Effect of Dietary Grapes on Female C57BL6/J Mice Consuming a High-Fat Diet: Behavioral and Genetic Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets and Freeze-Dried Grape Powder
2.2. Animals and Feeding
2.3. Behavioral Tests
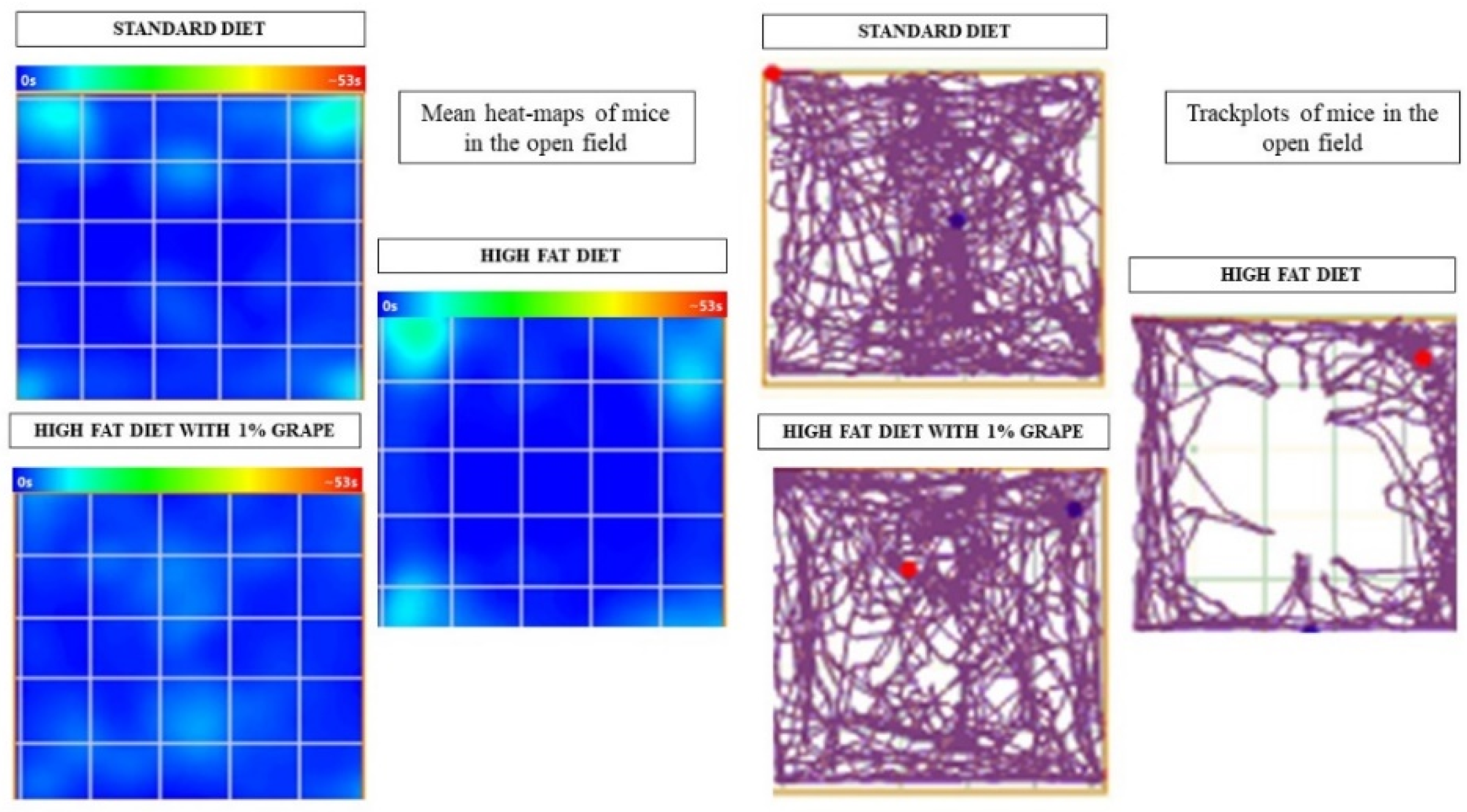
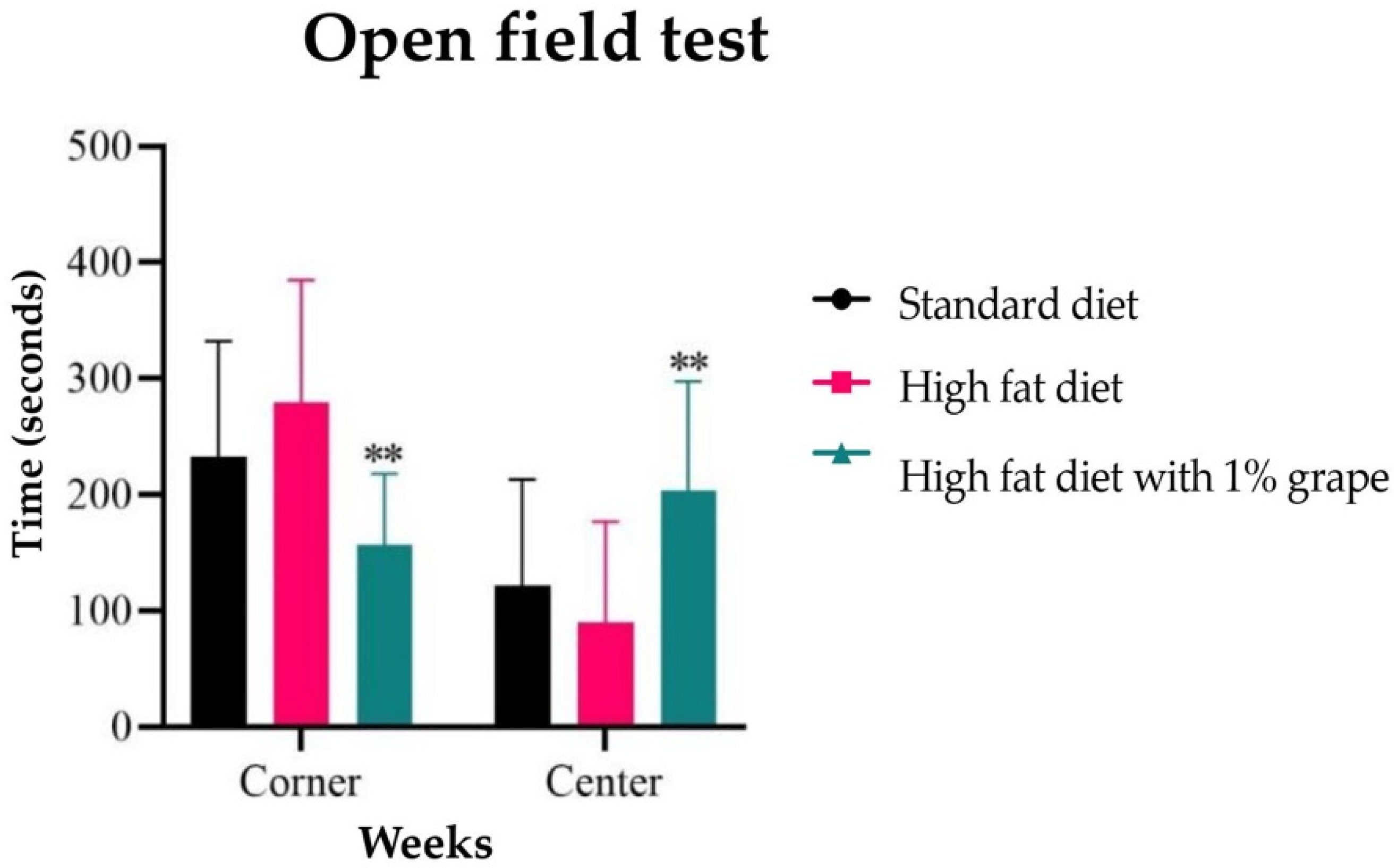
2.3.1. Open Field Test
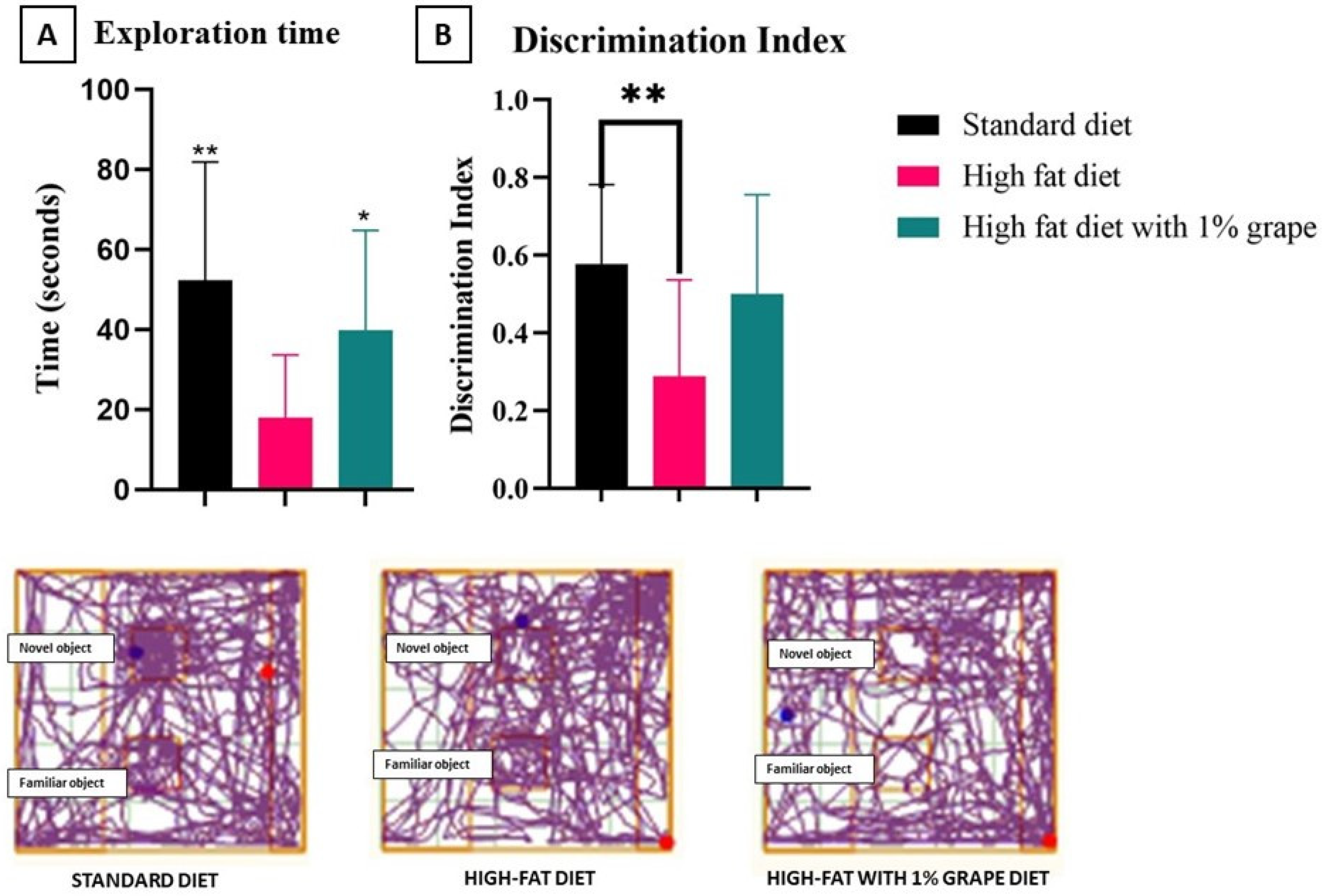
2.3.2. Novel Object Recognition Test (NORT)
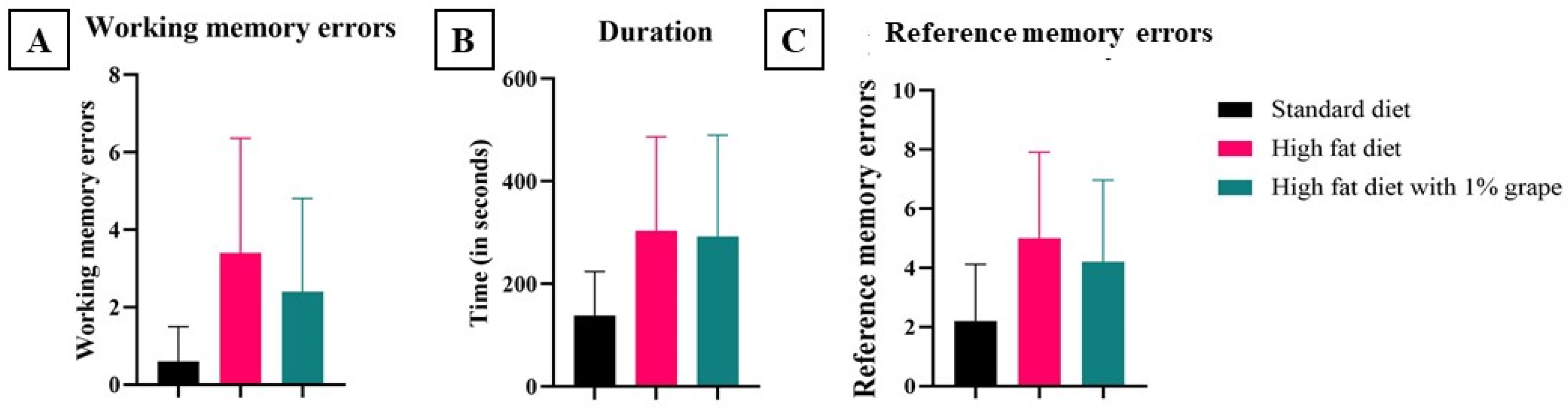
2.3.3. Radial Arm Maze
2.4. Brain Tissue Collection
2.5. RNA-Extraction and Sequencing
2.6. Differentially Expressed Genes
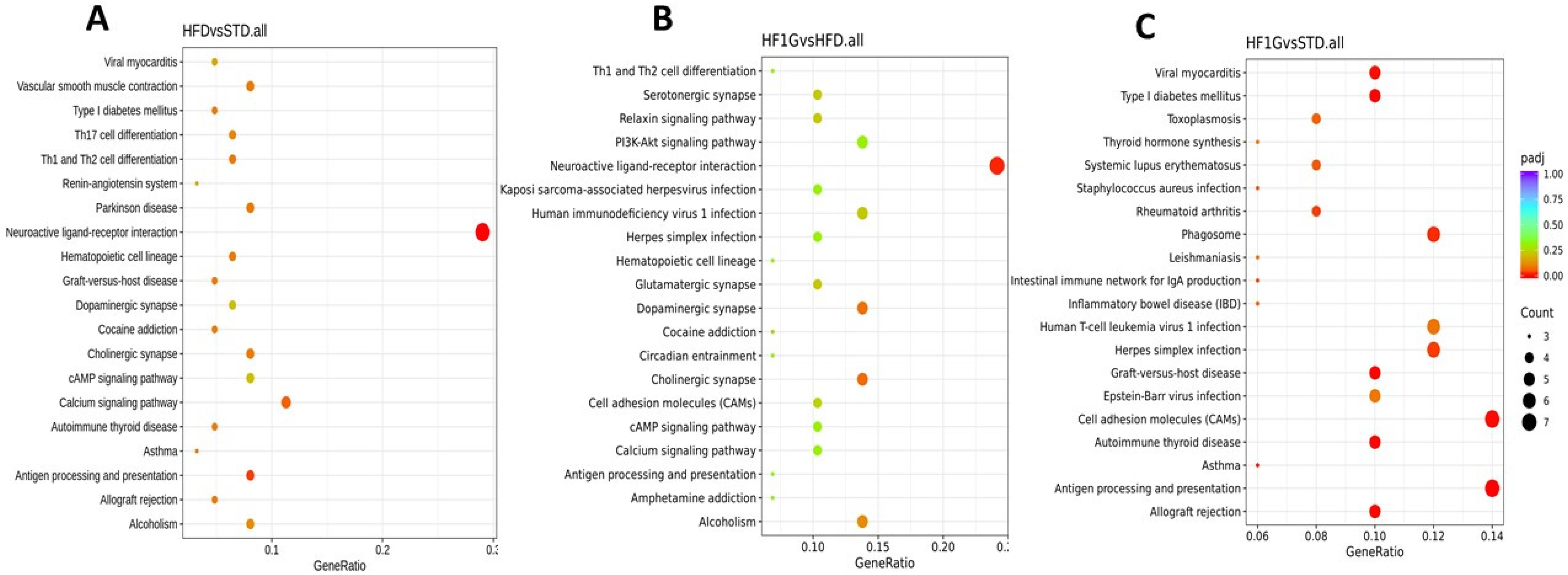
2.7. Gene Ontology
2.8. KEGG Pathway Analyses
2.9. Statistical Analyses
3. Results
3.1. Animal Body Weight
3.2. Behavioral Assays
3.2.1. Open Field Test
3.2.2. Novel Object Recognition Test (NORT)
3.2.3. Radial Arm Maze
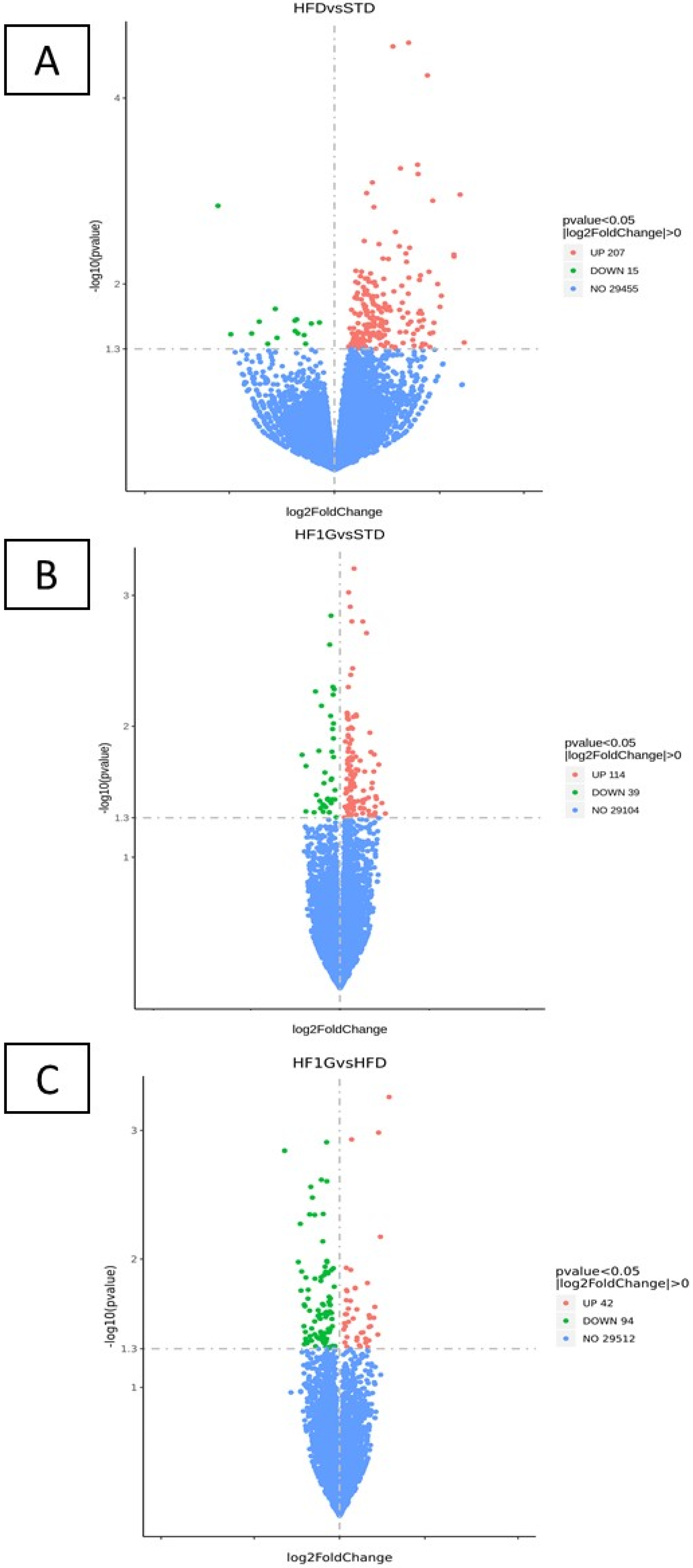
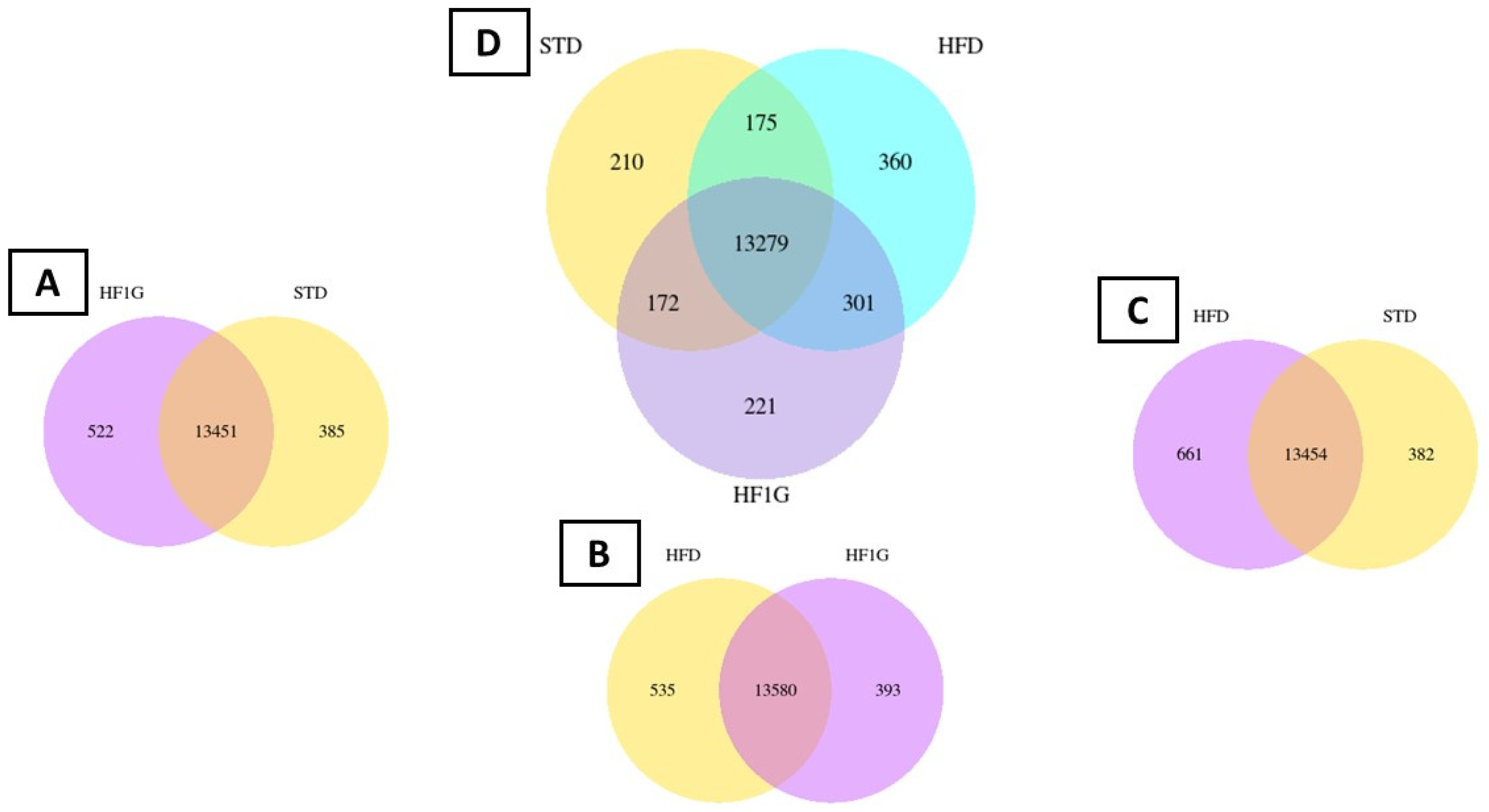
3.3. RNA-Seq Data Analysis
3.3.1. Differentially Expressed Genes
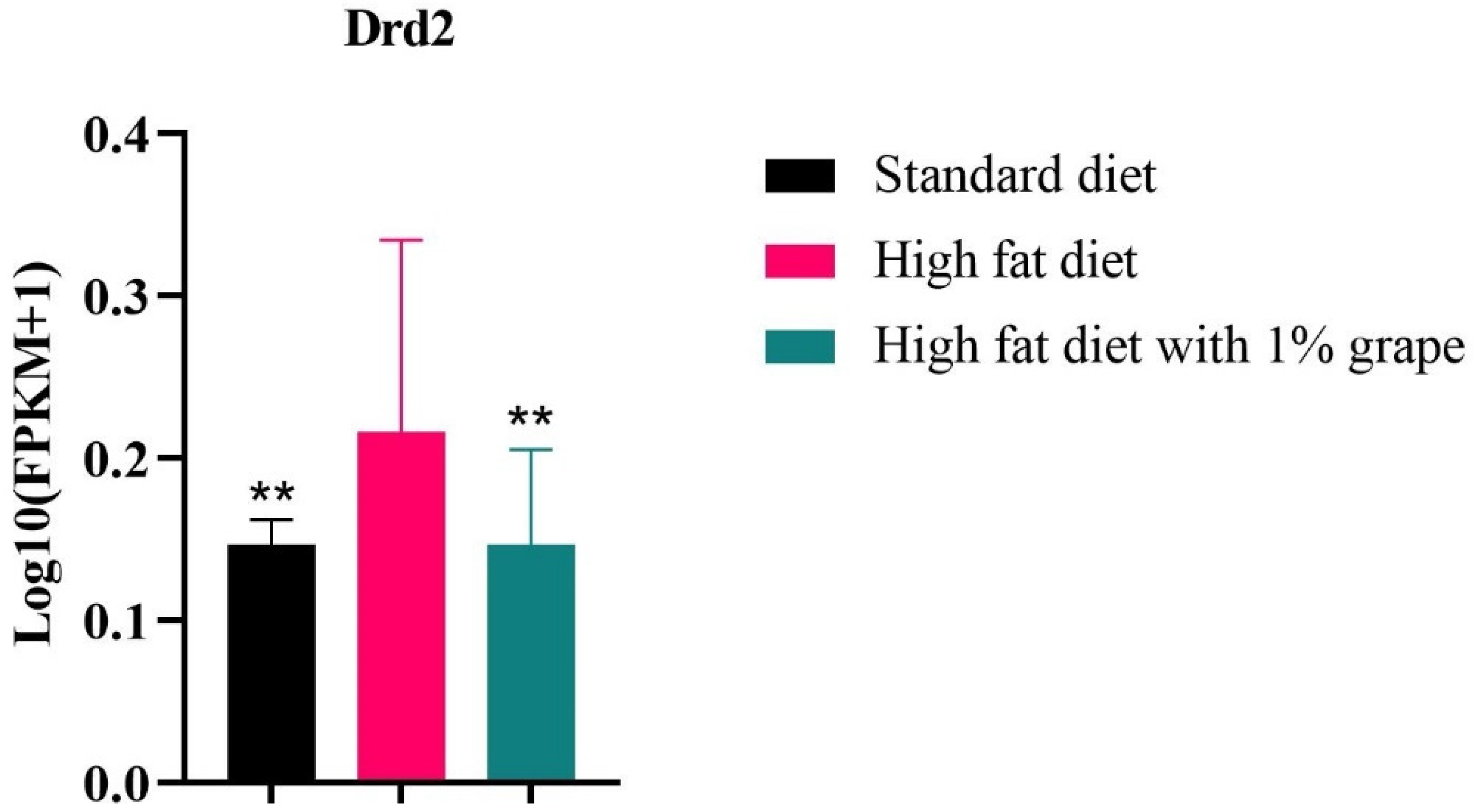
3.3.2. Dopamine Receptor 2 in Whole Brain
3.3.3. Genes Associated with Feeding Behavior
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shook, L.L.; Kislal, S.; Edlow, A.G. Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat. Diagn. 2020, 40, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Tantot, F.; Parkes, S.L.; Marchand, A.R.; Boitard, C.; Naneix, F.; Layé, S.; Trifilieff, P.; Coutureau, E.; Ferreira, G. The effect of high-fat diet consumption on appetitive instrumental behavior in rats. Appetite 2017, 108, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M. The phenomenon of resveratrol: Redefining the virtues of promiscuity. Ann. N. Y. Acad. Sci. 2011, 1215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bulloj, A.; Finnemann, S.C. Grapes and Vision. In Grapes and Health; Pezzuto, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 213–235. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Ali, Q.; Pokkunuri, I.; Asghar, M.; Salim, S. Grape powder treatment prevents anxiety-like behavior in a rat model of aging. Nutr. Res. 2015, 35, 504–511. [Google Scholar] [CrossRef]
- Calapai, G.; Bonina, F.; Bonina, A.; Rizza, L.; Mannucci, C.; Arcoraci, V.; Laganà, G.; Alibrandi, A.; Pollicino, C.; Inferrera, S.; et al. A Randomized, Double-Blinded, Clinical Trial on Effects of a Vitis vinifera Extract on Cognitive Function in Healthy Older Adults. Front. Pharmacol. 2017, 8, 776. [Google Scholar] [CrossRef] [Green Version]
- Pezzuto, J.M. Grapes and human health: A perspective. J. Agric. Food Chem. 2008, 56, 6777–6784. [Google Scholar] [CrossRef]
- Maher, P. Grapes and the Brain. In Grapes and Health; Pezzuto, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–161. [Google Scholar]
- van Breemen, R.B.; Wright, B.; Li, Y.; Nosal, D.; Burton, T. Standardized Grape Powder for Basic and Clinical Research. In Grapes and Health; Pezzuto, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–26. [Google Scholar]
- Meziane, H.; Ouagazzal, A.M.; Aubert, L.; Wietrzych, M.; Krezel, W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes Brain Behav. 2007, 6, 192–200. [Google Scholar] [CrossRef]
- Joshi, T.; Patel, I.; Kumar, A.; Donovan, V.; Levenson, A.S. Grape Powder Supplementation Attenuates Prostate Neoplasia Associated with Pten Haploinsufficiency in Mice Fed High-Fat Diet. Mol. Nutr. Food Res. 2020, 64, e2000326. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, J.Á.; Zafrilla, M.P.; Marhuenda, J. Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toribio-Mateas, M. Harnessing the Power of Microbiome Assessment Tools as Part of Neuroprotective Nutrition and Lifestyle Medicine Interventions. Microorganisms 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J substrain differences in response to high-fat diet intervention. Sci. Rep. 2020, 10, 14052. [Google Scholar] [CrossRef]
- Bryant, C.D. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann. N. Y. Acad. Sci. 2011, 1245, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.S. Memory decline: The boundary between aging and age-related disease. Ann. Neurol. 2002, 51, 282–284. [Google Scholar] [CrossRef]
- Berahmand, F.; Anoush, G.; Hosseini, M.J.; Anoush, M. Grape Seed Oil as a Natural Therapy in Male Rats with Alzheimer’s Diseases. Adv. Pharm. Bull. 2020, 10, 430–436. [Google Scholar] [CrossRef]
- Lin, B.; Hasegawa, Y.; Takane, K.; Koibuchi, N.; Cao, C.; Kim-Mitsuyama, S. High-Fat-Diet Intake Enhances Cerebral Amyloid Angiopathy and Cognitive Impairment in a Mouse Model of Alzheimer’s Disease, Independently of Metabolic Disorders. J. Am. Heart Assoc. 2016, 5, e003154. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.M.; Ryan, B.; Kyrke-Smith, M.; Logan, B.; Tate, W.P.; Abraham, W.C.; Williams, J.M. Temporal profiling of gene networks associated with the late phase of long-term potentiation in vivo. PLoS ONE 2012, 7, e40538. [Google Scholar] [CrossRef] [PubMed]
- Dudek, S.M.; Fields, R.D. Gene Expression in Hippocampal Long-Term Potentiation. Neuroscientist 1999, 5, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Lisachev, P.D.; Shtark, M.B. Long-Term Potentiation-Associated Gene Expression: Involvement of the Tumour Protein p53. In The Hippocampus: Plasticity and Functions; Stuchlik, A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Haleem, D.J.; Mahmood, K. Brain serotonin in high-fat diet-induced weight gain, anxiety and spatial memory in rats. Nutr. Neurosci. 2021, 24, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Keralapurath, M.M.; Lin, Z.; Wagner, J.J.; de La Serre, C.B.; Harn, D.A.; Filipov, N.M. Neurochemical and electrophysiological deficits in the ventral hippocampus and selective behavioral alterations caused by high-fat diet in female C57BL/6 mice. Neuroscience 2015, 297, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Alkadhi, I.; Atrooz, F.; Patki, G.; Salim, S. Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr. Res. 2015, 35, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Allam, F.; Dao, A.T.; Chugh, G.; Bohat, R.; Jafri, F.; Patki, G.; Mowrey, C.; Asghar, M.; Alkadhi, K.A.; Salim, S. Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. J. Nutr. 2013, 143, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Cagniard, B.; Balsam, P.D.; Brunner, D.; Zhuang, X. Mice with Chronically Elevated Dopamine Exhibit Enhanced Motivation, but not Learning, for a Food Reward. Neuropsychopharmacology 2006, 31, 1362–1370. [Google Scholar] [CrossRef] [Green Version]
- Phillips, T.J.; Brown, K.J.; Burkhart-Kasch, S.; Wenger, C.D.; Kelly, M.A.; Rubinstein, M.; Grandy, D.K.; Low, M.J. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat. Neurosci. 1998, 1, 610–615. [Google Scholar] [CrossRef]
- Han, J.; Nepal, P.; Odelade, A.; Freely, F.D.; Belton, D.M.; Graves, J.L., Jr.; Maldonado-Devincci, A.M. High-Fat Diet-Induced Weight Gain, Behavioral Deficits, and Dopamine Changes in Young C57BL/6J Mice. Front. Nutr. 2021, 7, 591161. [Google Scholar] [CrossRef]
- Bertelli, M.; Cecchin, S.; Lapucci, C.; Jacomelli, G.; Jinnah, H.A.; Pandolfo, M.; Micheli, V. Study of the adenosinergic system in the brain of HPRT knockout mouse (Lesch–Nyhan disease). Clin. Chim. Acta 2006, 373, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Pankevich, D.E.; Teegarden, S.L.; Hedin, A.D.; Jensen, C.L.; Bale, T.L. Caloric Restriction Experience Reprograms Stress and Orexigenic Pathways and Promotes Binge Eating. J. Neurosci. 2010, 30, 16399–16407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.; Lee, G.; Kong, J.; Choi, G.; Park, Y.J.; Kim, J.B. Feeding Period Restriction Alters the Expression of Peripheral Circadian Rhythm Genes without Changing Body Weight in Mice. PLoS ONE 2012, 7, e49993. [Google Scholar] [CrossRef] [Green Version]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, R.J.; Hoggard, N.; Aceves-Martins, M. The effect of grape interventions on cognitive and mental performance in healthy participants and those with mild cognitive impairment: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef] [PubMed]



| Standard Diet | High-Fat Diet | High-Fat Diet with 1% Grape Powder | |
|---|---|---|---|
| STD | HFD | HF1G | |
| g/kg Diet | |||
| Casein | 195 | 195 | 191 |
| DL-Methionine | 3 | 3 | 3 |
| Sucrose | 191 | 191 | 191 |
| Dextrose (anhydrous) | 66 | 65 | 62.454 |
| Fructose | 66 | 65 | 62.454 |
| Corn Starch | 235 | 167.43 | 166.22 |
| Anhydrous Milkfat | 30 | 210 | 210 |
| Cholesterol | 0 | 1.5 | 1.5 |
| Cellulose | 50 | 50 | 50 |
| Mineral Mix, AIN-76 (170915) | 35 | 35 | 35 |
| Calcium Carbonate | 4 | 4 | 4 |
| Potassium Citrate (monohydrate) | 4 | 4 | 4 |
| Vitamin Mix, Teklad (40060) | 10 | 10 | 10 |
| Ethoxyquin (antioxidant) | 0.04 | 0.04 | 0.04 |
| Grape Powder, freeze-dried | 0 | 0 | 10 |
| GO ID | Annotation | HFD vs. STD | HF1G vs. HFD | |
|---|---|---|---|---|
| GO:0014059 | Biological Process | Regulation of dopamine secretion by D2 receptor | Upregulated | Downregulated |
| GO:0098691 | Cellular Component | Dopaminergic synapse | Upregulated | Downregulated |
| GO:0035240 | Molecular Function | Dopamine binding | Upregulated | Downregulated |
| P05912 | Panther pathways | Dopamine receptor-mediated signaling pathway | Upregulated | Downregulated |
| P00026 | Panther Pathways | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | Upregulated | Downregulated |
| GO Annotation | Gene Name | HFD vs. STD | HF1G vs. HFD | ||
|---|---|---|---|---|---|
| Fold Change | p-Value | Fold Change | p-Value | ||
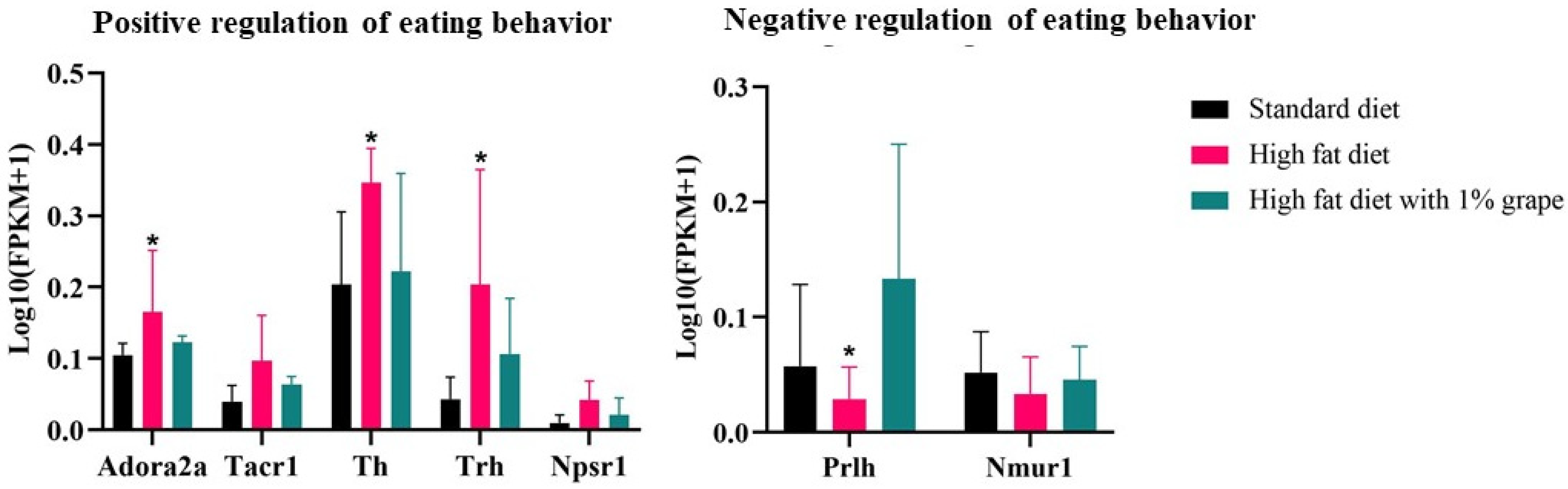
| Positive feeding behavior | Trh | 4.42 | 0.00 | −2.43 | 0.03 |
| Th | 2.12 | 0.00 | −1.52 | 0.07 | |
| Adora2a | 1.57 | 0.02 | −1.23 | 0.08 | |
| Tacr1 | 1.85 | 0.01 | −1.07 | 0.12 | |
| Npsr1 | 2.40 | 0.03 | −0.34 | 0.3 | |
| Negative feeding behavior | Prlh | −1.50 | 0.48 | 3.37 | 0.04 |
| Nmur1 | −0.71 | 0.53 | 0.68 | 0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parande, F.; Dave, A.; Park, E.-J.; McAllister, C.; Pezzuto, J.M. Effect of Dietary Grapes on Female C57BL6/J Mice Consuming a High-Fat Diet: Behavioral and Genetic Changes. Antioxidants 2022, 11, 414. https://doi.org/10.3390/antiox11020414
Parande F, Dave A, Park E-J, McAllister C, Pezzuto JM. Effect of Dietary Grapes on Female C57BL6/J Mice Consuming a High-Fat Diet: Behavioral and Genetic Changes. Antioxidants. 2022; 11(2):414. https://doi.org/10.3390/antiox11020414
Chicago/Turabian StyleParande, Falguni, Asim Dave, Eun-Jung Park, Christopher McAllister, and John M. Pezzuto. 2022. "Effect of Dietary Grapes on Female C57BL6/J Mice Consuming a High-Fat Diet: Behavioral and Genetic Changes" Antioxidants 11, no. 2: 414. https://doi.org/10.3390/antiox11020414
APA StyleParande, F., Dave, A., Park, E.-J., McAllister, C., & Pezzuto, J. M. (2022). Effect of Dietary Grapes on Female C57BL6/J Mice Consuming a High-Fat Diet: Behavioral and Genetic Changes. Antioxidants, 11(2), 414. https://doi.org/10.3390/antiox11020414






