Metabolomics-Based Profiling of Clerodendrum speciosum (Lamiaceae) Leaves Using LC/ESI/MS-MS and In Vivo Evaluation of Its Antioxidant Activity Using Caenorhabditis elegans Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of the Extract and Different Fractions of C. speciosum Leaves
2.3. Determination of Total Phenolic Content of the Extract and Different Fractions of C. speciosum Leaves
2.4. In Vitro Antioxidant Determination of the Extract and Different Fractions of C. speciosum Leaves
2.4.1. 2,2-Diphenyl-1-picrylhydrazyl Radicle (DPPH•) Scavenging Capacity Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. In Vivo Antioxidant Determination of the Extract and Different Fractions of C. speciosum Leaves Using C. elegans
2.5.1. C. elegans Strains and Culture Conditions
2.5.2. Survival Assay of C. elegans Using Juglone Induced Oxidative Stress
2.5.3. Assessment of Reactive Oxygen Species (ROS) in C. elegans
2.5.4. Quantitation of hsp-16.2/GFP Expression in Caenorhabditis elegans
2.6. Phytochemical Profiling of C. speciosum Leaves Total Methanol Extract Using LC/ESI/MS-MS
2.7. Computer Aided Drug Design Studies
2.7.1. In Silico Molecular Docking
2.7.2. ADME/TOPAKT Prediction
3. Results and Discussion
3.1. Determination of the Total Phenolic Content of C. speciosum Leaves Extract and Different Fractions
3.2. In Vitro Antioxidant Determination of the Extract and Different Fractions of C. speciosum Leaves
3.3. In Vivo Antioxidant Determination of the Extract and Different Fractions of C. speciosum Leaves Using Caenorhabditis elegans Model
3.3.1. Survival Assay of Caenorhabditis elegans Using Juglone Induced Oxidative Stress
3.3.2. Assessment of Intracellular Reactive Oxygen Species (ROS) in Caenorhabditis elegans
3.3.3. Quantitation of hsp-16.2/GFP Expression in Caenorhabditis elegans
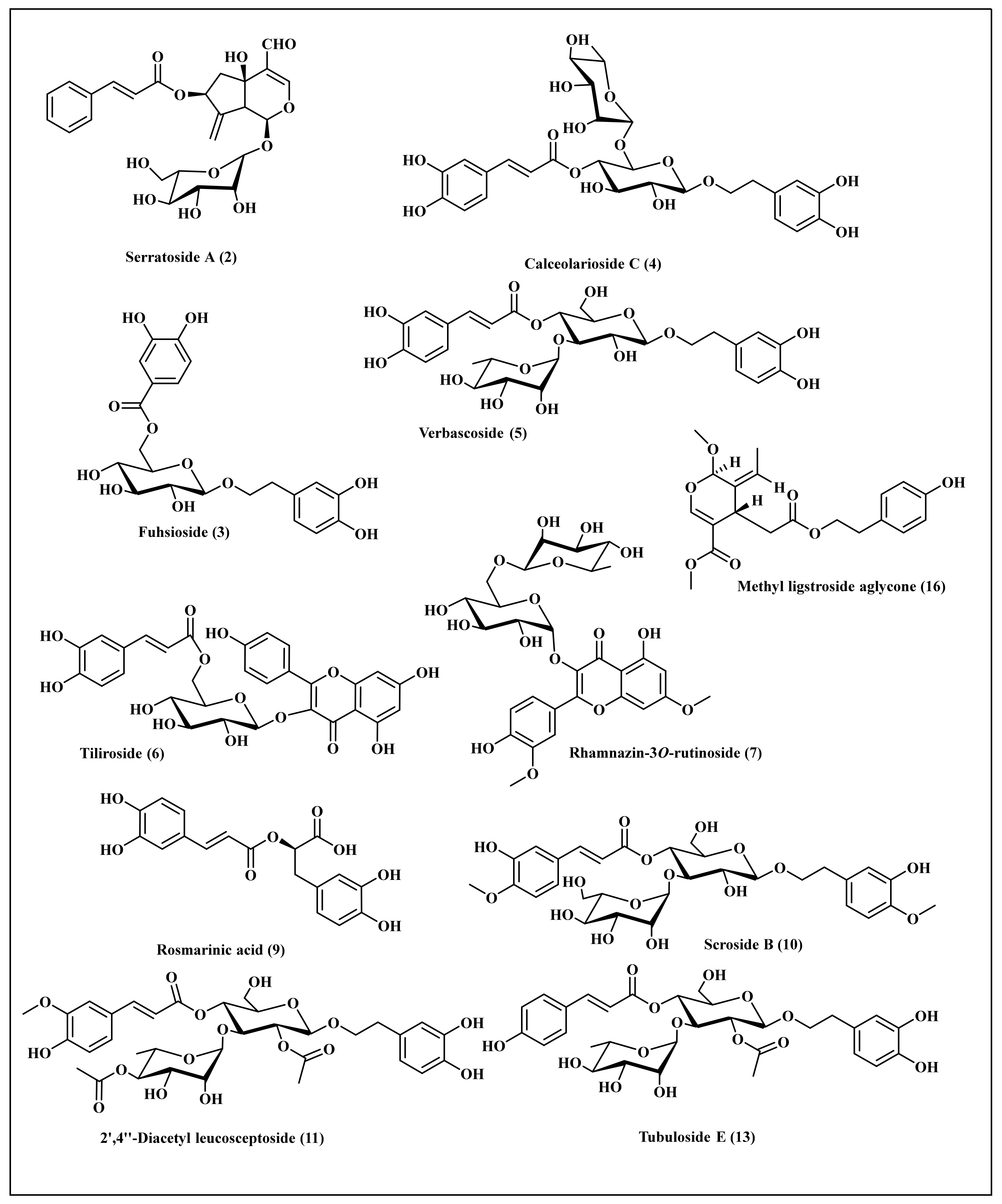
3.4. Phytochemical Profiling of C. speciosum Leaves Total Methanol Extract Using LC/ESI/MS-MS
3.5. Computer Aided Drug Design Studies
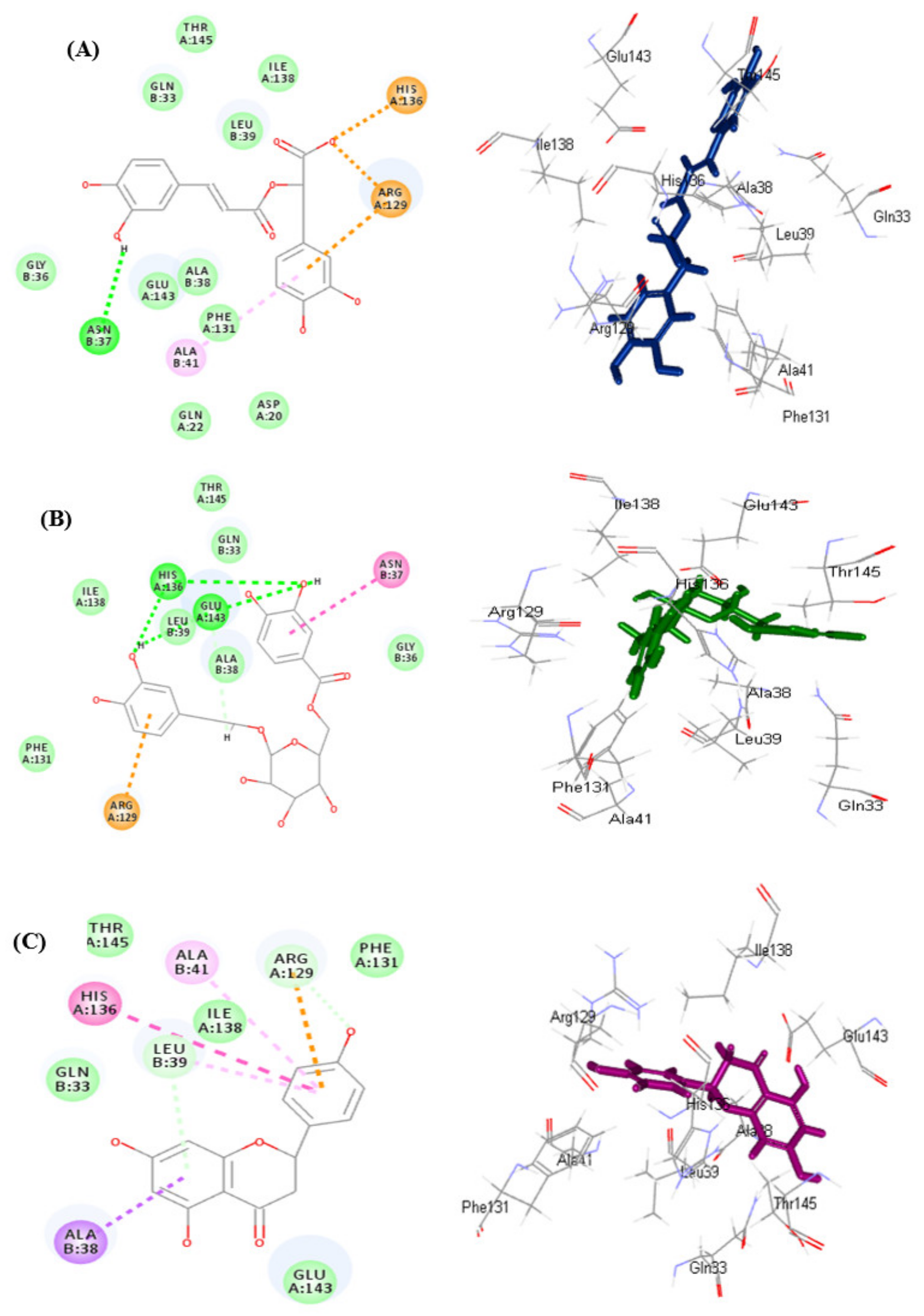
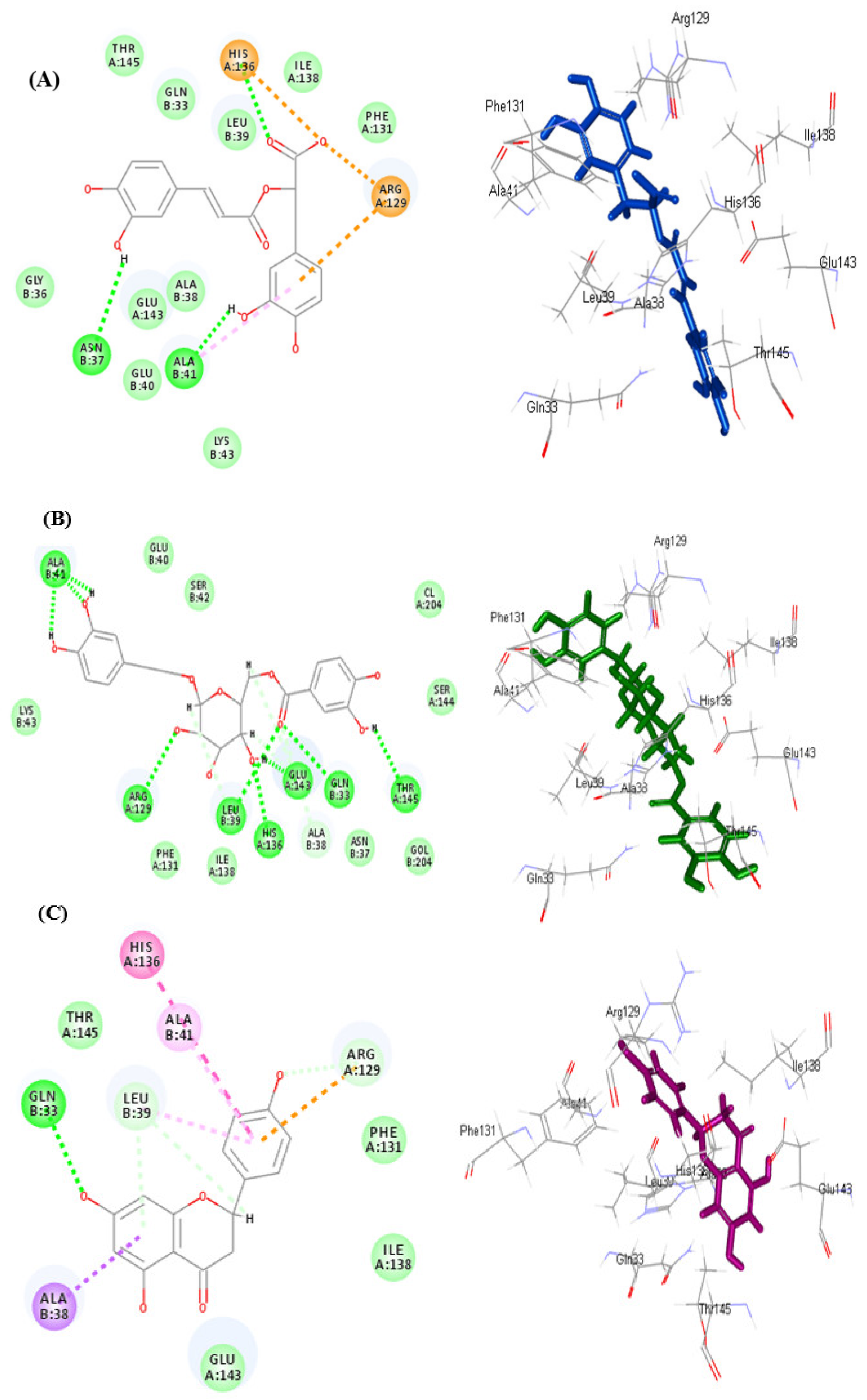
3.5.1. In Silico Molecular Docking
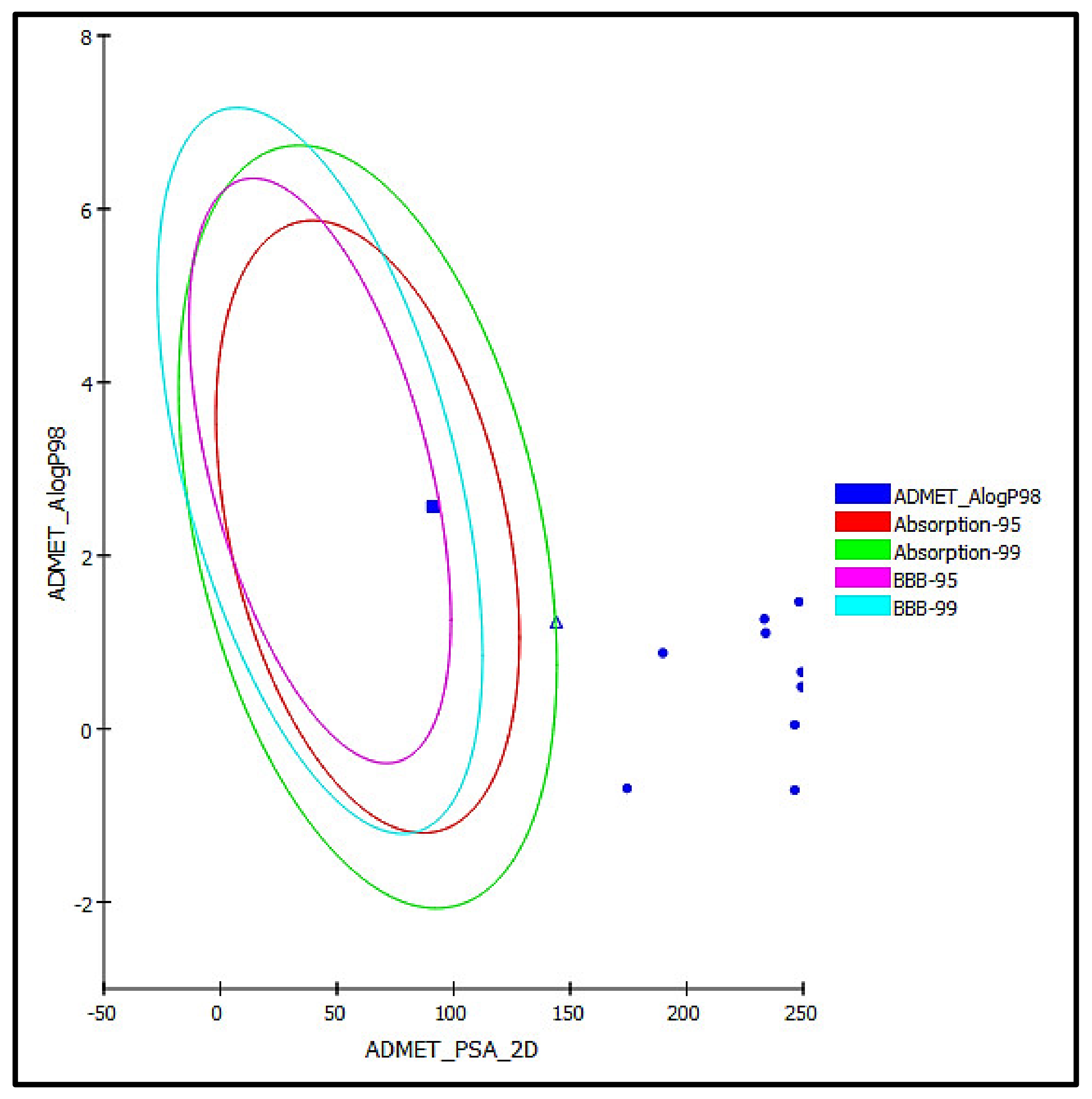
3.5.2. ADME/TOPAKT Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Youssef, F.S.; Labib, R.M.; Eldahshan, O.A. Synergistic hepatoprotective and antioxidant effect of artichoke, fig, mulberry herbal mixture on HepG2 cells and their metabolic profiling using NMR coupled with chemometrics. Chem. Biodivers. 2017, 14, e1700206. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [PubMed] [Green Version]
- Chen, W.; Sudji, I.R.; Wang, E.; Joubert, E.; van Wyk, B.-E.; Wink, M. Ameliorative effect of aspalathin from rooibos (Aspalathus linearis) on acute oxidative stress in Caenorhabditis elegans. Phytomedicine 2013, 20, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caenorhabditis elegans as a model organism to evaluate the antioxidant effects of phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef]
- Abbas, S.; Wink, M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009, 75, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 2010, 17, 902–909. [Google Scholar] [CrossRef]
- Kar, P.; Goyal, A.; Das, A.; Sen, A. Antioxidant and pharmaceutical potential of Clerodendrum L.: An overview. Int. J. Green Pharm. 2014, 8, 210–216. [Google Scholar]
- Okwu, D.E.; Iroabuchi, F. Phytochemical composition and biological activities of Uvaria chamae and Clerodendoron splendens. J. Chem. 2009, 6, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Bum, E.N.; Taiwe, G.; Moto, F.; Ngoupaye, G.; Vougat, R.; Sakoue, V.; Gwa, C.; Ayissi, E.; Dong, C.; Rakotonirina, A. Antiepileptic medicinal plants used in traditional medicine to treat epilepsy. In Clinical and Genetic Aspects of Epilepsy; InTechOpen: London, UK, 2011. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiernagle, T. Maintenance of Caenorhabditis elegans. Int. J. Biomed. Health Sci. 2021, 10, 55–67. [Google Scholar]
- Abbas, S.; Wink, M. Green tea extract induces the resistance of Caenorhabditis elegans against oxidative stress. Antioxidants 2014, 3, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Sobeh, M.; ElHawary, E.; Peixoto, H.; Labib, R.M.; Handoussa, H.; Swilam, N.; El-Khatib, A.H.; Sharapov, F.; Mohamed, T.; Krstin, S. Identification of phenolic secondary metabolites from Schotia brachypetala Sond.(Fabaceae) and demonstration of their antioxidant activities in Caenorhabditis elegans. PeerJ 2016, 4, e2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobeh, M.; Youssef, F.S.; Esmat, A.; Petruk, G.; El-Khatib, A.H.; Monti, D.M.; Ashour, M.L.; Wink, M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018, 113, 145–153. [Google Scholar] [CrossRef]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crops Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ovidi, E.; Musayeib, N.M.A.; Ashour, M.L. Morphology, anatomy and secondary metabolites investigations of Premna odorata Blanco and evaluation of its anti-tuberculosis activity using in vitro and in silico studies. Plants 2021, 10, 1953. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Hussain, H.; Zengin, G.; Mollica, A.; Al Musayeib, N.M.; Ashour, M.L.; Westermann, B.; Wessjohann, L.A. Validation of the antioxidant and enzyme inhibitory potential of selected triterpenes using in vitro and in silico studies, and the evaluation of their ADMET properties. Molecules 2021, 26, 6331. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2017. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV-Visible, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2018, 164, 653–658. [Google Scholar] [CrossRef]
- Saling, S.C.; Comar, J.F.; Mito, M.S.; Peralta, R.M.; Bracht, A. Actions of juglone on energy metabolism in the rat liver. Toxicol. Appl. Pharmacol. 2011, 257, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Swindell, W.R. Heat shock proteins in long-lived worms and mice with insulin/insulin-like signaling mutations. Aging 2009, 1, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Poyatos, M.d.P.; Ruiz-Medina, A.; Zengin, G.; Llorent-Martínez, E.J. Phenolic characterization, antioxidant activity, and enzyme inhibitory properties of Berberis thunbergii DC. leaves: A valuable source of phenolic acids. Molecules 2019, 24, 4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asraoui, F.; Kounnoun, A.; Cadi, H.E.; Cacciola, F.; Majdoub, Y.O.E.; Alibrando, F.; Mandolfino, F.; Dugo, P.; Mondello, L.; Louajri, A. Phytochemical Investigation and Antioxidant Activity of Globularia alypum L. Molecules 2021, 26, 759. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Luan, F.; He, X.-D.; Wang, Y.; Li, M.-X. Traditional uses and pharmacological properties of Clerodendrum phytochemicals. J. Tradit. Complement. Med. 2018, 8, 24–38. [Google Scholar] [PubMed]
- Ozipek, M.; Saracoglu, I.; Kojima, K.; Ogihara, Y.; Calis, I. Fuhsioside, a new phenylethanoid glucoside from Veronica fuhsii. Chem. Pharm. Bull. 1999, 47, 561–562. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, M.; Galeffi, C.; Messana, I.; Marini-Bettolo, G.; Garbarino, J.; Gambaro, V. Phenylpropanoid glycosides from Calceolaria hypericina. Phytochemistry 1988, 27, 639–641. [Google Scholar] [CrossRef]
- Elaskary, H.I.; Sabry, O.M.; Khalil, A.M.; El Zalabani, S.M. UPLC-PDA-ESI-MS/MS Profiling of Clerodendrum inerme and Clerodendrum splendens and significant activity against Mycobacterium tuberculosis. Pharmacogn. J. 2020, 12, 1518–1524. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Kalinowska, E.; Popowski, D.; Bazylko, A. Lamalbid, chlorogenic acid, and verbascoside as tools for standardization of Lamium album flowers—development and validation of HPLC–DAD method. Molecules 2020, 25, 1721. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A polyphenol-rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front. Pharmacol. 2018, 9, 566. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tong, C.; Fu, Q.; Guo, K.; Shi, S.; Xiao, Y. Comprehensive polyphenolic profile of Plantago depressa using high-speed countercurrent chromatography off-line with high-performance liquid chromatography–Diode Array Detector–Quadrupole Time-of-flight Tandem Mass Spectrometry. eFood 2019, 1, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Munkombwe, N.M. Acetylated phenolic glycosides from Harpagophytum procumbens. Phytochemistry 2003, 62, 1231–1234. [Google Scholar] [CrossRef]
- Veitch, N.C.; Elliott, P.C.; Kite, G.C.; Lewis, G.P. Flavonoid glycosides of the black locust tree, Robinia pseudoacacia (Leguminosae). Phytochemistry 2010, 71, 479–486. [Google Scholar] [CrossRef]
- Ahn, J.; Chae, H.-S.; Chin, Y.-W.; Kim, J. Dereplication-guided isolation of new phenylpropanoid-substituted diglycosides from Cistanche salsa and their inhibitory activity on NO production in macrophage. Molecules 2017, 22, 1138. [Google Scholar] [CrossRef] [Green Version]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Kasai, R.; Oinaka, T.; Yang, C.-R.; Zhou, J.; Tanaka, O. Saponins from Chinese folk medicine,“Liang Wang Cha,” leaves and stems of Nothopanax delavayi, Araliaceae. Chem. Pharm. Bull. 1987, 35, 1486–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantino, P.D.; Sanders, R.W. Subfamilial classification of Labiatae. Syst. Bot. 1986, 11, 163–185. [Google Scholar] [CrossRef]
- Uddin, M.J.; Çiçek, S.S.; Willer, J.; Shulha, O.; Abdalla, M.A.; Sönnichsen, F.; Girreser, U.; Zidorn, C. Phenylpropanoid and flavonoid glycosides from the leaves of Clerodendrum infortunatum (Lamiaceae). Biochem. Syst. Ecol. 2020, 92, 104131. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Wu, H.; Han, Y.; Zhang, J.; Ma, E.; Guo, Y. Molecular basis for antioxidant enzymes in mediating copper detoxification in the nematode Caenorhabditis elegans. PLoS ONE 2014, 9, e107685. [Google Scholar]
- Barsyte, D.; Lovejoy, D.A.; LITHGOW, G.J. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001, 15, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, H.; Wang, J.; Liu, G.; Cui, S.W.; Kang, J.; Jiang, Y.; Wang, H. Naringenin prolongs lifespan and delays aging mediated by IIS and MAPK in Caenorhabditis elegans. Food Funct. 2021, 12, 12127–12141. [Google Scholar] [CrossRef]
- Zhu, Q.; Qu, Y.; Zhou, X.-G.; Chen, J.-N.; Luo, H.-R.; Wu, G.-S. A dihydroflavonoid naringin extends the lifespan of C. elegans and delays the progression of aging-related diseases in PD/AD models via DAF-16. Oxidative Med. Cell. Longev. 2020, 2020, 6069354. [Google Scholar] [CrossRef]
- Fadel, O.; El Kirat, K.; Morandat, S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim. Biophys. Acta BBA-Biomembr. 2011, 1808, 2973–2980. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Cheng, W.-X.; Li, C.; Pan, X.-L.; Xie, X.-G.; Li, T.-H. DFT study on the antioxidant activity of rosmarinic acid. J. Mol. Struct. 2005, 719, 177–183. [Google Scholar] [CrossRef]
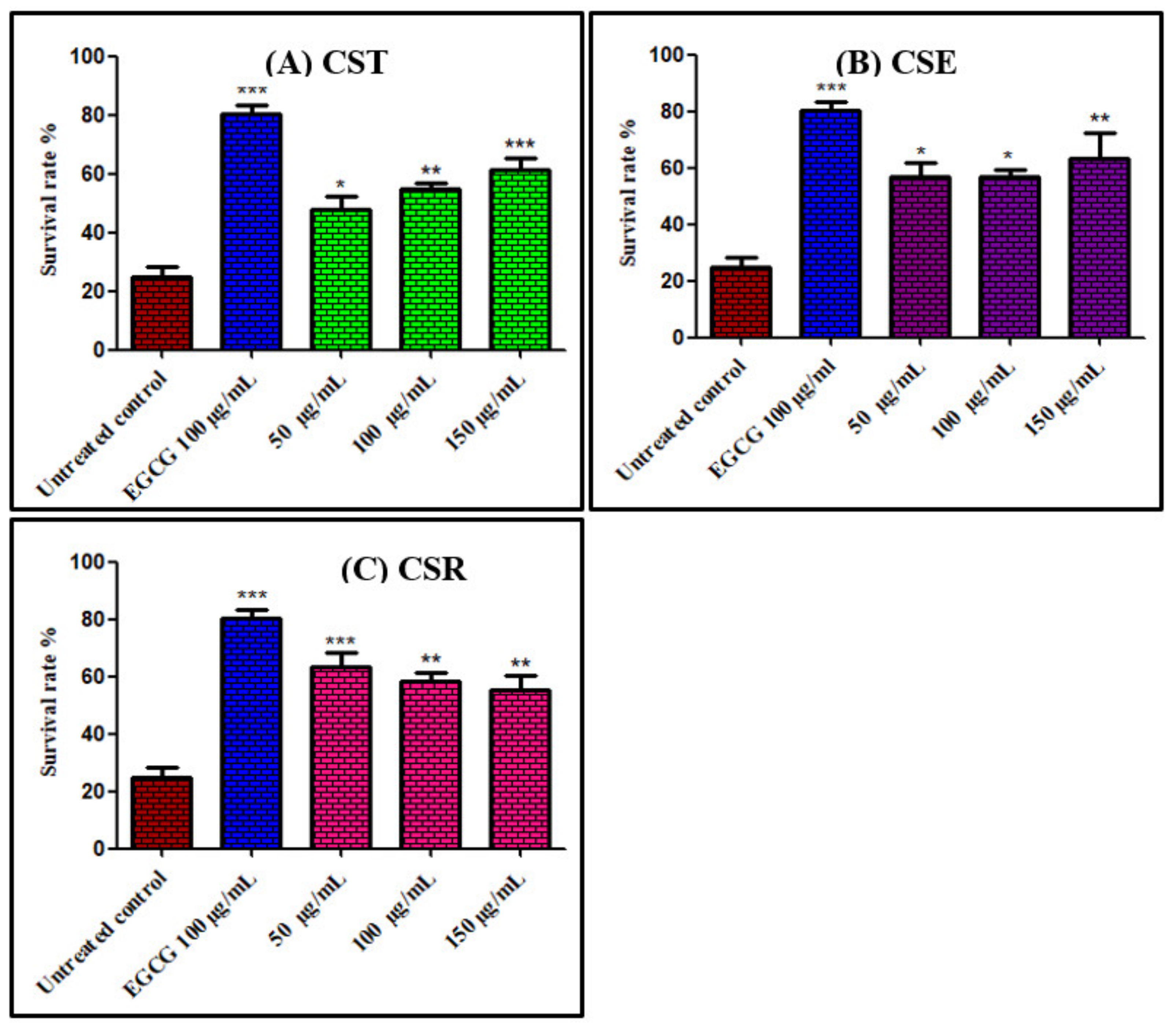

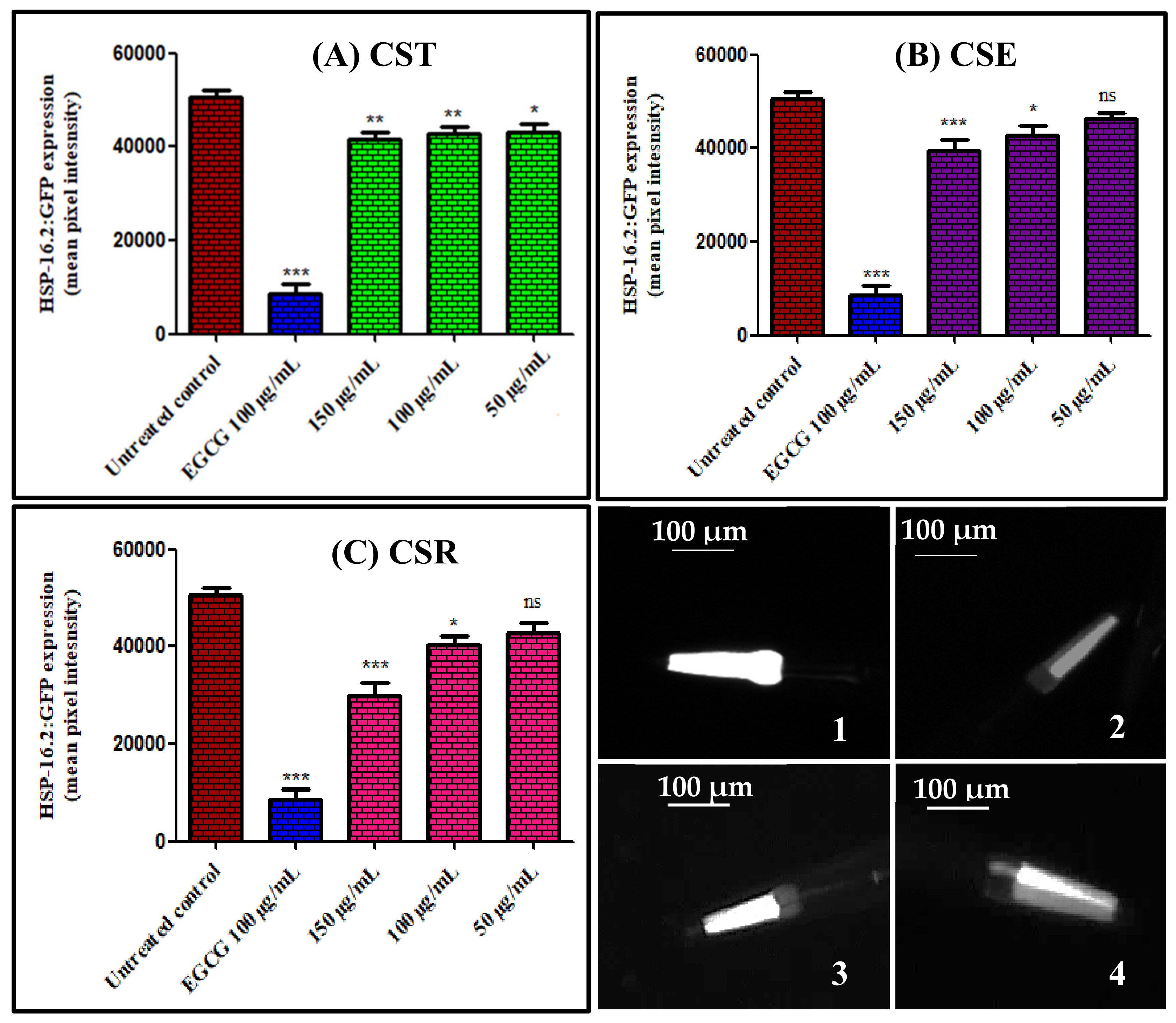

| Sample | DPPH (EC50 µg/mL) | FRAP (Fe2+ Equivalents/mg of Sample) |
|---|---|---|
| CST | 27.1 ± 1.7 | 11.44 ± 0.68 |
| CSE | 16.2 ± 1.2 | 16.27 ± 0.86 |
| CSR | 21.3 ± 2.0 | 12.16 ± 0.68 |
| Ascorbic acid | 2.92 ± 0.29 | - |
| Quercetin | - | 24.04 ± 1.23 |
| Peak No. | tR (min) | Name | [M − H]– (m/z) | MS2 (m/z) | References |
|---|---|---|---|---|---|
| 1 | 8.10 | Quinic acid derivative | 569 | 389, 371, 327, 265, 191, 173 | [30] |
| 2 | 16.10 | 7-Cinnamoyloxyugandoside (Serratoside A) | 503 | 435, 341, 241, 179, 163 | [31,32] |
| 3 | 22.81 | Fuhsioside | 451 | 405, 315, 345 | [33] |
| 4 | 23.33 | Calceolarioside C | 609 | 179, 161 | [34] |
| 5 | 27.70 | Verbascoside | 623 | 605, 577, 517, 507, 461, 179, 161 | [35] |
| 6 | 31.85 | Tiliroside | 593 | 447, 285 | [36] |
| 7 | 33.09 | Rhamnazin-3-O-rutinoside | 637 | 475, 329, 297 | [35] |
| 8 | 36.42 | Quercetin methyl galloyl-hexoside | 629 | 179, 301, 463, 477 | [37] |
| 9 | 38.20 | Rosmarinic acid | 359 | 197, 179, 161, 133 | [35] |
| 10 | 39.66 | Scroside B | 667 | 623, 505, 491, 461, 401, 329, 193, 175 | [38] |
| 11 | 81.18 | 2′,4″-Diacetyl leucosceptoside | 721 | 175, 193 | [39] |
| 12 | 83.19 | Acacetin-7-O-β-d-hexosyl-(1 → 2)[α-l-rhamnopyranosyl-(1 → 6)]-β-d-hexoside (Acacetin trioside) | 723 | 283 | [40] |
| 13 | 87.92 | Tubuloside E | 649 | 147, 351, 497 | [41] |
| 14 | 90.70 | Coumaric acid derivative | 561 | 439, 163 | [42] |
| 15 | 99.39 | 3-Hydroxy-12-oleanene-28,29-dioic acid 3-O-α-L-pentosyl, 28-O-β-D-hexosyl ester | 779 | 733 | [43] |
| 16 | 118.54 | Methyl ligstroside aglycone | 375 | 361 | [37] |
| Compounds | pH-Based Ionization Mode | Number of Formed Hydrogen Bonds with the Amino Acid Residues | Rule-Based Ionization Mode | Number of Formed Hydrogen Bonds with the Amino Acid Residues |
|---|---|---|---|---|
| Serratoside A (2) | 39.83 | - | 27.20 | 4; Ala41, Arg129, Lys43 |
| Fuhsioside (3) | −17.7 | 4; His136, Leu39, Glu143 | −28.73 | 9; His136, Leu39, Glu143, Gln33, Ala41, Arg129, Thr145 |
| Calceolarioside C (4) | FD | - | FD | - |
| Verbascoside (5) | FD | - | FD | - |
| Tiliroside (6) | −17.5 | 6; Gln33, Ala41, His136, Leu39, Glu143 | FD | - |
| Rhamnazin-3O-rutinoside (7) | FD | - | FD | - |
| Rosmarinic acid (9) | −41.99 | 1; Asn37 | −41.24 | 1; Asn37 |
| Scroside B (10) | FD | - | FD | - |
| 2,4 Diacetyl leucopstoside (11) | FD | - | FD | - |
| Tubuloside E (13) | FD | - | FD | - |
| Methyl ligstroside aglycone (16) | −0.19 | 3; His136, Leu39, Glu143 | −3.34 | 2; Gly36, Gln33 |
| Naringenin | −19.45 | - | −19.31 | 1; Gln33 |
| Compounds | Absorption Level | Solubility Level | BBB Level | PPB Level | CPY2D6 | Hepatotoxic | PSA-2D | Alog p98 |
|---|---|---|---|---|---|---|---|---|
| Serratoside A (2) | 3 | 4 | 4 | False | NI | NT | 174.40 | −0.69 |
| Fuhsioside (3) | 3 | 3 | 4 | False | NI | NT | 189.80 | 0.88 |
| Calceolarioside C (4) | 3 | 2 | 4 | False | NI | NT | 249.29 | 0.66 |
| Verbascoside (5) | 3 | 2 | 4 | False | NI | NT | 249.29 | 0.48 |
| Tiliroside (6) | 3 | 2 | 4 | False | NI | NT | 233.33 | 1.27 |
| Rhamnazin-3O-rutinoside (7) | 3 | 2 | 4 | False | NI | NT | 246.34 | −0.71 |
| Rosmarinic acid (9) | 2 | 3 | 4 | False | NI | NT | 144.09 | 1.23 |
| Scroside B (10) | 3 | 2 | 4 | False | NI | NT | 246.34 | 0.05 |
| 2,4 Diacetyl leucopstoside (11) | 3 | 2 | 4 | False | NI | NT | 248.24 | 1.47 |
| Tubuloside E (13) | 3 | 2 | 4 | False | NI | NT | 233.89 | 1.11 |
| Methyl ligstroside aglycone (16) | 0 | 3 | 3 | False | NI | NT | 91.14 | 2.56 |
| Compounds | Ames Prediction | Rat Oral LD50 | Rat Chronic LOAEL | Skin Irritancy | Ocular Irritancy | Rat Female NTP | Rat Male NTP |
|---|---|---|---|---|---|---|---|
| Serratoside A (2) | Non-Mutagen | 0.77 | 0.01 | Mild | None | Non-Carcinogen | Non-Carcinogen |
| Fuhsioside (3) | Non-Mutagen | 5.82 | 0.16 | None | None | Non-Carcinogen | Non-Carcinogen |
| Calceolarioside C (4) | Non-Mutagen | 10.32 | 0.08 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
| Verbascoside (5) | Non-Mutagen | 10.57 | 0.10 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
| Tiliroside (6) | Non-Mutagen | 0.67 | 0.03 | None | Moderate | Non-Carcinogen | Non-Carcinogen |
| Rhamnazin-3O-rutinoside (7) | Non-Mutagen | 2.36 | 0.04 | Mild | Mild | Non-Carcinogen | Carcinogen |
| Rosmarinic acid (9) | Non-Mutagen | 3.17 | 0.13 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
| Scroside B (10) | Non-Mutagen | 9.60 | 0.04 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
| 2,4 Diacetyl leucopstoside (11) | Non-Mutagen | 8.46 | 0.05 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
| Tubuloside E (13) | Non-Mutagen | 8.97 | 0.10 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
| Methyl ligstroside aglycone (16) | Non-Mutagen | 2.11 | 0.03 | Mild | Mild | Non-Carcinogen | Non-Carcinogen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, F.S.; Sobeh, M.; Dmirieh, M.; Bogari, H.A.; Koshak, A.E.; Wink, M.; Ashour, M.L.; Elhady, S.S. Metabolomics-Based Profiling of Clerodendrum speciosum (Lamiaceae) Leaves Using LC/ESI/MS-MS and In Vivo Evaluation of Its Antioxidant Activity Using Caenorhabditis elegans Model. Antioxidants 2022, 11, 330. https://doi.org/10.3390/antiox11020330
Youssef FS, Sobeh M, Dmirieh M, Bogari HA, Koshak AE, Wink M, Ashour ML, Elhady SS. Metabolomics-Based Profiling of Clerodendrum speciosum (Lamiaceae) Leaves Using LC/ESI/MS-MS and In Vivo Evaluation of Its Antioxidant Activity Using Caenorhabditis elegans Model. Antioxidants. 2022; 11(2):330. https://doi.org/10.3390/antiox11020330
Chicago/Turabian StyleYoussef, Fadia S., Mansour Sobeh, Malak Dmirieh, Hanin A. Bogari, Abdulrahman E. Koshak, Michael Wink, Mohamed L. Ashour, and Sameh S. Elhady. 2022. "Metabolomics-Based Profiling of Clerodendrum speciosum (Lamiaceae) Leaves Using LC/ESI/MS-MS and In Vivo Evaluation of Its Antioxidant Activity Using Caenorhabditis elegans Model" Antioxidants 11, no. 2: 330. https://doi.org/10.3390/antiox11020330
APA StyleYoussef, F. S., Sobeh, M., Dmirieh, M., Bogari, H. A., Koshak, A. E., Wink, M., Ashour, M. L., & Elhady, S. S. (2022). Metabolomics-Based Profiling of Clerodendrum speciosum (Lamiaceae) Leaves Using LC/ESI/MS-MS and In Vivo Evaluation of Its Antioxidant Activity Using Caenorhabditis elegans Model. Antioxidants, 11(2), 330. https://doi.org/10.3390/antiox11020330










