The Role of Nitric Oxide in Stem Cell Biology
Abstract
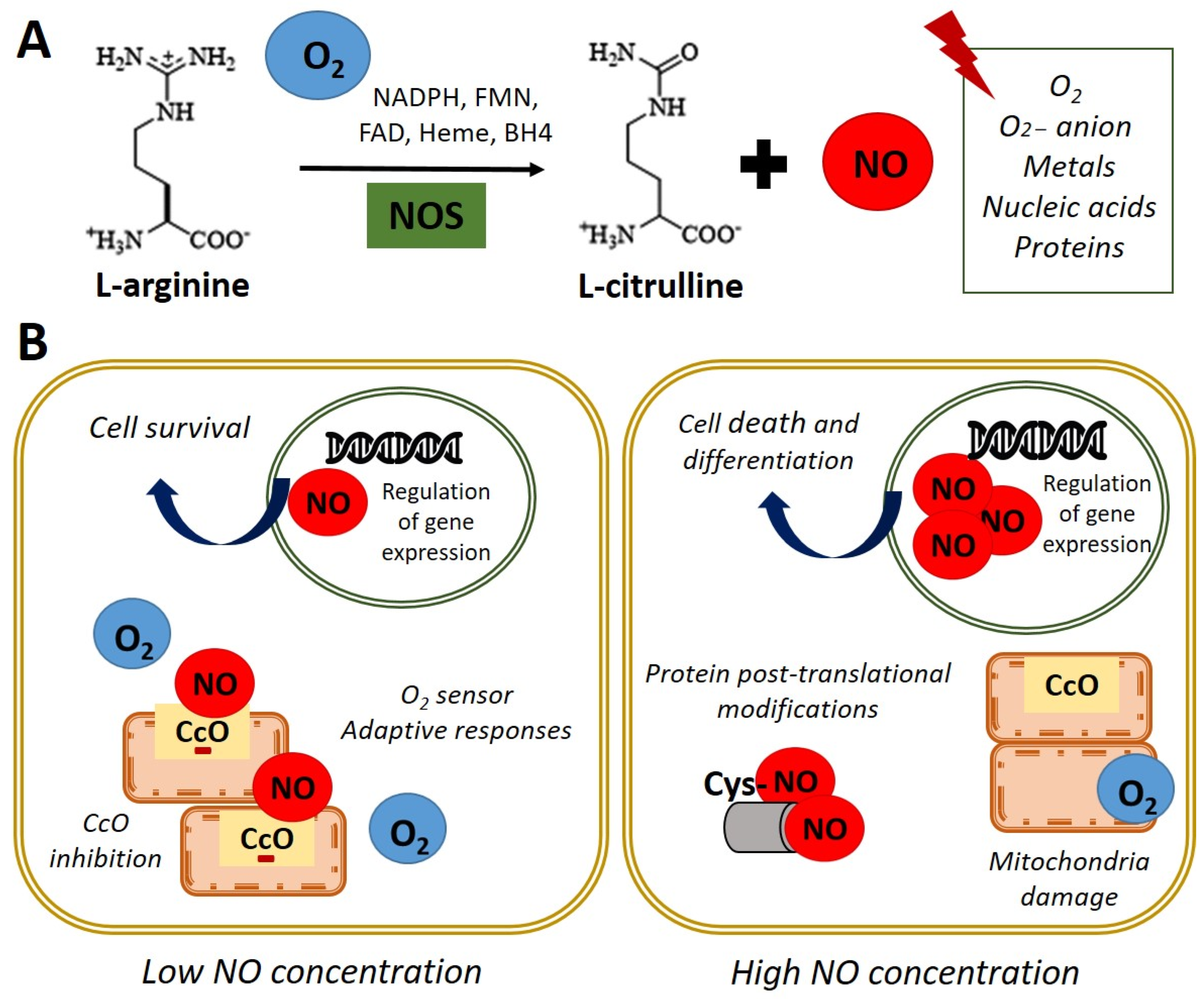
:1. Biological Functions of Nitric Oxide
2. Nitric Oxide Signaling Pathways in Stem Cell
3. Nitric Oxide in the Embryonic Development
4. Nitric Oxide and Stemness of Pluripotent Cells
4.1. Nitric Oxide and Stem Cell Differentiation
4.2. Nitric Oxide and Pluripotency
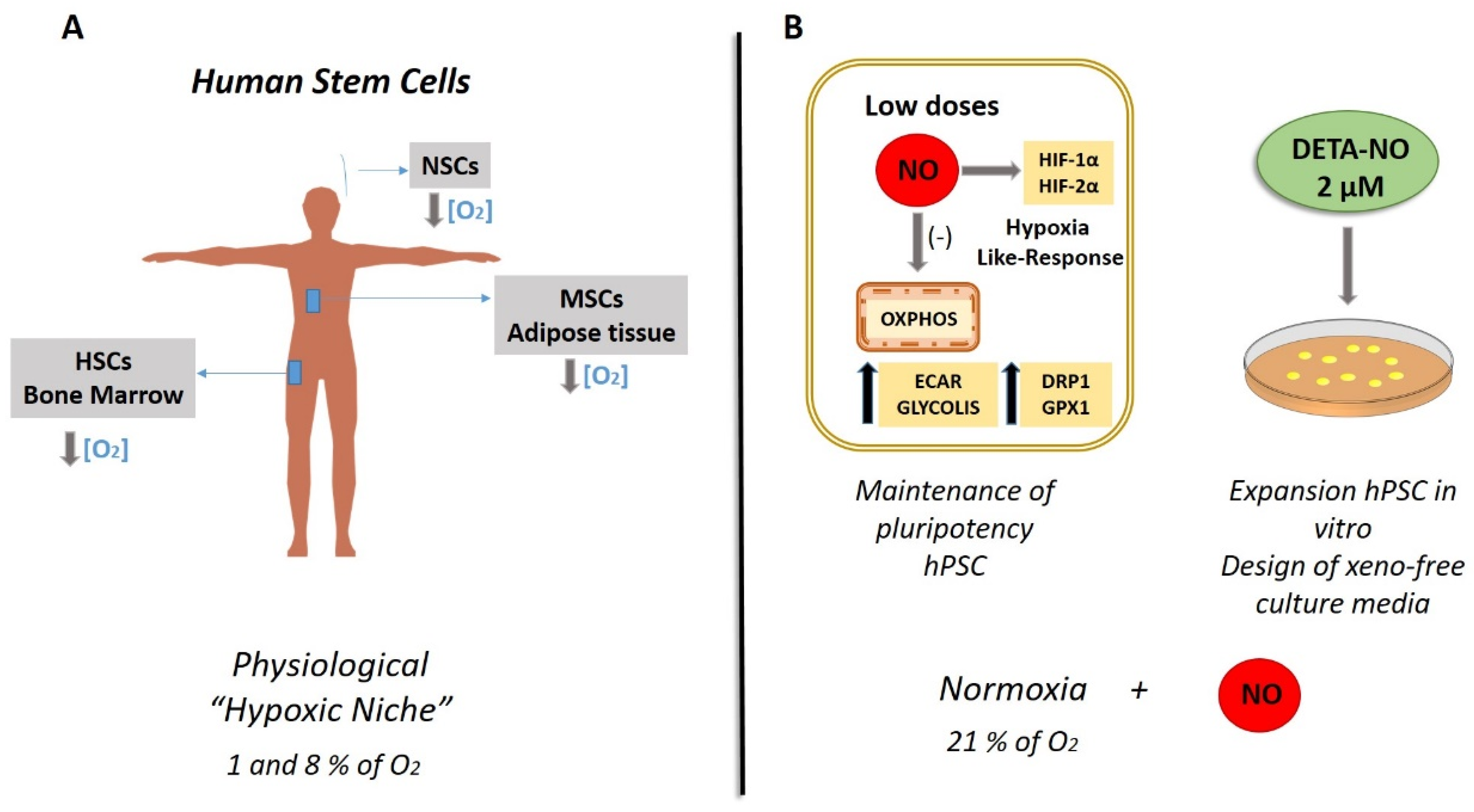
5. The Role of Nitric Oxide in Metabolic Signature in Stem Cells: Hypoxia-like Response Encouraged by Low Nitric Oxide
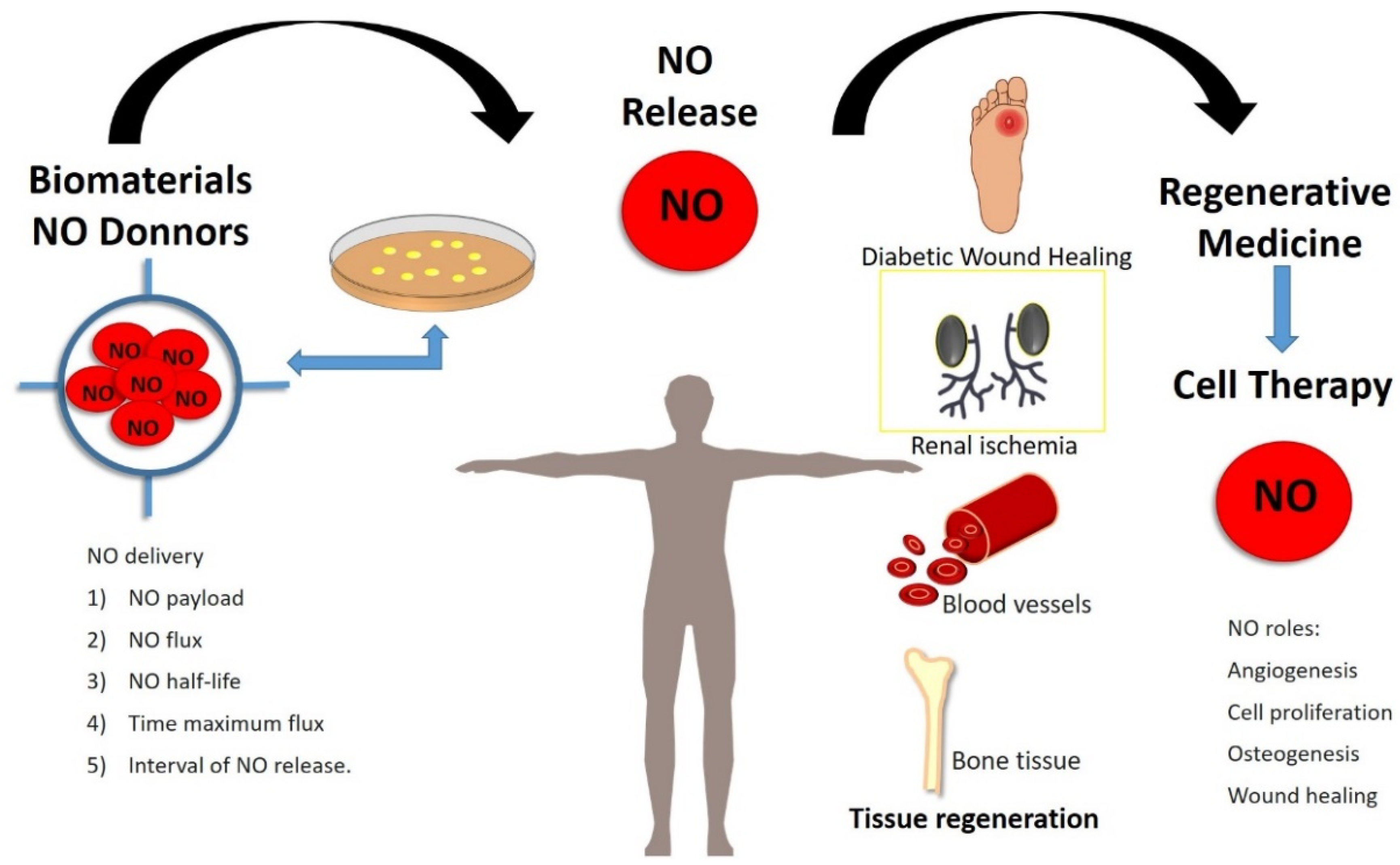
6. Exogenous Nitric Oxide in Regenerative Medicine
- NO distribution from small molecule NO donors
- NO transfer from injectable materials:
- Liposomes
- Micelles
- Dendrimers
- Silica and gold nanoparticles
- Polymer particles
- Metal–organic frameworks
- NO delivery from implantable materials
- Localized synthesis of NO using natural enzymes and synthetic prodrugs
- Enzyme mimics for conversion of endogenous prodrugs of NO
7. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Marletta, M.A. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell 1994, 78, 927–930. [Google Scholar] [CrossRef] [Green Version]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Tejedo, J.R.; Tapia-Limonchi, R.; Mora-Castilla, S.; Cahuana, G.M.; Hmadcha, A.; Martin, F.; Bedoya, F.J.; Soria, B. Low concentrations of nitric oxide delay the differentiation of embryonic stem cells and promote their survival. Cell Death Dis. 2010, 1, e80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Castilla, S.; Tejedo, J.R.; Hmadcha, A.; Cahuana, G.M.; Martín, F.; Soria, B.; Bedoya, F.J. Nitric oxide repression of Nanog promotes mouse embryonic stem cell differentiation. Cell Death Differ. 2010, 17, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Krumenacker, J.S.; Hanafy, K.A.; Murad, F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res. Bull. 2004, 62, 505–515. [Google Scholar] [CrossRef]
- Tanioka, T.; Tamura, Y.; Fukaya, M.; Shinozaki, S.; Mao, J.; Kim, M.; Shimizu, N.; Kitamura, T.; Kaneki, M. Inducible nitric-oxide synthase and nitric oxide donor decrease insulin receptor substrate-2 protein expression by promoting proteasome-dependent degradation in pancreatic beta-cells: Involvement of glycogen synthase kinase-3beta. J. Biol. Chem. 2011, 286, 29388–29396. [Google Scholar] [CrossRef] [Green Version]
- Bloch, W.; Fleischmann, B.K.; Lorke, D.E.; Andressen, C.; Hops, B.; Hescheler, J.; Addicks, K. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc. Res. 1999, 43, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994, 356, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.W.; Stamler, J.S. New insights into protein S-nitrosylation. Mitochondria as a model system. J. Biol. Chem. 2004, 279, 25891–25897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo, J.; García-Lecea, M.; Cadenas, S.; Hernández, C.; Moncada, S. Regulation of hypoxia-inducible factor-1α by nitric oxide through mitochondria-dependent and -independent pathways. Biochem. J. 2003, 376, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.; Moncada, S.; Bolaños, J.P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004, 6, 45–51. [Google Scholar] [CrossRef]
- Caballano-Infantes, E.; Díaz, I.; Hitos, A.B.; Cahuana, G.M.; Martínez-Ruiz, A.; Soria-Juan, B.; Rodríguez-Griñolo, R.; Hmadcha, A.; Martín, F.; Soria, B.; et al. Stemness of human pluripotent cells: Hypoxia-like response induced by low nitric oxide. Antioxidants 2021, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Dawson, V.L. Nitric Oxide Signaling in Neurodegeneration and Cell Death. Adv. Pharmacol. 2018, 82, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Cahuana, G.M.; Tejedo, J.R.; Hmadcha, A.; Ramírez, R.; Cuesta, A.L.; Soria, B.; Martin, F.; Bedoya, F.J. Nitric oxide mediates the survival action of IGF-1 and insulin in pancreatic β cells. Cell. Signal. 2008, 20, 301–310. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Cabrales, P.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009, 5, 865–869. [Google Scholar] [CrossRef]
- Salguero-Aranda, C.; Tapia-Limonchi, R.; Cahuana, G.M.; Hitos, A.B.; Diaz, I.; Hmadcha, A.; Fraga, M.; Martín, F.; Soria, B.; Tejedo, J.R.; et al. Differentiation of Mouse Embryonic Stem Cells toward Functional Pancreatic β-Cell Surrogates through Epigenetic Regulation of Pdx1 by Nitric Oxide. Cell Transplant. 2016, 25, 1879–1892. [Google Scholar] [CrossRef] [Green Version]
- Krumenacker, J.S.; Katsuki, S.; Kots, A.; Murad, F. Differential expression of genes involved in cGMP-dependent nitric oxide signaling in murine embryonic stem (ES) cells and ES cell-derived cardiomyocytes. Nitric Oxide Biol. Chem. 2006, 14, 1–11. [Google Scholar] [CrossRef]
- Krumenacker, J.S.; Murad, F. NO-cGMP signaling in development and stem cells. Mol. Genet. Metab. 2006, 87, 311–314. [Google Scholar] [CrossRef]
- Mujoo, K.; Krumenacker, J.S.; Murad, F. Nitric oxide-cyclic GMP signaling in stem cell differentiation. Free Radic. Biol. Med. 2011, 51, 2150–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol. Cell Physiol. 1996, 271, 1424–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef]
- Tranguch, S.; Huet-Hudson, Y. Decreased viability of nitric oxide synthase double knockout mice. Mol. Reprod. Dev. 2003, 65, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Zhao, Y.D.; Courtman, D.W.; Stewart, D.J. Abnormal Aortic Valve Development in Mice Lacking Endothelial Nitric Oxide Synthase. Circulation 2000, 101, 2345–2348. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Song, W.; Lu, X.; Hamilton, J.A.; Lei, M.; Peng, T.; Yee, S.P. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation 2002, 106, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.M.; Mistry, R.; John Challiss, R.A.; Straub, V.A. Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy. J. Neurosci. 2013, 33, 5626–5637. [Google Scholar] [CrossRef] [Green Version]
- Jisha, J.; Schwaerzer, G.K.; Kalyanaraman, H.; Cory, E.; Sah, R.L.; Li, M.; Vaida, F.; Boss, G.R.; Pilz, R.B. Soluble guanylate cyclase as a novel treatment target for osteoporosis. Endocrinology 2014, 155, 4720–4730. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, H.; Schall, N.; Pilz, R.B. Nitric oxide and cyclic GMP functions in bone. Nitric Oxide 2018, 76, 62–70. [Google Scholar] [CrossRef]
- Tesfamariam, B. Targeting heme-oxidized soluble guanylate cyclase to promote osteoblast function. Drug Discov. Today 2020, 25, 422–429. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.J.; Lee, J.W.; Lee, S.C.; Heo, J.S. Osteogenic Effect of Inducible Nitric Oxide Synthase (iNOS)-Loaded Mineralized Nanoparticles on Embryonic Stem Cells. Cell. Physiol. Biochem. 2018, 51, 746–762. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, V.; Jovanovic, A.; Lehinant, S. Soluble guanylate cyclase modulators in heart failure. Curr. Heart Fail. Rep. 2011, 8, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 225–247. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Roessig, L.; Patel, M.J.; Anstrom, K.J.; Butler, J.; Voors, A.A.; Lam, C.S.P.; Ponikowski, P.; Temple, T.; Pieske, B.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial. JACC Heart Fail. 2018, 6, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, N.; Malhi, N.; Toma, M. Impact of oral soluble guanylate cyclase stimulators in heart failure: A systematic review and Meta-analysis of randomized controlled trials. Am. Heart J. 2021, 241, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Fujimoto, S.; Hayashi, D.; Suzuki, T.; Sakaue, M.; Miyazaki, Y.; Tanaka, K.; Usami, M.; Takizawa, T. Valproic acid promotes mature neuronal differentiation of adipose tissue-derived stem cells through iNOS–NO–sGC signaling pathway. Nitric Oxide 2019, 93, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bandara, N.; Gurusinghe, S.; Kong, A.; Mitchell, G.; Wang, L.X.; Lim, S.Y.; Strappe, P. Generation of a nitric oxide signaling pathway in mesenchymal stem cells promotes endothelial lineage commitment. J. Cell. Physiol. 2019, 234, 20392–20407. [Google Scholar] [CrossRef]
- Reynaert, N.L.; Ckless, K.; Korn, S.H.; Vos, N.; Guala, A.S.; Wouters, E.F.M.; Van Der Vliet, A.; Janssen-Heininger, Y.M.W. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc. Natl. Acad. Sci. USA 2004, 101, 8945–8950. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Jiang, Y.; Hao, H.; Xia, Y.; Xu, J.; Liu, Z.; Verfaillie, C.M.; Zweier, J.L.; Liu, Z. Nitric oxide enhances Oct-4 expression in bone marrow stem cells and promotes endothelial differentiation. Eur. J. Pharmacol. 2008, 591, 59–65. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional Expression Cloning of Nanog, a Pluripotency Sustaining Factor in Embryonic Stem Cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Chanda, P.K.; Meng, S.; Lee, J.; Leung, H.E.; Chen, K.; Cooke, J.P. Nuclear S-nitrosylation defines an optimal zone for inducing pluripotency. Circulation 2019, 140, 1081–1099. [Google Scholar] [CrossRef] [PubMed]
- Peunova, N.; Scheinker, V.; Cline, H.; Enikolopov, G. Nitric oxide is an essential negative regulator of cell proliferation in Xenopus brain. J. Neurosci. 2001, 21, 8809–8818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enikolopov, G.; Banerji, J.; Kuzin, B. Nitric oxide and Drosophila development. Cell Death Differ. 1999, 6, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Tranguch, S.; Steuerwald, N.; Huet-Hudson, Y.M. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol. Reprod. 2003, 68, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Leri, A.; Kajstura, J.; Anversa, P. Role of cardiac stem cells in cardiac pathophysiology: A paradigm shift in human myocardial biology. Circ. Res. 2011, 109, 941–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, M.; Shimokawa, H.; Morishita, T.; Nakashima, Y.; Yanagihara, N. Development of genetically engineered mice lacking all three nitric oxide synthases. J. Pharmacol. Sci. 2006, 102, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Van Voorhis, B.J.; Dunn, M.S.; Snyder, G.D.; Weiner, C.P. Nitric oxide: An autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology 1994, 135, 1799–1806. [Google Scholar] [CrossRef]
- Kagabu, S.; Kodama, H.; Fukuda, J.; Karube, A.; Murata, M.; Tanaka, T. Inhibitory effects of nitric oxide on the expression and activity of aromatase in human granulosa cells. Mol. Hum. Reprod. 1999, 5, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Dineva, J.D.; Vangelov, I.M.; Nikolov, G.G.; Konakchieva, R.T.; Ivanova, M.D. Nitric oxide stimulates the production of atrial natriuretic peptide and progesteron by human granulosa luteinized cells with an antiapoptotic effect. Endocr. Regul. 2008, 42, 45–51. [Google Scholar]
- Bergandi, L.; Basso, G.; Evangelista, F.; Canosa, S.; Dalmasso, P.; Aldieri, E.; Revelli, A.; Benedetto, C.; Ghigo, D. Inducible nitric oxide synthase and heme oxygenase 1 are expressed in human cumulus cells and may be used as biomarkers of oocyte competence. Reprod. Sci. 2014, 21, 1370–1377. [Google Scholar] [CrossRef]
- Krause, B.J.; Hanson, M.A.; Casanello, P. Role of nitric oxide in placental vascular development and function. Placenta 2011, 32, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shi, J.; Chen, S.; Dong, Y.; Zhang, L.; Midgley, A.C.; Kong, D.; Wang, S. Polycaprolactone/gelatin degradable vascular grafts simulating endothelium functions modified by nitric oxide generation. Regen. Med. 2019, 14, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Jin, L.; Tian, Z.; Wang, J.; Yang, Y.; Liu, J.F.; Chen, Y.; Hu, C.H.; Chen, T.Y.; Zhao, Y.R.; et al. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer Sci. 2019, 110, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahuana, G.M.; Tejedo, J.R.; Jiménez, J.; Ramírez, R.; Sobrino, F.; Bedoya, F.J. Nitric oxide-induced carbonylation of Bcl-2, GAPDH and ANT precedes apoptotic events in insulin-secreting RINm5F cells. Exp. Cell Res. 2004, 293, 22–30. [Google Scholar] [CrossRef]
- Li, J.; Bombeck, C.A.; Yang, S.; Kim, Y.M.; Billiar, T.R. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J. Biol. Chem. 1999, 274, 17325–17333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, S.; Kim, P.K.M.; Sallam, K.; Lei, J.; Billiar, T.R.; Shears, L.L. Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 12277–12281. [Google Scholar] [CrossRef] [Green Version]
- Krischel, V.; Bruch-Gerharz, D.; Suschek, C.; Kröncke, K.D.; Ruzicka, T.; Kolb-Bachofen, V. Biphasic effect of exogenous nitric oxide on proliferation and differentiation in skin derived keratinocytes but not fibroblasts. J. Investig. Dermatol. 1998, 111, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Størling, J.; Binzer, J.; Andersson, A.K.; Züllig, R.A.; Tonnesen, M.; Lehmann, R.; Spinas, G.A.; Sandler, S.; Billestrup, N.; Mandrup-Poulsen, T. Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia 2005, 48, 2039–2050. [Google Scholar] [CrossRef] [Green Version]
- Tejedo, J.; Bernabé, J.C.; Ramírez, R.; Sobrino, F.; Bedoya, F.J. NO induces a cGMP-independent release of cytochrome c from mitochondria which precedes caspase 3 activation in insulin producing RINm5F cells. FEBS Lett. 1999, 459, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Vodovotz, Y.; Kim, P.K.M.; Bagci, E.Z.; Ermentrout, G.B.; Chow, C.C.; Bahar, I.; Billiar, T.R. Inflammatory Modulation of Hepatocyte Apoptosis by Nitric Oxide: In Vivo, In Vitro, and In Silico Studies. Curr. Mol. Med. 2005, 4, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Tejedo, J.R.; Cahuana, G.M.; Ramírez, R.; Esbert, M.; Jiménez, J.; Sobrino, F.; Bedoya, F.J. nitric oxide triggers the phosphatidylinositol 3-kinase/Akt survival pathway in insulin-producing RINm5F cells by arousing Src to activate insulin receptor substrate-1. Endocrinology 2004, 145, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhang, K.; Zhang, S.; Wang, D.; Han, Z.; Che, Y.; Kong, D.; Zhao, Q.; Han, Z.; He, Z.X.; et al. Nitric oxide releasing hydrogel promotes endothelial differentiation of mouse embryonic stem cells. Acta Biomater. 2017, 63, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Limonchi, R.; Cahuana, G.M.; Caballano-Infantes, E.; Salguero-Aranda, C.; Beltran-Povea, A.; Hitos, A.B.; Hmadcha, A.; Martin, F.; Soria, B.; Bedoya, F.J.; et al. Nitric Oxide Prevents Mouse Embryonic Stem Cell Differentiation through Regulation of Gene Expression, Cell Signaling and Control of Cell Proliferation. J. Cell Biochem. 2016, 117, 2078–2088. [Google Scholar] [CrossRef]
- Chung, H.T.; Pae, H.O.; Choi, B.M.; Billiar, T.R.; Kim, Y.M. Nitric Oxide as a Bioregulator of Apoptosis. Biochem. Biophys. Res. Commun. 2001, 282, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Dylla, S.J.; Muijtjens, M.; Weissman, I.L. Enforced Bcl-2 expression overrides serum and feeder cell requirements for mouse embryonic stem cell self-renewal. Proc. Natl. Acad. Sci. USA 2005, 102, 3312–3317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Pan, Q.; Zhao, W.; Wu, X.; Yu, S.; Shen, Q.; Zhang, J.; Yue, W.; Peng, S.; Li, N.; et al. BCL2 enhances survival of porcine pluripotent stem cells through promoting FGFR2. Cell Prolif. 2021, 54, e12932. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; De Vera, M.E.; Watkins, S.C.; Billiar, T.R. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-α-induced apoptosis by inducing heat shock protein 70 expression. J. Biol. Chem. 1997, 272, 1402–1411. [Google Scholar] [CrossRef] [Green Version]
- Mannick, J.B.; Miao, X.Q.; Stamler, J.S. Nitric oxide inhibits Fas-induced apoptosis. J. Biol. Chem. 1997, 272, 24125–24128. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.C.; Fiscus, R.R. Essential roles of the nitric oxide (no)/cGMP/protein kinase G type-Iα (PKG-Iα) signaling pathway and the atrial natriuretic peptide (ANP)/cGMP/PKG-Iα autocrine loop in promoting proliferation and cell survival of OP9 bone marrow stromal cells. J. Cell. Biochem. 2011, 112, 829–839. [Google Scholar] [CrossRef]
- Parmar, K.; Mauch, P.; Vergilio, J.A.; Sackstein, R.; Down, J.D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. USA 2007, 104, 5431–5436. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, J.; Zhou, W.; Xing, Y.; Sperber, H.; Ferreccio, A.; Agoston, Z.; Kuppusamy, K.T.; Moon, R.T.; Ruohola-Baker, H. Hypoxia-Inducible Factors Have Distinct and Stage-Specific Roles during Reprogramming of Human Cells to Pluripotency. Cell Stem Cell 2014, 14, 592–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, J.; Zhang, Z.; Nelson, A.; Lamba, D.A.; Reh, T.A.; Ware, C.; Ruohola-Baker, H. Hypoxia induces re-entry of committed cells into pluripotency. Stem Cells 2013, 31, 1737–1748. [Google Scholar] [CrossRef] [Green Version]
- Takubo, K.; Goda, N.; Yamada, W.; Iriuchishima, H.; Ikeda, E.; Kubota, Y.; Shima, H.; Johnson, R.S.; Hirao, A.; Suematsu, M.; et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010, 7, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezashi, T.; Das, P.; Roberts, R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4783–4788. [Google Scholar] [CrossRef] [Green Version]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Takahashi, K.; Okita, K.; Ichisaka, T.; Yamanaka, S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 2009, 5, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Bayat-Mokhtari, R.; Tsui, M.; Lotfi, S.; Tsuchida, R.; Felsher, D.W.; Yeger, H. HIF-2α suppresses p53 to enhance the stemness and regenerative potential of human embryonic stem cells. Stem Cells 2012, 30, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Folmes, C.D.L.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.M.; Kwon, S.; Pak, Y.K.; Seol, H.W.; Choi, Y.M.; Park, D.J.; Park, K.S.; Lee, H.K. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2006, 348, 1472–1478. [Google Scholar] [CrossRef]
- Gu, H.; Huang, X.; Xu, J.; Song, L.; Liu, S.; Zhang, X.B.; Yuan, W.; Li, Y. Optimizing the method for generation of integration-free induced pluripotent stem cells from human peripheral blood. Stem Cell Res. Ther. 2018, 9, 163. [Google Scholar] [CrossRef]
- Morrison, S.J.; Csete, M.; Groves, A.K.; Melega, W.; Wold, B.; Anderson, D.J. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000, 20, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Danet, G.H.; Pan, Y.; Luongo, J.L.; Bonnet, D.A.; Simon, M.C. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Investig. 2003, 112, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garita-Hernández, M.; Diaz-Corrales, F.; Lukovic, D.; González-Guede, I.; Diez-Lloret, A.; Valdés-Sánchez, M.L.; Massalini, S.; Erceg, S.; Bhattacharya, S.S. Hypoxia increases the yield of photoreceptors differentiating from mouse embryonic stem cells and improves the modeling of retinogenesis in vitro. Stem Cells 2013, 31, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Tóth, A.; Méhes, G.; Trencsényi, G.; Paragh, G.; Jeney, V. Hypoxia Triggers Osteochondrogenic Differentiation of Vascular Smooth Muscle Cells in an HIF-1 (Hypoxia-Inducible Factor 1)-Dependent and Reactive Oxygen Species-Dependent Manner. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1088–1099. [Google Scholar] [CrossRef]
- Bapat, A.; Schippel, N.; Shi, X.; Jasbi, P.; Gu, H.; Kala, M.; Sertil, A.; Sharma, S. Hypoxia promotes erythroid differentiation through the development of progenitors and proerythroblasts. Exp. Hematol. 2021, 97, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Sandau, K.B.; Fandrey, J.; Brüne, B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood 2001, 97, 1009–1015. [Google Scholar] [CrossRef]
- Sandau, K.B.; Faus, H.G.; Brüne, B. Induction of hypoxia-inducible-factor 1 by nitric oxide is mediated via the PI 3K pathway. Biochem. Biophys. Res. Commun. 2000, 278, 263–267. [Google Scholar] [CrossRef]
- Knowles, H.J.; Mole, D.R.; Ratcliffe, P.J.; Harris, A.L. Normoxic stabilization of hypoxia-inducible factor-1alpha by modulation of the labile iron pool in differentiating U937 macrophages: Effect of natural resistance-associated macrophage protein 1. Cancer Res. 2006, 66, 2600–2607. [Google Scholar] [CrossRef] [Green Version]
- Agani, F.H.; Puchowicz, M.; Chavez, J.C.; Pichiule, P.; Lamanna, J. Role of nitric oxide in the regulation of HIF-1alpha expression during hypoxia. Am. J. Physiol. Cell Physiol. 2002, 283, C178–C186. [Google Scholar] [CrossRef] [Green Version]
- Qingquan, C.; Wenlan, L.; Xi, S.; Jian, L.K.; Rong, P.; Qingquan, C.; Wenlan, L.; Xi, S.; Jian, L.K.; Rong, P. Endogenous reactive oxygen species and nitric oxide have opposite roles in regulating HIF-1alpha expression in hypoxic astrocytes. Biophys. Rep. 2021, 7, 239–249. [Google Scholar] [CrossRef]
- Covello, K.L.; Kehler, J.; Yu, H.; Gordan, J.D.; Arsham, A.M.; Hu, C.J.; Labosky, P.A.; Simon, M.C.; Keith, B. HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006, 20, 557–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Ruan, H.; Himmati, F.; Zhao, M.T.; Chen, C.C.; Makar, M.; Chen, I.Y.; Sallam, K.; Mocarski, E.S.; Sayed, D.; et al. HIF1α Regulates Early Metabolic Changes due to Activation of Innate Immunity in Nuclear Reprogramming. Stem Cell Rep. 2020, 14, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.R.; Calder, P.C.; Houghton, F.D. GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic culture of human embryonic stem cells. Sci. Rep. 2015, 5, 17500. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jang, H.; Kim, T.W.; Kang, B.H.; Lee, S.E.; Jeon, Y.K.; Chung, D.H.; Choi, J.; Shin, J.; Cho, E.J.; et al. Core Pluripotency Factors Directly Regulate Metabolism in Embryonic Stem Cell to Maintain Pluripotency. Stem Cells 2015, 33, 2699–2711. [Google Scholar] [CrossRef]
- Tengan, C.H.; Moraes, C.T. NO control of mitochondrial function in normal and transformed cells. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 573–581. [Google Scholar] [CrossRef]
- Prieto, J.; León, M.; Ponsoda, X.; Sendra, R.; Bort, R.; Ferrer-Lorente, R.; Raya, A.; López-Garciá, C.; Torres, J. Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nat. Commun. 2016, 7, 11124. [Google Scholar] [CrossRef]
- Cui, P.; Zhang, P.; Yuan, L.; Wang, L.; Guo, X.; Cui, G.; Zhang, Y.; Li, M.; Zhang, X.; Li, X.; et al. HIF-1α Affects the Neural Stem Cell Differentiation of Human Induced Pluripotent Stem Cells via MFN2-Mediated Wnt/β-Catenin Signaling. Front. Cell Dev. Biol. 2021, 9, 1624. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Liu, Z.S.; Wang, J.; Wang, H.X.; Li, A.; Yang, Y.; Wang, X.Z.; Zhao, Y.Q.; Han, Q.Y.; Cai, H.; et al. Glutathione peroxidase-1 is required for self-renewal of murine embryonic stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 454–460. [Google Scholar] [CrossRef]
- Palmer, L.A.; Gaston, B.; Johns, R.A. Normoxic Stabilization of Hypoxia-Inducible Factor-1 Expression and Activity: Redox-Dependent Effect of Nitrogen Oxides. Mol. Pharmacol. 2000, 58, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Metzen, E.; Zhou, J.; Jelkmann, W.; Fandrey, J.; Brüne, B. Nitric Oxide Impairs Normoxic Degradation of HIF-1α by Inhibition of Prolyl Hydroxylases. Mol. Biol. Cell 2003, 14, 3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Su, K.; Yang, X.; Bowe, D.B.; Paterson, A.J.; Kudlow, J.E. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 2003, 115, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Tsihlis, N.D.; Kapadia, M.R.; Vavra, A.K.; Jiang, Q.; Fu, B.; Martinez, J.; Kibbe, M.R. Nitric oxide decreases activity and levels of the 11S proteasome activator PA28 in the vasculature. Nitric Oxide Biol. Chem. 2012, 27, 50–58. [Google Scholar] [CrossRef]
- Valek, L.; Heidler, J.; Scheving, R.; Wittig, I.; Tegeder, I. Nitric oxide contributes to protein homeostasis by S-nitrosylations of the chaperone HSPA8 and the ubiquitin ligase UBE2D. Redox Biol. 2019, 20, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.C.; Wei, Y.; Li, Z.; Kong, D.; Zhao, Q. Nitric-Oxide-Releasing Biomaterial Regulation of the Stem Cell Microenvironment in Regenerative Medicine. Adv. Mater. 2020, 32, 1805818. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as stem cell regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef] [Green Version]
- Shabani, M.; Pulfer, S.K.; Bulgrin, J.P.; Smith, D.J. Enhancement of wound repair with a topically applied nitric oxide-releasing polymer. Wound Repair Regen. 1996, 4, 353–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, K.; Chen, B.; Zhou, S.; Li, N.; Liu, C.; Yang, J.; Lin, R.; Zhang, T.; He, W. A polyethylenimine-based diazeniumdiolate nitric oxide donor accelerates wound healing. Biomater. Sci. 2019, 7, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Klein-Nulend, J.; Van Oers, R.F.M.; Bakker, A.D.; Bacabac, R.G. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos. Int. 2014, 25, 1427–1437. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Paniagua, A.; Diamond, C.; Ferlauto, H.R.; Fernandez-Moure, J.S. Nanomaterial Nitric Oxide Delivery in Traumatic Orthopedic Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 8, 1512. [Google Scholar] [CrossRef]
- Armour, K.E.; Armour, K.J.; Gallagher, M.E.; Gödecke, A.; Helfrich, M.H.; Reid, D.M.; Ralston, S.H. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 2001, 142, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.B.; Barbul, A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef]
- Damoulis, P.D.; Drakos, D.E.; Gagari, E.; Kaplan, D.L. Osteogenic Differentiation of Human Mesenchymal Bone Marrow Cells in Silk Scaffolds Is Regulated by Nitric Oxide. Ann. N. Y. Acad. Sci. 2007, 1117, 367–376. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Guo, L.; Su, Y.; Wen, J.; Du, J.; Li, X.; Liu, Y.; Feng, J.; Xie, Y.; et al. Nitric oxide balances osteoblast and adipocyte lineage differentiation via the JNK/MAPK signaling pathway in periodontal ligament stem cells. Stem Cell Res. Ther. 2018, 9, 118. [Google Scholar] [CrossRef]
- Moon, C.Y.; Nam, O.H.; Kim, M.; Lee, H.S.; Kaushik, S.N.; Walma, D.A.C.; Jun, H.W.; Cheon, K.; Choi, S.C. Effects of the nitric oxide releasing biomimetic nanomatrix gel on pulp-dentin regeneration: Pilot study. PLoS ONE 2018, 13, e0205534. [Google Scholar] [CrossRef] [Green Version]
- Sonoda, S.; Mei, Y.F.; Atsuta, I.; Danjo, A.; Yamaza, H.; Hama, S.; Nishida, K.; Tang, R.; Kyumoto-Nakamura, Y.; Uehara, N.; et al. Exogenous nitric oxide stimulates the odontogenic differentiation of rat dental pulp stem cells. Sci. Rep. 2018, 8, 3419. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Qin, K.; Wu, Y.; Tian, Y.; Wang, J.; Zhang, J.; Hou, J.; Cui, Y.; Wang, K.; et al. Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Control. Release 2015, 210, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Brouki Milan, P.; Zamanian, A.; Heying, R.; Mozafari, M. Nitric oxide-releasing vascular grafts: A therapeutic strategy to promote angiogenic activity and endothelium regeneration. Acta Biomater. 2019, 92, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Qi, P.; Liu, J.; Yang, Y.; Tan, X.; Xiao, Y.; Maitz, M.F.; Huang, N.; Yang, Z. Biomimetic engineering endothelium-like coating on cardiovascular stent through heparin and nitric oxide-generating compound synergistic modification strategy. Biomaterials 2019, 207, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; Forrester, M.T.; Hess, D.T.; Stamler, J.S. S-Nitrosylation in Cardiovascular Signaling. Circ. Res. 2010, 106, 633. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Hou, J.; Qian, M.; Jin, D.; Hao, T.; Pan, Y.; Wang, H.; Wu, S.; Liu, S.; Wang, F.; et al. Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat. Commun. 2021, 12, 4501. [Google Scholar] [CrossRef]
- Farrell, K.; Simmers, P.; Mahajan, G.; Boytard, L.; Camardo, A.; Joshi, J.; Ramamurthi, A.; Pinet, F.; Kothapalli, C.R. Alterations in phenotype and gene expression of adult human aneurysmal smooth muscle cells by exogenous nitric oxide. Exp. Cell Res. 2019, 384, 111589. [Google Scholar] [CrossRef] [Green Version]
- Pálóczi, J.; Varga, Z.V.; Apáti, Á.; Szebényi, K.; Sarkadi, B.; Madonna, R.; De Caterina, R.; Csont, T.; Eschenhagen, T.; Ferdinandy, P.; et al. Exogenous Nitric Oxide Protects Human Embryonic Stem Cell-Derived Cardiomyocytes against Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 4298945. [Google Scholar] [CrossRef]
- Tang, Y.L.; Tang, Y.; Zhang, Y.C.; Qian, K.; Shen, L.; Phillips, M.I. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005, 46, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Marino, F.; Scalise, M.; Cianflone, E.; Salerno, L.; Cappetta, D.; Salerno, N.; De Angelis, A.; Torella, D.; Urbanek, K. Physical exercise and cardiac repair: The potential role of nitric oxide in boosting stem cell regenerative biology. Antioxidants 2021, 10, 1002. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Xiong, K.; Wang, J.; Lee, H.; Huang, N. Metal-Phenolic Surfaces for Generating Therapeutic Nitric Oxide Gas. Chem. Mater. 2018, 30, 5220–5226. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, D.; Oh, Y.; Heo, J.; Hong, J. A Nanocoating Co-localizing Nitric Oxide and Growth Factor onto Individual Endothelial Cells Reveals Synergistic Effects on Angiogenesis. Adv. Healthc. Mater. 2021, 2102095. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.T.; Weller, R.B.; Paganotti, A.; Seabra, A.B. Delivering nitric oxide into human skin from encapsulated S-nitrosoglutathione under UV light: An in vitro and ex vivo study. Nitric Oxide Biol. Chem. 2020, 94, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Najafi, H.; Abolmaali, S.S.; Heidari, R.; Valizadeh, H.; Jafari, M.; Tamaddon, A.M.; Azarpira, N. Nitric oxide releasing nanofibrous Fmoc-dipeptide hydrogels for amelioration of renal ischemia/reperfusion injury. J. Control. Release 2021, 337, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, S.; Xin, X.; Li, P.; Dou, J.; Han, X.; Kang, I.K.; Yuan, J.; Chi, B.; Shen, J. S-nitrosated keratin composite mats with NO release capacity for wound healing. Chem. Eng. J. 2020, 400, 125964. [Google Scholar] [CrossRef]
| Sections | Main Findings | References |
|---|---|---|
| Section 1. Biological Functions of Nitric Oxide | NO’s biosynthesis NO’s functions in cells | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] |
| Section 2. Nitric Oxide Signaling Pathways in Stem Cell | Molecular mechanism underlying NO role in stem cell NO-cGMP pathway NO and posttranslational modifications | [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| Section 3. Nitric Oxide in the Embryonic Development | NO in embryogenesis NO role in oocytes maturation | [42,43,44,45,46,47,48,49,50,51] |
| Section 4. Nitric Oxide and Stemness of Pluripotent Cells Section 4.1. Nitric Oxide and Stem Cell Differentiation Section 4.2. Nitric Oxide and Pluripotency | The dual role of NO in stemness. High NO doses induce cell differentiation Low NO doses maintain pluripotency | [5,6,14,16,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] |
| Section 5. The Role of Nitric Oxide in Metabolic Signature in Stem Cells: Hypoxia-like Response Encouraged by Low Nitric Oxide | NO as a hypoxic mimetic in stem cells under physiological conditions | [14,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] |
| Section 6. Exogenous Nitric Oxide in Regenerative Medicine | Recent advances in NO applications in the tissue bioengineered field: wound healing, bone regeneration and cardiovascular disease. Novel biomaterials to control delivery of NO in situ | [106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballano-Infantes, E.; Cahuana, G.M.; Bedoya, F.J.; Salguero-Aranda, C.; Tejedo, J.R. The Role of Nitric Oxide in Stem Cell Biology. Antioxidants 2022, 11, 497. https://doi.org/10.3390/antiox11030497
Caballano-Infantes E, Cahuana GM, Bedoya FJ, Salguero-Aranda C, Tejedo JR. The Role of Nitric Oxide in Stem Cell Biology. Antioxidants. 2022; 11(3):497. https://doi.org/10.3390/antiox11030497
Chicago/Turabian StyleCaballano-Infantes, Estefanía, Gladys Margot Cahuana, Francisco Javier Bedoya, Carmen Salguero-Aranda, and Juan R. Tejedo. 2022. "The Role of Nitric Oxide in Stem Cell Biology" Antioxidants 11, no. 3: 497. https://doi.org/10.3390/antiox11030497
APA StyleCaballano-Infantes, E., Cahuana, G. M., Bedoya, F. J., Salguero-Aranda, C., & Tejedo, J. R. (2022). The Role of Nitric Oxide in Stem Cell Biology. Antioxidants, 11(3), 497. https://doi.org/10.3390/antiox11030497







