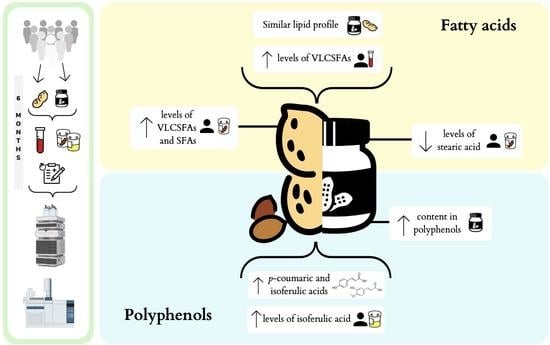
Effect of Crushing Peanuts on Fatty Acid and Phenolic Bioaccessibility: A Long-Term Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Anthropometric, Clinical, and Biochemical Measurements
2.4. Dietary Intake and Physical Activity
2.5. Determination of Fatty Acids in Peanut Products, Plasma and Feces
2.5.1. Reagents and Standards
2.5.2. Sample Preparation
2.5.3. Chromatographic Analysis
2.6. Determination of Phenolic Compounds in Peanut Products and Urine
2.6.1. Extraction and Quantification of Phenolic Compounds from Peanut Products
Reagents and Standards
Sample Preparation
Chromatographic Analysis
2.6.2. Extraction and Quantification of Phenolic Acids in Urine
Reagents and Standards
Sample Preparation
Chromatographic Analysis
2.7. Statistical Analyses
3. Results and Discussion
3.1. Participant Characteristics
3.2. Dietary Intake and Physical Activity
3.3. Fatty Acid Profile in Whole Peanuts and Peanut Butter
3.4. Fatty Acid Bioavailability in Participants
3.4.1. Plasma Fatty Acids
3.4.2. Fecal Fatty Acids
3.5. Polyphenol Composition of Whole Peanuts and Peanut Butter
3.6. Polyphenol Bioavailability in Participants
Urinary Polyphenols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Whole Peanuts (25 g) | Peanut Butter (32 g) | |
|---|---|---|
| Energy (kcal) | 147 | 190 |
| Protein (g) | 6.25 | 8 |
| Carbohydrate (g) | 5.33 | 6.08 |
| Fiber (g) | 2.05 | 2.24 |
| Fat (g) | 15.40 | 18.97 |
| Nutrient Intake | Whole Peanuts | Peanut Butter | p-Value WP vs. PB | ||
|---|---|---|---|---|---|
| Baseline | Post- intervention | Baseline | Post- Intervention | ||
| Energy (kcal/day) | 2771 ± 594 | 1663 ± 499 | 2706 ± 602 | 2669 ± 478 | 0.816 |
| Carbohydrates (g/day) | 257 ± 80.74 | 238 ± 65.18 | 241 ± 73.92 | 226 ± 53.41 | 0.658 |
| Fiber (g/day) | 45.17 ± 21.95 | 43.80 ± 18.22 | 42.12 ± 42.12 | 40.56 ± 10.07 | 0.925 |
| Protein (g/day) | 104 ± 29.43 | 106 ± 26.77 | 110 ± 31.86 | 111 ± 24.14 | 0.442 |
| Total fat (g/day) | 145 ± 29.17 | 146 ± 28.43 | 142 ± 35.35 | 151 ± 31.06 | 0.963 |
| SFAs (g/day) | 37.62 ± 10.00 | 36.76 ± 10.62 | 38.18 ± 11.05 | 37.28 ± 10.71 | 0.834 |
| MUFAs (g/day) | 67.76 ± 15.90 | 70.37 ± 16.12 | 69.06 ± 17.18 | 69.73 ± 15.96 | 0.899 |
| PUFAs (g/day) | 25.91 ± 6.77 | 22.45 ± 4.80 | 23.99 ± 7.25 | 21.90 ± 4.87 | 0.926 |
| Food intake | |||||
| Fruits (g/day) | 315 ± 156 | 293 ± 227 | 295 ± 160 | 237 ± 133 | 0.330 |
| Vegetables (g/day) | 291 ± 127 | 292 ± 109 | 290 ± 89.17 | 303 ± 101 | 0.499 |
| Dairy products (g/day) | 259 ± 178 | 241 ± 177 | 266 ± 153 | 226 ± 138 | 0.968 |
| Olive oil (g/day) | 42.86 ± 20.89 | 50.00 ± 14.92 | 46.09 ± 14.53 | 43.11 ± 14.60 | 0.064 |
| Nuts (g/day) | 19.79 ± 17.97 | 0.20 ± 0.40 * | 19.68 ± 14.96 | 0.34 ± 0.98 * | 0.267 |
| Cereals (g/day) | 165 ± 102 | 148 ± 74.49 | 155 ± 90.61 | 132 ± 70.78 | 0.456 |
| Legumes (g/day) | 40.03 ± 33.00 | 39.46 ± 28.88 | 33.59 ± 17.40 | 28.57 ± 16.53 | 0.190 |
| Meat (g/day) | 82.62 ± 56.19 | 81.24 ± 55.10 | 113 ± 70.44 | 96.49 ± 49.36 | 0.366 |
| White meat (g/day) | 34.76 ± 26.85 | 38.98 ± 29.58 | 52.30 ± 27.34 | 49.94 ± 28.04 | 0.263 |
| Red meat (g/day) | 47.86 ± 35.78 | 42.26 ± 34.41 | 60.83 ± 57.14 | 46.55 ± 36.35 | 0.713 |
| Eggs (g/day) | 28.15 ± 16.15 | 31.22 ± 16.04 | 45.65 ± 61.76 | 29.25 ± 14.02 | 0.780 |
| White fish (g/day) | 23.21 ± 26.81 | 19.84 ± 20.43 | 20.76 ± 20.74 | 19.67 ± 19.48 | 0.957 |
| Blue fish (g/day) | 16.04 ± 17.37 | 21.49 ± 16.52 | 18.42 ± 19.87 | 20.02 ± 21.32 | 0.303 |
| Physical activity (METs/week) | 4850 ± 2124 | 3269 ± 1613 * | 4704 ± 2382 | 3736 ± 1838 | 0.415 |
References
- Ros, E.; Hu, F.B. Consumption of Plant Seeds and Cardiovascular Health: Epidemiologic and Clinical Trial Evidence Emilio. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M. Consumption of Tree Nuts Worldwide in 2018. Available online: https://www.statista.com/statistics/1030815/tree-nut-global-consumption-by-type/ (accessed on 20 July 2021).
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in Peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Paz-Graniel, I.; Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Liu, X.; Malik, V.S.; Sun, Q.; Willett, W.C.; Manson, J.A.E.; Rexrode, K.M.; Li, Y.; Hu, F.B.; Bhupathiraju, S.N. Nut Consumption and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2017, 70, 2519–2532. [Google Scholar] [CrossRef]
- Jones, J.B.; Provost, M.; Keaver, L.; Breen, C.; Ludy, M.J.; Mattes, R.D. A randomized trial on the effects of flavorings on the health benefits of daily peanut consumption. Am. J. Clin. Nutr. 2014, 99, 490–496. [Google Scholar] [CrossRef]
- Amba, V.; Murphy, G.; Etemadi, A.; Wang, S.; Abnet, C.C.; Hashemian, M. Nut and peanut butter consumption and mortality in the national institutes of health-AARP diet and health study. Nutrients 2019, 11, 1508. [Google Scholar] [CrossRef]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of Nut Consumption with Total and Cause-Specific Mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef]
- Hashemian, M.; Murphy, G.; Etemadi, A.; Dawsey, S.M.; Liao, L.M.; Abnet, C.C. Nut and peanut butter consumption and the risk of esophageal and gastric cancer subtypes. Am. J. Clin. Nutr. 2017, 106, 858–864. [Google Scholar] [CrossRef]
- González, C.A.; Salas-Salvadó, J. The potential of nuts in the prevention of cancer. Br. J. Nutr. 2006, 96 (Suppl. 2), S87–S94. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Lamarche, B.; Banach, M.S.; Srichaikul, K.; Vidgen, E.; Mitchell, S.; Parker, T.; Nishi, S.; Bashyam, B.; et al. Correction to: Nuts as a replacement for carbohydrates in the diabetic diet: A reanalysis of a randomised controlled trial (Diabetologia, (2019), 10.1007/s00125-018-4628-9). Diabetologia 2019, 62, 549–552. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Ojo, O.; Wang, L.L.; Wang, Q.; Jiang, Q.; Shao, X.Y.; Wang, X.H. A randomized controlled trial to compare the effect of peanuts and almonds on the cardio-metabolic and inflammatory parameters in patients with type 2 diabetes mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E. Qualified Health Claims: Letter of Enforcement Discretion—Nuts and Coronary Heart Disease. Available online: https://www.weightwatchers.com/images/1033/dynamic/articles/2010/03/FDA_Approved_Qualified_Health_Claim_on_Nuts.pdf (accessed on 8 August 2021).
- Toomer, O.T. Nutritional chemistry of the peanut (Arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.; Oda, K.; Ros, E. Nut consumption and blood lipid levels: A pooled analysis of 25 intervention trials. Arch. Intern. Med. 2010, 170, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Pelkman, C.L.; Fishell, V.K.; Maddox, D.H.; Pearson, T.A.; Mauger, D.T.; Kris-Etherton, P.M. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am. J. Clin. Nutr. 2004, 79, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.S.; Silvis, S.E. Absorption of Whole Peanuts, Peanut Oil, and Peanut Butter. N. Engl. J. Med. 1980, 303, 917–918. [Google Scholar] [CrossRef]
- Berry, S.E.E.; Tydeman, E.A.; Lewis, H.B.; Phalora, R.; Rosborough, J.; Picout, D.R.; Ellis, P.R. Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects. Am. J. Clin. Nutr. 2008, 88, 922–929. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Carrière, F.; Mackie, A.R.; Gray, D.A.; Butterworth, P.J.; Ellis, P.R. The role of plant cell wall encapsulation and porosity in regulating lipolysis during the digestion of almond seeds. Food Funct. 2016, 7, 69–78. [Google Scholar] [CrossRef]
- Mandalari, G.; Grundy, M.M.L.; Grassby, T.; Parker, M.L.; Cross, K.L.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Laganà, G.; et al. The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 2014, 112, 1521–1529. [Google Scholar] [CrossRef]
- Mandalari, G.; Nueno-Palop, C.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. [Google Scholar] [CrossRef]
- Grassby, T.; Mandalari, G.; Grundy, M.M.-L.; Edwards, C.H.; Bisignano, C.; Trombetta, D.; Smeriglio, A.; Chessa, S.; Ray, S.; Sanderson, J.; et al. In vitro and in vivo modeling of lipid bioaccessibility and digestion from almond muffins: The importance of the cell-wall barrier mechanism. J. Funct. Foods 2017, 37, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Taylor, A.M.; Swanson, K.S.; Novotny, J.A.; Baer, D.J. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: A randomized controlled trial. Nutrients 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pan, Y. Influence of food matrix and food processing on the chemical interaction and bioaccessibility of dietary phytochemicals: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. In Vitro Digestion of Apple Tissue Using a Dynamic Stomach Model: Grinding and Crushing Effects on Polyphenol Bioaccessibility. J. Agric. Food Chem. 2020, 68, 574–583. [Google Scholar] [CrossRef]
- Liu, D.; Lopez-Sanchez, P.; Gidley, M.J. Cellular barriers in apple tissue regulate polyphenol release under different food processing and: In vitro digestion conditions. Food Funct. 2019, 10, 3008–3017. [Google Scholar] [CrossRef]
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.M.M.; Noort, M.W.J.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Kuijsten, A.; Arts, I.C.W.; Van’t Veer, P.; Hollman, P.C.H. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J. Nutr. 2005, 135, 2812–2816. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Domínguez-López, I.; Trius-Soler, M.; Castellví, M.; Bosch, B.; Castro-Barquero, S.; Estruch, R.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin. Nutr. 2021, 40, 5556–5567. [Google Scholar] [CrossRef]
- Juton, C.; Castro-barquero, S.; Casas, R.; Freitas, T.; Ruiz-león, A.M.; Crovetto, F.; Domenech, M.; Crispi, F.; Vieta, E.; Gratacós, E.; et al. Reliability and concurrent and construct validity of a food frequency questionnaire for pregnant women at high risk to develop fetal growth restriction. Nutrients 2021, 13, 1629. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.-I.; Marrugat, J. Validation of the Minnesota Leisure Time Spanish Women. Med. Sci. Sport. Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Castellote, A.I.; López-Sabater, M.C. Comparison of conventional and fast gas chromatography in human plasma fatty acid determination. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 809, 339–344. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Castellote-Bargalló, A.I.; López-Sabater, M.C. Comparison of two direct methods for the determination of fatty acids in infant feces. Anal. Biochem. 2000, 282, 250–255. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; López-Sabater, M.C.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargalló, A.I. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur. J. Clin. Nutr. 2002, 56, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole Orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Tulipani, S.; Jáuregui, O.; Valderas-Martinez, P.; Vallverdú-Queralt, A.; Estruch, R.; Torrado, X.; Lamuela-Raventós, R.M. Sensitive and rapid UHPLC-MS/MS for the analysis of tomato Phenolics in human biological samples. Molecules 2015, 20, 20409–20425. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Physician 2009, 80, 345–350. [Google Scholar] [CrossRef]
- Brufau, G.; Boatella, J.; Rafecas, M. Nuts: Source of energy and macronutrients. Br. J. Nutr. 2006, 96, 24–28. [Google Scholar] [CrossRef]
- Hinds, M.J. Fatty acid composition of Caribbean-grown peanuts (Arachis hypogaea L.) at three maturity stages. Food Chem. 1995, 53, 7–14. [Google Scholar] [CrossRef]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; O’Brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Vavra Ambrozova, J.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Ardisson Korat, A.V.; Malik, V.S.; Furtado, J.D.; Sacks, F.; Rosner, B.; Rexrode, K.M.; Willett, W.C.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating very-long-chain SFA concentrations are inversely associated with incident type 2 diabetes in US men and women. J. Nutr. 2020, 150, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Wong, D.; Cederbaum, S.; Lim, B.; Qu, Y. Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol. Genet. Metab. 2012, 107, 620–622. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Fretts, A.M.; Sitlani, C.M.; Biggs, M.L.; Mukamal, K.; King, I.B.; Song, X.; Djoussé, L.; Siscovick, D.S.; McKnight, B.; et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: The cardiovascular health study. Am. J. Clin. Nutr. 2015, 101, 1047–1054. [Google Scholar] [CrossRef]
- Kröger, J.; Zietemann, V.; Enzenbach, C.; Weikert, C.; Jansen, E.H.; Döring, F.; Joost, H.-G.; Boeing, H.; Schulze, M.B. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 2011, 93, 127–142. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.E.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; Djousse, L.; Heckbert, S.R.; King, I.B.; Mcknight, B.; Sitlani, C.; Sacks, F.M.; Song, X.; et al. Plasma Phospholipid Saturated Fatty Acids and Incident Atrial Fibrillation: The Cardiovascular Health Study. J. Am. Heart Assoc. 2014, 3, e000889. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; King, I.B.; Rice, K.; McKnight, B.; Sotoodehnia, N.; Rea, T.D.; Johnson, C.O.; Raghunathan, T.E.; Cobb, L.A.; Mozaffarian, D.; et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins. Leukot. Essent. Fatty Acids 2014, 91, 149–153. [Google Scholar] [CrossRef]
- Traoret, C.J.; Lokko, P.; Cruz, A.C.R.F.; Oliveira, C.G.; Costa, N.M.B.; Bressan, J.; Alfenas, R.C.G.; Mattes, R.D. Peanut digestion and energy balance. Int. J. Obes. 2008, 32, 322–328. [Google Scholar] [CrossRef]
- Mandalari, G.; Parker, M.L.; Grundy, M.M.L.; Grassby, T.; Smeriglio, A.; Bisignano, C.; Raciti, R.; Trombetta, D.; Baer, D.J.; Wilde, P.J. Understanding the effect of particle size and processing on almond lipid bioaccessibility through microstructural analysis: From mastication to faecal collection. Nutrients 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.K.; Kendall, C.W.C.; Bazinet, R.P.; Hanley, A.J.; Comelli, E.M.; Jenkins, D.J.A.; Sievenpiper, J.L. Almond Bioaccessibility in a Randomized Crossover Trial: Is a Calorie a Calorie? Mayo Clin. Proc. 2021, 96, 2386–2397. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.R.; Kendall, C.W.C.; Ren, Y.; Parker, C.; Pacy, J.F.; Waldron, K.W.; Jenkins, D.J.A. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am. J. Clin. Nutr. 2004, 80, 604–613. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Grassby, T.; Mandalari, G.; Waldron, K.W.; Butterworth, P.J.; Berry, S.E.E.; Ellis, P.R. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015, 101, 25–33. [Google Scholar] [CrossRef]
- McKiernan, F.; Mattes, R.D. Effects of peanut processing on masticatory performance during variable appetitive states. J. Nutr. Metab. 2010, 2010, 487301. [Google Scholar] [CrossRef]
- Talcott, S.T.; Duncan, C.E.; Del Pozo-Insfran, D.; Gorbet, D.W. Polyphenolic and antioxidant changes during storage of normal, mid, and high oleic acid peanuts. Food Chem. 2005, 89, 77–84. [Google Scholar] [CrossRef]
- Talcott, S.T.; Passeretti, S.; Duncan, C.E.; Gorbet, D.W. Polyphenolic content and sensory properties of normal and high oleic acid peanuts. Food Chem. 2005, 90, 379–388. [Google Scholar] [CrossRef]
- Stevens-Barrón, J.C.; de la Rosa, L.A.; Wall-Medrano, A.; Álvarez-Parrilla, E.; Rodríguez-Ramirez, R.; Robles-Zepeda, R.E.; Astiazaran-García, H. Chemical Composition and In Vitro Bioaccessibility. Nutrients 2019, 11, 2303. [Google Scholar] [CrossRef]
- Christman, L.M.; Dean, L.L.; Bueno Almeida, C.; Weissburg, J.R. Acceptability of Peanut Skins as a Natural Antioxidant in Flavored Coated Peanuts. J. Food Sci. 2018, 83, 2571–2577. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Alkaltham, M.S.; Özcan, M.M.; Uslu, N.; Hayat, K. Effect of maturing stages on bioactive properties, fatty acid compositions, and phenolic compounds of peanut (Arachis hypogaea L.) kernels harvested at different harvest times. J. Oleo Sci. 2021, 70, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Isanga, J.; Zhang, G.-N. Biologically Active Components and Nutraceuticals in Peanuts and Related Products: Review. Food Rev. Int. 2007, 23, 123–140. [Google Scholar] [CrossRef]
- Hathorn, C.S.; Sanders, T.H. Flavor and Antioxidant Capacity of Peanut Paste and Peanut Butter Supplemented with Peanut Skins. J. Food Sci. 2012, 77, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kerr, W.L.; Swanson, R.B.; Hargrove, J.L.; Pegg, R.B. Peanut skins-fortified peanut butters: Effect of processing on the phenolics content, fibre content and antioxidant activity. Food Chem. 2014, 145, 883–891. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R.M. Urinary isoxanthohumol is a specific and accurate biomarker of beer consumption. J. Nutr. 2014, 144, 484–488. [Google Scholar] [CrossRef][Green Version]
- Hurtado-Barroso, S.; Quifer-Rada, P.; Marhuenda-Muñoz, M.; Rinaldi de Alvarenga, J.F.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M. Increase of 4-hydroxybenzoic, a bioactive phenolic compound, after an organic intervention diet. Antioxidants 2019, 8, 340. [Google Scholar] [CrossRef]
- Campesi, I.; Romani, A.; Romani, A. The sex-gender effects in the road to tailored botanicals. Nutrients 2019, 11, 1637. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Achaintre, D.; Rothwell, J.A.; Rinaldi, S.; Assi, N.; Ferrari, P.; Leitzmann, M.; Boutron-Ruault, M.C.; Fagherazzi, G.; Auffret, A.; et al. Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study. Sci. Rep. 2016, 6, 26905. [Google Scholar] [CrossRef]
- Laveriano-santos, E.P.; Parilli-moser, I.; Ramírez-garza, S.L.; Tresserra-rimbau, A.; Storniolo, C.E.; Ruiz-león, A.M.; Estruch, R.; Bodega, P.; de Miguel, M.; de Cos-Gandoy, A.; et al. Polyphenols in urine and cardiovascular risk factors: A cross-sectional analysis reveals gender differences in Spanish adolescents from the SI! program. Antioxidants 2020, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Martinez-Sanz, M.; Lopez-Sanchez, P.; Gilbert, E.P.; Gidley, M.J. Adsorption behaviour of polyphenols on cellulose is affected by processing history. Food Hydrocoll. 2017, 63, 496–507. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Langston, F.M.A.; Nash, G.R.; Bows, J.R. The retention and bioavailability of phytochemicals in the manufacturing of baked snacks. Crit. Rev. Food Sci. Nutr. 2021, 1–37. [Google Scholar] [CrossRef]
| Whole Peanuts (n = 21) | Peanut Butter (n = 23) | p-Value | |
|---|---|---|---|
| Female, n (%) | 14 (66) | 18 (78) | 0.388 |
| Education level, n (%) | 0.652 | ||
| University students | 11 (52%) | 11 (48%) | |
| Graduated | 10 (48%) | 12 (52%) | |
| Age (years) | 22.28 ± 3.20 | 23.43 ± 2.90 | 0.142 |
| Body composition | |||
| Weight (kg) | 63.26 ± 10.12 | 60.10 ± 7.72 | 0.240 |
| BMI (kg/m2) | 22.12 ± 3.52 | 22.19 ± 2.59 | 0.541 |
| Waist circumference (cm) | 72.73 ± 8.31 | 71.28 ± 5.53 | 0.796 |
| Hip circumference (cm) | 98.74 ± 6.35 | 95.85 ± 6.24 | 0.120 |
| Waist to hip ratio | 0.73 ± 0.06 | 0.74 ± 0.05 | 0.415 |
| Body fat (%) | 26.66 ± 8.07 | 28.45 ± 7.88 | 0.404 |
| Muscle mass (%) | 32.09 ± 5.71 | 31.04 ± 5.81 | 0.459 |
| Physical activity (METs/week) | 4850 ± 2124 | 4703 ± 2382 | 0.751 |
| Blood pressure | |||
| SBP (mmHg) | 112 ± 7.34 | 110 ± 8.87 | 0.235 |
| DBP (mmHg) | 72.63 ± 7.63 | 72.87 ± 6.20 | 0.698 |
| Blood analytes | |||
| Glucose (mmol/L) | 4.54 ± 0.44 | 4.59 ± 0.35 | 0.760 |
| Triglycerides (mmol/L) | 0.71 ± 0.20 | 0.85 ± 0.35 | 0.152 |
| Total cholesterol (mmol/L) | 4.33 ± 0.52 | 4.60 ± 0.88 | 0.318 |
| LDL-cholesterol (mmol/L) | 2.22 ± 0.39 | 2.60 ± 0.69 | 0.070 |
| HDL-cholesterol (mmol/L) | 1.75 ± 0.30 | 1.69 ± 0.40 | 0.459 |
| Dietary intake | |||
| Energy (kcal/day) | 2771 ± 594 | 2706 ± 602 | 0.816 |
| Carbohydrates (g/day) | 257 ± 80.74 | 241 ± 73.92 | 0.642 |
| Protein (g/day) | 104 ± 29.43 | 110 ± 31.86 | 0.388 |
| Total fat (g/day) | 145 ± 29.17 | 142 ± 35.35 | 0.816 |
| SFAs (g/day) | 37.62 ± 10.00 | 38.18 ± 11.05 | 0.514 |
| MUFAs (g/day) | 67.76 ± 15.90 | 69.06 ± 17.18 | 0.852 |
| PUFAs (g/day) | 25.91 ± 6.77 | 23.99 ± 7.25 | 0.499 |
| Fatty Acids | Whole Peanuts | Peanut Butter | p-Value | ||
|---|---|---|---|---|---|
| % | mg FA/g Peanut | % | mg FA/g Peanut Butter | ||
| Myristic acid (C14:0) | 0.03 | 0.16 ± 0.02 | 0.02 | 0.14 ± 0.01 | 0.101 |
| Palmitic acid (C16:0) | 9.84 | 60.59 ± 8.07 | 8.38 | 49.71 ± 3.61 | 0.100 |
| Palmitoleic acid (C16:1 n-7) | 0.08 | 0.48 ± 0.05 | 0.05 | 0.27 ± 0.01 | 0.003 |
| Heptadecanoic acid (C17:0) | 0.10 | 0.59 ± 0.08 | 0.09 | 0.55 ± 0.02 | 0.447 |
| Stearic acid (C18:0) | 3.02 | 18.61 ± 2.45 | 2.81 | 16.69 ± 1.17 | 0.288 |
| Oleic acid (C18:1 n-9) | 55.11 | 339 ± 45.90 | 61.49 | 364 ± 27.48 | 0.461 |
| Linoleic acid (C18:2 n-6) | 24.23 | 149 ± 20.36 | 20.24 | 120 ± 9.22 | 0.086 |
| Arachidic acid (C20:0) | 1.44 | 8.90 ± 1.18 | 1.38 | 8.19 ± 0.57 | 0.406 |
| Alpha-linolenic acid (C18:3 n-3) | 0.05 | 0.31 ± 0.04 | 0.04 | 0.25 ± 0.02 | 0.081 |
| Eicosenoic acid (C20:1 n-9) | 1.11 | 6.86 ± 0.93 | 1.19 | 7.05 ± 0.49 | 0.768 |
| Eicosadienoic acid (C20:2 n-6) | 0.10 | 0.61 ± 0.08 | 0.02 | 0.10 ± 0.02 | <0.001 |
| Behenic acid (C22:0) | 3.11 | 19.15 ± 2.58 | 2.64 | 15.66 ± 1.03 | 0.095 |
| Erucic acid (C22:1 n-9) | 0.08 | 0.52 ± 0.09 | 0.08 | 0.47 ± 0.03 | 0.423 |
| Tricosanoic acid (C23:0) | 0.10 | 0.63 ± 0.10 | 0.04 | 0.24 ± 0.02 | 0.002 |
| Lignoceric acid (C24:0) | 1.52 | 9.40 ± 1.34 | 1.43 | 8.50 ± 0.48 | 0.335 |
| SFAs | 19.16 | 118 ± 15.81 | 16.81 | 99.67 ± 6.90 | 0.139 |
| VLCSFAs | 6.18 | 38.07 ± 5.19 | 5.50 | 32.59 ± 2.10 | 0.165 |
| MUFAs | 56.42 | 347 ± 46.99 | 62.85 | 372 ± 28.02 | 0471 |
| PUFAs | 24.42 | 150 ± 20.51 | 20.34 | 120 ± 9.26 | 0.083 |
| Total fatty acids | 616 ± 83.31 | 593 ± 44.18 | 0.634 | ||
| Plasma Fatty Acids (µg/mL) | Whole Peanuts | Peanut Butter | p-Value WP vs. PB | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Post- Intervention | Post-Intervention–Baseline | Baseline | Post- Intervention | Post-Intervention–Baseline | ||
| Myristic acid (C14:0) | 15.46 ± 5.89 | 16.05 ± 7.74 | 0.59 ± 6.17 | 21.62 ± 16.81 | 19.72 ± 11.17 | −2.76 ± 16.74 | 0.355 |
| Palmitic acid (C16:0) | 519 ± 275 | 541 ± 172 | 22.50 ± 285 | 643 ± 194 | 651 ± 165 | 8.12 ± 224 | 0.128 |
| Palmitoleic acid (C16:1 n-7) | 41.80 ± 14.78 | 47.26 ± 15.59 | 5.47 ± 14.99 | 55.01 ± 33.51 | 87.61 ± 132.38 | 28.80 ± 134.57 | 0.878 |
| Stearic acid (C18:0) | 190 ± 64.88 | 216 ± 150 | 25.65 ± 134 | 221 ± 46.80 | 212 ± 59.05 | −18.01 ± 60.73 | 0.200 |
| Vaccenic acid (C18:1 n-7) | 32.24 ± 12.04 | 40.50 ± 10.08 | 8.26 ± 12.61 | 49.31 ± 16.46 | 78.38 ± 124 | 25.66 ± 123 | 0.474 |
| Oleic acid (C18:1 n-9) | 469 ± 169 | 544 ± 143 | 74.45 ± 135 | 691 ± 155 | 669 ± 207 | −51.38 ± 203 | 0.040 |
| Linoleic acid (C18:2 n-6) | 757 ± 272 | 886 ± 215 | 129 ± 186 | 999 ± 194 | 982 ± 280 | −60.04 ± 325 | 0.049 |
| Alpha-linolenic acid (C18:3 n-3) | 22.35 ± 53.82 | 8.53 ± 4.23 | −13.82 ± 54.10 | 13.48 ± 9.92 | 11.56 ± 4.25 | −2.43 ± 8.75 | 0.630 |
| Gamma-linolenic acid (C18:3 n-6) | 6.47 ± 4.48 | 8.41 ± 4.24 * | 1.94 ± 3.64 | 10.12 ± 4.48 | 53.23 ± 204 | 40.79 ± 201 | 0.065 |
| Arachidic acid (C20:0) | 2.54 ± 1.32 | 3.06 ± 1.03 * | 0.51 ± 1.51 | 3.06 ± 1.01 | 4.23 ± 1.58 * | 1.12 ± 1.66 | 0.264 |
| Eicosenoic acid (C20:1 n-9) | 7.39 ± 7.66 | 6.63 ± 3.03 | −0.76 ± 8.65 | 9.65 ± 7.77 | 40.59 ± 88.16 | 29.17 ± 86.44 | 0.378 |
| Eicosadienoic acid (C20:2 n-6) | 8.30 ± 8.90 | 7.75 ± 2.70 | −0.55 ± 8.84 | 9.25 ± 3.29 | 8.52 ± 3.57 | −1.11 ± 4.27 | 0.148 |
| Eicosapentanoic acid (C20:5 n-3) | 25.60 ± 20.54 | 24.69 ± 9.91 | −0.91 ± 19.11 | 28.04 ± 19.65 | 44.93 ± 50.75 | 14.94 ± 53.92 | 0.235 |
| Arachidonic acid (C20:4 n-6) | 321 ± 159 | 202 ± 51.45 * | −119 ± 120 | 222 ± 59.51 | 214 ± 75.14 | −17.21 ± 80.51 | 0.062 |
| Behenic acid (C22:0) | 2.09 ± 1.03 | 4.66 ± 1.69 * | 2.57 ± 1.71 | 2.63 ± 1.51 | 5.33 ± 1.52 * | 2.58 ± 2.26 | 0.953 |
| Docosatetraenoic acid (C22: 4 n-6) | 17.78 ± 16.92 | 16.21 ± 5.42 | −1.57 ± 18.51 | 18.76 ± 3.19 | 17.99 ± 3.22 | −1.54 ± 5.67 | 0.062 |
| Docosapentaenoic acid (C22:5 n-3) | 21.97 ± 13.64 | 20.15 ± 4.45 | −1.82 ± 15.63 | 22.68 ± 5.08 | 22.21 ± 5.14 | −1.44 ± 7.35 | 0.431 |
| Docosahexaenoic acid (C22:6 n-3) | 69.42 ± 25.55 | 81.06 ± 22.47 | 11.65 ± 30.77 | 85.65 ± 33.33 | 92.80 ± 38.31 | 3.12 ± 44.40 | 0.474 |
| Lignoceric acid (C24:0) | 3.45 ± 0.77 | 9.28 ± 3.93 * | 5.83 ± 3.81 | 3.93 ± 2.16 | 11.18 ± 4.02 * | 6.93 ± 4.18 | 0.318 |
| Tetracosenoic acid (C24:1 n-9) | 11.44 ± 6.06 | 11.31 ± 3.68 | −0.13 ± 8.00 | 13.34 ± 3.01 | 13.09 ± 5.74 | −0.82 ±7.51 | 0.431 |
| SFAs | 755 ± 215 | 769 ± 351 | 12.65 ± 255 | 854 ± 254 | 895 ± 214 | 39.50 ± 204 | 0.990 |
| VLCSFAs | 8.09 ± 2.54 | 17.00 ± 5.70 * | 8.92 ± 5.37 | 9.62 ± 3.48 | 20.73 ± 5.55 * | 11.11 ± 5.69 | 0.157 |
| MUFAs | 563 ± 185 | 650 ± 167 | 87.29 ± 152 | 819 ± 199 | 889 ± 306 | 66.26 ± 293 | 0.507 |
| PUFAs | 1240 ± 847 | 1247 ± 273 | 7.37 ± 844 | 1402 ± 232 | 1439 ± 253 | 37.11 ± 243 | 0.222 |
| Total fatty acids | 2558 ± 949 | 2666 ± 663 | 107 ± 615 | 3116 ± 593 | 3043 ± 907 | 66.88 ± 539 | 0.419 |
| Fecal Fatty Acids (µg/100 mg) | Whole Peanuts | Peanut Butter | p-Value WP vs. PB | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Post- Intervention | Post-Intervention–Baseline | Baseline | Post- Intervention | Post-Intervention–Baseline | ||
| Myristic acid (C14:0) | 38.68 ± 41.81 | 59.51 ± 60.79 | 20.83 ± 65.41 | 30.99 ± 18.28 | 95.57 ± 256 | 64.57 ± 255 | 0.842 |
| Pentadecanoic acid (C15:0) | 59.65 ± 49.12 | 83.15 ± 85.52 | 23.49 ± 93.10 | 54.42 ± 32.78 | 49.61 ± 32.82 | −4.81 ± 41.10 | 0.534 |
| Palmitic acid (C16:0) | 730 ± 791 | 1108 ± 766 * | 378 ± 724 | 578 ± 277 | 936 ± 828 | 358 ± 825 | 0.445 |
| Palmitoleic acid (C16:1 n7) | 33.03 ± 65.92 | 45.63 ± 90.08 | 12.60 ± 34.91 | 14.01 ± 6.35 | 25.52 ± 26.52 | 11.51 ± 25.56 | 0.935 |
| Heptadecanoic acid (C17:0) | 31.60 ± 30.29 | 45.82 ± 64.29 | 14.22 ± 67.00 | 21.36 ± 9.77 | 24.66 ± 13.33 | 3.30 ± 14.73 | 0.823 |
| Stearic acid (C18:0) | 1074 ± 130 | 1885 ± 408 | 811 ± 605 | 1232 ± 784 | 1132 ± 1110 | −100 (733) | 0.026 |
| Oleic acid (C18:1 n9) | 593 ± 664 | 2202 ± 551 * | 1680 ± 308 | 376 ± 523 | 1250 ± 994 * | 873 (137) | 0.264 |
| Vaccenic acid (C18:1 n7) | 188 ± 740 | 90.47 ± 218 | −97.62 ± 526 | 31.09 ± 22.91 | 45.00 ± 62.29 | 13.90 (66.19) | 0.663 |
| Linoleic acid (C18:2 n6) | 348 ± 592 | 660 ± 977 | 312 ± 792 | 349 ± 697 | 587 ± 702 | 238 (883) | 0.860 |
| Arachidic acid (C20:0) | 31.45 ± 26.45 | 61.57 ± 39.17 * | 30.11 ± 32.52 | 25.30 ± 10.74 | 41.13 ± 22.67 * | 15.84 (22.27) | 0.103 |
| Alpha-linolenic acid (C18:3 n3) | 48.16 ± 92.41 | 55.26 ± 116 | 7.10 ± 133 | 160 ± 397 | 42.13 ± 68.34 | −118 (383) | 0.445 |
| Eicosenoic acid (C20:1 n9) | 16.11 ± 17.47 | 35.97 ± 7.92 * | 19.86 ± 42.22 | 7.51 ± 4.60 | 19.53 ± 17.45 * | 12.02 (18.12) | 0.664 |
| Eicosadienoic acid (C20:2 n6) | 5.87 ± 5.52 | 7.31 ± 10.54 | 1.43 ± 9.93 | 5.12 ± 9.30 | 4.06 ± 5.72 | −1.06 ± 10.70 | 0.630 |
| Behenic acid (C22:0) | 26.72 ± 26.95 | 76.13 ± 39.37 * | 49.41 ± 41.60 | 21.35 ± 11.53 | 42.18 ± 32.67 * | 20.83 ± 33.55 | 0.006 |
| Arachidonic acid (C20:4 n6) | 3.54 ± 2.53 | 4.01 ± 3.47 | 0.47 ± 3.35 | 0.87 ± 0.72 | 1.59 ± 1.90 | 0.72 ± 2.23 | 0.953 |
| Eicosapentanoic acid (C20:5 n3) | 2.48 ± 4.80 | 1.14 ± 1.21 | −1.33 ± 4.99 | 1.03 ± 1.58 | 4.70 ± 11.33 | 3.67 ± 11.47 | 0.053 |
| Docosatetraenoic acid (C22: 4 n6) | 10.55 ± 9.77 | 13.84 ± 11.70 | 3.29 ± 9.19 | 20.53 ± 9.46 | 20.62 ± 10.29 | 0.09 ± 10.47 | 0.378 |
| Lignoceric acid (C24:0) | 24.81 ± 24.50 | 54.21 ± 28.51 * | 29.39 ± 34.47 | 21.39 ± 8.33 | 35.49 ± 18.85 * | 14.10 ± 20.96 | 0.026 |
| Tetracosenoic acid (C24:1 n9) | 6.92 ± 3.81 | 10.77 ± 11.51 | 3.84 ± 12.02 | 7.93 ± 4.23 | 7.58 ± 4.57 | −0.68 ± 6.91 | 0.341 |
| SFAs | 2017 ± 1775 | 3975 ± 4997 * | 1957 ± 1609 | 1986 ± 1036 | 2357 ± 1924 | 371 ± 1623 | 0.040 |
| VLCSFAs | 82.99 ± 73.05 | 192 ± 102 * | 108 ± 106 | 68.03 ± 28.51 | 119 ± 69.06 * | 50.76 ± 71.99 | 0.012 |
| MUFAs | 838 ± 1199 | 2385 ± 5872 * | 1547 ± 4873 | 437 ± 538 | 1347 ± 2075 * | 910 ± 2217 | 0.727 |
| PUFAs | 419 ± 684 | 742 ± 1043 | 324 ± 850 | 538 ± 821 | 661 ± 805 | 123 ± 1183 | 0.889 |
| Total fatty acids | 3274 ± 2953 | 7102 ± 1439 * | 3828 ± 2777 | 2960 ± 1832 | 4366 ± 3216 | 1405 ± 7362 | 0.560 |
| Polyphenols (mg/100 g) | Whole Peanuts | Peanut Butter | p-Value WP vs. PB |
|---|---|---|---|
| Flavonoids | 0.99 ± 0.04 | 9.38 ± 0.64 | 0.002 |
| Catechin | 0.23 ± 0.01 | 3.24 ± 0.13 | <0.001 |
| Epicatechin | 0.21 ± 0.02 | 5.65 ± 0.71 | 0.005 |
| Quercetin 3-β-d-glucoside | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.001 |
| Quercetin 3-O-glucuronide | 0.03 ± 0.00 | 0 | 0.001 |
| Kaempferol-O-glucoside | 0.08 ± 0.00 | 0.07 ± 0.01 | 0.237 |
| Rutin | 0.41 ± 0.01 | 0.40 ± 0.03 | 0.719 |
| 8-prenylnaringenin | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.290 |
| Stilbenes | |||
| Resveratrol | 0.32 ± 0.01 | 0.34 ± 0.02 | 0.432 |
| Phenolic acids | 47.82 ± 1.34 | 98.69 ± 11.26 | 0.015 |
| Protocatechuic acid | 1.52 ± 0.09 | 0.26 ± 0.03 | <0.001 |
| 2,5-dihydroxybenzoic acid | 0.16 ± 0.14 | 0.38 ± 0.05 | 0.098 |
| Vanillic acid | 1.90 ± 0.09 | 0.88 ± 0.10 | <0.001 |
| Caffeic acid | 0.49 ± 0.05 | 0.26 ± 0.02 | 0.011 |
| p-coumaric acid | 24.53 ± 0.35 | 41.15 ± 3.33 | 0.037 |
| m-coumaric acid | 1.65 ± 0.03 | 1.59 ± 0.05 | 0.357 |
| o-coumaric acid | 0.06 ± 0.00 | 0.02 ± 0.00 | 0.002 |
| Ferulic acid | 2.13± 0.08 | 1.71 ± 0.18 | 0.040 |
| Isoferulic acid | 13.91 ± 0.60 | 48.81 ± 5.53 | 0.008 |
| Sinapic acid | 1.35 ± 0.09 | 1.80 ± 0.31 | 0.116 |
| Urine Polyphenols (mg/day) | Whole Peanuts | Peanut Butter | p-Value WP vs. PB | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Post- Intervention | Post-Intervention–Baseline | Baseline | Post- Intervention | Post-Intervention–Baseline | ||
| Stilbenes | |||||||
| Dihidroresveratrol | 0.05 ± 0.18 | 0.01 ± 0.01 | −0.04 ± 0.18 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.01 ± 0.02 | 0.397 |
| Phenolic Acids | |||||||
| Protocatechuic acid | 1.13 ± 0.86 | 1.10 ± 1.14 | −0.02 ± 1.17 | 2.08 ± 1.80 | 1.72 ± 1.71 | −0.35 ± 2.21 | 0.787 |
| 2,5 dihidroxibenzoic | 1.09 ± 0.78 | 1.18 ± 1.17 | 0.08 ± 1.08 | 1.87 ± 1.79 | 1.71 ± 1.75 | −0.16 ± 2.11 | 0.935 |
| Vanillic acid | 8.10 ± 6.02 | 6.63 ± 5.78 | −1.46 ± 4.89 | 16.69 ± 25.82 | 13.69 ± 21.19 | −3.33 ± 14.70 | 0.860 |
| p-Coumaric acid | 0.54 ± 1.34 | 0.37 ± 0.33 | −0.18 ± 1.30 | 0.18 ± 0.21 | 0.43 ± 0.47 | 0.23 ± 0.50 | 0.431 |
| m-Coumaric acid | 0.53 ± 0.95 | 0.36 ± 0.57 | −0.17 ± 0.76 | 0.39 ± 1.12 | 0.40 ± 0.49 | 0.01 ± 1.23 | 0.378 |
| o-Coumaric acid | 0.33 ± 0.84 | 0.12 ± 0.14 | −0.21 ± 0.82 | 0.19 ± 0.22 | 0.22 ± 0.30 | 0.03 ± 0.18 | 0.431 |
| Isoferulic | 0.78 ± 0.64 | 1.49 ± 1.22 * | 0.71 ± 1.11 | 0.79 ± 0.53 | 2.95 ± 4.32 * | 2.17 ± 4.26 | 0.916 |
| Sinapic acid | 0.58 ± 0.68 | 0.38 ± 0.47 | −0.20 ± 0.77 | 4.23 ± 12.84 | 8.36 ± 27.73 | 4.14 ± 14.94 | 0.200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parilli-Moser, I.; Domínguez-López, I.; Arancibia-Riveros, C.; Marhuenda-Muñoz, M.; Vallverdú-Queralt, A.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Effect of Crushing Peanuts on Fatty Acid and Phenolic Bioaccessibility: A Long-Term Study. Antioxidants 2022, 11, 423. https://doi.org/10.3390/antiox11020423
Parilli-Moser I, Domínguez-López I, Arancibia-Riveros C, Marhuenda-Muñoz M, Vallverdú-Queralt A, Hurtado-Barroso S, Lamuela-Raventós RM. Effect of Crushing Peanuts on Fatty Acid and Phenolic Bioaccessibility: A Long-Term Study. Antioxidants. 2022; 11(2):423. https://doi.org/10.3390/antiox11020423
Chicago/Turabian StyleParilli-Moser, Isabella, Inés Domínguez-López, Camila Arancibia-Riveros, María Marhuenda-Muñoz, Anna Vallverdú-Queralt, Sara Hurtado-Barroso, and Rosa M. Lamuela-Raventós. 2022. "Effect of Crushing Peanuts on Fatty Acid and Phenolic Bioaccessibility: A Long-Term Study" Antioxidants 11, no. 2: 423. https://doi.org/10.3390/antiox11020423
APA StyleParilli-Moser, I., Domínguez-López, I., Arancibia-Riveros, C., Marhuenda-Muñoz, M., Vallverdú-Queralt, A., Hurtado-Barroso, S., & Lamuela-Raventós, R. M. (2022). Effect of Crushing Peanuts on Fatty Acid and Phenolic Bioaccessibility: A Long-Term Study. Antioxidants, 11(2), 423. https://doi.org/10.3390/antiox11020423