Depletion of Homeostatic Antibodies against Malondialdehyde-Modified Low-Density Lipoprotein Correlates with Adverse Events in Major Vascular Surgery
Abstract
:1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Blood Samples
2.4. Generation of MDA-LDL
2.5. ELISA to Detect MDA-LDL and Apolipoprotein B-100 (ApoB)
2.6. ELISA to Detect Antibodies to MDA-LDL
2.7. ELISA to Detect Total IgG and IgM Antibodies
2.8. ELISA to Detect C3
2.9. ELISA to Detect IgG/MDA-LDL, IgM/MDA-LDL and C3/MDA-LDL Complexes
2.10. Statistical Analysis
2.11. Role of the Funding Sources
3. Results
3.1. Baseline Characteristics
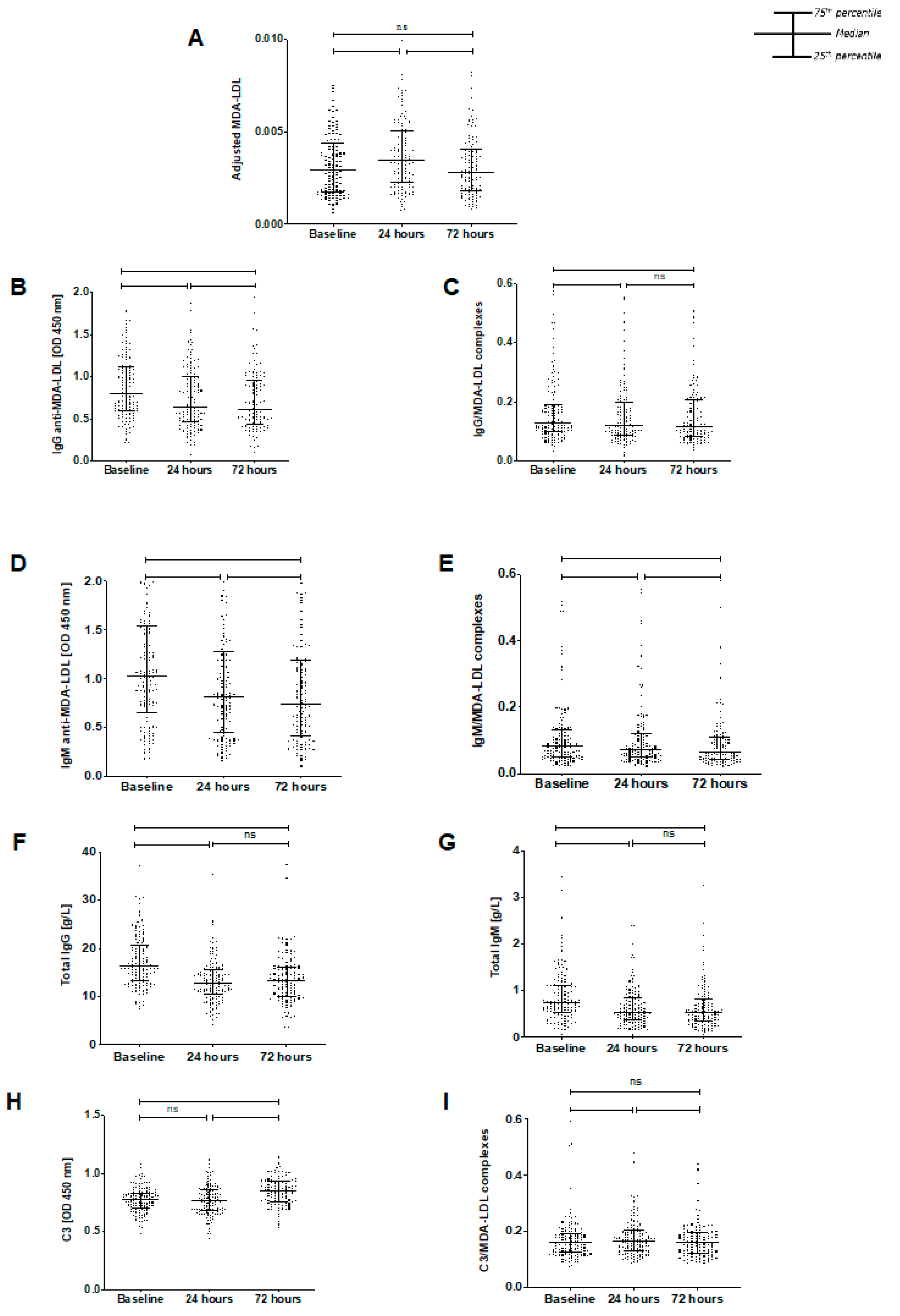
3.2. Temporal Changes in Biomarkers
3.3. Antibody Relationships
3.4. Relationships between Biomarkers and Clinical Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ApoB | apolipoprotein B-100 |
| ELISA | enzyme-linked immunosorbent assay |
| HRP | horseradish peroxidase |
| LDL | low-density lipoprotein |
| MDA | malondialdehyde |
| MDA-LDL | malondialdehyde-conjugated LDL |
| OD | optical density |
| OxLDL | oxidized low-density lipoprotein |
| PBS | phosphate-buffered saline |
| SIMIAN | Stress Induced Myocardial Infarction After Non-cardiac vascular surgery |
References
- Naito, M.; Kuzuya, M.; Iguchi, A. Mechanisms of endothelial cell injury induced by oxidatively modified LDL. J. Jpn. Atheroscler. Soc. 1994, 22, 257–262. [Google Scholar] [CrossRef]
- Hartley, A.; Haskard, D.; Khamis, R. Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis-Novel insights and future directions in diagnosis and therapy. Trends Cardiovasc. Med. 2019, 29, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, V.J.; Haskard, D.O.; Fedorowski, A.; Hartley, A.; Kardys, I.; Caga-Anan, M.; Akkerhuis, K.M.; Oemrawsingh, R.M.; van Geuns, R.J.; de Jaegere, P.; et al. IgM anti-malondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the Nordic Diltiazem (NORDIL) study and the Integrated Imaging and Biomarker Study 3 (IBIS-3). EBioMedicine 2018, 36, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khamis, R.Y.; Hughes, A.D.; Caga-Anan, M.; Chang, C.L.; Boyle, J.J.; Kojima, C.; Welsh, P.; Sattar, N.; Johns, M.; Sever, P.; et al. High Serum Immunoglobulin G and M Levels Predict Freedom From Adverse Cardiovascular Events in Hypertension: A Nested Case-Control Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. EBioMedicine 2016, 9, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Iseme, R.A.; McEvoy, M.; Kelly, B.; Agnew, L.; Walker, F.R.; Handley, T.; Oldmeadow, C.; Attia, J.; Boyle, M. A role for autoantibodies in atherogenesis. Cardiovasc. Res. 2017, 113, 1102–1112. [Google Scholar] [CrossRef]
- Van den Berg, V.J.; Vroegindewey, M.M.; Kardys, I.; Boersma, E.; Haskard, D.; Hartley, A.; Khamis, R. Anti-Oxidized LDL Antibodies and Coronary Artery Disease: A Systematic Review. Antioxidants 2019, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Brilakis, E.S.; Lennon, R.J.; Miller, E.R.; Witztum, J.L.; McConnell, J.P.; Kornman, K.S.; Berger, P.B. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 2007, 48, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.J.; Malik, T.H.; Ehrenstein, M.R.; Boyle, J.J.; Botto, M.; Haskard, D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009, 120, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Karvonen, J.; Paivansalo, M.; Kesaniemi, Y.A.; Horkko, S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 2003, 108, 2107–2112. [Google Scholar] [CrossRef]
- Soto, Y.; Conde, H.; Aroche, R.; Brito, V.; Luaces, P.; Nasiff, A.; Obregón, A.; López, A.M.V. Autoantibodies to oxidized low density lipoprotein in relation with coronary artery disease. Hum. Antibodies 2009, 18, 109–117. [Google Scholar] [CrossRef]
- Oksjoki, R.; Kovanen, P.T.; Lindstedt, K.A.; Jansson, B.; Pentikainen, M.O. OxLDL-IgG immune complexes induce survival of human monocytes. Arter. Thromb. Vasc. Biol. 2006, 26, 576–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, D.A.; Tenner, A.J. Innate immune proteins C1q and mannan-binding lectin enhance clearance of atherogenic lipoproteins by human monocytes and macrophages. J. Immunol. 2010, 185, 3932–3939. [Google Scholar] [CrossRef] [Green Version]
- Malik, T.H.; Cortini, A.; Carassiti, D.; Boyle, J.J.; Haskard, D.O.; Botto, M. The alternative pathway is critical for pathogenic complement activation in endotoxin- and diet-induced atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2010, 122, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Tanaka, T.; Nagoshi, T.; Sekiyama, H.; Arase, S.; Minai, K.; Ogawa, T.; Yoshimura, M. Increase in the oxidised low-density lipoprotein level by smoking and the possible inhibitory effect of statin therapy in patients with cardiovascular disease: A retrospective study. BMJ Open 2015, 5, e005455. [Google Scholar] [CrossRef]
- Strzyżewski, K.W.; Pioruńska-Stolzmann, M.; Majewski, W.; Kasprzak, M.; Strzyżewski, W. Effect of surgical treatment on lipid peroxidation parameters and antioxidant status in the serum of patients with peripheral arterial disease. Dis. Markers 2013, 35, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Arató, E.; Jancsó, G.; Sínay, L.; Kürthy, M.; Lantos, J.; Ferencz, S.; Horváth, S.; Shafiei, M.; Kasza, G.; Verzár, Z.; et al. Reperfusion injury and inflammatory responses following acute lower limb revascularization surgery. Clin. Hemorheol. Microcirc. 2008, 39, 79–85. [Google Scholar] [CrossRef]
- Eagle, K.A.; Rihal, C.S.; Mickel, M.C.; Holmes, D.R.; Foster, E.D.; Gersh, B.J. Cardiac risk of noncardiac surgery: Influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study. Circulation 1997, 96, 1882–1887. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.S.; Hartley, A.; Caga-Anan, M.; Ammari, T.; Khan, A.H.A.; Nguyen, B.A.V.; Kojima, C.; Anderson, J.; Lynham, S.; Johns, M.; et al. A Novel Immunoassay for Malondialdehyde-Conjugated Low-Density Lipoprotein Measures Dynamic Changes in the Blood of Patients Undergoing Coronary Artery Bypass Graft Surgery. Antioxidants 2021, 10, 1298. [Google Scholar] [CrossRef]
- Palinski, W.; Ylä-Herttuala, S.; Rosenfeld, M.E.; Butler, S.W.; A Socher, S.; Parthasarathy, S.; Curtiss, L.K.; Witztum, J.L. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis 1990, 10, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Lau, H.K.; Han, K.R.; Shortal, B.; Miller, E.R.; Segev, A.; Curtiss, L.K.; Witztum, J.L.; Strauss, B.H. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): Short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation 2004, 109, 3164–3170. [Google Scholar] [CrossRef] [Green Version]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weismann, D.; Hartvigsen, K.; Lauer, N.; Bennett, K.L.; Scholl, H.P.N.; Issa, P.C.; Cano, M.; Brandstätter, H.; Tsimikas, S.; Skerka, C.; et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 2011, 478, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, V.W.; Yun, S.; Botto, M.; Mason, J.C.; Malik, T.H.; Song, W.; Paixao-Cavalcante, D.; Pickering, M.C.; Boyle, J.J.; Haskard, D.O. Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice. Am. J. Pathol. 2009, 175, 1757–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cominacini, L.; Pasini, A.F.; Garbin, U.; Davoli, A.; Tosetti, M.L.; Campagnola, M.; Rigoni, A.; Pastorino, A.M.; Lo Cascio, V.; Sawamura, T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 2000, 275, 12633–12638. [Google Scholar] [CrossRef] [Green Version]
| SIMIAN Study Baseline Characteristics | |
|---|---|
| Characteristic | Study Population (n = 131) |
| Age, years (median, IQR) | 71.5 (65–77) |
| Sex | |
| Male | 105 (80.2%) |
| Female | 26 (19.8%) |
| Ethnicity | |
| White | 130 (99.2%) |
| Asian | 1 (0.8%) |
| Hypertension | 97 (74.0%) |
| IHD | 35 (26.7%) |
| Previous MI | 34 (26.0%) |
| Previous CABG | 16 (12.2%) |
| Diabetes | 28 (21.4%) |
| CKD Stages | |
| I | 51 (38.9%) |
| II | 48 (36.6%) |
| III | 29 (22.1%) |
| IV | 3 (2.3%) |
| Previous CVA | 12 (9.2%) |
| Previous TIA | 12 (9.2%) |
| Smoking status | |
| Current smoker | 42 (32.1%) |
| Ex-smoker | 78 (59.5%) |
| Non-smoker | 11 (8.4%) |
| Operation | |
| Open AAA | 4 (3.1%) |
| Endovascular AAA | 61 (46.6%) |
| Arterial Bypass | 58 (44.3%) |
| Limb Amputation | 7 (5.3%) |
| Unavailable Information | 1 (0.8%) |
| Other co-morbidities | |
| Neurological disease | 4 (3.1%) |
| Rheumatological disease | 28 (21.4%) |
| Malignancy | 22 (16.8) |
| Asthma | 6 (4.6%) |
| COPD | 39 (29.8%) |
| TB | 3 (2.3%) |
| GORD | 32 (24.4%) |
| Peptic Ulcer Disease | 12 (9.2%) |
| Hepatic Disease | 3 (2.3%) |
| 24-Hour Change | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Per% change in MDA-LDL | 0.99 (0.97–1.01) | 0.48 | |||
| MDA-LDL | Smallest increase | 1.00 (Ref.) | |||
| Mid | 1.33 (0.46–3.8) | 0.6 | |||
| Largest increase | 0.7 (0.22–2.26) | 0.55 | |||
| Trend | 0.57 | ||||
| Per% change in IgG anti-MDA-LDL | 0.94 (0.9–0.98) | <0.001 | 0.95 (0.9–0.99) | 0.03 | |
| IgG anti-MDA-LDL | Largest decrease | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Mid | 0.23 (0.07–0.7) | 0.01 | 0.26 (0.08–0.9) | 0.03 | |
| Smallest decrease | 0.13 (0.03–0.5) | <0.001 | 0.16 (0.04–0.66) | 0.01 | |
| Trend | <0.001 | 0.008 | |||
| Per% change in IgM anti-MDA-LDL | 0.96 (0.93–0.99) | 0.01 | 0.98 (0.94–1.02) | 0.25 | |
| IgM anti-MDA-LDL | Largest decrease | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Mid | 0.33 (0.11–0.96) | 0.04 | 0.58 (0.16–2.08) | 0.41 | |
| Smallest decrease | 0.21 (0.06–0.71) | 0.01 | 0.41 (0.1–1.71) | 0.22 | |
| Trend | 0.01 | 0.25 | |||
| Per% change in IgG/MDA-LDL complexes | 0.99 (0.97–1.01) | 0.49 | |||
| IgG/MDA-LDL complexes | Largest decrease | 1.00 (Ref.) | |||
| Mid | 0.2 (0.05–0.78) | 0.02 | |||
| Smallest decrease | 0.73 (0.27–1.99) | 0.54 | |||
| Trend | 0.48 | ||||
| Per% change in IgM/MDA-LDL complexes | 0.99 (0.97–1.01) | 0.24 | |||
| IgM/MDA-LDL complexes | Largest decrease | 1.00 (Ref.) | |||
| Mid | 0.34 (0.11–1.08) | 0.07 | |||
| Smallest decrease | 0.52 (0.18–1.48) | 0.22 | |||
| Trend | 0.18 | ||||
| 24-Hour Change | |||
|---|---|---|---|
| Model 1 | |||
| OR (95% CI) | p Value | ||
| Per% change in total IgG | 0.97 (0.94–0.99) | 0.02 | |
| Total IgG | Largest decrease | 1.00 (Ref.) | |
| Mid | 0.29 (0.09–0.9) | 0.03 | |
| Smallest decrease | 0.36 (0.12–1.04) | 0.06 | |
| Trend | 0.04 | ||
| Per% change in total IgM | 0.96 (0.93–0.99) | <0.001 | |
| Total IgM | Largest decrease | 1.00 (Ref.) | |
| Mid | 0.37 (0.13–1.04) | 0.06 | |
| Smallest decrease | 0.15 (0.04–0.56) | <0.001 | |
| Trend | <0.001 | ||
| Per% change in complement C3 | 0.99 (0.96–1.03) | 0.6 | |
| Complement C3 | Smallest increase | 1.00 (Ref.) | |
| Mid | 0.48 (0.16–1.46) | 0.19 | |
| Largest increase | 0.59 (0.2–1.76) | 0.35 | |
| Trend | 0.32 | ||
| Per% change in C3/MDA-LDL complexes | 1.21 (1.04–1.41) | 0.01 | |
| C3/MDA-LDL complexes | Smallest increase | 1.00 (Ref.) | |
| Mid | 0.8 (0.2–3.26) | 0.76 | |
| Largest increase | 4.19 (1.35–12.97) | 0.01 | |
| Trend | 0.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartley, A.; Pradeep, M.; Van den Berg, V.; Khan, A.H.A.; Shah, H.A.; Allaf, M.; Chow, A.; Caga-Anan, M.; Shalhoub, J.; Koenig, W.; et al. Depletion of Homeostatic Antibodies against Malondialdehyde-Modified Low-Density Lipoprotein Correlates with Adverse Events in Major Vascular Surgery. Antioxidants 2022, 11, 271. https://doi.org/10.3390/antiox11020271
Hartley A, Pradeep M, Van den Berg V, Khan AHA, Shah HA, Allaf M, Chow A, Caga-Anan M, Shalhoub J, Koenig W, et al. Depletion of Homeostatic Antibodies against Malondialdehyde-Modified Low-Density Lipoprotein Correlates with Adverse Events in Major Vascular Surgery. Antioxidants. 2022; 11(2):271. https://doi.org/10.3390/antiox11020271
Chicago/Turabian StyleHartley, Adam, Magapu Pradeep, Victor Van den Berg, Ameer Hamid Ahmed Khan, Hasan Ali Shah, Mohammed Allaf, Anna Chow, Mikhail Caga-Anan, Joseph Shalhoub, Wolfgang Koenig, and et al. 2022. "Depletion of Homeostatic Antibodies against Malondialdehyde-Modified Low-Density Lipoprotein Correlates with Adverse Events in Major Vascular Surgery" Antioxidants 11, no. 2: 271. https://doi.org/10.3390/antiox11020271
APA StyleHartley, A., Pradeep, M., Van den Berg, V., Khan, A. H. A., Shah, H. A., Allaf, M., Chow, A., Caga-Anan, M., Shalhoub, J., Koenig, W., Fisher, M., Haskard, D. O., & Khamis, R. Y. (2022). Depletion of Homeostatic Antibodies against Malondialdehyde-Modified Low-Density Lipoprotein Correlates with Adverse Events in Major Vascular Surgery. Antioxidants, 11(2), 271. https://doi.org/10.3390/antiox11020271






