CoenzymeQ10 and Ischemic Preconditioning Potentially Prevent Tourniquet-Induced Ischemia/Reperfusion in Knee Arthroplasty, but Combined Pretreatment Possibly Neutralizes Their Beneficial Effects
Abstract
1. Introduction
2. Materials and Methods
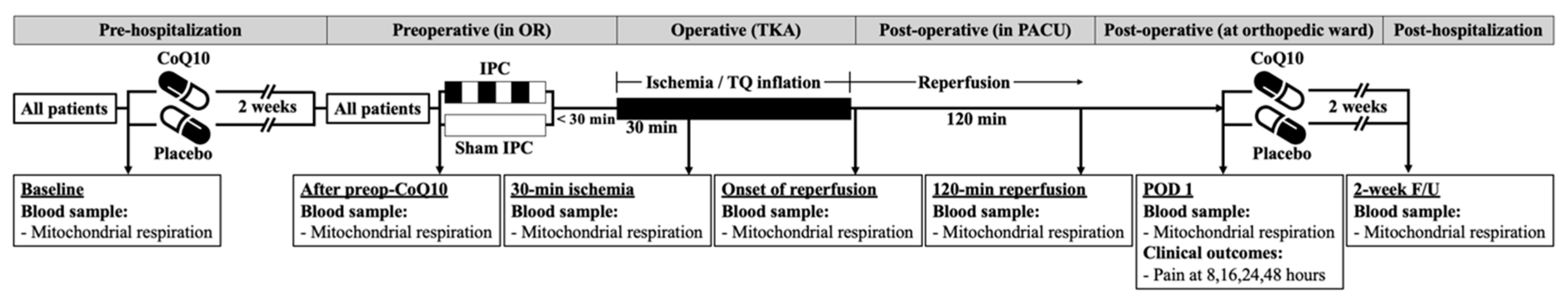
2.1. Study Design
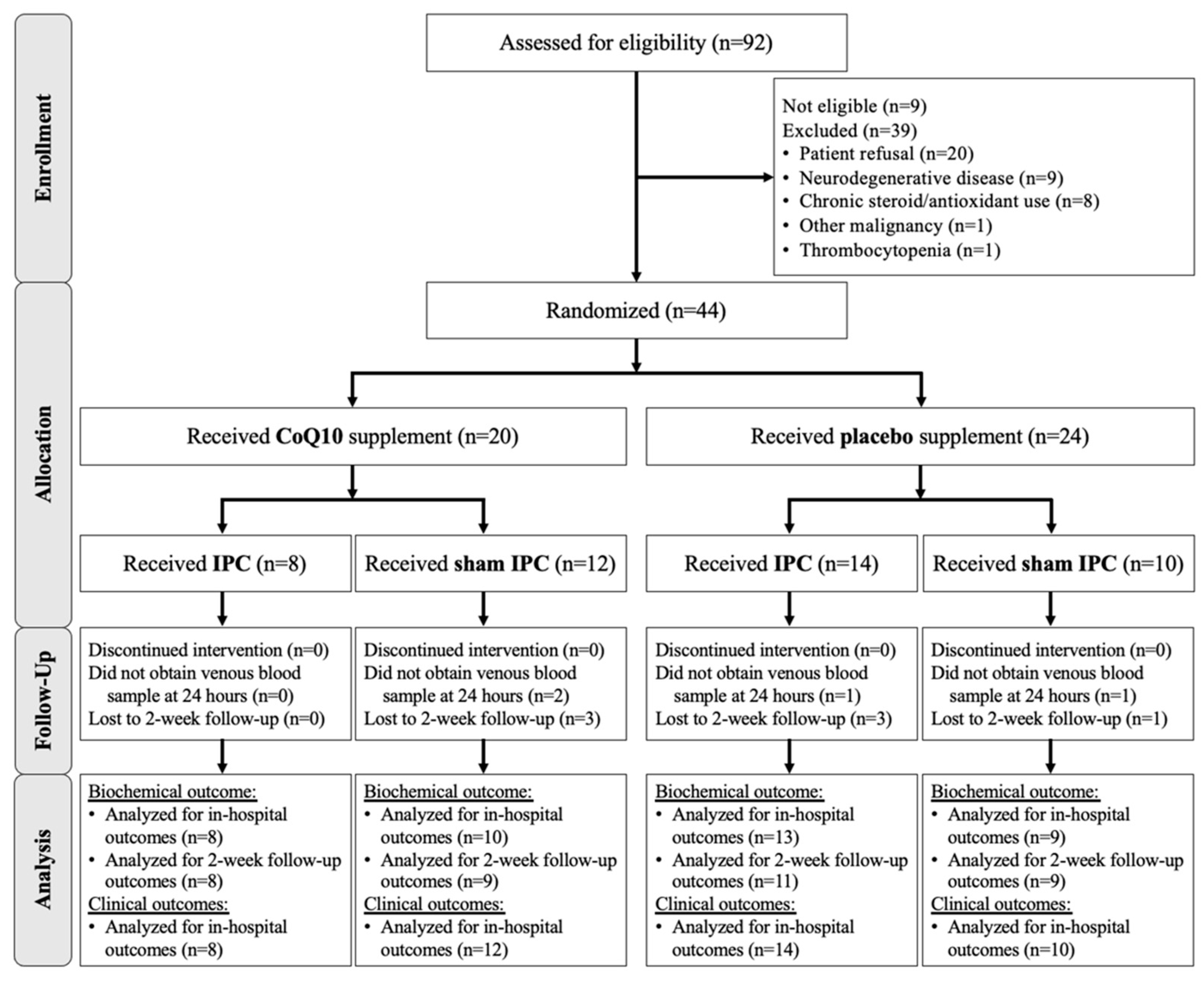
2.2. Participants
2.3. Study Interventions, Randomization and Blinding
2.4. Anesthesia Protocol and Operative Procedure
2.5. Postoperative Analgesic Protocol
2.6. Biochemical Outcomes
2.7. Clinical Outcomes
2.8. Statistical Analysis
3. Results
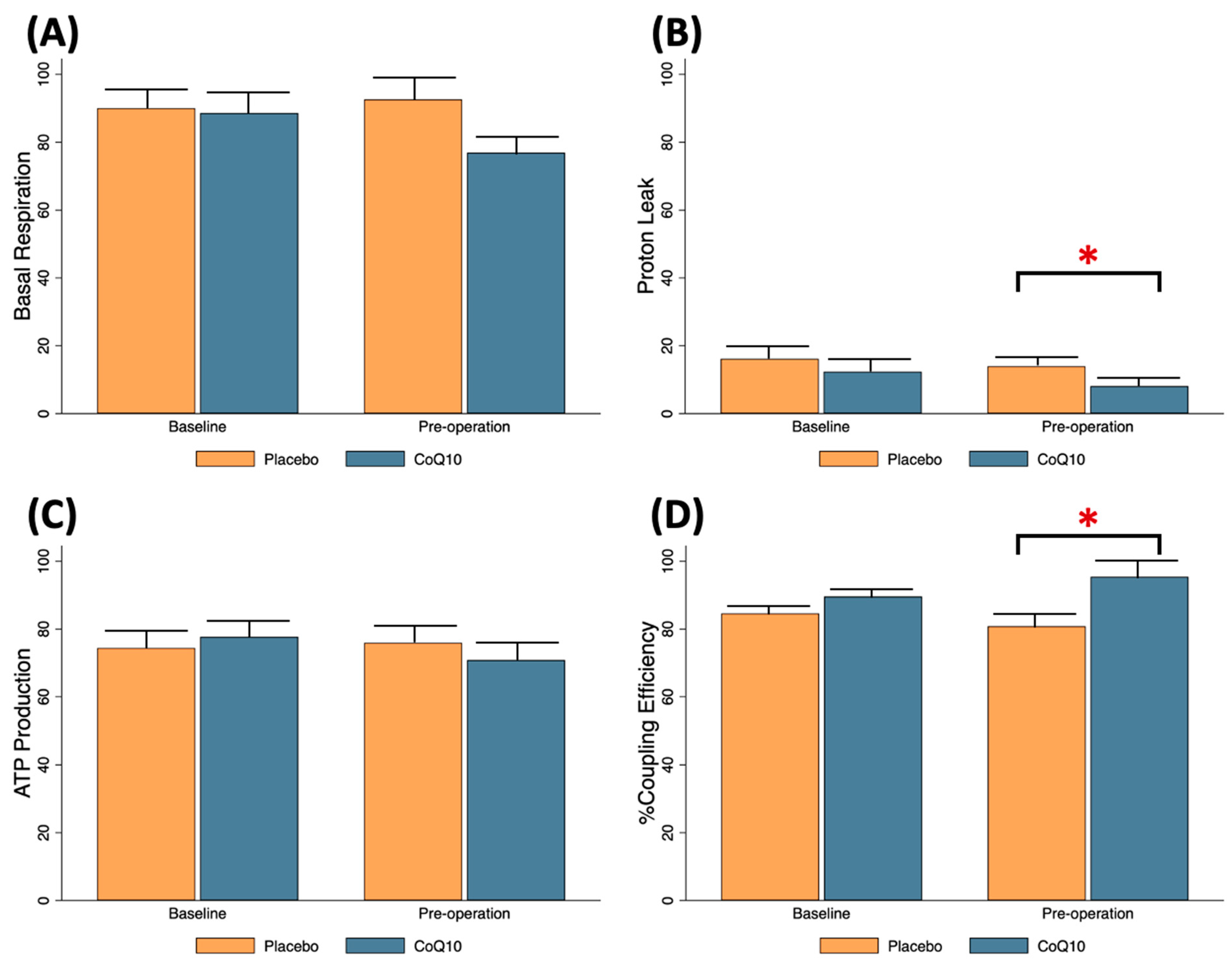
3.1. Effects of Preoperative CoQ10 Supplementation on Baseline Conditions of Knee OA Patients
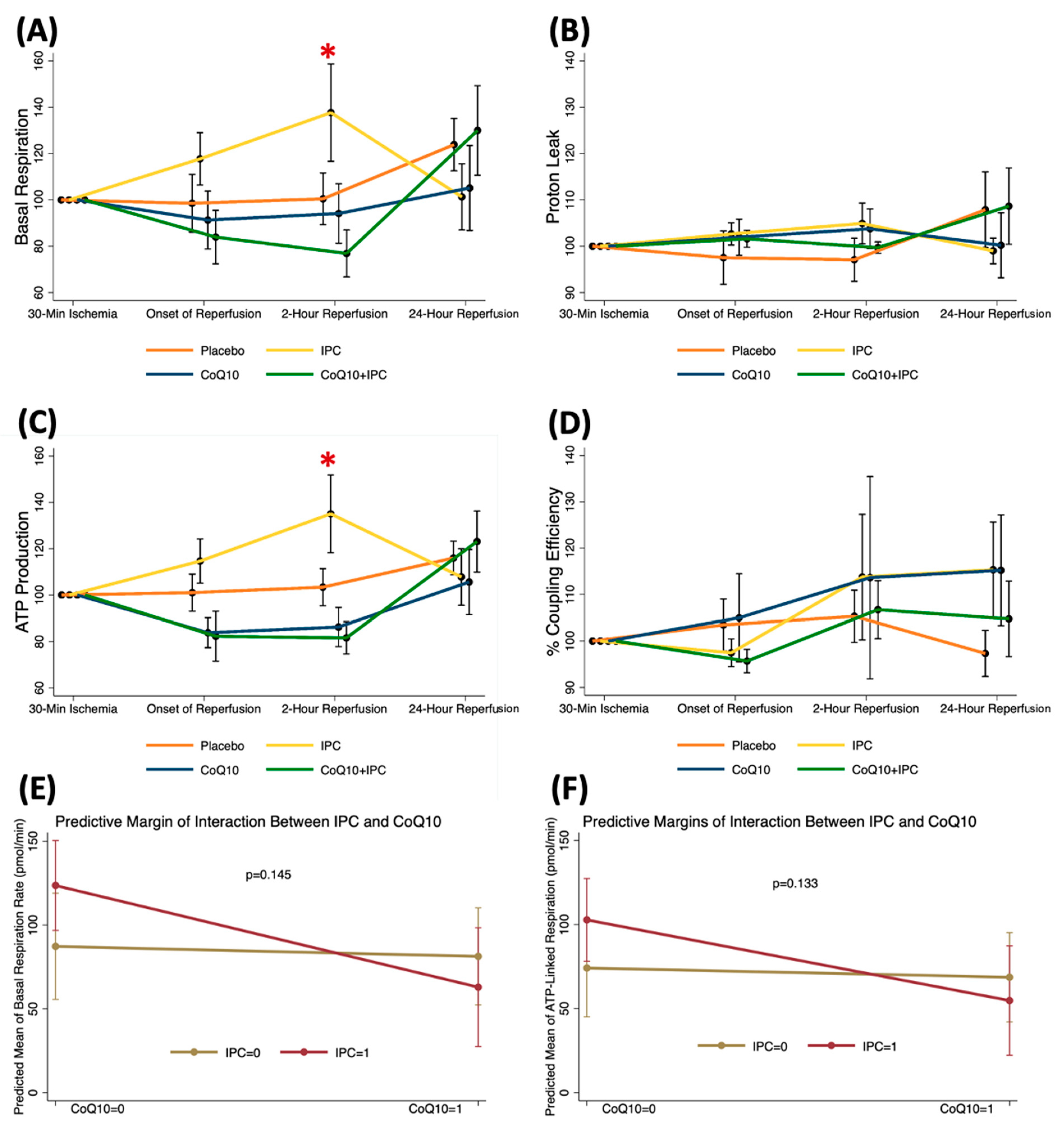
3.2. Effects of CoQ10, IPC, and CoQ10 + IPC on Early Phase of TQ-Induced I/R
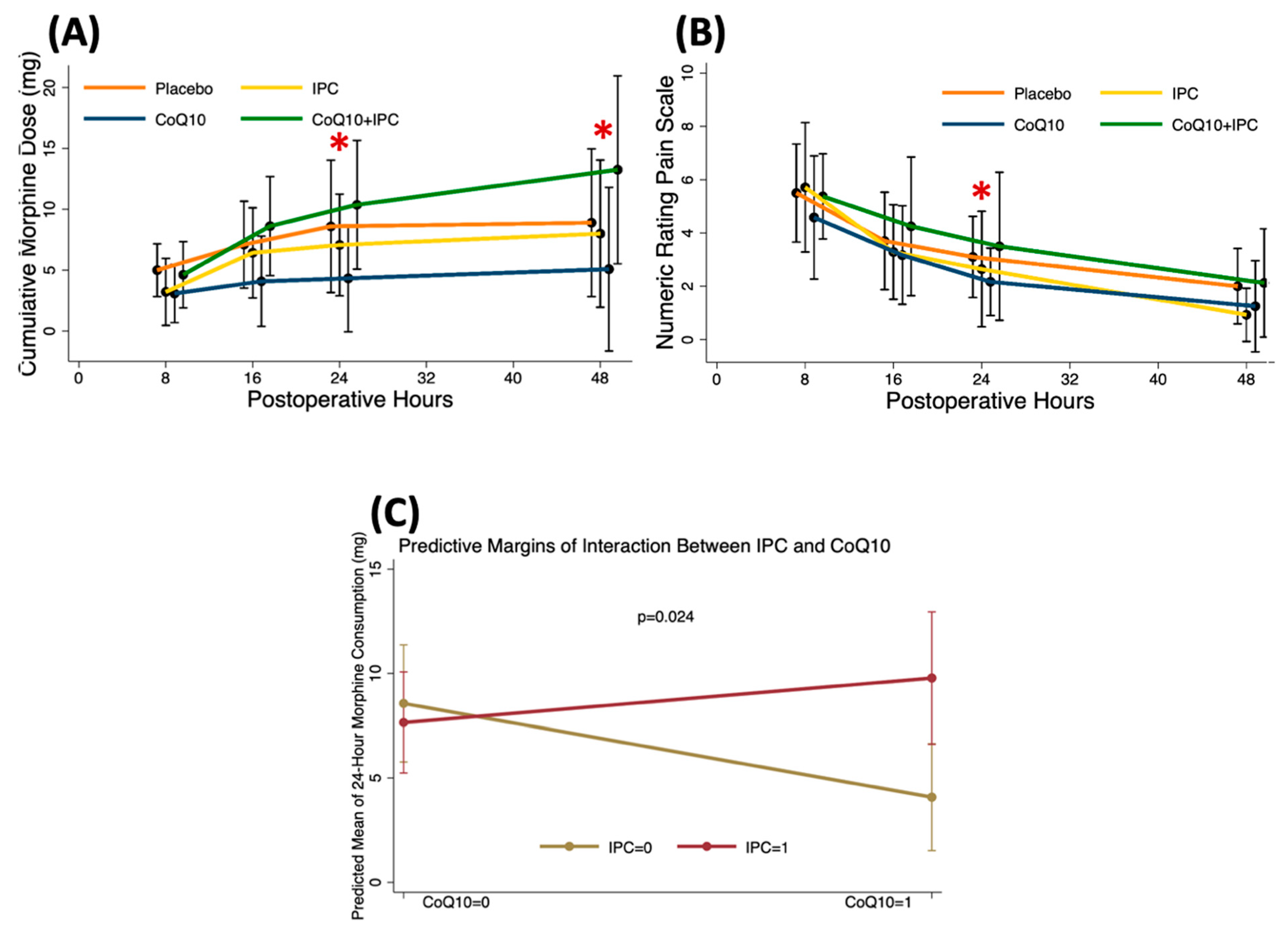
3.3. Effects of CoQ10, IPC, and CoQ10 + IPC on Postoperative Analgesia
3.4. Effects of Perioperative CoQ10 Supplementation on Late Phase of TQ-Induced I/R
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| ETC | electron transport chain |
| IPACK | interspace between the popliteal artery and capsule of the knee |
| IPC | ischemic preconditioning |
| I/R | ischemia and reperfusion |
| mPTP | mitochondrial permeability transition pores |
| NRS | numeric rating scale |
| OA | osteoarthritis |
| OCR | oxygen consumption rate |
| OXPHOS | oxidative phosphorylation |
| PBMC | peripheral blood mononuclear cell |
| ROS | reactive oxygen species |
| SBP | systolic blood pressure |
| TKA | total knee arthroplasty |
| TQ | tourniquet |
References
- Palazzuolo, M.; Antoniadis, A.; Mahlouly, J.; Wegrzyn, J. Total knee arthroplasty improves the quality-adjusted life years in patients who exceeded their estimated life expectancy. Int. Orthop. 2021, 45, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hinton, Z.W.; Fletcher, A.N.; Ryan, S.P.; Wu, C.J.; Bolognesi, M.P.; Seyler, T.M. Body Mass Index, American Society of Anesthesiologists Score, and Elixhauser Comorbidity Index Predict Cost and Delay of Care During Total Knee Arthroplasty. J Arthroplast. 2020, 36, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Keulen, M.H.F.; Asselberghs, S.; Boonen, B.; Hendrickx, R.P.M.; van Haaren, E.H.; Schotanus, M.G.M. Predictors of (Un)successful Same-Day Discharge in Selected Patients Following Outpatient Hip and Knee Arthroplasty. J. Arthroplast. 2020, 35, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Mascioli, A.A.; Shaw, M.L.; Boykin, S.; Mahadevan, P.; Wilder, J.H.; Bell, J.W.; Dabov, G.D.; Toy, P.C. Total Knee Arthroplasty in Freestanding Ambulatory Surgery Centers: 5-Year Retrospective Chart Review of 90-Day Postsurgical Outcomes and Health Care Resource Utilization. J. Am. Acad. Orthop. Surg. 2021, 29, e1184–e1192. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Chawla, A.; Underwood, M.; Price, A.J.; Metcalfe, A.; Hutchinson, C.; Warwick, J.; Seers, K.; Parsons, H.; Wall, P.D. Tourniquet use for knee replacement surgery. Cochrane Database Syst. Rev. 2020, 12, CD012874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Migliorini, F.; Maffulli, N.; Aretini, P.; Trivellas, A.; Tingart, M.; Eschweiler, J.; Baroncini, A. Impact of tourniquet during knee arthroplasty: A bayesian network meta-analysis of peri-operative outcomes. Arch. Orthop. Trauma Surg. 2021, 141, 1007–1023. [Google Scholar] [CrossRef]
- Cai, D.F.; Fan, Q.H.; Zhong, H.H.; Peng, S.; Song, H. The effects of tourniquet use on blood loss in primary total knee arthroplasty for patients with osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2019, 14, 348. [Google Scholar] [CrossRef]
- McCarthy Deering, E.; Hu, S.Y.; Abdulkarim, A. Does Tourniquet Use in TKA Increase Postoperative Pain? A Systematic Review and Meta-analysis. Clin. Orthop. Relat. Res. 2019, 477, 547–558. [Google Scholar] [CrossRef]
- Arthur, J.R.; Spangehl, M.J. Tourniquet Use in Total Knee Arthroplasty. J. Knee Surg. 2019, 32, 719–729. [Google Scholar] [CrossRef]
- Leurcharusmee, P.; Sawaddiruk, P.; Punjasawadwong, Y.; Chattipakorn, N.; Chattipakorn, S.C. The Possible Pathophysiological Outcomes and Mechanisms of Tourniquet-Induced Ischemia-Reperfusion Injury during Total Knee Arthroplasty. Oxid. Med. Cell Longev. 2018, 2018, 8087598. [Google Scholar] [CrossRef]
- Paradis, S.; Charles, A.L.; Meyer, A.; Lejay, A.; Scholey, J.W.; Chakfe, N.; Zoll, J.; Geny, B. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am. J. Physiol. Cell Physiol. 2016, 310, C968–C982. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.P.; Tu, H.; Pipinos, I.I.; Muelleman, R.L.; Albadawi, H.; Li, Y.L. Tourniquet-induced acute ischemia-reperfusion injury in mouse skeletal muscles: Involvement of superoxide. Eur. J. Pharmacol. 2011, 650, 328–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mansour, Z.; Charles, A.L.; Kindo, M.; Pottecher, J.; Chamaraux-Tran, T.N.; Lejay, A.; Zoll, J.; Mazzucotelli, J.P.; Geny, B. Remote effects of lower limb ischemia-reperfusion: Impaired lung, unchanged liver, and stimulated kidney oxidative capacities. Biomed Res. Int. 2014, 2014, 392390. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, A.; Ponelies, N.; Schild, L. Effect of limited ischemia time on the amount and function of mitochondria within human skeletal muscle cells. Eur. J. Trauma Emerg. Surg. 2016, 42, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef]
- Lass, A.; Kwong, L.; Sohal, R.S. Mitochondrial coenzyme Q content and aging. Biofactors 1999, 9, 199–205. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef]
- Schniertshauer, D.; Gebhard, D.; Bergemann, J. Age-Dependent Loss of Mitochondrial Function in Epithelial Tissue Can Be Reversed by Coenzyme Q10. J. Aging Res. 2018, 2018, 6354680. [Google Scholar] [CrossRef]
- Rosenfeldt, F.; Marasco, S.; Lyon, W.; Wowk, M.; Sheeran, F.; Bailey, M.; Esmore, D.; Davis, B.; Pick, A.; Rabinov, M.; et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J. Thorac. Cardiovasc. Surg. 2005, 129, 25–32. [Google Scholar] [CrossRef]
- de Frutos, F.; Gea, A.; Hernandez-Estefania, R.; Rabago, G. Prophylactic treatment with coenzyme Q10 in patients undergoing cardiac surgery: Could an antioxidant reduce complications? A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 254–259. [Google Scholar] [CrossRef]
- Carden, D.L.; Granger, D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Korthuis, R.J.; Gute, D.C.; Cepinskas, G.; Kvietys, P.R. Cellular mechanisms of acute versus delayed preconditioning. Pathophysiology 1998, 5, 35–48. [Google Scholar] [CrossRef]
- Pac-Soo, C.K.; Mathew, H.; Ma, D. Ischaemic conditioning strategies reduce ischaemia/reperfusion-induced organ injury. Br. J. Anaesth. 2015, 114, 204–216. [Google Scholar] [CrossRef]
- Torregroza, C.; Raupach, A.; Feige, K.; Weber, N.C.; Hollmann, M.W.; Huhn, R. Perioperative Cardioprotection: General Mechanisms and Pharmacological Approaches. Anesth. Analg. 2020, 131, 1765–1780. [Google Scholar] [CrossRef]
- Murphy, T.; Walsh, P.M.; Doran, P.P.; Mulhall, K.J. Transcriptional responses in the adaptation to ischaemia-reperfusion injury: A study of the effect of ischaemic preconditioning in total knee arthroplasty patients. J. Transl. Med. 2010, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Memtsoudis, S.G.; Stundner, O.; Yoo, D.; Gonzalez Della Valle, A.; Boettner, F.; Bombardieri, A.M.; Jules-Elysee, K.; Poultsides, L.; Ma, Y.; Sculco, T.P. Does limb preconditioning reduce pain after total knee arthroplasty? A randomized, double-blind study. Clin. Orthop. Relat. Res. 2014, 472, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Memtsoudis, S.G.; Valle, A.G.; Jules-Elysse, K.; Poultsides, L.; Reid, S.; Starcher, B.; Ma, Y.; Sculco, T.P. Perioperative inflammatory response in total knee arthroplasty patients: Impact of limb preconditioning. Reg. Anesth. Pain Med. 2010, 35, 412–416. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, M.; Riou, M.; Charles, A.L.; Talha, S.; Meyer, A.; Andres, E.; Chakfe, N.; Lejay, A.; Geny, B. The Rise of Mitochondria in Peripheral Arterial Disease Physiopathology: Experimental and Clinical Data. J. Clin. Med. 2019, 8, 2125. [Google Scholar] [CrossRef]
- Blaisdell, F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc. Surg. 2002, 10, 620–630. [Google Scholar] [CrossRef]
- Martou, G.; O’Blenes, C.A.; Huang, N.; McAllister, S.E.; Neligan, P.C.; Ashrafpour, H.; Pang, C.Y.; Lipa, J.E. Development of an in vitro model for study of the efficacy of ischemic preconditioning in human skeletal muscle against ischemia-reperfusion injury. J. Appl. Physiol. 2006, 101, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Marriage, B.J.; Clandinin, M.T.; Macdonald, I.M.; Glerum, D.M. Cofactor treatment improves ATP synthetic capacity in patients with oxidative phosphorylation disorders. Mol. Genet. Metab. 2004, 81, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, M.B.; Khayat, Z.K.; Anbari, K.; Niapour, A.; Gholami, M.; Gharravi, A.M. Coenzyme Q10 protects skeletal muscle from ischemia-reperfusion through the NF-kappa B pathway. Perfusion 2017, 32, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Betul, A.Y.; Adoum, B.A. The investigation of the preventive effects of Coenzyme Q10 and Berberine for tourniquet induced ischemia-reperfusion injury on skeletal muscle in rat hindlimb. GSC Biol. Pharm. Sci. 2019, 9, 127–133. [Google Scholar] [CrossRef]
- Hernandez-Camacho, J.D.; Bernier, M.; Lopez-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Duberley, K.E.; Heales, S.J.; Abramov, A.Y.; Chalasani, A.; Land, J.M.; Rahman, S.; Hargreaves, I.P. Effect of Coenzyme Q10 supplementation on mitochondrial electron transport chain activity and mitochondrial oxidative stress in Coenzyme Q10 deficient human neuronal cells. Int. J. Biochem. Cell Biol. 2014, 50, 60–63. [Google Scholar] [CrossRef]
- Bergamini, C.; Moruzzi, N.; Sblendido, A.; Lenaz, G.; Fato, R. A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS ONE 2012, 7, e33712. [Google Scholar] [CrossRef]
- Miles, M.V. The uptake and distribution of coenzyme Q10. Mitochondrion 2007, 7, S72–S77. [Google Scholar] [CrossRef]
- Lee, J.; Hong, Y.S.; Jeong, J.H.; Yang, E.J.; Jhun, J.Y.; Park, M.K.; Jung, Y.O.; Min, J.K.; Kim, H.Y.; Park, S.H.; et al. Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS ONE 2013, 8, e69362. [Google Scholar] [CrossRef]
- Thaveau, F.; Zoll, J.; Rouyer, O.; Chafke, N.; Kretz, J.G.; Piquard, F.; Geny, B. Ischemic preconditioning specifically restores complexes I and II activities of the mitochondrial respiratory chain in ischemic skeletal muscle. J. Vasc. Surg. 2007, 46, 541–547; discussion 547. [Google Scholar] [CrossRef][Green Version]
- Mansour, Z.; Bouitbir, J.; Charles, A.L.; Talha, S.; Kindo, M.; Pottecher, J.; Zoll, J.; Geny, B. Remote and local ischemic preconditioning equivalently protects rat skeletal muscle mitochondrial function during experimental aortic cross-clamping. J. Vasc. Surg. 2012, 55, 497–505.e1. [Google Scholar] [CrossRef][Green Version]
- Kocman, E.A.; Ozatik, O.; Sahin, A.; Guney, T.; Kose, A.A.; Dag, I.; Alatas, O.; Cetin, C. Effects of ischemic preconditioning protocols on skeletal muscle ischemia-reperfusion injury. J. Surg. Res. 2015, 193, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Koca, K.; Yurttas, Y.; Cayci, T.; Bilgic, S.; Kaldirim, U.; Durusu, M.; Cekli, Y.; Ozkan, H.; Hanci, V.; Purtuloglu, T.; et al. The role of preconditioning and N-acetylcysteine on oxidative stress resulting from tourniquet-induced ischemia-reperfusion in arthroscopic knee surgery. J. Trauma 2011, 70, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.; Sutter, P.M.; Marx, A.; Stierli, P.; Heberer, M.; Gurke, L. Preconditioning with short cycles improves ischemic tolerance in rat fast- and slow-twitch skeletal muscle. Eur. Surg. Res. 2000, 32, 297–304. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Liu, X. Effects of various protocols of ischemic preconditioning on rat tram flaps. Microsurgery 2008, 28, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Skyschally, A.; Schulz, R.; Gres, P.; Korth, H.G.; Heusch, G. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals with ascorbic acid. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H698–H703. [Google Scholar] [CrossRef] [PubMed]
- Tsovolas, K.; Iliodromitis, E.K.; Andreadou, I.; Zoga, A.; Demopoulou, M.; Iliodromitis, K.E.; Manolaki, T.; Markantonis, S.L.; Kremastinos, D.T. Acute administration of vitamin C abrogates protection from ischemic preconditioning in rabbits. Pharmacol. Res. 2008, 57, 283–289. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Tsovolas, K.; Aggeli, I.K.; Zoga, A.; Gaitanaki, C.; Paraskevaidis, I.A.; Markantonis, S.L.; Beis, I.; Kremastinos, D.T. Acute administration of vitamin E triggers preconditioning via K(ATP) channels and cyclic-GMP without inhibiting lipid peroxidation. Free. Radic. Biol. Med. 2006, 41, 1092–1099. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Bofilis, E.; Zoga, A.; Constantinou, M.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. Melatonin does not prevent the protection of ischemic preconditioning in vivo despite its antioxidant effect against oxidative stress. Free. Radic. Biol. Med. 2004, 37, 500–510. [Google Scholar] [CrossRef]
- Hoole, S.P.; Heck, P.M.; Sharples, L.; Khan, S.N.; Duehmke, R.; Densem, C.G.; Clarke, S.C.; Shapiro, L.M.; Schofield, P.M.; O’Sullivan, M.; et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: A prospective, randomized control trial. Circulation 2009, 119, 820–827. [Google Scholar] [CrossRef]
- Lee, B.J.; Huang, Y.C.; Chen, S.J.; Lin, P.T. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition 2012, 28, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Y.; Huang, S.; He, M.; Liu, Q.; Chen, W.; Liu, M.; Xu, D.; He, P. Coenzyme Q10 Prevents the Interleukin-1 Beta Induced Inflammatory Response via Inhibition of MAPK Signaling Pathways in Rat Articular Chondrocytes. Drug Dev. Res. 2017, 78, 403–410. [Google Scholar] [CrossRef] [PubMed]

| Placebo (n = 24) | CoQ10 (n = 20) | Mean Difference (95% CI) | |
|---|---|---|---|
| Female, n (%) | 20 (83%) | 16 (80%) | |
| Age (y), mean ± SD | 66.5 ± 5.8 | 70.4 ± 6.3 | 3.9 (0.2, 7.6) |
| BMI (kg/m2), mean ± SD | 26.0 ± 3.6 | 24.4 ± 3.9 | −1.6 (−3.9, 0.7) |
| ASA, n (%) | |||
| II/III | 24 (100%)/0 (0%) | 19 (95%)/1 (5%) | |
| Co-morbidity, n (%) | |||
| Hypertension | 15 (63%) | 15 (75%) | |
| Diabetic mellites | 3 (13%) | 2 (10%) | |
| Dyslipidemia | 11 (46%) | 9 (45%) | |
| Current medication, n (%) | |||
| β-blocker | 1 (4%) | 2 (10%) | |
| Metformin | 2 (8%) | 1 (5%) | |
| Statin | 9 (38%) | 8 (40%) | |
| Side of operation, n (%) | |||
| Right/left | 13 (54%)/11 (46%) | 14 (70%)/6 (30%) | |
| Surgeon 1/surgeon 2 | 11 (46%)/13 (54%) | 11 (55%)/9 (45%) | |
| Operation time (min), mean ± SD | 115.5 ± 29.3 | 111.3 ± 22.9 | −4.2 (−20.5, 12.0) |
| TQ duration (min), mean ± SD | 87.6 ± 17.0 | 87.1 ± 15.9 | −0.6 (−10.7, 9.5) |
| TQ pressure (mmHg), mean ± SD | 252.9 ± 21.2 | 250.5 ± 13.2 | −2.4 (−13.4, 8.6) |
| Intraoperative oxygen use, n (%) | 7 (29%) | 2 (10%) | |
| Intraoperative hypotension, n (%) | 6 (25%) | 4 (20%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leurcharusmee, P.; Sawaddiruk, P.; Punjasawadwong, Y.; Sugundhavesa, N.; Klunklin, K.; Tongprasert, S.; Sitilertpisan, P.; Jaiwongkam, T.; Apaijai, N.; Chattipakorn, N.; et al. CoenzymeQ10 and Ischemic Preconditioning Potentially Prevent Tourniquet-Induced Ischemia/Reperfusion in Knee Arthroplasty, but Combined Pretreatment Possibly Neutralizes Their Beneficial Effects. Antioxidants 2022, 11, 419. https://doi.org/10.3390/antiox11020419
Leurcharusmee P, Sawaddiruk P, Punjasawadwong Y, Sugundhavesa N, Klunklin K, Tongprasert S, Sitilertpisan P, Jaiwongkam T, Apaijai N, Chattipakorn N, et al. CoenzymeQ10 and Ischemic Preconditioning Potentially Prevent Tourniquet-Induced Ischemia/Reperfusion in Knee Arthroplasty, but Combined Pretreatment Possibly Neutralizes Their Beneficial Effects. Antioxidants. 2022; 11(2):419. https://doi.org/10.3390/antiox11020419
Chicago/Turabian StyleLeurcharusmee, Prangmalee, Passakorn Sawaddiruk, Yodying Punjasawadwong, Nantawit Sugundhavesa, Kasisin Klunklin, Siam Tongprasert, Patraporn Sitilertpisan, Thidarat Jaiwongkam, Nattayaporn Apaijai, Nipon Chattipakorn, and et al. 2022. "CoenzymeQ10 and Ischemic Preconditioning Potentially Prevent Tourniquet-Induced Ischemia/Reperfusion in Knee Arthroplasty, but Combined Pretreatment Possibly Neutralizes Their Beneficial Effects" Antioxidants 11, no. 2: 419. https://doi.org/10.3390/antiox11020419
APA StyleLeurcharusmee, P., Sawaddiruk, P., Punjasawadwong, Y., Sugundhavesa, N., Klunklin, K., Tongprasert, S., Sitilertpisan, P., Jaiwongkam, T., Apaijai, N., Chattipakorn, N., & Chattipakorn, S. C. (2022). CoenzymeQ10 and Ischemic Preconditioning Potentially Prevent Tourniquet-Induced Ischemia/Reperfusion in Knee Arthroplasty, but Combined Pretreatment Possibly Neutralizes Their Beneficial Effects. Antioxidants, 11(2), 419. https://doi.org/10.3390/antiox11020419






