Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Materials
2.2. Lyophilized Sesame Seed Coats Extract (LSSCE)
2.3. Refined Olive Oil and LSSCE
2.4. Phenolic and Flavonoid Contents of LSSCE
2.5. Antioxidant Ability of LSSCE
2.6. HPLC-DAD of LSSCE
2.6.1. Determination of Phenolics
2.6.2. Determinations of Lignins (Sesamin and Sesamolin)
2.7. Fatty Acids Profile
2.8. Quality Parameters of Olive Oil and Chemical Kinetic
2.9. Antioxidant Indices
2.10. Organoleptic Assessment
2.11. Data Analysis
3. Results and Discussion
3.1. ROO Profile and Phenolic Compounds
3.2. Phytochemicals and Antioxidant Activity of Lyophilized Sesame Seed Coats Extract (LSSCE)
3.3. HPLC Fingerprint of LSSCE
3.4. Effect of LSSCE on Stability of ROO
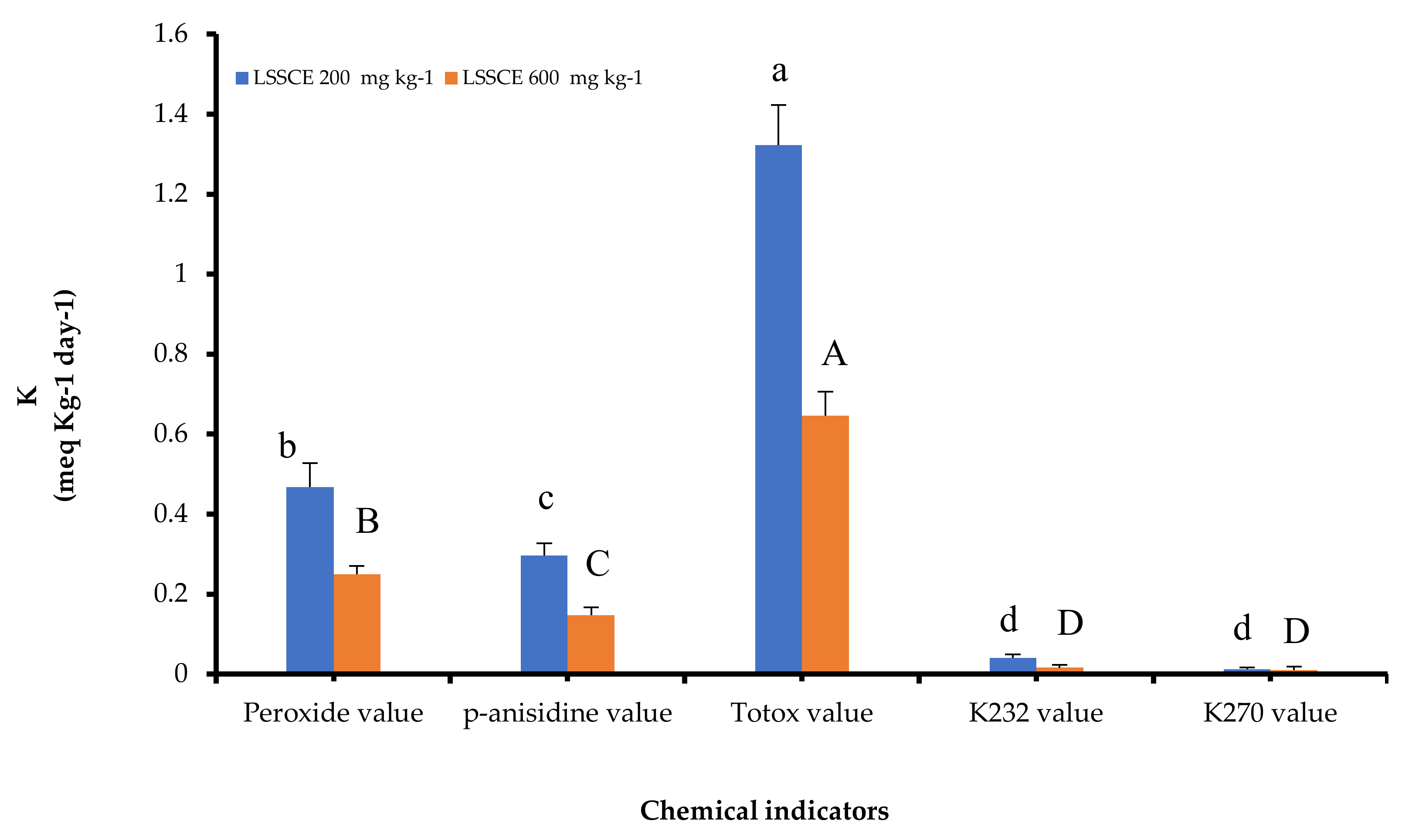
3.4.1. Peroxide Value (PV)
3.4.2. p-Anisidine Value (AV) and Totox Value (TV)
3.4.3. K232 and K270 Values
3.5. LSSCE and Chemical Kinetic of ROO Oxidation
3.6. Antioxidant Remarks
3.7. Organoleptic Assessment of ROO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durante de Oliveira, S.; Araújo, C.M.; da Silva Campelo Borges, G.; dos Santos Lima, M.; Viera, V.B.; Garcia, E.F.; de Souza, E.L.; de Oliveira, M.E.G. Improvement in physicochemical characteristics, bioactive compounds and antioxidant activity of acerola (Malpighia emarginata DC) and guava (Psidium guajava L.) fruit by-products fermented with potentially probiotic lactobacilli. LWT 2020, 134, 110200. [Google Scholar] [CrossRef]
- Monteiro, G.C.; Minatel, I.O.; Pimentel, A., Jr.; Gomez-Gomez, H.A.; de Camargo, J.P.C.; Diamante, M.S.; Basílio, L.S.P.; Tecchio, M.A.; Lima, G.P.P. Bioactive compounds and antioxidant capacity of grape pomace flours. LWT 2021, 135, 110053. [Google Scholar] [CrossRef]
- Azabou, S.; Sebii, H.; Taheur, F.B.; Abid, Y.; Jridi, M.; Nasri, M. Phytochemical profile and antioxidant properties of tomato by-products as affected by extraction solvents and potential application in refined olive oils. Food Biosci. 2020, 36, 100664. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.-L.; Ferreira, T.A. Characterization of tomato processing by-product for use as a potential functional food ingredient: Nutritional composition, antioxidant activity and bioactive compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of antioxidants for enhancing oxidative stability of bulk edible oils: A review. Int. J. Food Sci. Technol. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging processing technologies for the recovery of valuable bioactive compounds from potato peels. Foods 2020, 9, 1598. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Felice, F.; Fabiano, A.; De Leo, M.; Piras, A.M.; Beconcini, D.; Cesare, M.M.; Braca, A.; Zambito, Y.; Di Stefano, R. Antioxidant effect of cocoa by-product and cherry polyphenol extracts: A comparative study. Antioxidants 2020, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2021, 10, 11. [Google Scholar] [CrossRef]
- Mujtaba, M.; Cho, H.M.; Masjuki, H.; Kalam, M.; Ong, H.; Gul, M.; Harith, M.; Yusoff, M. Critical review on sesame seed oil and its methyl ester on cold flow and oxidation stability. Energy Rep. 2020, 6, 40–54. [Google Scholar] [CrossRef]
- Hamza, M.; Abd El-Salam, R. Optimum planting date for three sesame cultivars growing under sandy soil conditions in Egypt. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 868–877. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization Statistics; Production Yearbook. 2017. Available online: http://www.fao.org/faostat/en/#data/QD/visualize (accessed on 12 November 2021).
- El-Roby, A.M.; Hammad, K.S.M.; Galal, S.M. Enhancing oxidative stability of sunflower oil with sesame (Sesamum indicum) coat ultrasonic extract rich in polyphenols. J. Food Processing Preserv. 2020, 44, e14564. [Google Scholar] [CrossRef]
- Catargiu, A.D.; Raican, D.-D.; Poiana, M.-A. Innovative approaches to improve the quality attributes of halva: A review. J. Agroaliment. Processes Technol. 2017, 23, 188–193. [Google Scholar]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Zouari, R.; Besbes, S.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Cookies from composite wheat–sesame peels flours: Dough quality and effect of Bacillus subtilis SPB1 biosurfactant addition. Food Chem. 2016, 194, 758–769. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre characteristics and antioxidant activity of sesame seed coats (testae). Int. J. Food Prop. 2012, 15, 25–37. [Google Scholar] [CrossRef]
- Wang, B.-S.; Chang, L.-W.; Yen, W.-J.; Duh, P.-D. Antioxidative effect of sesame coat on LDL oxidation and oxidative stress in macrophages. Food Chem. 2007, 102, 351–360. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Coello-Oliemans, C.; Ornelas-Cravioto, A.; Santacruz, A.; Becerra-Moreno, A.; Jacobo-Velázquez, D.A. Phytochemical characterization of sesame bran: An unexploited by-product rich in bioactive compounds. CyTA-J. Food 2018, 16, 814–821. [Google Scholar] [CrossRef]
- Mehdi, L.; Ahmed, Z.; Ouassila, L.; Abdeslam-Hassen, M. Supereritical fluid extraction of oil from (de-hulled and ground) sesame seeds: Statistical and phenomenological modelling. In Proceedings of the 2020 11th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 29–31 October 2020; pp. 1–6. [Google Scholar]
- Prakash, K.; Naik, S.N.; Yadav, U. Effects of Sesame Seed Oil (Black/White) as a Natural Antioxidant on the Oxidative and Frying Stability of Linseed Oil. Eur. J. Nutr. Food Saf. 2020, 12, 133–146. [Google Scholar] [CrossRef]
- ES. Egyptian Standards. Vegetable Edible Oils Part 2-No. 49, Olive Oils and Olive Pomace Oils; Egyptian Organization for Standardization and Quality: Cairo, Egypt, 2005. [Google Scholar]
- FAO; WHO. Codex No. 33-1981; Standard for Olive Oils and Olive Pomace Oils. FAO: Rome, Italy; WHO: Geneva, Switzerland, 2013.
- Medeiros, D.M.; Hampton, M. Olive oil and health benefits. In Handbook of Nutraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2019; pp. 211–222. [Google Scholar]
- Haldar, S.; Wong, L.H.; Tay, S.L.; Jacoby, J.J.; He, P.; Osman, F.; Ponnalagu, S.; Jiang, Y.R.; Lian, H.P.R.; Henry, C.J. Two Blends of Refined Rice Bran, Flaxseed, and Sesame Seed Oils Affect the Blood Lipid Profile of Chinese Adults with Borderline Hypercholesterolemia to a Similar Extent as Refined Olive Oil. J. Nutr. 2020, 150, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Morsy, O.M.; Elbarbary, H.A.; Saad, M.A. Enhancing of oxidative stability and quality attributes of olive oil using spirulina (Arthrospira platensis) nanoparticles. LWT 2019, 101, 444–455. [Google Scholar] [CrossRef]
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod. Processing 2019, 117, 321–328. [Google Scholar] [CrossRef]
- Hammouda, I.B.; Márquez-Ruiz, G.; Holgado, F.; Sonda, A.; Skalicka-Wozniak, K.; Bouaziz, M. RP-UHPLC–DAD-QTOF-MS As a Powerful Tool of Oleuropein and Ligstroside Characterization in Olive-Leaf Extract and Their Contribution to the Improved Performance of Refined Olive-Pomace Oil during Heating. J. Agric. Food Chem. 2020, 68, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Appl. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Volobuff, C.R.F.; Santiago, M.; Cardoso, C.A.L.; Vieira, M.D.C.; Valdevina Pereira, Z. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in Psychotria leaf extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT-Food Sci. Technol. 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–34. [Google Scholar]
- Kim, K.W.; Thomas, R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Chang, L.-W.; Yen, W.-J.; Huang, S.C.; Duh, P.-D. Antioxidant activity of sesame coat. Food Chem. 2002, 78, 347–354. [Google Scholar] [CrossRef]
- Sukumar, D.; Arimboor, R.; Arumughan, C. HPTLC fingerprinting and quantification of lignans as markers in sesame oil and its polyherbal formulations. J. Pharm. Biomed. Anal. 2008, 47, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Ackman, R.G. The gas chromatograph in practical analyses of common and uncommon fatty acids for the 21st century. Anal. Chim. Acta 2002, 465, 175–192. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 2009. [Google Scholar]
- Paquot, C.; Hautfenne, A. Standard Methods for the Analysis of Oils, Fats, and Derivatives; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar]
- Gómez-Alonso, S.; Mancebo-Campos, V.; Desamparados Salvador, M.; Fregapane, G. Oxidation kinetics in olive oil triacylglycerols under accelerated shelf-life testing (25–75 °C). Eur. J. Lipid Sci. Technol. 2004, 106, 369–375. [Google Scholar] [CrossRef]
- Steele, R. Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Cambridge, UK, 2004. [Google Scholar]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of selected chemical characteristics of cold-pressed oils on their oxidative stability determined using the rancimat and pressure differential scanning calorimetry method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- International Olive Oil Council. Decision COI/T.15/NC No 3/Rev. 8; Trade Standard Applying to Olive Oil and Olive-Pomace Oil. International Olive Oil Council: Madrid, Spain, 2015.
- International Olive Oil Council. Document COI/T.20/Doc, No 15/Rev.3; Sensory Analysis of Olive Oil Method for the Organoleptic Assessment of Virgin Olive Oil. International Olive Oil Council: Madrid, Spain, 2010.
- Bongartz, A.; Oberg, D. Sensory evaluation of Extra Virgin Olive Oil (EVOO) extended to include the quality factor “harmony”. J. Agric. Sci. Technol. 2011, 422–435. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J. Principles and Procedures of Statistics: A Biometrical Approach; McGrawiHill: New York, NY, USA, 1980. [Google Scholar]
- Alavi, N.; Golmakani, M.-T. Improving oxidative stability of virgin olive oil by addition of microalga Chlorella vulgaris biomass. J. Food Sci. Technol. 2017, 54, 2464–2473. [Google Scholar] [CrossRef]
- Saad, A.B.; Jerbi, A.; Khlif, I.; Ayedi, M.; Allouche, N. Stabilization of Refined Olive Oil with Phenolic Monomers Fraction and Purified Hydroxytyrosol from Olive Mill Wastewater. Chem. Afr. 2020, 3, 657–665. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Villani, V.; Paduano, A.; Sacchi, R. Olive oil phenolic compounds affect the release of aroma compounds. Food Chem. 2015, 181, 284–294. [Google Scholar] [CrossRef]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Michel, I.; Tessaro, I.C.; Marczak, L.D.F. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Elhanafi, L.; Benkhadda, Z.B.; Rais, C.; Houhou, M.; Lebtar, S.; Channo, A.; Greche, H. Biochemical Composition, Antioxidant Power and Antiinflammatory of Dehulled Sesamum indicum Seeds and Its Coat Fraction. Jordan J. Biol. Sci. 2020, 13, 289–294. [Google Scholar]
- Lin, X.; Zhou, L.; Li, T.; Brennan, C.; Fu, X.; Liu, R.H. Phenolic content, antioxidant and antiproliferative activities of six varieties of white sesame seeds (Sesamum indicum L.). Rsc. Adv. 2017, 7, 5751–5758. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, Y.; Li, P.; Wang, X.; Zhang, W.; Wei, W.; Zhang, X. HPLC analysis of seed sesamin and sesamolin variation in a sesame germplasm collection in China. J. Am. Oil Chem. Soc. 2012, 89, 1011–1020. [Google Scholar] [CrossRef]
- Shi, L.K.; Liu, R.J.; Jin, Q.Z.; Wang, X.G. The contents of lignans in sesame seeds and commercial sesame oils of China. J. Am. Oil Chem. Soc. 2017, 94, 1035–1044. [Google Scholar] [CrossRef]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Tabee, E.; Jägerstad, M.; Dutta, P.C. Frying quality characteristics of French fries prepared in refined olive oil and palm olein. J. Am. Oil Chem. Soc. 2009, 86, 885–893. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, Y.; Li, J.; Long, Q.; Zhong, H. Effects of Seed Coat on Oxidative Stability and Antioxidant Activity of Apricot (Prunus armeniaca L.) Kernel Oil at Different Roasting Temperatures. J. Am. Oil Chem. Soc. 2018, 95, 1297–1306. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Mamouni, R.; Matthäus, B.; Charrouf, Z. Chemical Characterization and Kinetic parameter determination under Rancimat test conditions of four monovarietal virgin olive oils grown in Morocco. OCL 2016, 23, A401. [Google Scholar] [CrossRef] [Green Version]






| Fatty Acids | ROO |
|---|---|
| Palmitic acid (C 16:0) | 12.32 ± 0.07 |
| Palmitoleic acid (C 16:1) | 0.38 ± 0.03 |
| Margaric acid (C 17:0) | 0.05 ± 0.01 |
| Margaroleic (C 17:1) | 0.04 ± 0.01 |
| Stearic acid (C 18:0) | 3.12 ± 0.04 |
| Oleic acid (C 18:1) | 73.35 ± 0.01 |
| Linoleic acid (C 18:2) | 9.12 ± 0.14 |
| α-Linolenic acid (C 18:3n-3) | 0.61 ± 0.05 |
| Arachidic acid (C 20:0) | 0.6 ± 0.01 |
| Eicosenoic acid (C 20:1) | 0.41 ± 0.02 |
| Saturated fatty acid ΣSFA | 16.09 ± 0. 23 |
| Monounsaturated fatty acid ΣMUFA | 74.18 ± 0.22 |
| Polyunsaturated fatty acid ΣPUFA | 9.73 ± 0.12 |
| Sample | TP (mg g−1 dw) | TF (mg g−1 dw) | IC50 (μg mL−1) |
|---|---|---|---|
| Sesame seed coats (SSCs) | 85.12 ± 1.33 b | 7.45 ± 0.23 b | 15.64 ± 0.96 a |
| Lyophilized sesame seed coats extract (LSSCE) | 105.9 ± 2.98 a | 9.00 ± 0.41 a | 9.90 ± 0.65 c |
| α-tocopherol | “—” | “—” | 14.28 ± 0.82 b |
| BHT | “—” | “—” | 5.05 ± 0.87 d |
| Samples | IP (h) | AOP (%) | IOS (%) |
|---|---|---|---|
| Control | 15.81 ± 0.23 e | - | - |
| LSSCE 200 mg kg−1 | 17.76 ± 0.42 b | 10.98 ± 0.22 d | 12.33 ± 0.25 b |
| LSSCE 400 mg kg−1 | 20.23 ± 0.50 c | 21.85 ± 0.48 c | 27.96 ± 0.38 c |
| LSSCE 600 mg kg−1 | 23.17 ± 0.35 b | 31.77 ± 0.66 b | 46.55 ± 0.52 b |
| BHT 200 mg kg−1 | 27.15 ± 0.6 a | 41.77 ± 0.53 a | 71.73 ± 0.85 a |
| Attributes | Treatments | Storage Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | ||
| Color | Control | 7.00 ± 0.00 aA | 5.20 ± 0.82 bB | 3.37 ± 0.40 cC | 2.16 ± 0.33 dC | *R | R | R | R | R |
| LSSCE 200 mg kg−1 | 7.00 ± 0.00 aA | 5.90 ± 0.32 bB | 5.00 ± 0.22 cB | 4.23 ± 0.47 dB | 3.40 ± 0.33 eC | 2.57 ± 0.18 fC | R | R | R | |
| LSSCE 400 mg kg−1 | 7.00 ± 0.00 aA | 6.25 ± 0.63 aA | 5.83 ± 0.38 bA | 5.11 ± 0.32 bB | 4.85 ± 0.42 cB | 4.40 ± 0.56 cB | 3.22 ± 0.31 dB | R | R | |
| LSSCE 600 mg kg−1 | 7.00 ± 0.00 aA | 6.60 ± 0.30 aA | 6.00 ± 0.44 bA | 5.77 ± 0.54 bA | 5.47 ± 0.32 bA | 5.15 ± 0.43 bA | 4.88 ± 0.23 cA | 4.13 ± 0.12 cA | 3.70 ± 0.22 dA | |
| BHT 200 mg kg−1 | 7.00 ± 0.00 aA | 6.87 ± 0.55 aA | 6.28 ± 0.37 aA | 5.94 ± 0.53 bA | 5.65 ± 0.40 bA | 5.36 ± 0.33 bA | 4.95 ± 0.47 cA | 4.58 ± 0.26 cA | 3.95 ± 0.35 dA | |
| Aroma | Control | 7.00 ± 0.00 aA | 6.90 ± 0.11 aA | 4.75 ± 0.44 bB | 2.10 ± 0.33 cC | R | R | R | R | R |
| LSSCE 200 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.25 ± 0.18 aA | 4.50 ± 0.38 bB | 3.15 ± 0.34 cC | 2.40 ± 0.23 cC | R | R | R | |
| LSSCE 400 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.86 ± 0.00 aA | 6.45 ± 0.40 aA | 5.90 ± 0.57 bB | 4.40 ± 0.41 cB | 3.55 ± 0.27 dB | R | R | |
| LSSCE 600 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.90 ± 0.45 aA | 6.73 ± 0.33 aA | 6.20 ± 0.24 aA | 5.66 ± 0.37 bA | 4.58 ± 0.45 cA | |
| BHT 200 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.92 ± 0.42 aA | 6.45 ± 0.30 aA | 5.10 ± 0.24 bA | |
Acceptability | Control | 7.00 ± 0.00 aA | 6.85 ± 0.13 aA | 4.56 ± 0.33 bC | 3.12 ± 0.26 cC | R* | R | R | R | R |
| LSSCE 200 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.15 ± 0.25 bB | 5.73 ± 0.41 bB | 4.22 ± 0.25 cC | 3.23 ± 0.15 dC | R | R | R | |
| LSSCE 400 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.45 ± 0.32 aA | 5.86 ± 0.23 bB | 5.12 ± 0.34 bB | 4.85 ± 0.37 cB | 3.42 ± 0.18 dB | R | R | |
| LSSCE 600 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.90 ± 0.22 aA | 6.35 ± 0.38 aA | 5.95 ± 0.23 bA | 5.32 ± 0.32 bA | 4.93 ± 0.35 cA | 3.84 ± 0.12 dA | |
| BHT 200 mg kg−1 | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 7.00 ± 0.00 aA | 6.92 ± 0.25 aA | 6.65 ± 0.30 aA | 5.74 ± 0.22 bA | 5.25 ± 0.16 bA | 4.36 ± 0.25 cA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsy, M.K.; Sami, R.; Algarni, E.; Al-Mushhin, A.A.M.; Benajiba, N.; A., A.; Almasoudi, A.G.; Mekawi, E. Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil. Antioxidants 2022, 11, 338. https://doi.org/10.3390/antiox11020338
Morsy MK, Sami R, Algarni E, Al-Mushhin AAM, Benajiba N, A. A, Almasoudi AG, Mekawi E. Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil. Antioxidants. 2022; 11(2):338. https://doi.org/10.3390/antiox11020338
Chicago/Turabian StyleMorsy, Mohamed K., Rokayya Sami, Eman Algarni, Amina A. M. Al-Mushhin, Nada Benajiba, Almasoudi A., Abeer G. Almasoudi, and Enas Mekawi. 2022. "Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil" Antioxidants 11, no. 2: 338. https://doi.org/10.3390/antiox11020338
APA StyleMorsy, M. K., Sami, R., Algarni, E., Al-Mushhin, A. A. M., Benajiba, N., A., A., Almasoudi, A. G., & Mekawi, E. (2022). Phytochemical Profile and Antioxidant Activity of Sesame Seed (Sesamum indicum) By-Products for Stability and Shelf Life Improvement of Refined Olive Oil. Antioxidants, 11(2), 338. https://doi.org/10.3390/antiox11020338






