Fruit-Derived Anthocyanins: Effects on Cycling-Induced Responses and Cycling Performance
Abstract
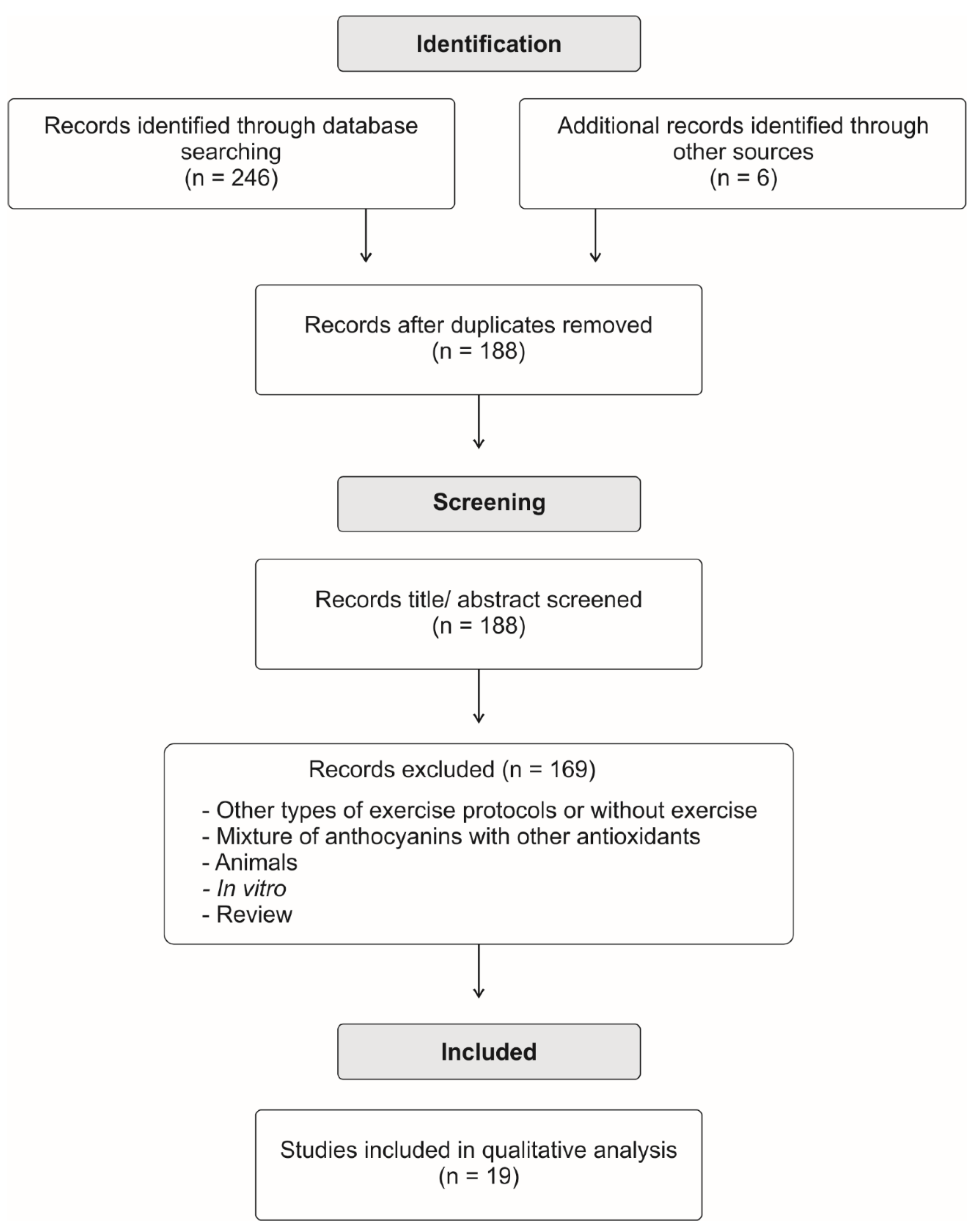
1. Introduction
2. Research Strategy
3. Fruit-Derived Anthocyanins on Cycling-Induced Responses and Cycling Performance
3.1. Oxidative Stress, Inflammation, Muscle Damage, and Fatigue
3.2. Nitric Oxide Biomarkers, Vascular Function, Muscle Oxygenation, and Performance
3.3. Substrate Oxidation and Cardiometabolic Markers
4. Interpretation of Research Findings
5. Methodological Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Harborne, J.B.; Grayer, R.J. The anthocyanins. In The Flavonoids: Advances in Research since 1980; Chapmam & Hall: London, UK, 1988; pp. 1–20. [Google Scholar]
- Brouillard, R. Chemical structure of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 1–40. [Google Scholar]
- Khoo, H.E.; Alzan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2020, 104, 67–90. [Google Scholar] [CrossRef]
- Lima, V.L.A.G.; Pinheiro, I.O.; Nascimento, M.S.; Gomes, P.B.; Guerra, N.B. Identificação de antocianidinas em acerolas do banco ativo de germoplasma da Universidade Federal Rural de Pernambuco. Ciênc. Tecnol. Aliment. 2006, 26, 927–935. [Google Scholar] [CrossRef][Green Version]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 3rd ed.; Springer: Garching, Germany, 2004. [Google Scholar]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency Cherries Reduce the Oxidative Stress and Inflammatory Responses to Repeated Days High-Intensity Stochastic Cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- Toscano, L.T.; Tavares, R.L.; Toscano, L.T.; da Silva, C.S.O.; de Almeida, A.E.M.; Biasoto, A.C.T.; Gonçalves, M.C.R.; Silva, A.S. Potential ergogenic activity of grape juice in runners. Appl. Physiol. Nutr. Metab. 2015, 40, 899–906. [Google Scholar] [CrossRef]
- Bloedon, T.K.; Braithwaite, R.E.; Carson, I.A.; Klimis-Zacas, D.; Lehnhard, R.A. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 630–645. [Google Scholar] [CrossRef]
- Copetti, C.L.K.; Orssatto, L.B.R.; Diefenthaeler, F.; Silveira, T.T.; da Silva, E.L.; de Liz, S.; Mendes, B.C.; Rieger, D.K.; Vieira, F.G.K.; Hinnig, P.F.; et al. Acute effect of juçara juice (Euterpe edulis Martius) on oxidative stress biomarkers and fatigue in a high-intensity interval training session: A single-blind cross-over randomized study. J. Funct. Foods 2020, 67, 103835. [Google Scholar] [CrossRef]
- Desai, T.; Bottoms, L.; Roberts, M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur. J. Appl. Physiol. 2018, 118, 2523–2539. [Google Scholar] [CrossRef]
- Petrovic, S.; Arsic, A.; Glibetic, M.; Cikiriz, N.; Jakovljevic, V.; Vucic, V. The effects of polyphenol-rich chokeberry juice on fatty acid profiles and lipid peroxidation of active handball players: Results from a randomized, double-blind, placebo-controlled study. Can. J. Physiol. Pharmacol. 2016, 94, 1058–1063. [Google Scholar] [CrossRef]
- Sadowska-Kreppa, E.; Klapcinska, B.; Podgórski, T.; Szade, B.; Tyl, K.; Hadzik, A. Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol. Sport 2015, 32, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, S.I.B.M.; Galan, B.S.M.; De Carvalho, F.G.; Venancio, V.P.; Antunes, L.M.G.; Papoti, M.; Toro, M.J.U.; Da Costa, I.F.; De Freitas, E.C. Açai pulp supplementation as a nutritional strategy to prevent oxidative damage, improve oxidative status, and modulate blood lactate of male cyclists. Eur. J. Nutr. 2019, 59, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate supplementation accelerates recovery of muscle damage and soreness and inflammatory markers after a weightlifting training session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.C.; Copetti, C.L.K.; Panza, V.S.P.; Orsatto, L.B.R.; Rosa, J.S.; Diefenthaeler, F.; Dalmarco, E.M.; Di Pietro, P.F.; Rieger, D.K. Effects of Euterpe edulis Martius on inflammatory responses to high-intensity intermittent exercise: Cross-over randomized trial: Effect of juçara juice on inflammatory state after HIIE. Nutrition 2021, 91–92, 111344. [Google Scholar] [CrossRef]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Cruz, I.A.; Mendes, R.R.; Gomes, J.H.; Oliveira e Silva, A.M.; Souza, R.F.; Oliveira, A.S. Effects of chronic supplementation of açaí on the muscle damage in track runners. J. Phys. Educ. 2019, 30, e3012. [Google Scholar] [CrossRef]
- Godwin, C.; Cook, M.D.; Willems, M.E.T. Effect of New Zealand Blackcurrant Extract on Performance during the Running Based Anaerobic Sprint Test in Trained Youth and Recreationally Active Male Football Players. Sports 2017, 5, 69. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Cousins, L.; Williams, D.; Blacker, S.D. Beneficial Effects of New Zealand Blackcurrant Extract on Maximal Sprint Speed during the Loughborough Intermittent Shuttle Test. Sports 2016, 4, 42. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Dose effects of New Zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.A.; Willems, M.E.T.; Shepherd, S.O. New Zealand blackcurrant extract enhances fat oxidation during prolonged cycling in endurance-trained females. Eur. J. Appl. Physiol. 2018, 118, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Kelly, V. Fruit Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Cook, M.D.; Willems, M.E.T. Dietary Anthocyanins: A Review of the Exercise Performance Effects and Related Physiological Responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of montmorency tart cherry (L. Prunus cerasus) consumption on nitric oxide biomarkers and exercise performance. Scand. J. Med. Sci. Sports 2018, 28, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.T.; Barton, M.J.; Bowtell, J.L. Montmorency cherry supplementation improves 15-km cycling time trial performance. Eur. J. Appl. Physiol. 2019, 119, 675–684. [Google Scholar] [CrossRef]
- Murphy, C.A.; Cook, M.D.; Willems, M.E.T. Effect of New Zealand Blackcurrant Extract on Repeated Cycling Time Trial Performance. Sports 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Cycling. In Nutrition in Sport; Maughan, R.J., Ed.; Blackwell Science Ltd.: Oxford, UK, 2000. [Google Scholar]
- 2021 Outdoor Participation Trends Report. Available online: https://outdoorindustry.org/wp-content/uploads/2015/03/2021-Outdoor-Participation-Trends-Report.pdf (accessed on 19 January 2022).
- Walking and Cycling Statistics, England. 2020. Available online: https://www.gov.uk/government/statistics/walking-and-cycling-statistics-england-2020/walking-and-cycling-statistics-england-2020 (accessed on 19 January 2022).
- Union Cycliste Internationale (UCI) Annual Report. Available online: https://assets.ctfassets.net/761l7gh5x5an/6vcY4Oi10QENlnBqoPOnxi/ce34be6a60bdeb394d93a680a69ebb68/2020-uci-rapport-annuel-inside-english-web.pdf (accessed on 19 January 2022).
- Lucia, A.; Hoyos, J.; Chicharro, J.L. Physiology of Professional Road Cycling. Sports Med. 2013, 31, 325–337. [Google Scholar] [CrossRef]
- De Pauw, K.; Roelans, B.; Cheung, S.S.; Geus, B.; de Rietjens, G.; Meeusen, R. Guidelines to Classify Subject Groups in Sport-Science Research. Int. J. Sports Physiol. Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef]
- Santilla, M.; Häkkinen, K.; Pihlainen, K.; Kyröläinen, H. Comparison between direct and predicted maximal oxygen uptake measurement during cycling. Mil. Med. 2013, 178, 234–238. [Google Scholar] [CrossRef]
- Torregrosa-García, A.; Ávila-Gandía, V.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; López-Román, F.J. Pomegranate Extract Improves Maximal Performance of Trained Cyclists after an Exhausting Endurance Trial: A Randomised Controlled Trial. Nutrients 2019, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Crum, E.M.; Muhamed, A.M.C.; Barnes, M.; Stannard, S.R. The effect of acute pomegranate extract supplementation on oxygen uptake in highly-trained cyclists during high-intensity exercise in a high-altitude environment. J. Int. Soc. Sports Nutr. 2017, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Scott, A.; Mitchell, N. The influence of different sources of polyphenols on submaximal cycling and time trial performance. J. Athl. Enhanc. 2013, 2, S10. [Google Scholar]
- Willems, M.E.T.; Sahin, M.A.; Berendsen, T.; Cook, M.D. Effect of New Zealand Blackcurrant Extract on Cycling Performance and Substrate Oxidation in Normobaric Hypoxia in Trained Cyclists. Sports 2019, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.E.T.; Myers, S.D.; Gault, M.L.; Cook, M.D. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Montanari, S.; Sahin, M.A.; Lee, B.J.; Blacker, S.D.; Willems, M.E.T. No Effects of New Zealand Blackcurrant Extract on Physiological and Performance Responses in Trained Male Cyclists Undertaking Repeated Testing across a Week Period. Sports 2020, 8, 114. [Google Scholar] [CrossRef]
- Montanari, S.; Sahin, M.A.; Lee, B.J.; Blacker, S.D.; Willems, M.E.T. No Effects of Different Doses of New Zealand Blackcurrant Extract on Cardiovascular Responses During Rest and Submaximal Exercise Across a Week in Trained Male Cyclists. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 66–72. [Google Scholar] [CrossRef]
- Bell, P.G.; Gaze, D.C.; Davison, G.W.; George, T.W.; Scotter, M.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. J. Funct. Foods 2014, 11, 82–90. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Singh, B.; Pal, J.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Borges, G.S.C.; Gonzaga, L.V.; Seraglio, S.K.T.; Olivo, I.S.; Azevedo, M.S.; Nehring, P.; De Gois, J.S.; De Almeida, T.S.; Vitali, L.; et al. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res. Int. 2015, 77, 125–131. [Google Scholar] [CrossRef]
- Borges, G.S.C.; Vieira, F.G.K.; Copetti, C.; Gonzaga, L.V.; Zambiazi, R.C.; Mancini Filho, J.; Fett, R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Res. Int. 2011, 44, 2128–2133. [Google Scholar] [CrossRef]
- Rosso, V.V.; Hillebrand, S.; Montill, A.E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and acai (Euterpe oleracea Mart.) by HPLC-PD A–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; De Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar] [CrossRef]
- Paschalis, V.; Theodorou, A.A.; Margaritelis, N.V.; Kyparos, A.; Nikolaidis, M.G. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic. Biol. Med. 2018, 1, 288–297. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Kalt, W.; Liu, Y.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Anthocyanin metabolites are abundant and persistent in human urine. J. Agric. Food Chem. 2014, 62, 3926–3934. [Google Scholar] [CrossRef]
- Shanmuganayagam, D.; Beahm, M.R.; Osman, H.E.; Krueger, C.G.; Reed, J.D.; Folts, J.D. Grape seed and grape skin extracts elicit a greater antiplatelet effect when used in combination than when used individually in dogs and humans. J. Nutr. 2002, 132, 3592–3598. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copetti, C.L.K.; Diefenthaeler, F.; Hansen, F.; Vieira, F.G.K.; Di Pietro, P.F. Fruit-Derived Anthocyanins: Effects on Cycling-Induced Responses and Cycling Performance. Antioxidants 2022, 11, 387. https://doi.org/10.3390/antiox11020387
Copetti CLK, Diefenthaeler F, Hansen F, Vieira FGK, Di Pietro PF. Fruit-Derived Anthocyanins: Effects on Cycling-Induced Responses and Cycling Performance. Antioxidants. 2022; 11(2):387. https://doi.org/10.3390/antiox11020387
Chicago/Turabian StyleCopetti, Cândice L. K., Fernando Diefenthaeler, Fernanda Hansen, Francilene G. K. Vieira, and Patricia F. Di Pietro. 2022. "Fruit-Derived Anthocyanins: Effects on Cycling-Induced Responses and Cycling Performance" Antioxidants 11, no. 2: 387. https://doi.org/10.3390/antiox11020387
APA StyleCopetti, C. L. K., Diefenthaeler, F., Hansen, F., Vieira, F. G. K., & Di Pietro, P. F. (2022). Fruit-Derived Anthocyanins: Effects on Cycling-Induced Responses and Cycling Performance. Antioxidants, 11(2), 387. https://doi.org/10.3390/antiox11020387






