Enhanced Photosynthetic Capacity, Osmotic Adjustment and Antioxidant Defenses Contribute to Improve Tolerance to Moderate Water Deficit and Recovery of Triploid Citrus Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Water Deficit Treatment
2.3. Drought-Related Parameters
2.4. Leaf Gas Exchange and Chlorophyll Fluorescence
2.5. Oxidative Stress Indicators
2.6. Antioxidant Enzymatic Activities, Ascorbic Acid, and Proline Content
2.7. Statistical Analysis
3. Results
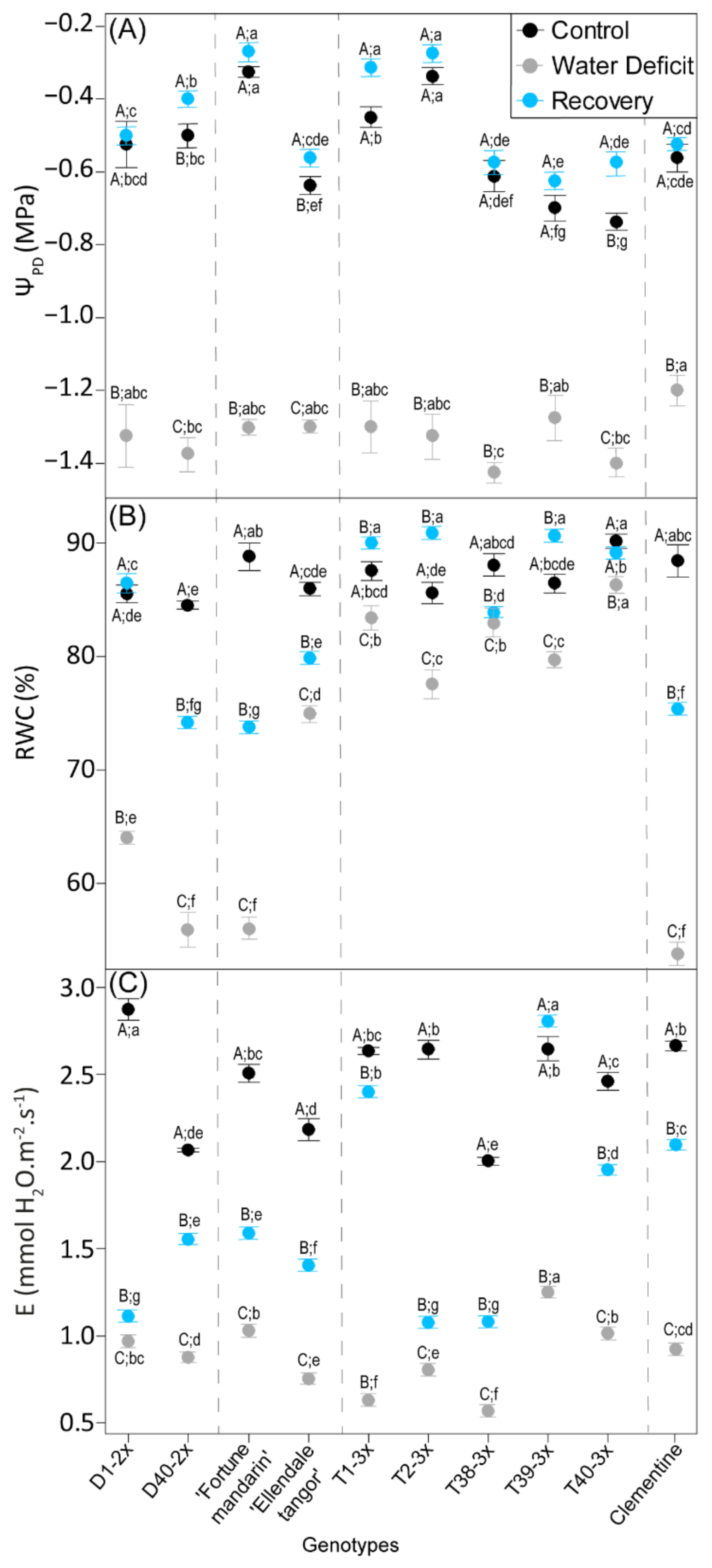
3.1. Plant Water Status
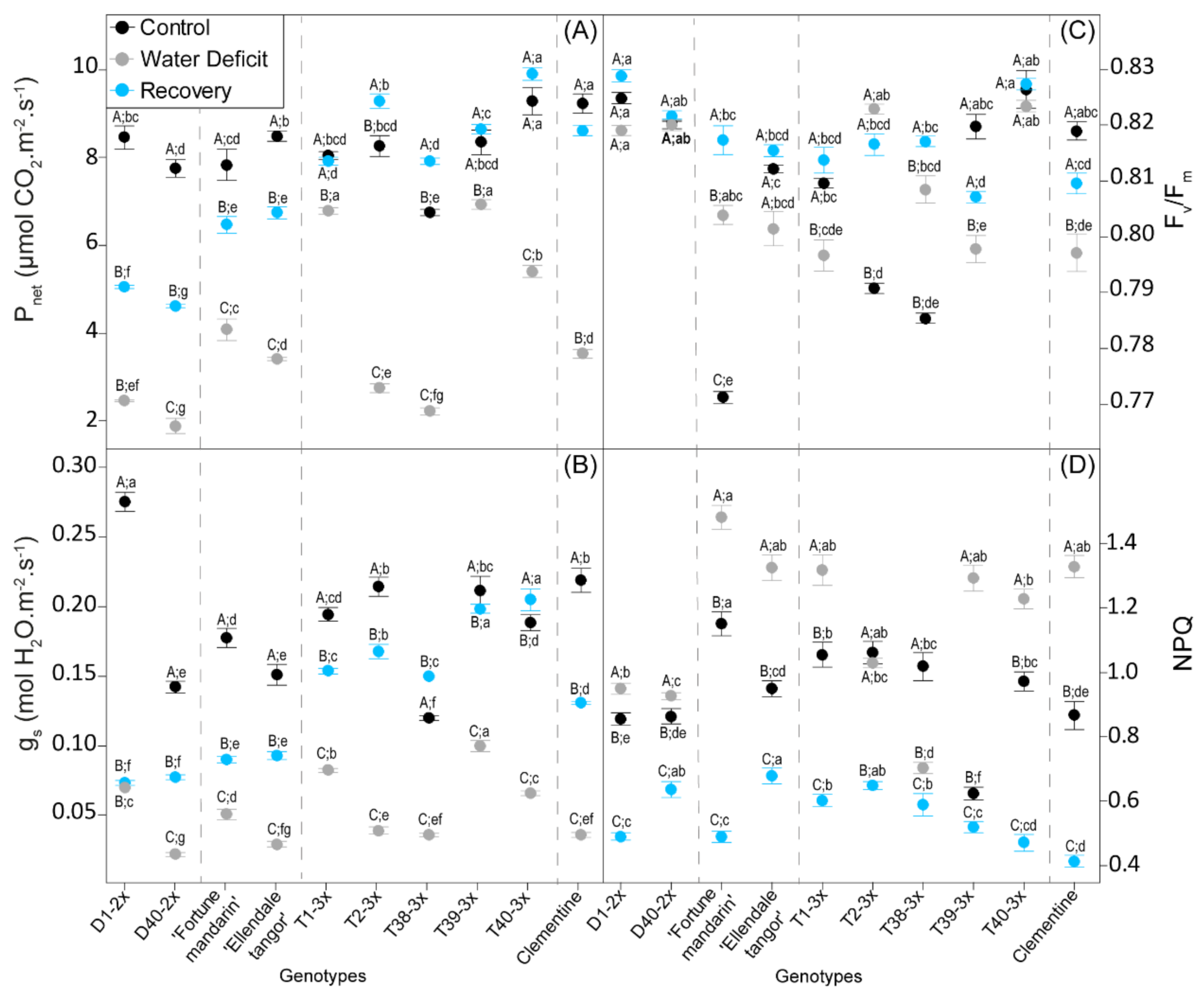
3.2. Leaf Gas Exchange and Photochemistry Parameters
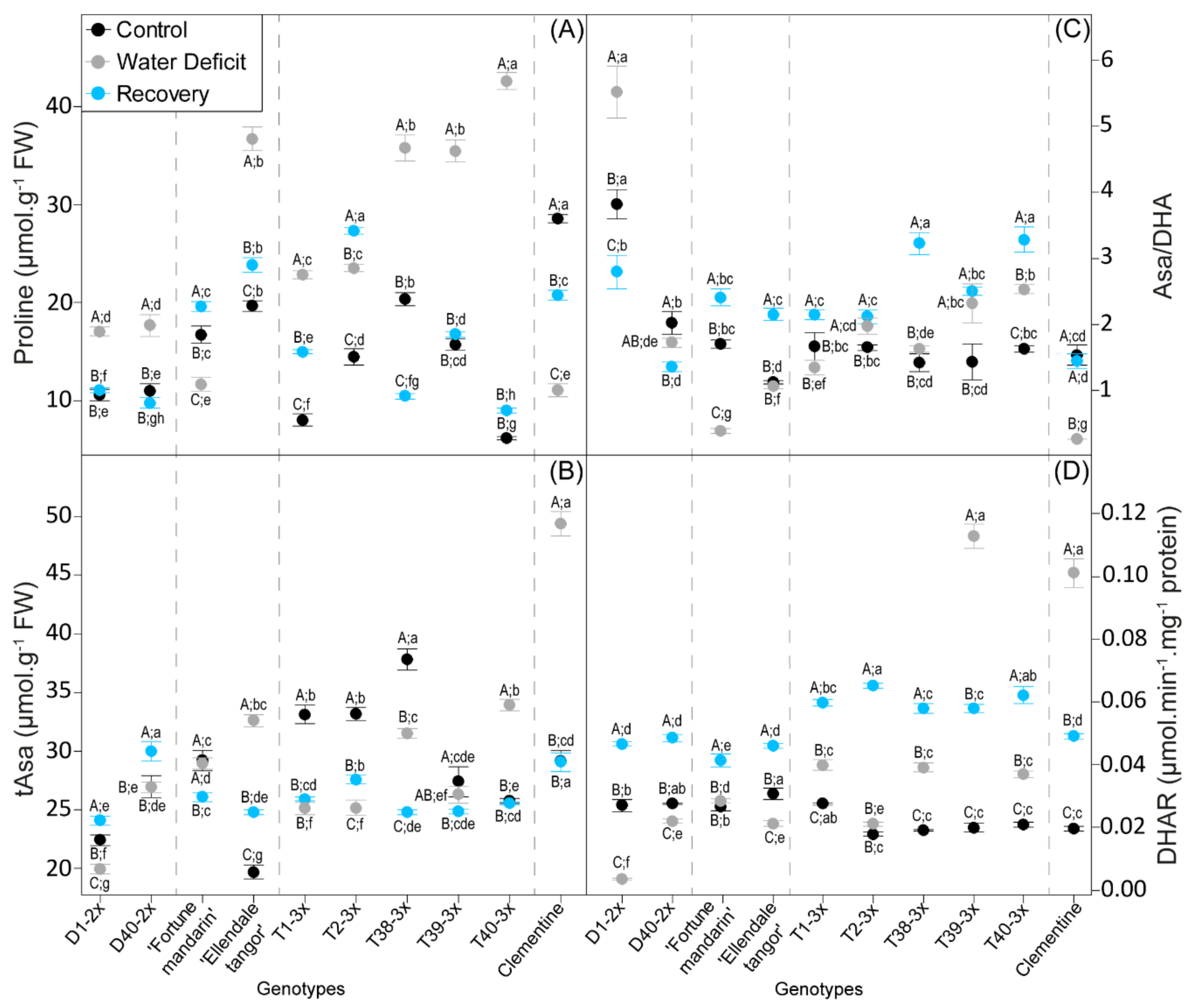
3.3. Oxidative Damages and Antioxidant Response
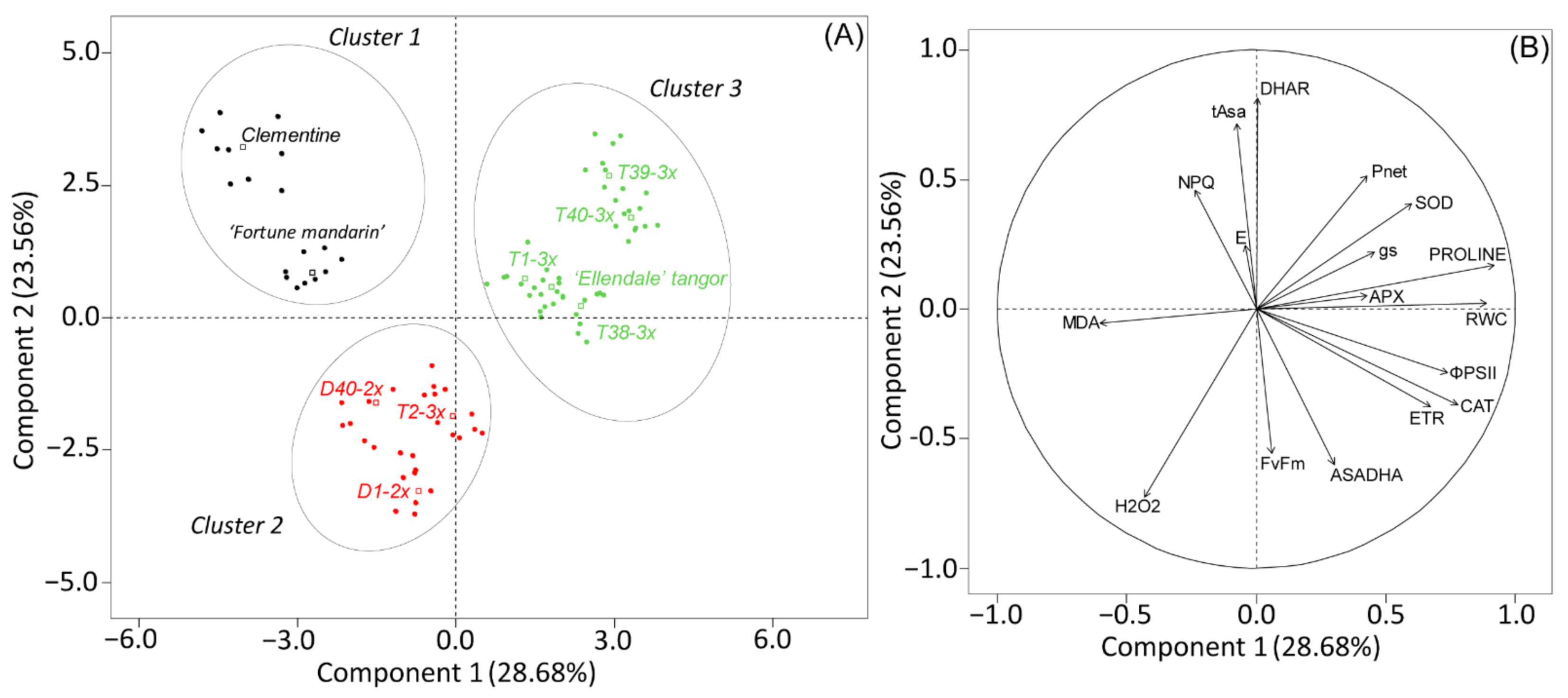
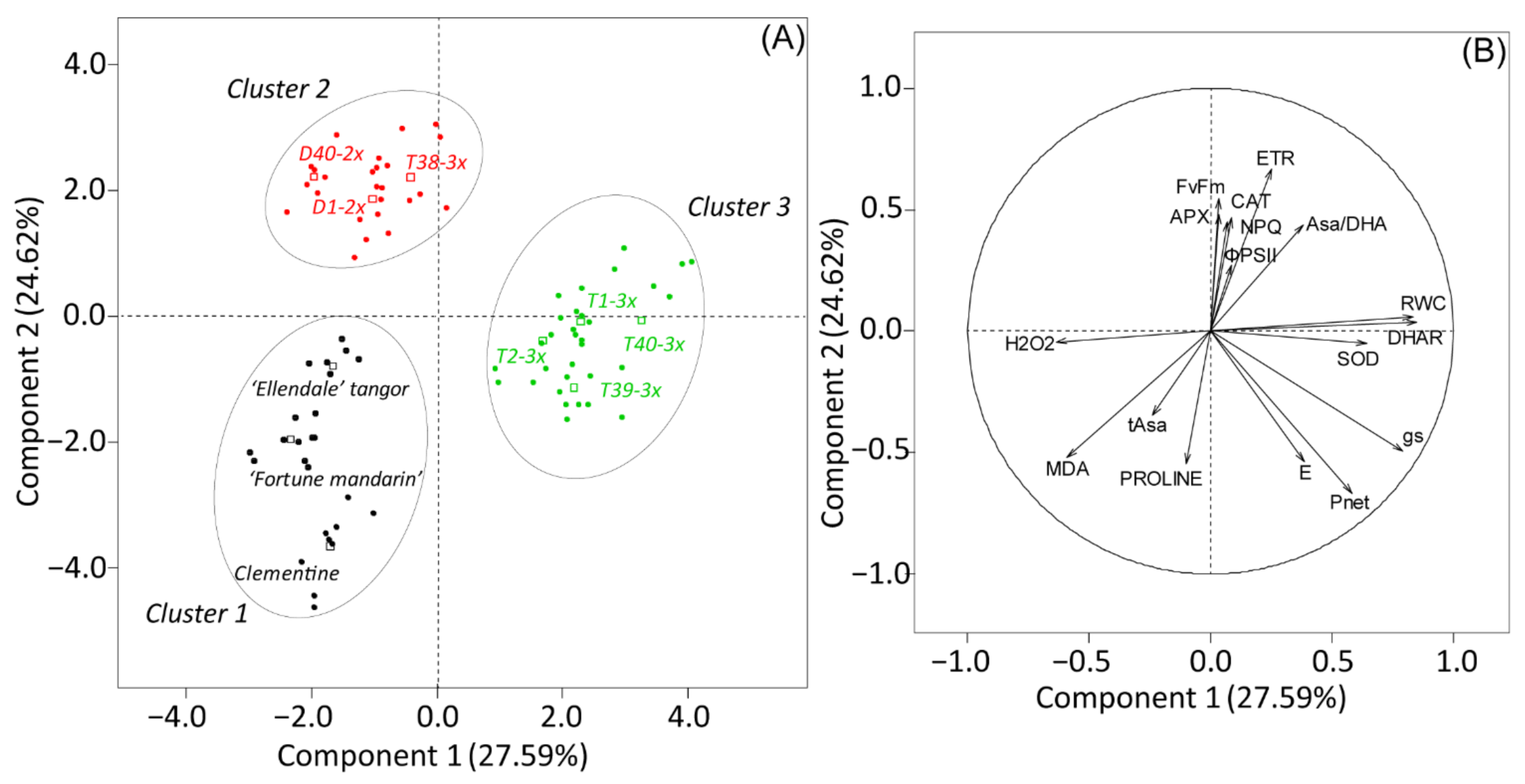
3.4. Overall Assessment of the Physiological and Biochemical Processes during Water Deficit and after Re-Watering
4. Discussion
4.1. Are Triploid Genotypes More Tolerant or Resilient to Water Deficit?
4.2. Does Osmotic Adjustment Explain the Better Sustain of Leaf Water Status in Triploid Genotypes?
4.3. Does Non-Photochemical Quenching Explain the Lower Oxidative Damage of the Photosynthetic Apparatus?
4.4. Does the Antioxidant Defence System Explain the Better Tolerance to Water Deficit in Triploid Genotypes?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambers, H.; Chapin, F.S.; Pons, T.L. Interactions Among Plants. In Plant Physiological Ecology; Lambers, H., Chapin, F.S., Pons, T.L., Eds.; Springer: New York, NY, USA, 2008; pp. 505–531. ISBN 978-0-387-78341-3. [Google Scholar]
- Core Writing Team; Pachauri, R.K.; Meyer, L.A. (Eds.) AR5 Synthesis Report: Climate Change: Synthesis Report 2014; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Dai, A. Increasing Drought under Global Warming in Observations and Models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Energy Dissipation in C3 Plants under Drought. Funct. Plant Biol. 2002, 29, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S.; Penuelas, J. Photo-and Antioxidative Protection, and a Role for Salicylic Acid during Drought and Recovery in Field-Grown Phillyrea Angustifolia Plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Arbona, V.; Flors, V.; Jacas, J.; García-Agustín, P.; Gómez-Cadenas, A. Enzymatic and Non-Enzymatic Antioxidant Responses of Carrizo Citrange, a Salt-Sensitive Citrus Rootstock, to Different Levels of Salinity. Plant Cell Physiol. 2003, 44, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Santini, J.; Giannettini, J.; Pailly, O.; Herbette, S.; Ollitrault, P.; Berti, L.; Luro, F. Comparison of Photosynthesis and Antioxidant Performance of Several Citrus and Fortunella Species (Rutaceae) under Natural Chilling Stress. Trees 2013, 27, 71–83. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed Priming and Transgenerational Drought Memory Improves Tolerance against Salt Stress in Bread Wheat. Plant Physiol. Biochem. 2017, 118, 362–369. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms Underlying Plant Resilience to Water Deficits: Prospects for Water-Saving Agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll Cycle—A Mechanism Protecting Plants against Oxidative Stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Lukić, N.; Kukavica, B.; Davidović-Plavšić, B.; Hasanagić, D.; Walter, J. Plant Stress Memory Is Linked to High Levels of Anti-Oxidative Enzymes over Several Weeks. Environ. Exp. Bot. 2020, 178, 104166. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Del Pozo, J.C.; Ramirez-Parra, E. Deciphering the Molecular Bases for Drought Tolerance in Arabidopsis Autotetraploids. Plant Cell Environ. 2014, 37, 2722–2737. [Google Scholar] [CrossRef]
- Deng, B.; Du, W.; Liu, C.; Sun, W.; Tian, S.; Dong, H. Antioxidant Response to Drought, Cold and Nutrient Stress in Two Ploidy Levels of Tobacco Plants: Low Resource Requirement Confers Polytolerance in Polyploids? Plant Growth Regul. 2012, 66, 37–47. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, Y.; Geng, X.N.; Zhang, Y.; Li, Y.; Kang, X.Y. Growth, Photosynthesis, and Reactive Oxygen System Responses of Allotriploid Populus Cathayana to Salt Stress. Photosynthetica 2020, 58, 944–950. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.-R.; Zhang, B.; Grierson, D.; Xu, C.-J.; Chen, K.-S. Transcriptomic and Metabolic Analyses Provide New Insights into Chilling Injury in Peach Fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef]
- Tan, F.-Q.; Zhang, M.; Xie, K.-D.; Fan, Y.-J.; Song, X.; Wang, R.; Wu, X.-M.; Zhang, H.-Y.; Guo, W.-W. Polyploidy Remodels Fruit Metabolism by Modifying Carbon Source Utilization and Metabolic Flux in Ponkan Mandarin (Citrus reticulata Blanco). Plant Sci. 2019, 289, 110276. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Cuenca, J.; Ollitrault, P.; Navarro, L. Extensive Citrus Triploid Hybrid Production by 2x × 4x Sexual Hybridizations and Parent-Effect on the Length of the Juvenile Phase. Plant Cell Rep. 2012, 31, 1723–1735. [Google Scholar] [CrossRef]
- Rouiss, H.; Bakry, F.; Froelicher, Y.; Navarro, L.; Aleza, P.; Ollitrault, P. Origin of C. Latifolia and C. Aurantiifolia Triploid Limes: The Preferential Disomic Inheritance of Doubled-Diploid ‘Mexican’ Lime Is Consistent with an Interploid Hybridization Hypothesis. Ann. Bot. 2018, 121, 571–585. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Z.-M.; Zhi, S.; Xu, F. Breeding Triploid Plants: A Review. Czech J. Genet. Plant Breed. 2016, 52, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Dutra de Souza, J.; de Andrade Silva, E.M.; Coelho Filho, M.A.; Morillon, R.; Bonatto, D.; Micheli, F.; da Silva Gesteira, A. Different Adaptation Strategies of Two Citrus Scion/Rootstock Combinations in Response to Drought Stress. PLoS ONE 2017, 12, e0177993. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ahmad, S.; Ali, M.A.; Ejaz, S.; Morillon, R. Better Salinity Tolerance in Tetraploid vs Diploid Volkamer Lemon Seedlings Is Associated with Robust Antioxidant and Osmotic Adjustment Mechanisms. J. Plant Physiol. 2020, 244, 153071. [Google Scholar] [CrossRef]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Martin, P.; Giannettini, J.; Berti, L.; Santini, J. Nutrient Deficiency Tolerance in Citrus Is Dependent on Genotype or Ploidy Level. Front. Plant Sci. 2019, 10, 127. [Google Scholar] [CrossRef]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Giannettini, J.; Berti, L.; Santini, J. Triploidy in Citrus Genotypes Improves Leaf Gas Exchange and Antioxidant Recovery from Water Deficit. Front. Plant Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Sivager, G.; Calvez, L.; Bruyere, S.; Boisne-Noc, R.; Brat, P.; Gros, O.; Ollitrault, P.; Morillon, R. Specific Physiological and Anatomical Traits Associated with Polyploidy and Better Detoxification Processes Contribute to Improved Huanglongbing Tolerance of the Persian Lime Compared with the Mexican Lime. Front. Plant Sci. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Ahmed, D.; Evrard, J.-C.; Ollitrault, P.; Froelicher, Y. The Effect of Cross Direction and Ploidy Level on Phenotypic Variation of Reciprocal Diploid and Triploid Mandarin Hybrids. Tree Genet. Genomes 2020, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Tomi, F.; Gibernau, M.; Giannettini, J.; Berti, L.; Santini, J. Triploid Citrus Genotypes Have a Better Tolerance to Natural Chilling Conditions of Photosynthetic Capacities and Specific Leaf Volatile Organic Compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative Hydrostatic Pressure Can Be Measured in Plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.; Weatherley, P. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Baker, N.R.; Harbinson, J.; Kramer, D.M. Determining the Limitations and Regulation of Photosynthetic Energy Transduction in Leaves. Plant Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Woollard, A.C.; Wolff, S.P. Lipid Hydroperoxide Measurement by Oxidation of Fe2+ in the Presence of Xylenol Orange. Comparison with the TBA Assay and an Iodometric Method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Stevens, R.; Page, D.; Gouble, B.; Garchery, C.; Zamir, D.; Causse, M. Tomato Fruit Ascorbic Acid Content Is Linked with Monodehydroascorbate Reductase Activity and Tolerance to Chilling Stress. Plant Cell Environ. 2008, 31, 1086–1096. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. PrometheusWiki 2011, 2011, 1–5. [Google Scholar]
- Yadollahi, A.; Arzani, K.; Ebadi, A.; Wirthensohn, M.; Karimi, S. The Response of Different Almond Genotypes to Moderate and Severe Water Stress in Order to Screen for Drought Tolerance. Sci. Hortic. 2011, 129, 403–413. [Google Scholar] [CrossRef]
- Poggi, I.; Polidori, J.J.; Gandoin, J.M.; Paolacci, V.; Battini, M.; Albertini, M.; Améglio, T.; Cochard, H. Stomatal Regulation and Xylem Cavitation in Clementine (Citrus clementina Hort) under Drought Conditions. J. Hortic. Sci. Biotechnol. 2007, 82, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Maherali, H.; Walden, A.E.; Husband, B.C. Genome Duplication and the Evolution of Physiological Responses to Water Stress. New Phytol. 2009, 184, 721–731. [Google Scholar] [CrossRef]
- Allario, T.; Brumos, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillon, R. Tetraploid Rangpur Lime Rootstock Increases Drought Tolerance via Enhanced Constitutive Root Abscisic Acid Production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.F.; Vincent, C.; Morillon, R.; Anjum, M.A.; Ahmad, S.; Hussain, S. Different Strategies Lead to a Common Outcome: Different Water-Deficit Scenarios Highlight Physiological and Biochemical Strategies of Water-Deficit Tolerance in Diploid versus Tetraploid Volkamer Lemon. Tree Physiol. 2021, 41, 2359–2374. [Google Scholar] [CrossRef]
- Lourkisti, R.; Oustric, J.; Quilichini, Y.; Froelicher, Y.; Herbette, S.; Morillon, R.; Berti, L.; Santini, J. Improved Response of Triploid Citrus Varieties to Water Deficit Is Related to Anatomical and Cytological Properties. Plant Physiol. Biochem. 2021, 162, 762–775. [Google Scholar] [CrossRef]
- Monda, K.; Araki, H.; Kuhara, S.; Ishigaki, G.; Akashi, R.; Negi, J.; Kojima, M.; Sakakibara, H.; Takahashi, S.; Hashimoto-Sugimoto, M. Enhanced Stomatal Conductance by a Spontaneous Arabidopsis Tetraploid, Me-0, Results from Increased Stomatal Size and Greater Stomatal Aperture. Plant Physiol. 2016, 170, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- De Souza, C.M.; Zorzatto, C.; Quinhones, C.G.; Lopes, J.M.L.; de Carvalho, H.H.; Araújo, W.L.; Viccini, L.F. Deciphering Ploidal Levels of Lippia Alba by Using Proteomics. Plant Physiol. Biochem. 2021, 167, 385–389. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Oustric, J.; Herbette, S.; Quilichini, Y.; Morillon, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Citrumelo 4475 Rootstocks Improve Diploid Common Clementine Tolerance to Long-Term Nutrient Deficiency. Sci. Rep. 2021, 11, 8902. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in Carotenoids, Tocopherols and Diterpenes during Drought and Recovery, and the Biological Significance of Chlorophyll Loss in Rosmarinus Officinalis Plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen Peroxide Metabolism and Functions in Plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a Positive Carbon Balance under Adverse Conditions: Responses of Photosynthesis and Respiration to Water Stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Li, W.-D.; Biswas, D.K.; Xu, H.; Xu, C.-Q.; Wang, X.-Z.; Liu, J.-K.; Jiang, G.-M. Photosynthetic Responses to Chromosome Doubling in Relation to Leaf Anatomy in Lonicera Japonica Subjected to Water Stress. Funct. Plant Biol. 2009, 36, 783–792. [Google Scholar] [CrossRef]
- Liao, T.; Wang, Y.; Xu, C.P.; Li, Y.; Kang, X.Y. Adaptive Photosynthetic and Physiological Responses to Drought and Rewatering in Triploid Populus Populations. Photosynthetica 2018, 56, 578–590. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Carter, J.E., Jr.; Patterson, R.P. Use of Relative Water Content as a Selection Tool for Drought Tolerance in Soybean. In Fide Agronomy Abstract, 77th Annual Meeting; Crop Science Society of America: Madison, WI, USA, 1985; Volume 77. [Google Scholar]
- Aparicio-Durán, L.; Gmitter, F.G., Jr.; Arjona-López, J.M.; Calero-Velázquez, R.; Hervalejo, Á.; Arenas-Arenas, F.J. Water-Stress Influences on Three New Promising HLB-Tolerant Citrus Rootstocks. Horticulturae 2021, 7, 336. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of Antioxidant Defense System Is Associated with Combined Drought and Heat Stress Tolerance in Citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef] [Green Version]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, Physiochemical and Antioxidant Responses of Maclura Pomifera to Drought Stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Fini, A.; Bellasio, C.; Pollastri, S.; Tattini, M.; Ferrini, F. Water Relations, Growth, and Leaf Gas Exchange as Affected by Water Stress in Jatropha Curcas. J. Arid Environ. 2013, 89, 21–29. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate Deficiency Can Limit Violaxanthin De-Epoxidase Activity in Vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulum, F.B.; Hadacek, F.; Hörandl, E. Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae). Biology 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Lourkisti, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Carrizo Citrange Rootstock (Citrus sinensis Osb. × Poncirus trifoliata L. Raf.) Enhances Natural Chilling Stress Tolerance of Common Clementine (Citrus clementina Hort. Ex Tan). J. Plant Physiol. 2017, 214, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of Various Drought Stresses and Subsequent Recovery on Proline, Total Soluble Sugar and Starch Metabolisms in Rice (Oryza sativa L.) Varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, C.; Hou, L.; Yang, W.; Liu, S.; Pang, X.; Li, Y. Multiple Responses Contribute to the Enhanced Drought Tolerance of the Autotetraploid Ziziphus Jujuba Mill. Var. Spinosa. Cell Biosci. 2021, 11, 119. [Google Scholar] [CrossRef]
- Arrigo, N.; Barker, M.S. Rarely Successful Polyploids and Their Legacy in Plant Genomes. Curr. Opin. Plant Biol. 2012, 15, 140–146. [Google Scholar] [CrossRef]
- Comai, L. The Advantages and Disadvantages of Being Polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.; Dong, Y.; Fan, G. Transcriptional and Post-Transcriptional Responses of Diploid and Autotetraploid Paulownia tomentosa × Paulownia fortunei under Water-Deficit Condition. Braz. J. Bot. 2019, 42, 623–641. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourkisti, R.; Froelicher, Y.; Morillon, R.; Berti, L.; Santini, J. Enhanced Photosynthetic Capacity, Osmotic Adjustment and Antioxidant Defenses Contribute to Improve Tolerance to Moderate Water Deficit and Recovery of Triploid Citrus Genotypes. Antioxidants 2022, 11, 562. https://doi.org/10.3390/antiox11030562
Lourkisti R, Froelicher Y, Morillon R, Berti L, Santini J. Enhanced Photosynthetic Capacity, Osmotic Adjustment and Antioxidant Defenses Contribute to Improve Tolerance to Moderate Water Deficit and Recovery of Triploid Citrus Genotypes. Antioxidants. 2022; 11(3):562. https://doi.org/10.3390/antiox11030562
Chicago/Turabian StyleLourkisti, Radia, Yann Froelicher, Raphaël Morillon, Liliane Berti, and Jérémie Santini. 2022. "Enhanced Photosynthetic Capacity, Osmotic Adjustment and Antioxidant Defenses Contribute to Improve Tolerance to Moderate Water Deficit and Recovery of Triploid Citrus Genotypes" Antioxidants 11, no. 3: 562. https://doi.org/10.3390/antiox11030562
APA StyleLourkisti, R., Froelicher, Y., Morillon, R., Berti, L., & Santini, J. (2022). Enhanced Photosynthetic Capacity, Osmotic Adjustment and Antioxidant Defenses Contribute to Improve Tolerance to Moderate Water Deficit and Recovery of Triploid Citrus Genotypes. Antioxidants, 11(3), 562. https://doi.org/10.3390/antiox11030562





