The Crosstalk between GPR81/IGFBP6 Promotes Breast Cancer Progression by Modulating Lactate Metabolism and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Pharmacological Treatment
2.2. Real-Time Monitoring of Cell Proliferation
2.3. Real-Time PCR for Gene Expression Analysis
2.4. Western Blot Analysis
2.5. Wound Healing Assay
2.6. Clonogenic Assay
2.7. Lactate Concentration Assay
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
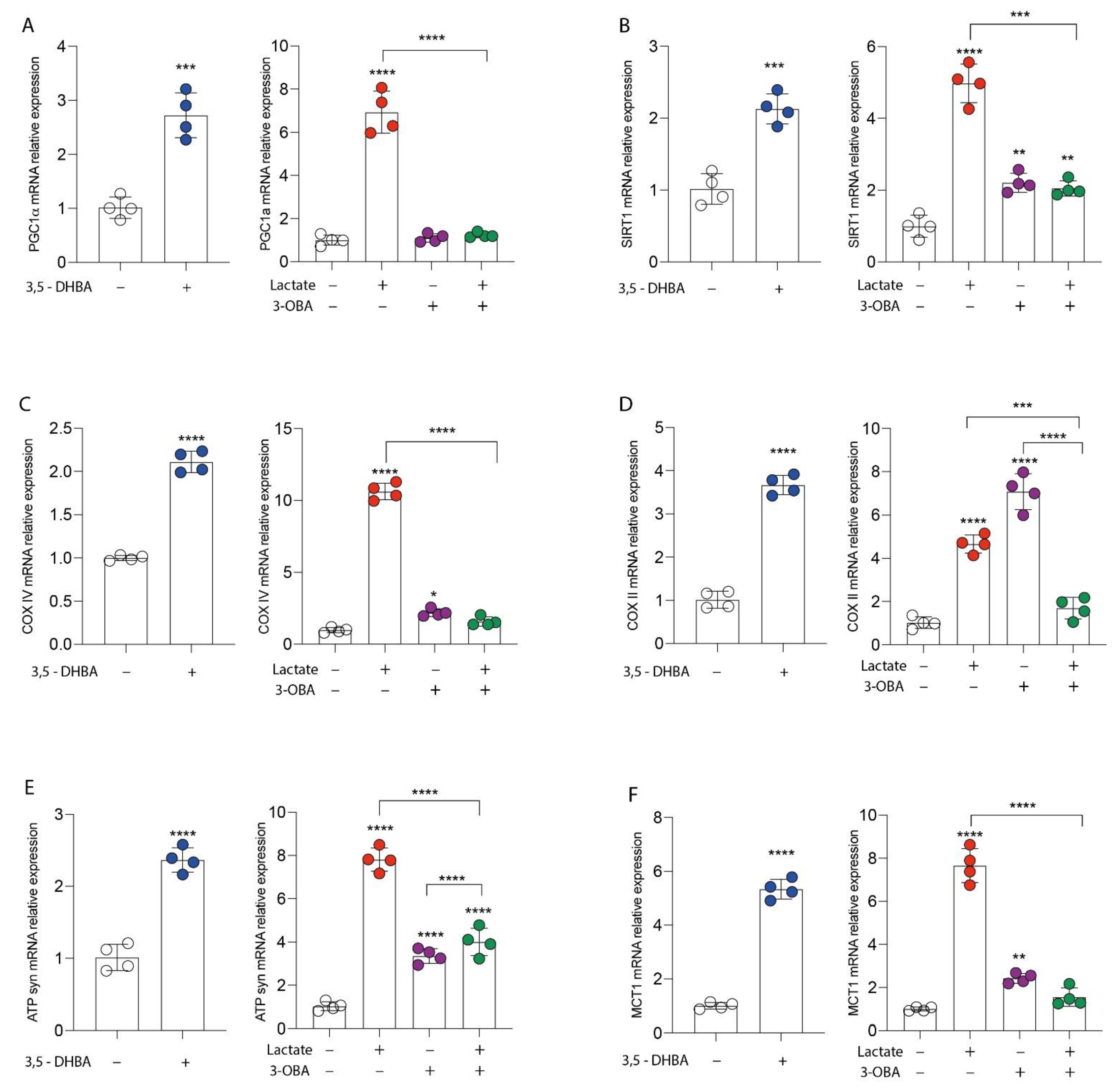
3.1. GPR81 Stimulation Promotes Breast Cancer Cell Growth and Modulates Mitochondrial Metabolism Gene Expression
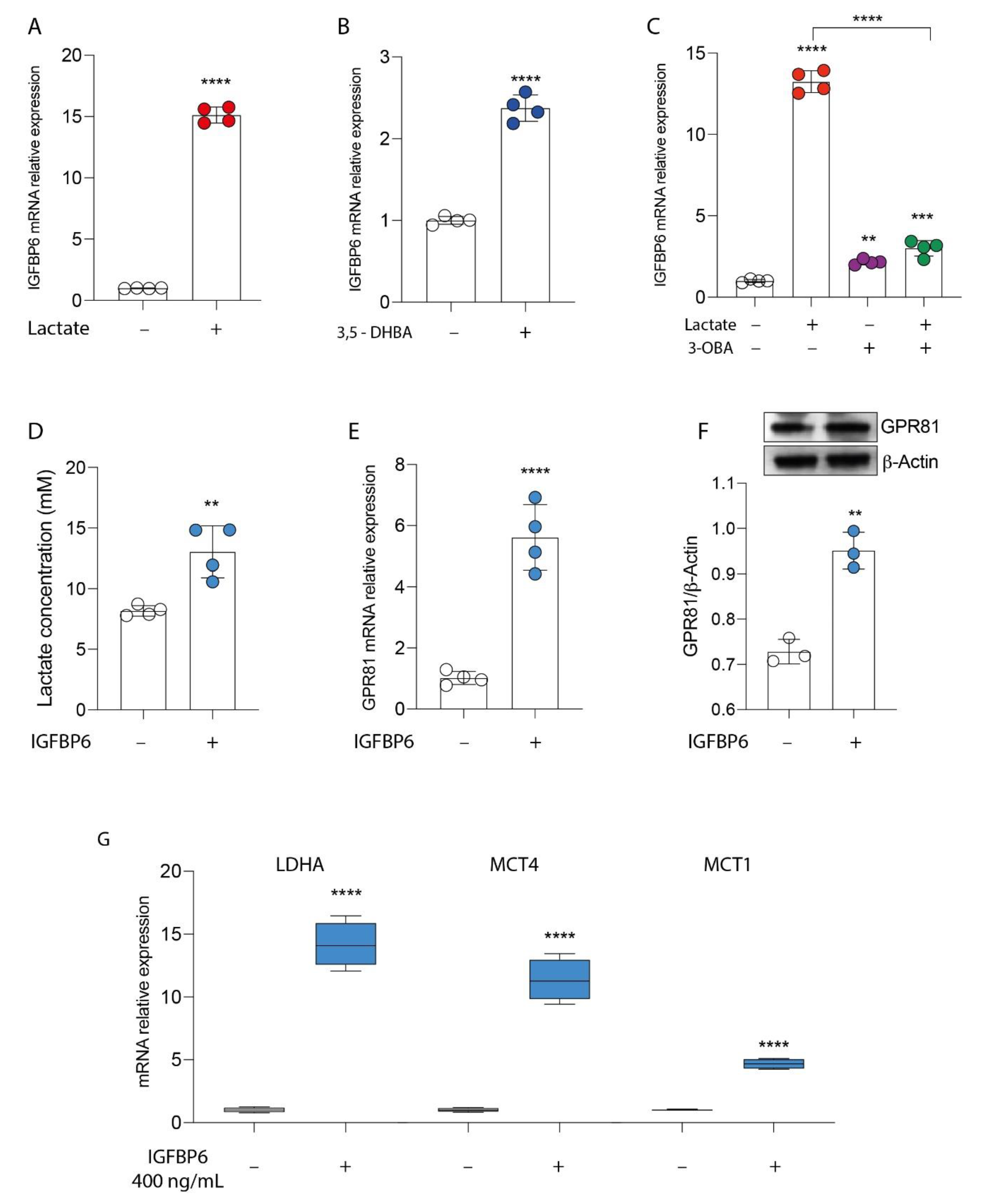
3.2. GPR81/IGFBP6 Axis Activation in Breast Cancer Cells
3.3. IGFBP6 Recapitulates Lactate/GPR81 Agonist Effects on MDA-MB-231 Cells
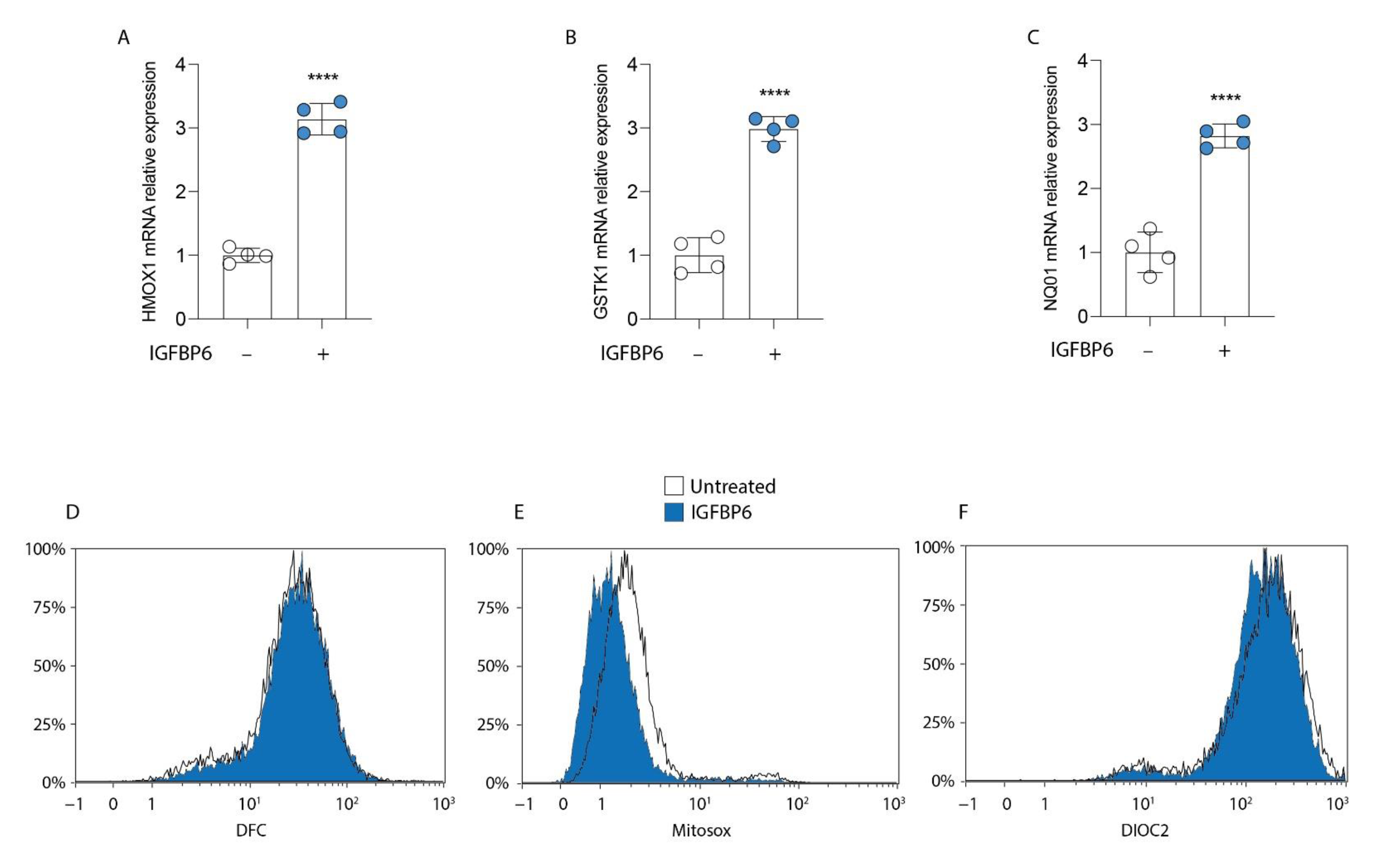
3.4. IGFBP6 Induces an Antioxidant Response in MDA-MB-231 Cells
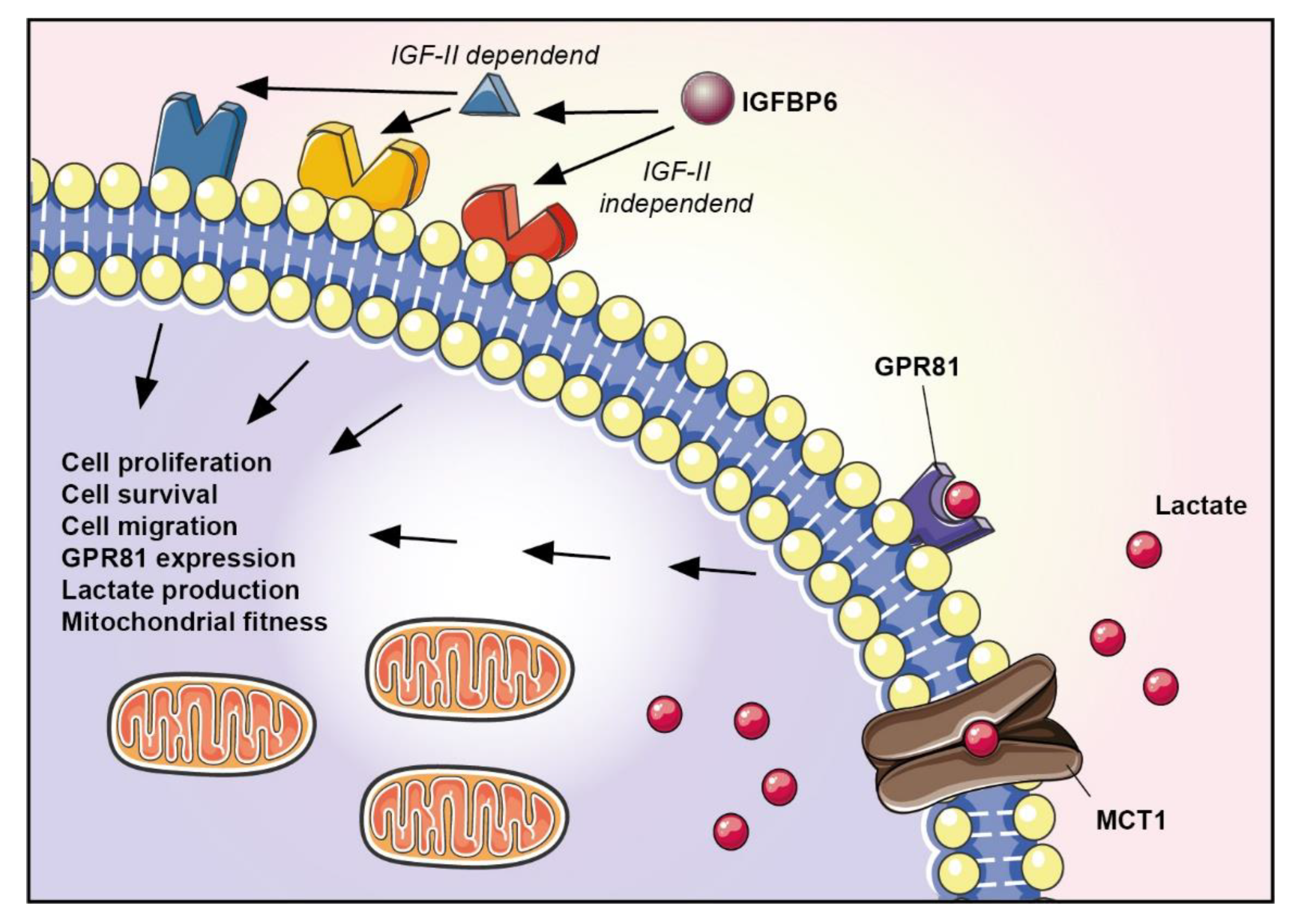
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef]
- Sachdev, J.C.; Sandoval, A.C.; Jahanzeb, M. Update on Precision Medicine in Breast cancer. Cancer Treat. Res. 2019, 178, 45–80. [Google Scholar]
- Braden, A.; Stankowski, R.; Engel, J.; Onitilo, A. Breast Cancer Biomarkers: Risk Assessment, Diagnosis, Prognosis, Prediction of Treatment Efficacy and Toxicity, and Recurrence. Curr. Pharm. Des. 2014, 20, 4879–4898. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sánchez, R.; Rodríguez-Enríquez, S.; Marín-Hernández, A.; Saavedra, E. Energy metabolism in tumor cells. FEBS J. 2007, 274, 1393–1418. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef]
- Sotgia, F.; Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Flomenberg, N.; Birbe, R.C.; Witkiewicz, A.K.; Howell, A.; Philp, N.J.; Pestell, R.G.; Lisanti, M.P. Mitochondrial metabolism in cancer metastasis: Visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle 2012, 11, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014, 74, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- Dhup, S.; Kumar Dadhich, R.; Ettore Porporato, P.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Morandi, A.; Giannoni, E.; Chiarugi, P. Lactate: A Metabolic Driver in the Tumour Landscape. Trends Biochem. Sci. 2019, 44, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Husain, Z.; Huang, Y.; Seth, P.; Sukhatme, V.P. Tumor-Derived Lactate Modifies Antitumor Immune Response: Effect on Myeloid-Derived Suppressor Cells and NK Cells. J. Immunol. 2013, 191, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Tuluc, M.; Whitaker-Menezes, D.; Ames, J.A.; Anantharaman, A.; Butera, A.; Leiby, B.; Cognetti, D.M.; Sotgia, F.; Lisanti, M.P.; et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013, 12, 1371–1384. [Google Scholar] [CrossRef]
- Brown, T.P.; Bhattacharjee, P.; Ramachandran, S.; Sivaprakasam, S.; Ristic, B.; Sikder, M.O.F.; Ganapathy, V. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 2020, 39, 3292–3304. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Xu, O.; Wang, J.; Dong, J.; Ren, X.; Jia, X.; Shan, C. Effects of GPR81 silencing combined with cisplatin stimulation on biological function in hypopharyngeal squamous cell carcinoma. Mol. Med. Rep. 2020, 22, 1727–1736. [Google Scholar] [CrossRef]
- Shen, Z.; Jiang, L.; Yuan, Y.; Deng, T.; Zheng, Y.R.; Zhao, Y.Y.; Li, W.L.; Wu, J.Y.; Gao, J.Q.; Hu, W.W.; et al. Inhibition of G Protein-Coupled Receptor 81 (GPR81) Protects Against Ischemic Brain Injury. CNS Neurosci. Ther. 2015, 21, 271–279. [Google Scholar] [CrossRef]
- Bach, L.A.; Fu, P.; Yang, Z. Insulin-like growth factor-binding protein-6 and cancer. Clin. Sci. 2013, 124, 215–229. [Google Scholar] [CrossRef]
- Bach, L.A. Recent insights into the actions of IGFBP-6. J. Cell Commun. Signal. 2015, 9, 189–200. [Google Scholar] [CrossRef]
- Bach, L.A. Current ideas on the biology of IGFBP-6: More than an IGF-II inhibitor? Growth Horm. IGF Res. 2016, 30, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.A.; Jackson, J.G.; McGuire, W.L.; Krywicki, R.F.; Yee, D. Expression of insulin-like growth factor binding proteins in human breast cancer correlates with estrogen receptor status. J. Cell. Biochem. 1993, 52, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, J.; Li, L.; Sun, Y.; Zhang, X.; Xue, Y.; Lv, J.; Gao, Y.; Li, S.; Yan, W.; et al. L-lactate preconditioning promotes plasticity-related proteins expression and reduces neurological deficits by potentiating GPR81 signaling in rat traumatic brain injury model. Brain Res. 2020, 1746, 146945. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Park, S.A.; Park, K.S.; Park, S.; Heo, K.; Seo, Y.K.; Noh, D.Y.; Ryu, S.H.; Suh, P.G. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget 2016, 7, 70898–70911. [Google Scholar] [CrossRef] [PubMed]
- Aboalola, D.; Han, V.K.M. Insulin-Like Growth Factor Binding Protein-6 Promotes the Differentiation of Placental Mesenchymal Stem Cells into Skeletal Muscle Independent of Insulin-Like Growth Factor Receptor-1 and Insulin Receptor. Stem Cells Int. 2019, 2019, 9245938. [Google Scholar] [CrossRef]
- Barbato, A.; Scandura, G.; Puglisi, F.; Cambria, D.; La Spina, E.; Palumbo, G.A.; Lazzarino, G.; Tibullo, D.; Di Raimondo, F.; Giallongo, C.; et al. Mitochondrial Bioenergetics at the Onset of Drug Resistance in Hematological Malignancies: An Overview. Front. Oncol. 2020, 10, 604143. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Banerji, V. Targeting mitochondrial bioenergetics as a therapeutic strategy for chronic lymphocytic leukemia. Oxid. Med. Cell. Longev. 2018, 2018, 2426712. [Google Scholar]
- Tibullo, D.; Giallongo, C.; Romano, A.; Vicario, N.; Barbato, A.; Puglisi, F.; Parenti, R.; Amorini, A.M.; Saab, M.W.; Tavazzi, B.; et al. Mitochondrial functions, energy metabolism and protein glycosylation are interconnected processes mediating resistance to bortezomib in multiple myeloma cells. Biomolecules 2020, 10, 696. [Google Scholar] [CrossRef]


| Gene of Interest | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| PGC1α | ATGAAGGGTACTTTTCTGCCCC | GGTCTTCACCAACCAGAGCA |
| SIRT1 | AGGCCACGGATAGGTCCATA | GTGGAGGTATTGTTTCCGGC |
| COX IV | GCGGCAGAATGTTGGCTAC | AGACAGGTGCTTGACATGGG |
| COX II | ACGACCTCGATGTTGGATCA | ATCATTTACGGGGGAAGGCG |
| ATPsyn | CCGCCTTCCGCGGTATAATC | ATGTACGCGGGCAATACCAT |
| MCT1 | TGTTGTTGCAAATGGAGTGT | AAGTCGATAATTGATGCCCATGCCAA |
| MCT4 | TATCCAGATCTACCTCACCAC | GGCCTGGCAAAGATGTCGATGA |
| IGFBP6 | GACCAGGAAAGAATGTGAAAGGA | GCTCTGCCAATTGACTTTCCTTAG |
| HCAR1 | TTCGTATTTGGTGGCAGGCA | TTTCGAGGGGTCCAGGTACA |
| LDHA | ||
| HMOX1 | AAGACTGCGTTCCTGCTCAAC | AAAGCCCTACAGCAACTGTCG |
| GSTK1 | CTGGGCTTCGAGATCCTGTG | GGCAGACAAACTTCCACTGTC |
| NQ01 | ||
| β-Actin | CCTTTGCCGATCCGCCG | AACATGATCTGGGTCATCTTCTCGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhitano, L.; Forte, S.; Orlando, L.; Grasso, S.; Barbato, A.; Vicario, N.; Parenti, R.; Fontana, P.; Amorini, A.M.; Lazzarino, G.; et al. The Crosstalk between GPR81/IGFBP6 Promotes Breast Cancer Progression by Modulating Lactate Metabolism and Oxidative Stress. Antioxidants 2022, 11, 275. https://doi.org/10.3390/antiox11020275
Longhitano L, Forte S, Orlando L, Grasso S, Barbato A, Vicario N, Parenti R, Fontana P, Amorini AM, Lazzarino G, et al. The Crosstalk between GPR81/IGFBP6 Promotes Breast Cancer Progression by Modulating Lactate Metabolism and Oxidative Stress. Antioxidants. 2022; 11(2):275. https://doi.org/10.3390/antiox11020275
Chicago/Turabian StyleLonghitano, Lucia, Stefano Forte, Laura Orlando, Stephanie Grasso, Alessandro Barbato, Nunzio Vicario, Rosalba Parenti, Paolo Fontana, Angela M. Amorini, Giuseppe Lazzarino, and et al. 2022. "The Crosstalk between GPR81/IGFBP6 Promotes Breast Cancer Progression by Modulating Lactate Metabolism and Oxidative Stress" Antioxidants 11, no. 2: 275. https://doi.org/10.3390/antiox11020275
APA StyleLonghitano, L., Forte, S., Orlando, L., Grasso, S., Barbato, A., Vicario, N., Parenti, R., Fontana, P., Amorini, A. M., Lazzarino, G., Li Volti, G., Di Rosa, M., Liso, A., Tavazzi, B., Lazzarino, G., & Tibullo, D. (2022). The Crosstalk between GPR81/IGFBP6 Promotes Breast Cancer Progression by Modulating Lactate Metabolism and Oxidative Stress. Antioxidants, 11(2), 275. https://doi.org/10.3390/antiox11020275

















