COVID-19 during Gestation: Maternal Implications of Evoked Oxidative Stress and Iron Metabolism Impairment
Abstract
:1. Introduction
2. Materials and Methods
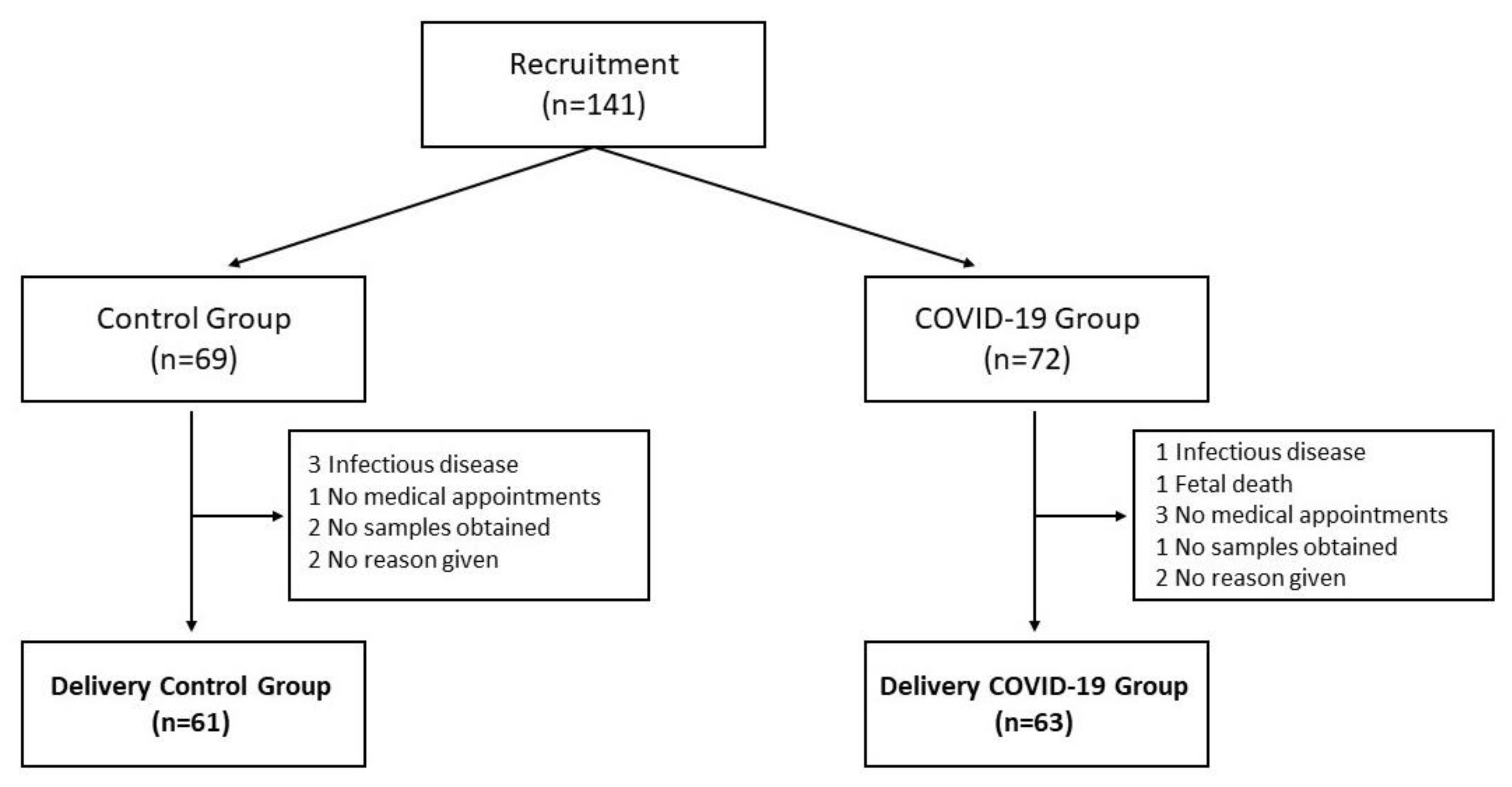
2.1. Subjects
2.2. Blood Sampling
2.3. Placenta Sampling
2.4. Antioxidant Capacity
2.5. Antioxidant Enzymes Activity
2.6. Protein Oxidation (Carbonyl Groups) Measurement
2.7. 8-Hydroxy-2′-deoxyguanosine (8-OHdG)
2.8. 15-F2t-Isoprostanes
2.9. Lipid Hydroperoxides
2.10. Antioxidant Vitamins
2.11. Multielemental Analysis by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
2.12. Western Blotting and Immunocytochemistry
2.13. Statistical Analysis
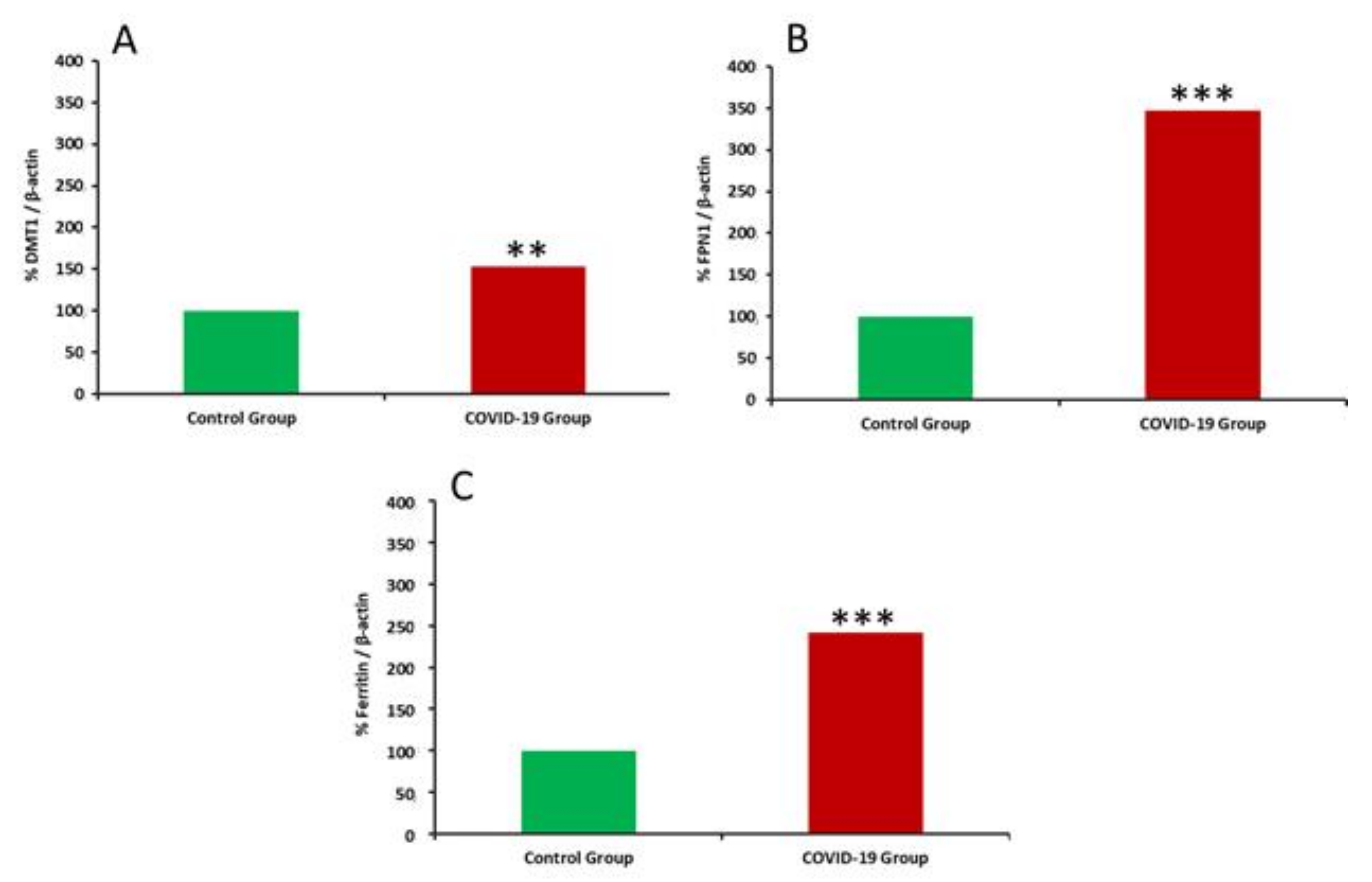
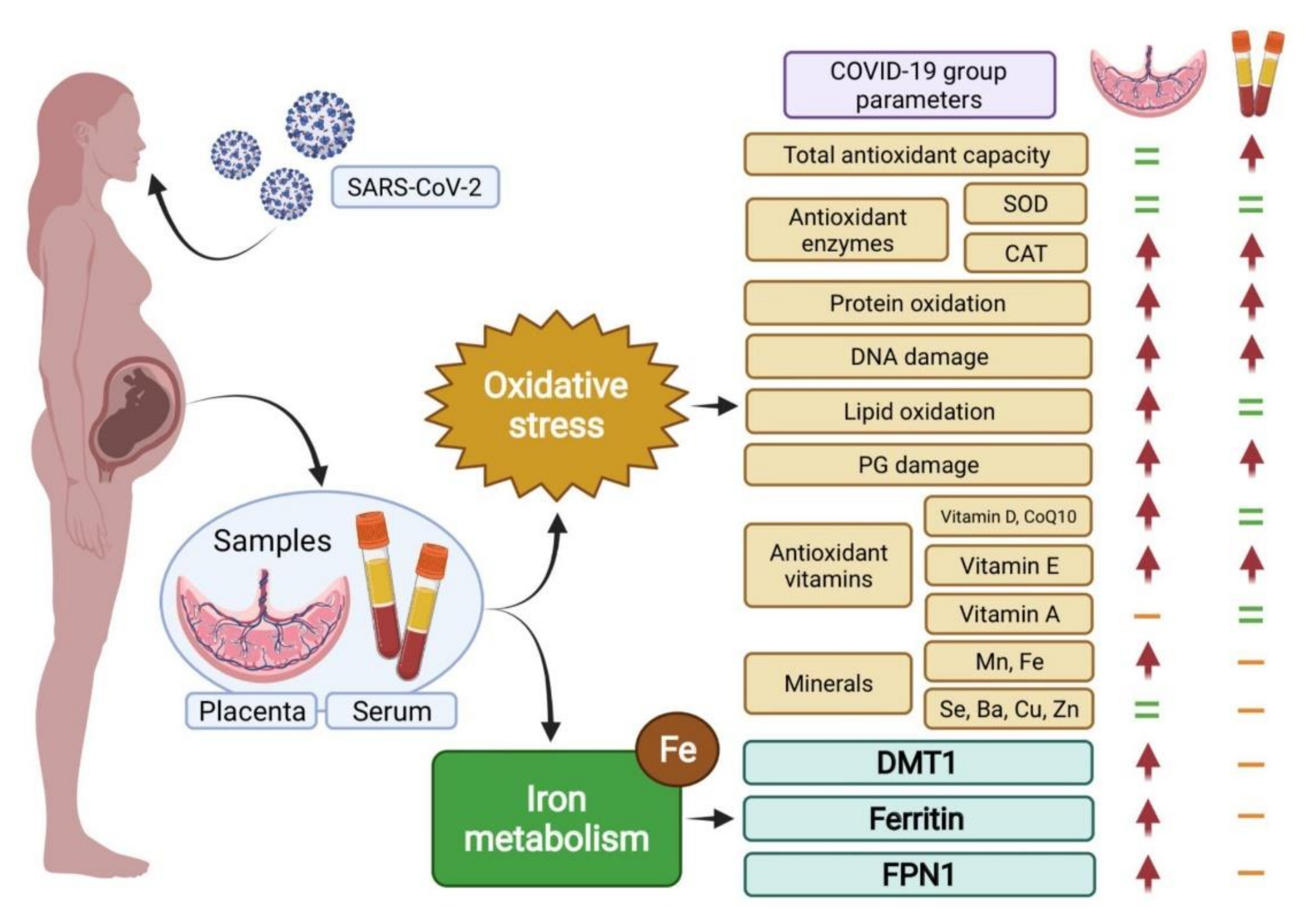
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Madinger, N.E.; Greenspoon, J.S.; Ellrodt, A.G. Pneumonia during pregnancy: Has modern technology improved maternal and fetal outcome? Am. J. Obstet. Gynecol. 1989, 161, 657–662. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Honein, M.A.; Rasmussen, S.A.; Williams, J.L.; Swerdlow, D.L.; Biggerstaff, M.S.; Lindstrom, S.; Louie, J.K.; Christ, C.M.; Bohm, S.R.; et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009, 374, 451–458. [Google Scholar] [CrossRef]
- Benedetti, T.J.; Valle, R.; Ledger, W.J. Antepartum pneumonia in pregnancy. Am. J. Obstet. Gynecol. 1982, 144, 413–417. [Google Scholar] [CrossRef]
- Yu, N.; Li, W.; Kang, Q.; Xiong, Z.; Wang, S.; Lin, X.; Liu, Y.; Xiao, J.; Liu, H.; Deng, D.; et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020, 20, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Di Toro, F.; Gjoka, M.; Di Lorenzo, G.; De Santo, D.; De Seta, F.; Maso, G.; Risso, F.M.; Romano, F.; Wiesenfeld, U.; Levi-D’Ancona, R.; et al. Impact of COVID-19 on maternal and neonatal outcomes: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.-L.; Zhao, S.-J.; Kwak-Kim, J.; Mor, G.; Liao, A.-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020, 139, 103122. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurina, Y.Y.; Bayir, H.; Chu, C.T.; Kapralov, A.A.; Vlasova, I.I.; Belikova, N.A.; Tyurin, V.A.; Amoscato, A.; Epperly, M.; et al. The “pro-apoptotic genies” get out of mitochondria: Oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem.-Biol. Interact. 2006, 163, 15–28. [Google Scholar] [CrossRef]
- Khairallah, R.J.; Kim, J.; O’Shea, K.M.; O’Connell, K.A.; Brown, B.H.; Galvão, T.; Daneault, C.; Rosiers, C.D.; Polster, B.; Hoppel, C.L.; et al. Improved Mitochondrial Function with Diet-Induced Increase in Either Docosahexaenoic Acid or Arachidonic Acid in Membrane Phospholipids. PLoS ONE 2012, 7, e34402. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Castro, J.; Pulido-Moran, M.; Moreno-Fernandez, J.; Kajarabille, N.; De Paco, C.; Garrido-Sanchez, M.; Prados, S.; Ochoa, J. Gender specific differences in oxidative stress and inflammatory signaling in healthy term neonates and their mothers. Pediatr. Res. 2016, 80, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Means, R.T. Iron Deficiency and Iron Deficiency Anemia: Implications and Impact in Pregnancy, Fetal Development, and Early Childhood Parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clin. Pract. 2020, 10, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.N.; Tan, T.K.; Abbas, M.; Wideman, S.K.; Bonadonna, M.; Stoffel, N.U.; Wray, K.; Kronsteiner, B.; Smits, G.; Campagna, D.R.; et al. Hepcidin-Mediated Hypoferremia Disrupts Immune Responses to Vaccination and Infection. Med 2020, 2, 164–179.e12. [Google Scholar] [CrossRef]
- Lorena, D.; Isabella, N.; Daniele, V.; Giovanni Battista, V.; Andrea, M.; Andrea, V.; Patrizio, C.; Giovanna, G.; Filippo, B. COVID-19, inflammatory response, iron homeostasis and toxicity: A prospective cohort study in the Emergency Department of Piacenza (Italy). Res. Sq. 2022. [Google Scholar] [CrossRef]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 5–38. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, G.; Saderi, L.; Aliberti, S.; Mondoni, M.; Piana, A.; Dessole, F.; Dessole, M.; Cherchi, P.L.; Dessole, S.; Sotgiu, G. COVID-19 in pregnant women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Kajarabille, N.; Hurtado, J.A.; Peña-Quintana, L.; Peña, M.; Ruiz, J.; Diaz-Castro, J.; Rodríguez-Santana, Y.; Martin-Alvarez, E.; López-Frias, M.; Soldado, O.; et al. Omega-3 LCPUFA supplement: A nutritional strategy to prevent maternal and neonatal oxidative stress. Matern. Child Nutr. 2017, 13, e12300. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.J.; Ekholm, J.E. The preparation of red cell ghosts (membranes). Methods Enzym. 1974, 31, 168–172. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Crapo, J.D.; McCord, J.M.; Fridovich, I. Preparation and assay of superioxide dismutases. Methods Enzymol. 1978, 53, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ntyonga-Pono, M.-P. COVID-19 infection and oxidative stress: An under-explored approach for prevention and treatment? Pan Afr. Med. J. 2020, 35, 12. [Google Scholar] [CrossRef]
- Pohanka, M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013, 58, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress in Infection and Consequent Disease. Oxidative Med. Cell. Longev. 2017, 2017, 3496043. [Google Scholar] [CrossRef]
- Rahman, I.; Adcock, I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006, 28, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabelo, L.A.; Alenina, N.; Bader, M. ACE2–angiotensin-(1–7)–Mas axis and oxidative stress in cardiovascular disease. Hypertens. Res. 2011, 34, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.; Yung, H.-W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta 2009, 30 (Suppl. A), 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxidative Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Qian, K.; Li, T.; Zhang, S.; Fu, W.; Ding, M.; Hu, S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020, 11, 2070. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gengler, C.; Dubruc, E.; Favre, G.; Greub, G.; de Leval, L.; Baud, D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin. Microbiol. Infect. 2021, 27, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Aziz, N.; Sekhon, L.; Agarwal, R.; Mansour, G.; Li, J.; Agarwal, A. Lipid Peroxidation and Antioxidant Status in Preeclampsia: A systematic review. Obstet. Gynecol. Surv. 2009, 64, 750–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental Origins of Chronic Disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef]
- Hung, T.-H.; Burton, G.J. Hypoxia and Reoxygenation: A Possible Mechanism for Placental Oxidative Stress in Preeclampsia. Taiwan. J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Scifres, C.M.; Nelson, D.M. Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 2009, 587, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Aranguren, L.C.; Prada, C.E.; Riãno-Medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Fukagawa, N.K. Age-Related Changes in Redox Signaling and VSMC Function. Antioxid. Redox Signal. 2010, 12, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Bochkov, V.N.; Philippova, M.; Oskolkova, O.; Kadl, A.; Furnkranz, A.; Karabeg, E.; Afonyushkin, T.; Gruber, F.; Breuss, J.; Minchenko, A.; et al. Oxidized Phospholipids Stimulate Angiogenesis Via Autocrine Mechanisms, Implicating a Novel Role for Lipid Oxidation in the Evolution of Atherosclerotic Lesions. Circ. Res. 2006, 99, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Delvin, E.; Ouellet, A.; Morin, L.; Dubé, J.; Boucoiran, I.; Moutquin, J.-M.; Fouron, J.-C.; Klam, S.; Levy, E.; et al. Oxidative conditions prevail in severe IUGR with vascular disease and Doppler anomalies. J. Matern.-Fetal Neonatal Med. 2015, 28, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Marchi, G.; Busti, F.; Vianello, A. Iron metabolism in infections: Focus on COVID-19. Semin. Hematol. 2021, 58, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Prentice, A. Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008, 6, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Fleming, R.E.; Minnick, M.F. Sequestration and Scavenging of Iron in Infection. Infect. Immun. 2013, 81, 3503–3514. [Google Scholar] [CrossRef] [Green Version]
- Ruscitti, P.; Cipriani, P.; Ciccia, F.; Di Benedetto, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Guggino, G.; Di Bartolomeo, S.; Triolo, G.; et al. H-ferritin and CD68+/H-ferritin+ monocytes/macrophages are increased in the skin of adult-onset Still’s disease patients and correlate with the multi-visceral involvement of the disease. Clin. Exp. Immunol. 2016, 186, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Colafrancesco, S.; Priori, R.; Alessandri, C.; Astorri, E.; Perricone, C.; Blank, M.; Agmon-Levin, N.; Shoenfeld, Y.; Valesini, G. sCD163 in AOSD: A biomarker for macrophage activation related to hyperferritinemia. Immunol. Res. 2014, 60, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Colafrancesco, S.; Alessandri, C.; Conti, F.; Priori, R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun. Rev. 2020, 19, 102573. [Google Scholar] [CrossRef]
- Pretorius, E.; Kell, D.B. Diagnostic morphology: Biophysical indicators for iron-driven inflammatory diseases. Integr. Biol. 2014, 6, 486–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lei, G.-S.; Zhang, C.; Cheng, B.-H.; Lee, C.-H. Mechanisms of Action of Vitamin D as Supplemental Therapy for Pneumocystis Pneumonia. Antimicrob. Agents Chemother. 2017, 61, e01226-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.; Kilby, M.D.; Moss, P.A.H.; Chakraverty, R. Differential Regulation of Vitamin D Receptor and Its Ligand in Human Monocyte-Derived Dendritic Cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewison, M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010, 321, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.; Spector, S.A. Autophagy induction by vitamin D inhibits bothMycobacterium tuberculosisand human immunodeficiency virus type 1. Autophagy 2012, 8, 1523–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.; Lin, E.; He, L.; Yu, J.; Tan, P.; Zhou, Y. Autophagy and Viral Infection. Adv. Exp. Med. Biol. 2019, 1209, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Kim, H.J.; Li, H.; Jo, K.D.; Lee, M.K.; Song, S.H.; Yang, H.O. 1,25-Dyhydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem. Biophys. Res. Commun. 2014, 451, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy 2008, 4, 947–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuk, J.-M.; Shin, D.-M.; Lee, H.-M.; Yang, C.-S.; Jin, H.S.; Kim, K.-K.; Lee, Z.-W.; Lee, S.-H.; Kim, J.M.; Jo, E.-K. Vitamin D3 Induces Autophagy in Human Monocytes/Macrophages via Cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagné, A.; Wei, S.Q.; Fraser, W.D.; Julien, P. Absorption, Transport, and Bioavailability of Vitamin E and its Role in Pregnant Women. J. Obstet. Gynaecol. Can. 2009, 31, 210–217. [Google Scholar] [CrossRef]
- Wu, D.; Meydani, S.N. Vitamin E, Immune Function, and Protection Against Infection. In Vitamin E in Human Health. Nutrition and Health; Weber, P., Birringer, M., Blumberg, J., Eggersdorfer, M., Frank, J., Eds.; Humana Press: Cham, Switzerland, 2019. [Google Scholar]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G. A critical appraisal of the mitochondrial coenzyme Q pool. FEBS Lett. 2001, 509, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Kaikkonen, J.; Nyyssönen, K.; Tuomainen, T.-P.; Ristonmaa, U.; Salonen, J.T. Determinants of plasma coenzyme Q10in humans. FEBS Lett. 1999, 443, 163–166. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, J.M.; Watts, G.F. Can coenzyme Q10 improve vascular function and blood pressure? Potential for effective therapeutic reduction in vascular oxidative stress. BioFactors 2003, 18, 129–136. [Google Scholar] [CrossRef]
- Cuffe, J.S.; Holland, O.; Salomon, C.; Rice, G.E.; Perkins, A. Review: Placental derived biomarkers of pregnancy disorders. Placenta 2017, 54, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbetti, N.; Cantero, M.R.; Dalghi, M.G.; Cantiello, H.F. Reactive Oxygen Species Inhibit Polycystin-2 (TRPP2) Cation Channel Activity in Term Human Syncytiotrophoblast. Placenta 2008, 29, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Jarvie, E.; Hauguel-De-Mouzon, S.; Nelson, S.; Sattar, N.; Catalano, P.M.; Freeman, D.J. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin. Sci. 2010, 119, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Control | COVID-19 | |
|---|---|---|
| Age (years) | 31.58 ± 1.09 | 31.96 ± 0.78 |
| Weight (kg) | 73.69 ± 2.55 | 74.12 ± 2.67 |
| Length (cm) | 166.83 ± 1.03 | 163.97 ± 0.68 |
| BMI (kg/m2) | 26.31 ± 1 | 27.3 ± 0.8 |
| Parity | Uni (%): 52.22 | 53.14 |
| Multi (%): 47.28 | 46.86 | |
| Delivery method | V (%): 56.2 | 58.7 |
| A (%): 21.8 | 19.9 | |
| C (%): 21.8 | 23.8 | |
| Hemoglobin 2nd T (g/L) | 11.88 ± 0.23 | 11.53 ± 0.13 |
| Hemoglobin 3rd T (g/L) | 11.96 ± 0.23 | 11.72 ± 0.17 |
| Hematocrit 2nd T (%) | 35.32 ± 0.61 | 34.11 ± 0.37 |
| Hematocrit 3rd T (%) | 35.70 ± 0.63 | 34.82 ± 0.47 |
| Serum Iron 3rd T (µg/dL) | 97.05 ± 14.35 | 60.14 ± 9.87 ** |
| Placenta | Serum | |||
|---|---|---|---|---|
| Control | COVID-19 | Control | COVID-19 | |
| ABTS (mmol/L Trolox) | 3.09 ± 0.15 | 3.11 ± 0.11 | 3.34 ± 0.175 | 2.78 ± 0.161 * |
| SOD (mU/mg protein) | 440.02 ± 40.14 | 430.25 ± 30.69 | 4.45 ± 0.43 | 3.92 ± 0.29 |
| CAT (mU/mg protein) | 186.18 × 103 ± 9.28 | 168.72 × 103 ± 5.10 ** | 77.01 ± 5.90 | 62.86 ± 2.86 * |
| 8-OhdG (ng/mL) | 283.70 ± 7.98 | 316.12 ± 6.95 ** | 75.82 ± 2.85 | 82.38 ± 1.51 ** |
| Hydroperoxides (µM) | 40.73 ± 2.47 | 48.02 ± 1.90 *** | 1.51± 0.27 | 1.12 ± 0.20 |
| Isoprostanes (ng/mL) | 35.30 ± 1.31 | 39.85 ± 0.57 ** | 4.35 ± 0.87 | 8.85 ± 0.22 ** |
| Carbonyl groups (nmol/mg protein) | 12.74 ± 0.67 | 16.37 ± 0.84 *** | 1.385 ± 0.026 | 1.601 ± 0.054 * |
| Control | COVID-19 | ||
|---|---|---|---|
| Placenta (µg/mg protein) | Vitamin D | 12.45 ± 0.58 | 14.06 ± 0.51 ** |
| Vitamin E | 64.80 ± 6.87 | 93.47 ± 7.27 ** | |
| Coenzyme Q10 | 82.09 ± 4.06 | 92.13 ± 2.84 * | |
| Serum (µmol/L) | Vitamin D | 46.35 ± 2.59 | 53.22 ± 3.00 |
| Vitamin E | 23.10 ± 2.35 | 29.47 ± 1.59 * | |
| Coenzyme Q10 | 0.44 ± 0.02 | 0.48 ± 0.03 | |
| Vitamin A | 2.29 ± 0.20 | 2.72 ± 0.28 |
| Control | COVID-19 | |
|---|---|---|
| Mn (µg/g DM) | 3.93 ± 0.92 | 7.47 ± 1.07 ** |
| Se (µg/g DM) | 7.47 ± 0.63 | 7.40 ± 0.64 |
| Ba (µg/g DM) | 0.47 ± 0.07 | 0.36 ± 0.03 |
| Cu (µg/g DM) | 8.66 ± 0.57 | 8.72 ± 0.38 |
| Zn (µg/g DM) | 49.18 ± 2.18 | 49.62 ± 1.51 |
| Fe (mg/g DM) | 3.54 ± 0.40 | 5.39 ± 0.45 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Fernandez, J.; Ochoa, J.J.; De Paco Matallana, C.; Caño, A.; Martín-Alvarez, E.; Sanchez-Romero, J.; Toledano, J.M.; Puche-Juarez, M.; Prados, S.; Ruiz-Duran, S.; et al. COVID-19 during Gestation: Maternal Implications of Evoked Oxidative Stress and Iron Metabolism Impairment. Antioxidants 2022, 11, 184. https://doi.org/10.3390/antiox11020184
Moreno-Fernandez J, Ochoa JJ, De Paco Matallana C, Caño A, Martín-Alvarez E, Sanchez-Romero J, Toledano JM, Puche-Juarez M, Prados S, Ruiz-Duran S, et al. COVID-19 during Gestation: Maternal Implications of Evoked Oxidative Stress and Iron Metabolism Impairment. Antioxidants. 2022; 11(2):184. https://doi.org/10.3390/antiox11020184
Chicago/Turabian StyleMoreno-Fernandez, Jorge, Julio J. Ochoa, Catalina De Paco Matallana, Africa Caño, Estefania Martín-Alvarez, Javier Sanchez-Romero, Juan M. Toledano, Maria Puche-Juarez, Sonia Prados, Susana Ruiz-Duran, and et al. 2022. "COVID-19 during Gestation: Maternal Implications of Evoked Oxidative Stress and Iron Metabolism Impairment" Antioxidants 11, no. 2: 184. https://doi.org/10.3390/antiox11020184
APA StyleMoreno-Fernandez, J., Ochoa, J. J., De Paco Matallana, C., Caño, A., Martín-Alvarez, E., Sanchez-Romero, J., Toledano, J. M., Puche-Juarez, M., Prados, S., Ruiz-Duran, S., Diaz-Meca, L., Carrillo, M. P., & Diaz-Castro, J. (2022). COVID-19 during Gestation: Maternal Implications of Evoked Oxidative Stress and Iron Metabolism Impairment. Antioxidants, 11(2), 184. https://doi.org/10.3390/antiox11020184










