obstructive pulmonary disease. Chin. Med. J. 2015, 128, 1584. [Google Scholar] [CrossRef]
- Dagouassat, M.; Gagliolo, J.-M.; Chrusciel, S.; Bourin, M.-C.; Duprez, C.; Caramelle, P.; Boyer, L.; Hue, S.; Stern, J.-B.; Validire, P. The cyclooxygenase-2–prostaglandin E2 pathway maintains senescence of chronic obstructive pulmonary disease fibroblasts. Am. J. Respir. Crit. Care Med. 2013, 187, 703–714. [Google Scholar] [CrossRef]
- Wei, J.; Xiong, X.-f.; Lin, Y.-h.; Zheng, B.-x.; Cheng, D.-y. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: A systematic review and meta-analysis. PeerJ 2015, 3, e1199. [Google Scholar] [CrossRef] [Green Version]
- Chung, K. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 18, 50s–59s. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-T.; Tumpey, T.M.; Kunkel, S.L.; Oakes, J.E.; Lausch, R.N. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investig. Ophthalmo. Vis. Sci. 1998, 39, 1854–1862. [Google Scholar]
- Zhou, X.; Gu, D.; Hou, G. Erythromycin attenuates metalloprotease/anti-metalloprotease imbalance in cigarette smoke-induced emphysema in rats via the mitogen-activated protein kinase/nuclear factor-κB activation pathway. Mol. Med. Rep. 2017, 15, 2983–2990. [Google Scholar] [CrossRef] [Green Version]
- Mercer, B.A.; D’Armiento, J.M. Emerging role of MAP kinase pathways as therapeutic targets in COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2006, 1, 137. [Google Scholar] [CrossRef] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-Kb response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-κB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef]
- Yang, S.-R.; Chida, A.S.; Bauter, M.R.; Shafiq, N.; Seweryniak, K.; Maggirwar, S.B.; Kilty, I.; Rahman, I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L46–L57. [Google Scholar] [CrossRef] [Green Version]
- Ishii, Y.; Itoh, K.; Morishima, Y.; Kimura, T.; Kiwamoto, T.; IIzuka, T.; Hegab, A.E.; Hosoya, T.; Nomura, A.; Sakamoto, T. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J. Immunol. 2005, 175, 6968–6975. [Google Scholar] [CrossRef]
- Kubo, H.; Asai, K.; Kojima, K.; Sugitani, A.; Kyomoto, Y.; Okamoto, A.; Yamada, K.; Ijiri, N.; Watanabe, T.; Hirata, K. Exercise ameliorates emphysema of cigarette smoke-induced COPD in mice through the exercise-Irisin-Nrf2 axis. Int. J. Chron. Obstruct. Pulm. Dis. 2019, 14, 2507. [Google Scholar] [CrossRef] [Green Version]
- Ueno, M.; Maeno, T.; Nishimura, S.; Ogata, F.; Masubuchi, H.; Hara, K.; Yamaguchi, K.; Aoki, F.; Suga, T.; Nagai, R. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, J.; Meng, Y.; Adcock, I.M.; Yao, X. Role of inflammatory cells in airway remodeling in COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2018, 13, 3341. [Google Scholar] [CrossRef] [Green Version]
- Adnan, A.-M.; Ammar, A.-Z.; Khalil, K. Role of eotaxin in chronic obstructive pulmonary disease (COPD). Int. J. Pharm. Sci. Rev. Res. 2013, 21, 10–14. [Google Scholar]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traves, S.; Culpitt, S.; Russell, R.; Barnes, P.; Donnelly, L. Increased levels of the chemokines GROα and MCP-1 in sputum samples from patients with COPD. Thorax 2002, 57, 590–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machida, H.; Inoue, S.; Shibata, Y.; Kimura, T.; Sato, K.; Abe, K.; Murano, H.; Yang, S.; Nakano, H.; Sato, M. Thymus and activation-regulated chemokine (TARC/CCL17) predicts decline of pulmonary function in patients with chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Göggel, R.; Chaudhary, N.; Wiedenmann, A.; Jung, B.; Weith, A.; Seither, P. Elevated expression of TARC (CCL17) and MDC (CCL22) in models of cigarette smoke-induced pulmonary inflammation. Biochem. Biophys. Res. Commun. 2005, 334, 254–262. [Google Scholar] [CrossRef]
- Dancer, R.; Wood, A.; Thickett, D. Metalloproteinases in idiopathic pulmonary fibrosis. Eur. Respir. J. 2011, 38, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Gilowska, I.; Kasper, Ł.; Bogacz, K.; Szczegielniak, J.; Szymasek, T.; Kasper, M.; Czerwinski, M.; Sładek, K.; Majorczyk, E. Impact of matrix metalloproteinase 9 on COPD development in Polish patients: Genetic polymorphism, protein level, and their relationship with lung function. BioMed Res. Int. 2018, 2018, 6417415. [Google Scholar] [CrossRef]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Ding, Y.; Yan, X.T.; Kim, Y.H.; Jang, H.D. Scopoletin and scopolin isolated from Artemisia iwayomogi suppress differentiation of osteoclastic macrophage RAW 264.7 cells by scavenging reactive oxygen species. J. Nat. Prod. 2013, 76, 615–620. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, D.M.; Lee, S.H.; Seong, A.R.; Gin, D.W.; Hwang, J.A.; Park, J.H. Chlorogenic acid suppresses pulmonary eosinophilia, IgE production, and Th2 type cytokine production in an ovalbumin-induced allergic asthma: Activation of STAT-6 and JNK is inhibited by chlorogenic acid. Int. Immunopharmacol. 2010, 10, 1242–1248. [Google Scholar] [CrossRef]
- Motaal, A.A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef] [Green Version]
- Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G. Antioxidant and Mitochondria-Targeted Activity of Caffeoylquinic-Acid-Rich Fractions of Wormwood (Artemisia absinthium L.) and Silver Wormwood (Artemisia ludoviciana Nutt.). Antiox 2021, 10, 1405. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids(acyl-quinic acids) in humans. Compr. Rev. Food. Sci. Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L.; Zhang, X. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef]
- Wang, Y.C.; Dong, J.; Nie, J.; Zhu, J.X.; Wang, H.; Chen, Q.; Chen, J.Y.; Xia, J.M.; Shuai, W.; Wang, Y.C. Amelioration of bleomycin-induced pulmonary fibrosis by chlorogenic acid through endoplasmic reticulum stress inhibition. Apoptosis 2017, 22, 1147–1156. [Google Scholar] [CrossRef]
- Zeng, J.; Wan, X.; Liu, T.; Xiong, Y.; Xiang, G.; Peng, Y.; Zhu, R.; Zhou, Y.; Liu, C.; Zeng, J. Chlorogenic acid ameliorates Klebsiella pneumoniae-induced pneumonia in immunosuppressed mice via inhibiting the activation of NLRP3 inflammasomes. Food Funct. 2021, 12, 9466–9475. [Google Scholar] [CrossRef]
- Zhang, J.; Onakpoya, I.J.; Posadzki, P.; Eddouks, M.; Zhang, J. The safety of herbal medicine: From prejudice to evidence. Evid. Based Complement. Altern. Med. 2015, 2015, 316706. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Han, P.; Yu, L.; Chen, Y.; Ye, B.; Qin, L.; Xin, H.; Han, T. Anti-allergic rhinitis effects of caffeoylquinic acids from the fruits of Xanthium strumarium in rodent animals via alleviating allergic and inflammatory reactions. Rev. Bras. Farmacogn. 2019, 29, 46–53. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358v. [Google Scholar] [CrossRef] [Green Version]
- Heghes, S.C.; Vostinaru, O.; Mogosan, C.I.; Miere, D.; Iuga, C.A.; Filip, L. Safety profile of nutraceuticals rich in coumarins: An update. Front. Pharmacol. 2022, 13, 803338. [Google Scholar] [CrossRef]
- Tamura, T.; Kobayashi, H.; Yamataka, A.; Lane, G.J.; Koga, H.; Miyano, T. Inchin-ko-to prevents medium-term liver fibrosis in postoperative biliary atresia patients. Pediatr. Surg. Int. 2007, 23, 343–347. [Google Scholar] [CrossRef]

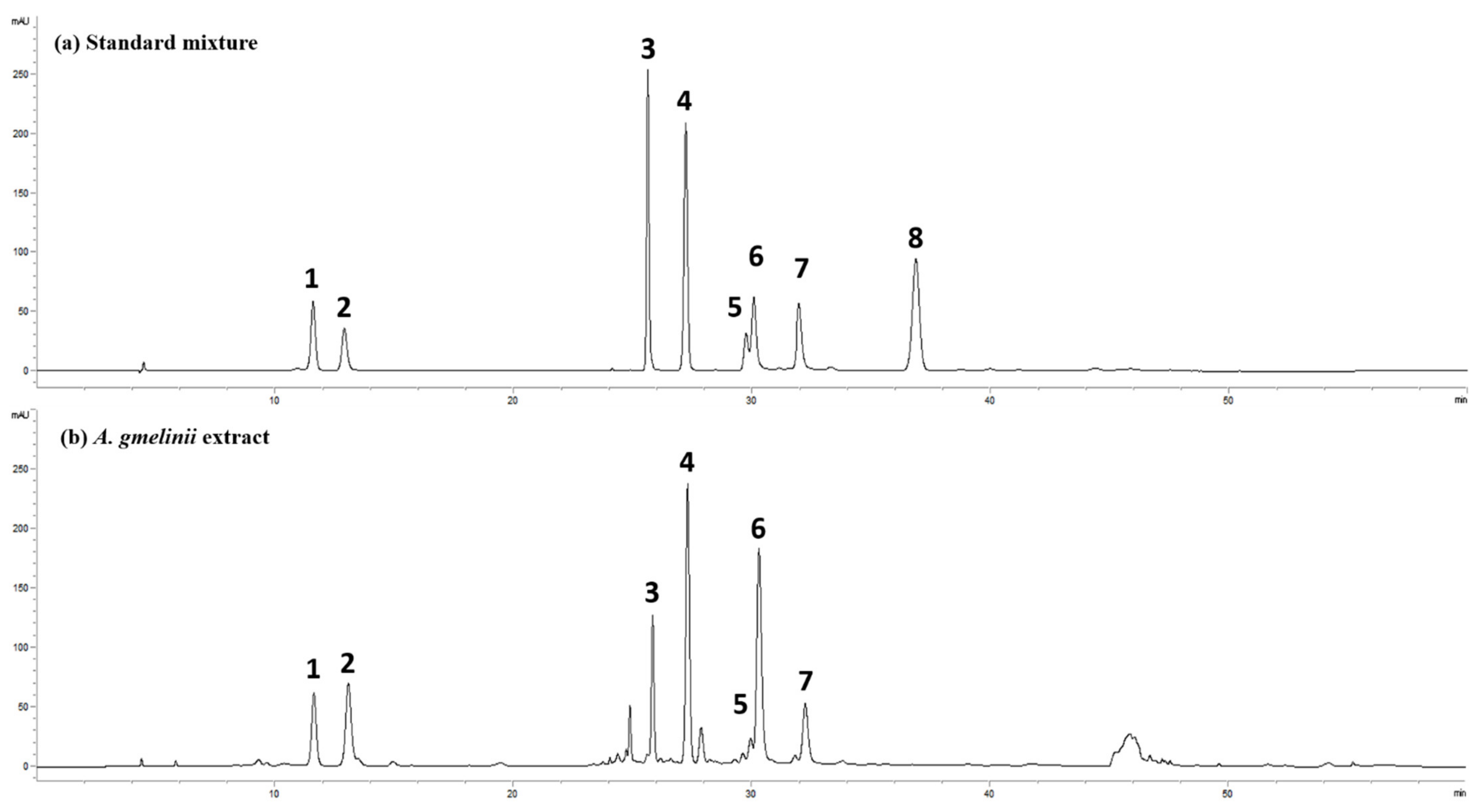
| Compound | Regression Equation | Regression Coefficient (R2) | Content (mg/g) |
|---|---|---|---|
| Scopolin | y = 8.1105x − 3.6018 | 0.9999 | 8.6 |
| Chlorogenic acid | y = 5.3291x − 42.167 | 1.0000 | 21.8 |
| Hyperoside | y = 17.799x + 3.6018 | 1.0000 | 5.2 |
| Scopoletin | y = 22.339x − 194.46 | 0.9990 | 7.3 |
| 4,5-di-O-caffeoylquinic acid | y = 3.8996x + 10.436 | 0.9996 | 5.2 |
| 3,5-di-O-caffeoylquinic acid | y = 7.2616x + 35.623 | 0.9998 | 34.6 |
| 3,4-di-O-caffeoylquinic acid | y = 8.2834x + 16.766 | 1.0000 | 9.5 |
| Scoparone | y = 24.082x − 114.77 | 0.9994 | ND |
| Identified Components | Il-6 (ρg/mL) | ||||
|---|---|---|---|---|---|
| Naive | CSE/LPS | 10 ng/mL | 100 ng/mL | 1000 ng/mL | |
| Scopolin | 784.38 ± 50.39 | 7029.17 ± 1214.07 ## | 5340.63 ± 726.96 ** | 5050.00 ± 641.53 ** | 5286.75 ± 753.36 ** |
| Chlorogenic acid | 762.50 ± 75.70 | 11,010.42 ± 1582.40 ## | 12,840.63 ± 762.50 | 8128.13 ± 590.90 ** | 8000.00 ± 718.10 ** |
| Hyperoside | 762.50 ± 75.70 | 11,010.42 ± 1582.40 ## | 7237.50 ± 1209.01 ** | 6406.25 ± 1620.76 ** | 4078.13 ± 318.42 ** |
| Scopoletin | 784.38 ± 50.39 | 7029.17 ± 1214.07 ## | 8515.63 ± 1092.70 | 6895.83 ± 1087.02 | 6641.67 ± 466.43 |
| 4,5-di-O-caffeoylquinic acid | 784.38 ± 50.39 | 7029.17 ± 1214.07 ## | 8090.63 ± 593.58 | 5537.50 ± 97.36 * | 4725.00 ± 234.30 ** |
| 3,5-di-O-caffeoylquinic acid | 762.50 ± 75.70 | 11,010.42 ± 1582.40 ## | 5346.88 ± 726.96 ** | 5122.92 ± 697.35 * | 4214.58 ± 750.66 ** |
| 3,4-di-O-caffeoylquinic acid | 762.50 ± 75.70 | 11,010.42 ± 1582.40 ## | 11,971.88 ± 210.50 | 8343.75 ± 696.90 ** | 6685.42 ± 914.50 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Shin, D.-U.; Eom, J.-E.; Jung, S.Y.; Song, H.-J.; Lim, K.m.; Kim, G.-D.; Yun, S.-I.; Kim, M.-Y.; Shin, H.S.; et al. Artemisia gmelinii Attenuates Lung Inflammation by Suppressing the NF-κB/MAPK Pathway. Antioxidants 2022, 11, 568. https://doi.org/10.3390/antiox11030568
Kim SY, Shin D-U, Eom J-E, Jung SY, Song H-J, Lim Km, Kim G-D, Yun S-I, Kim M-Y, Shin HS, et al. Artemisia gmelinii Attenuates Lung Inflammation by Suppressing the NF-κB/MAPK Pathway. Antioxidants. 2022; 11(3):568. https://doi.org/10.3390/antiox11030568
Chicago/Turabian StyleKim, Seung Yong, Dong-Uk Shin, Ji-Eun Eom, Sun Young Jung, Hyeon-Ji Song, Kyung min Lim, Gun-Dong Kim, Soon-Il Yun, Mi-Yeon Kim, Hee Soon Shin, and et al. 2022. "Artemisia gmelinii Attenuates Lung Inflammation by Suppressing the NF-κB/MAPK Pathway" Antioxidants 11, no. 3: 568. https://doi.org/10.3390/antiox11030568
APA StyleKim, S. Y., Shin, D.-U., Eom, J.-E., Jung, S. Y., Song, H.-J., Lim, K. m., Kim, G.-D., Yun, S.-I., Kim, M.-Y., Shin, H. S., & Lee, S.-Y. (2022). Artemisia gmelinii Attenuates Lung Inflammation by Suppressing the NF-κB/MAPK Pathway. Antioxidants, 11(3), 568. https://doi.org/10.3390/antiox11030568






