Vanadium Stress Alters Sweet Potato (Ipomoea batatas L.) Growth, ROS Accumulation, Antioxidant Defense System, Stomatal Traits, and Vanadium Uptake
Abstract
1. Introduction
2. Methodology
2.1. Seedling Collection, Growth Conditions, and Experimental Design
2.2. Growth Variables
2.3. Relative Water Content Analysis
2.4. Root Morphology
2.5. Gas Exchange Parameters
2.6. V Determination, Uptake, and Translocation
2.7. Measurement of Photosynthetic Pigments
2.8. Determination of Malondialdehyde (MDA)
2.9. Determination of Hydrogen Peroxide, Proteins, GSH, and Antioxidant Enzymes
2.10. Determination of Proline and Soluble Sugars
2.11. Determination of Total Polyphenols and Flavonoid Content
2.12. Scanning Electron Microscopy (SEM)
2.13. Statistical Analysis
3. Results
3.1. Growth Parameters, RWC, and Tolerance Index (TI)
3.2. Root Morphology
3.3. Leaf Gas Exchange Elements
3.4. Concentration, Uptake, and Translocation of Vanadium
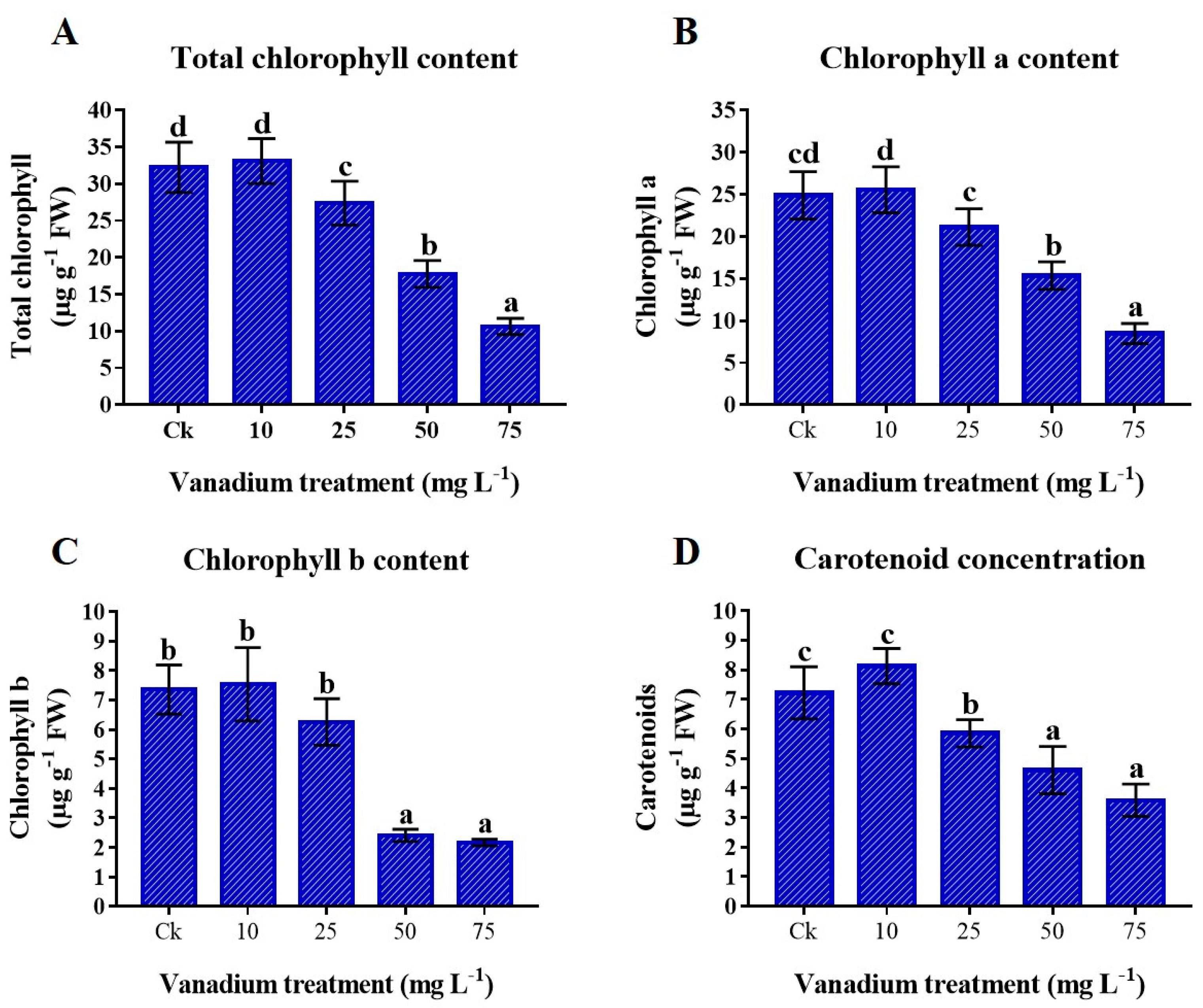
3.5. Photosynthetic Pigments
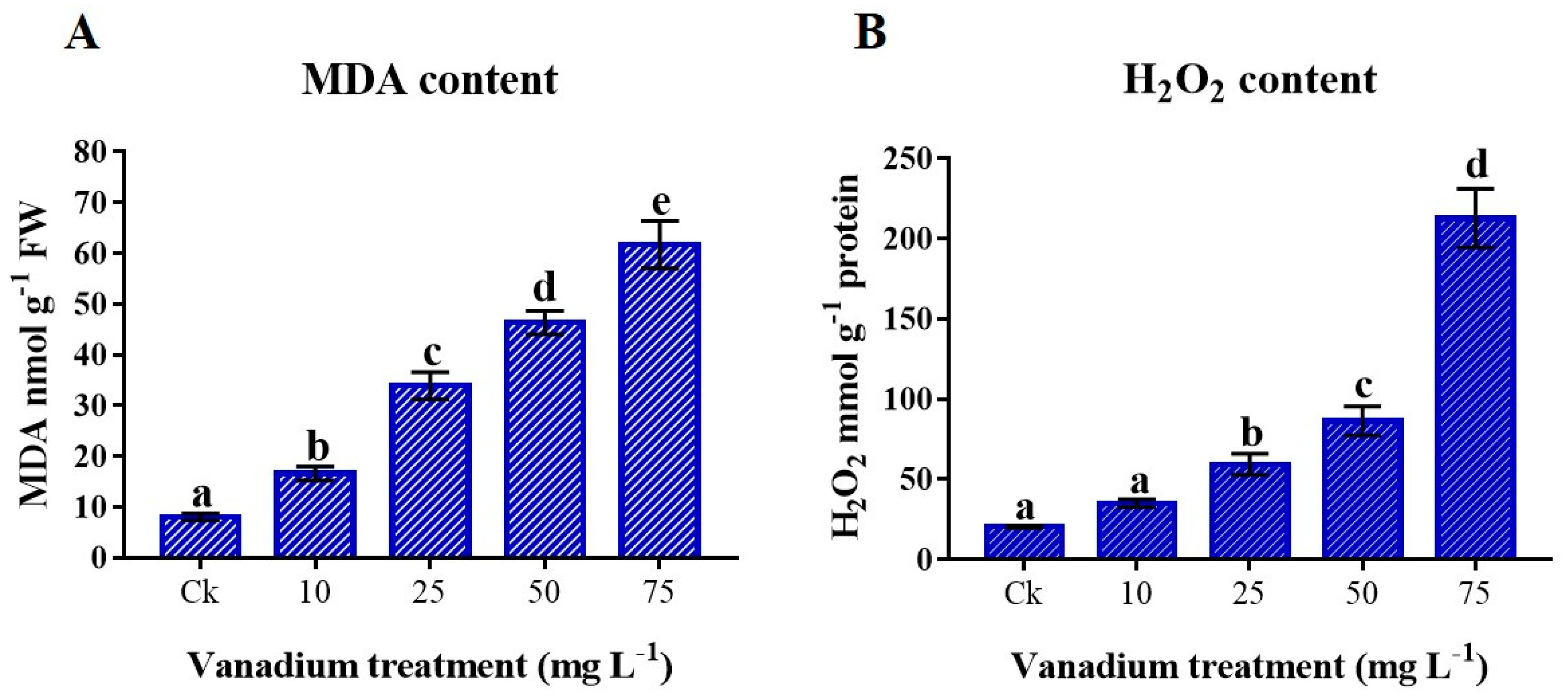
3.6. Lipid Peroxidation (MDA) and Reactive Oxygen Species (H2O2) Content
3.7. Osmolytes Production
3.8. Antioxidants
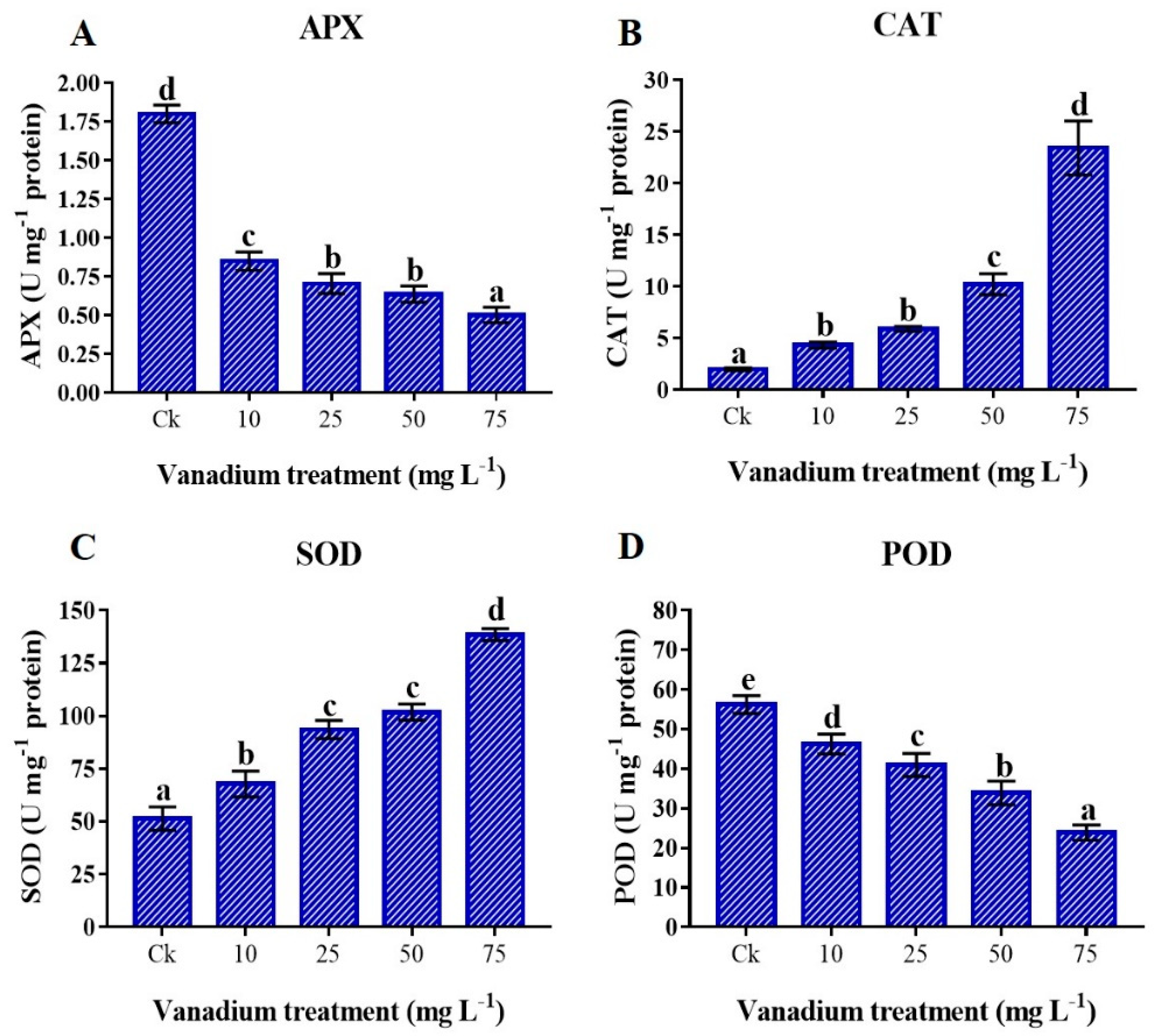
3.9. Antioxidant Enzymes
3.10. Effects on Leaf Morphology
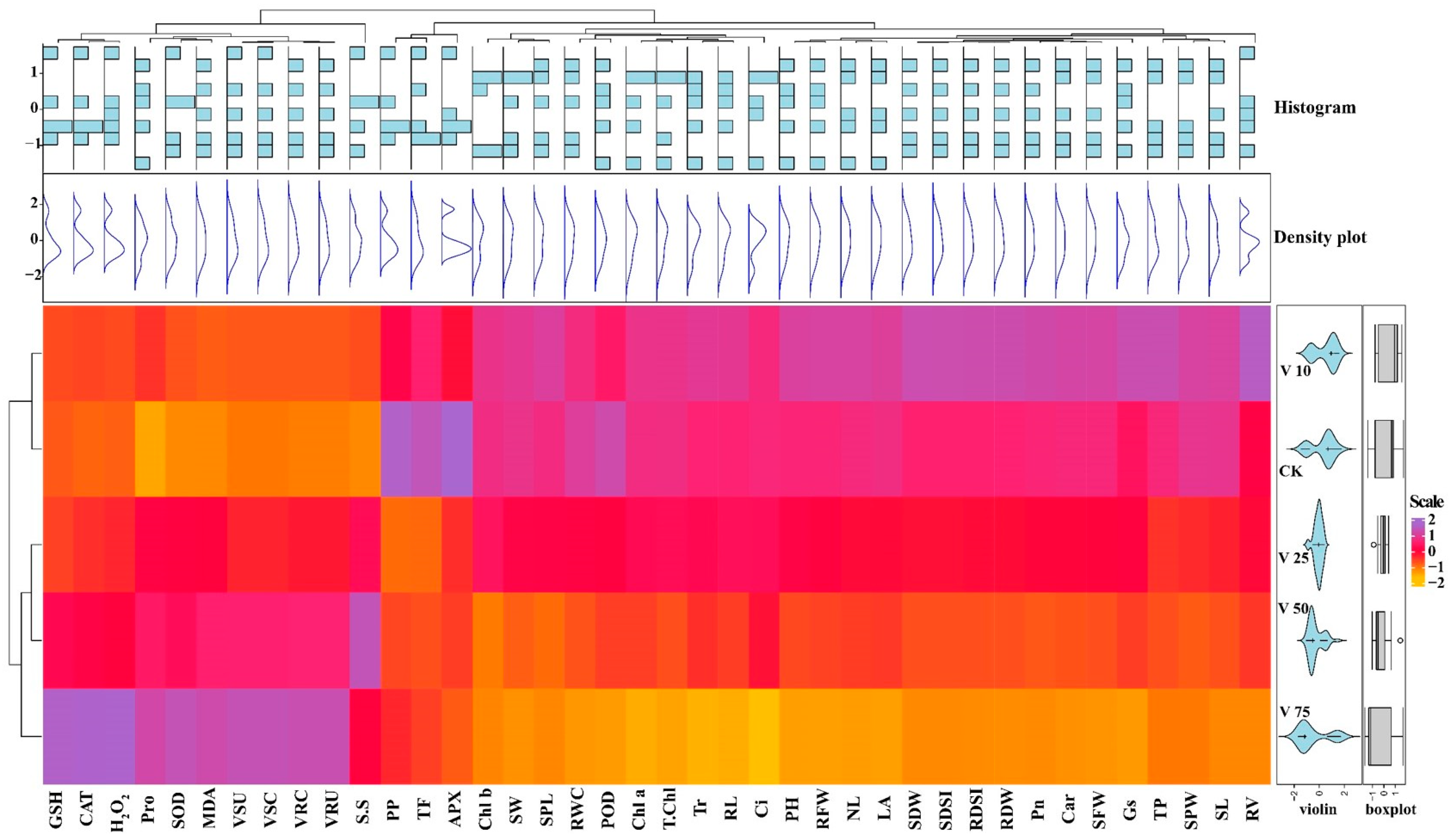
3.11. Pearson’s Correlation and Heat-map Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gan, C.; Chen, T.; Yang, J. Growth responses and accumulation of vanadium in alfalfa, milkvetch root, and swamp morning glory and their potential in phytoremediation. Bull. Environ. Contam. Toxicol. 2021, 107, 559–564. [Google Scholar] [CrossRef]
- Amorim, F.A.C.; Welz, B.; Costa, A.C.S.; Lepri, F.G.; Vale, M.G.R.; Ferreira, S.L.C. Determination of vanadium in petroleum and petroleum products using atomic spectrometric techniques. Talanta 2007, 72, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Teng, Y.; Wu, J.; Chen, H.; Wang, G.; Song, L.; Yue, W.; Zuo, R.; Zhai, Y. Current status and associated human health risk of vanadium in soil in China. Chemosphere 2017, 171, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.; Luo, H.; Yang, J. Characteristic of adsorption, desorption, and co-transport of vanadium on humic acid colloid. Ecotoxicol. Environ. Saf. 2020, 190, 110087. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.-Y.; Yang, J.-Y.; Huang, J.-H. Uptake and speciation of vanadium in the rhizosphere soils of rape (Brassica juncea L.). Environ. Sci. Pollut. Res. 2015, 22, 9215–9223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Y.; Yang, J.; Zhou, Y.; Wang, C. Effect of vanadium on testa, seed germination, and subsequent seedling growth of alfalfa (Medicago sativa L.). J. Plant Growth Regul. 2021, 40, 1566–1578. [Google Scholar] [CrossRef]
- He, W.; Liao, W.; Yang, J.; Jeyakumar, P.; Anderson, C. Removal of vanadium from aquatic environment using phosphoric acid modified rice straw. Bioremediat. J. 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef]
- Larsson, M.A.; Baken, S.; Gustafsson, J.P.; Hadialhejazi, G.; Smolders, E. Vanadium bioavailability and toxicity to soil microorganisms and plants. Environ. Toxicol. Chem. 2013, 32, 2266–2273. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Zhang, Y.; Wang, C.; Guo, S.; Yu, Y. Growth responses, accumulation, translocation and distribution of vanadium in tobacco and its potential in phytoremediation. Ecotoxicol. Environ. Saf. 2021, 207, 111297. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Zhou, Y.; Mumtaz, M.A.; Wang, Z. Vanadium toxicity induced changes in growth, antioxidant profiling, and vanadium uptake in pepper (Capsicum annum L.) seedlings. Horticulturae 2021, 8, 28. [Google Scholar] [CrossRef]
- Chen, T.; Li, T.-Q.; Yang, J.-Y. Damage suffered by swamp morning glory (Ipomoea aquatica Forsk) exposed to vanadium (V). Environ. Toxicol. Chem. 2016, 35, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.M.; Diao, X.P.; Ur Rehman, A.; Imtiaz, M.; Shakoor, A.; Altaf, M.A.; Younis, H.; Fu, P.; Ghani, M.U. Effect of vanadium on growth, photosynthesis, reactive oxygen species, antioxidant enzymes, and cell death of rice. J. Soil Sci. Plant Nutr. 2020, 20, 2643–2656. [Google Scholar] [CrossRef]
- Reiter, R.; Tan, D.-X.; Zhou, Z.; Cruz, M.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [PubMed]
- Sridhara Chary, N.; Kamala, C.T.; Samuel Suman Raj, D. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Li, G.; Huang, X.; Ji, Q.; Zhou, K.; Hou, H.; Ke, W.; Yang, J. Phenotypic, nutritional, and antioxidant characterization of blanched Oenanthe javanica for preferable cultivar. Front. Plant Sci. 2021, 12, 639639. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Zhou, K.; Khan, S.; Ke, W.; Hou, H. Investigation of an antioxidative system for salinity tolerance in Oenanthe javanica. Antioxidants 2020, 9, 940. [Google Scholar] [CrossRef]
- Imtiaz, M.; Mushtaq, M.A.; Rizwan, M.S.; Arif, M.S.; Yousaf, B.; Ashraf, M.; Shuanglian, X.; Rizwan, M.; Mehmood, S.; Tu, S. Comparison of antioxidant enzyme activities and DNA damage in chickpea (Cicer arietinum L.) genotypes exposed to vanadium. Environ. Sci. Pollut. Res. 2016, 23, 19787–19796. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J. Soil Sci. Plant Nutr. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Andresen, E.; Küpper, H. Cadmium Toxicity in Plants. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 11, pp. 395–413. ISBN 9789400751781. [Google Scholar]
- Aihemaiti, A.; Gao, Y.; Meng, Y.; Chen, X.; Liu, J.; Xiang, H.; Xu, Y.; Jiang, J. Review of plant-vanadium physiological interactions, bioaccumulation, and bioremediation of vanadium-contaminated sites. Sci. Total Environ. 2020, 712, 135637. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, M.; Liu, Y.; Fahad, S.; Qayyum, A.; Jadoon, S.A.; Chen, Y.; Zhu, G. Nickel toxicity alters growth patterns and induces oxidative stress response in sweetpotato. Front. Plant Sci. 2022, 13, 1054924. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.M.; Faber, M.; Claasen, N. Incorporating orange-fleshed sweet potato into the food system as a strategy for improved nutrition: The context of South Africa. Food Res. Int. 2018, 104, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mussoline, W.A.; Bohac, J.R.; Boman, B.J.; Trupia, S.; Wilkie, A.C. Agronomic productivity, bioethanol potential and postharvest storability of an industrial sweetpotato cultivar. Ind. Crops Prod. 2017, 95, 96–103. [Google Scholar] [CrossRef]
- Shekhar, S.; Mishra, D.; Buragohain, A.K.; Chakraborty, S.; Chakraborty, N. Comparative analysis of phytochemicals and nutrient availability in two contrasting cultivars of sweet potato (Ipomoea batatas L.). Food Chem. 2015, 173, 957–965. [Google Scholar] [CrossRef]
- Fu, Z.F.; Tu, Z.C.; Zhang, L.; Wang, H.; Wen, Q.H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef]
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2007, 55, 185–190. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, M.; Fahad, S.; Qayyum, A.; Chen, Y.; Zhu, G. Chromium induces toxicity at different phenotypic, physiological, biochemical, and ultrastructural levels in Sweet potato (Ipomoea batatas L.) plants. Int. J. Mol. Sci. 2022, 23, 13496. [Google Scholar] [CrossRef]
- García-Jiménez, A.; Trejo-Téllez, L.I.; Guillén-Sánchez, D.; Gómez-Merino, F.C. Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS ONE 2018, 13, e0201908. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, G.; Bishopp, A.; Heenatigala, P.P.M.; Hu, S.; Chen, Y.; Wu, Z.; Kumar, S.; Duan, P.; Yao, L.; et al. A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front. Chem. 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Huang, X.; Ji, Q.; Qayyum, A.; Zhou, K.; Ke, W.; Zhu, H.; Zhu, G. Influence of blanching on the gene expression profile of phenylpropanoid, flavonoid and vitamin biosynthesis, and their accumulation in Oenanthe javanica. Antioxidants 2022, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Chen, T.; Yang, J. Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacterial strain. Environ. Technol. Innov. 2020, 20, 101090. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Khan, L.U.; Altaf, M.M.; Jahan, M.S.; Nawaz, M.A.; Naz, S.; Shahid, S.; Lal, M.K.; et al. Protective mechanisms of melatonin against vanadium phytotoxicity in tomato seedlings: Insights into nutritional status, photosynthesis, root architecture system, and antioxidant machinery. J. Plant Growth Regul. 2021, 41, 1–17. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Liao, Y.; Yang, J. Effectiveness, stabilization, and potential feasible analysis of a biochar material on simultaneous remediation and quality improvement of vanadium contaminated soil. J. Clean. Prod. 2020, 277, 123506. [Google Scholar] [CrossRef]
- Xuebin, Q.; Yatao, X.; Ahmad, M.I.; Shehzad, M.; Zain, M. Silicon and its application methods improve physiological traits and antioxidants in Triticum aestivum (L.) under cadmium stress. J. Soil Sci. Plant Nutr. 2020, 20, 1110–1121. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Saldaña-Sánchez, W.D.; León-Morales, J.M.; López-Bibiano, Y.; Hernández-Hernández, M.; Langarica-Velázquez, E.C.; García-Morales, S. Effect of V, Se, and Ce on growth, photosynthetic pigments, and total phenol content of tomato and pepper seedlings. J. Soil Sci. Plant Nutr. 2019, 19, 678–688. [Google Scholar] [CrossRef]
- Yuan, Y.; Imtiaz, M.; Rizwan, M.; Dong, X.; Tu, S. Effect of vanadium on germination, growth and activities of amylase and antioxidant enzymes in genotypes of rice. Int. J. Environ. Sci. Technol. 2020, 17, 383–394. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Yang, J.; Jia, Z. Effect of vanadium on Lactuca sativa L. growth and associated health risk for human due to consumption of the vegetable. Environ. Sci. Pollut. Res. 2022, 29, 9766–9779. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Gil-Díaz, M.; Lobo, M.C. Response of two barley cultivars to increasing concentrations of cadmium or chromium in soil during the growing period. Biol. Trace Elem. Res. 2015, 163, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Wang, X.; Naveed, M.; Mustafa, A.; Ashraf, S.; Samreen, T.; Nadeem, S.M.; Jamil, M. Biochar mediated-alleviation of chromium stress and growth improvement of different maize cultivars in tannery polluted soils. Int. J. Environ. Res. Public Health 2021, 18, 4461. [Google Scholar] [CrossRef] [PubMed]
- Wissuwa, M.; Kretzschmar, T.; Rose, T.J. From promise to application: Root traits for enhanced nutrient capture in rice breeding. J. Exp. Bot. 2016, 67, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Meisch, H.-U.; Benzschawel, H.; Bielig, H.-J. The role of vanadium in green plants. Arch. Microbiol. 1977, 114, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, M.; Jia, Y.; Gou, M.; Zeyer, J. Toxicity of vanadium in soil on soybean at different growth stages. Environ. Pollut. 2017, 231, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Wang, R.; Wang, S.; Lu, X.; Wang, B. Effect of mercury stress on photosynthetic characteristics of two kinds of warm season turf grass. Int. J. Environ. Monit. Anal. 2015, 3, 293–297. [Google Scholar] [CrossRef]
- Abedini, M.; Mohammadian, F. Vanadium effects on phenolic content and photosynthetic pigments of sunflower. South-West. J. Hortic. Biol. Environ. 2018, 9, 77–86. [Google Scholar]
- Yang, J.Y.; Tang, Y. Accumulation and biotransformation of vanadium in Opuntia microdasys. Bull. Environ. Contam. Toxicol. 2015, 94, 448–452. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Balasaraswathi, K.; Jayaveni, S.; Sridevi, J.; Sujatha, D.; Phebe Aaron, K.; Rose, C. Cr–induced cellular injury and necrosis in Glycine max L.: Biochemical mechanism of oxidative damage in chloroplast. Plant Physiol. Biochem. 2017, 118, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- Gautam, V.; Kohli, S.K.; Kapoor, D.; Bakshi, P.; Sharma, P.; Arora, S.; Bhardwaj, R.; Ahmad, P. Stress protective effect of Rhododendron arboreum leaves (MEL) on chromium-treated Vigna radiata plants. J. Plant Growth Regul. 2021, 40, 423–435. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Adriano, D.C.; Carlson, C.L.; Sajwan, K.S. Vanadium: Toxicity and accumulation by beans. Water. Air. Soil Pollut. 1990, 49, 81–91. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Jiang, J.; Blaney, L.; Zou, Q.; Gao, Y.; Meng, Y.; Yang, M.; Xu, Y. The detoxification effect of liquid digestate on vanadium toxicity to seed germination and seedling growth of dog’s tail grass. J. Hazard. Mater. 2019, 369, 456–464. [Google Scholar] [CrossRef]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Hu, W.; Gao, J.; Yang, J. Vanadium in soil-plant system: Source, fate, toxicity, and bioremediation. J. Hazard. Mater. 2021, 405, 124200. [Google Scholar] [CrossRef]
- Tanyolaç, D.; Ekmekçi, Y.; Ünalan, Ş. Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper. Chemosphere 2007, 67, 89–98. [Google Scholar] [CrossRef]
- Imtiaz, M.; Tu, S.; Xie, Z.; Han, D.; Ashraf, M.; Rizwan, M.S. Growth, V uptake, and antioxidant enzymes responses of chickpea (Cicer arietinum L.) genotypes under vanadium stress. Plant Soil 2015, 390, 17–27. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Moreno-Sánchez, R. Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J. Theor. Biol. 2006, 238, 919–936. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Qiu, B.; Wu, X.; Niu, S.; Wu, F.; Zhang, G. Glutathione-mediated alleviation of chromium toxicity in rice plants. Biol. Trace Elem. Res. 2012, 148, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Izbiańska, K.; Arasimowicz-Jelonek, M.; Deckert, J. Phenylpropanoid pathway metabolites promote tolerance response of lupine roots to lead stress. Ecotoxicol. Environ. Saf. 2014, 110, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kisa, D.; Kayır, Ö.; Sağlam, N.; Şahin, S.; Öztürk, L.; Elmastaş, M. Changes of phenolic compounds in tomato associated with the heavy metal stress. Bartın Univ. Int. J. Nat. Appl. Sci. 2019, 2, 35–43. [Google Scholar]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Huang, Q.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Hina, F.; Zhou, W. Protective mechanisms of melatonin against selenium toxicity in Brassica napus: Insights into physiological traits, thiol biosynthesis and antioxidant machinery. BMC Plant Biol. 2019, 19, 507. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]







| Vanadium (mg L−1) | Height (cm) | Leaf Area (cm2) | Number of Leaf | Shoot FW (g) | Root FW (g) | RWC (%) |
|---|---|---|---|---|---|---|
| Ck | 42.67 ± 4.0 cd | 69.55 ± 8.0 c | 5.67 ± 0.6 c | 5.18 ± 0.4 d | 2.55 ± 0.5 cd | 92.98 ± 0.88 e |
| 10 | 46.33 ± 3.1 d | 74.86 ± 5.8 c | 6.33 ± 0.6 c | 5.90 ± 0.5 e | 2.97 ± 0.4 d | 90.18 ± 1.66 d |
| 25 | 38.00 ± 4.6 bc | 52.27 ± 4.3 b | 4.33 ± 0.6 b | 4.09 ± 0.4 c | 2.03 ± 0.3 bc | 83.89 ± 0.68 c |
| 50 | 31.67 ± 3.5 ab | 44.24 ± 5.6 b | 3.67 ± 0.6 b | 3.08 ± 0.3 b | 1.49 ± 0.2 b | 76.52 ± 1.33 b |
| 75 | 25.33 ± 4.2 a | 27.03 ± 4.7 a | 2.33 ± 0.6 a | 2.19 ± 0.2 a | 0.80 ± 0.1 a | 70.97 ± 1.12 a |
| Vanadium (mg L−1) | Shoot DW (g) | Root DW (g) | Root-Shoot Ratio | SDSI | RDSI |
|---|---|---|---|---|---|
| Ck | 0.518 ± 0.05 d | 0.324 ± 0.07 d | 0.623 ± 0.10 bc | 100 | 100 |
| 10 | 0.651 ± 0.09 e | 0.418 ± 0.05 e | 0.643 ± 0.01 c | 125.491 ± 12.3 d | 130.297 ± 10.5 d |
| 25 | 0.387 ± 0.03 c | 0.225 ±0.02 c | 0.582 ± 0.01 bc | 74.819 ± 5.5 c | 70.663 ± 8.8 c |
| 50 | 0.288 ± 0.02 b | 0.148 ± 0.03 b | 0.511 ± 0.05 b | 55.725 ± 4.6 b | 45.967 ± 3.6 b |
| 75 | 0.196 ± 0.02 a | 0.074 ± 0.01 a | 0.382 ± 0.07 a | 37.881 ± 2.8 a | 23.727 ± 6.7 a |
| Vanadium (mg L−1) | Concentration (mg kg−1 DW) | Uptake (mg kg−1 DW) | Translocation (Root to Shoot) | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| Ck | 0.07 ± 0.01 a | 0.89 ± 0.30 a | 0.01 ± 0.00 a | 0.09 ± 0.02 a | 0.08 ± 0.01 a |
| 10 | 3.53 ± 0.30 b | 7.91 ± 0.72 b | 0.37 ± 0.03 b | 0.82 ± 0.06 b | 0.45 ± 0.00 b |
| 25 | 9.50 ± 0.79 c | 19.19 ± 1.62 c | 0.99 ± 0.09 c | 1.99 ± 0.19 c | 0.50 ± 0.01 bc |
| 50 | 21.22 ± 1.49 d | 37.41 ± 3.02 d | 2.20 ± 0.17 d | 3.88 ± 0.26 d | 0.57 ± 0.08 cd |
| 75 | 32.03 ± 2.41 e | 52.68 ± 3.27 e | 3.32 ± 0.27 e | 5.46 ± 0.39 e | 0.61 ± 0.08 d |
| Vanadium (mg L−1) | Stomata Length (µm) | Stomata Width (µm) | Stomatal Pore Length (µm) | Stomatal Pore Width (µm) |
|---|---|---|---|---|
| Ck | 25.52 ± 2.45 c | 15.52 ± 1.92 c | 16.30 ± 2.11 c | 3.92 ± 1.08 b |
| 10 | 26.71 ± 2.97 c | 15.93 ± 1.78 c | 18.16 ± 1.31 c | 4.32 ± 0.92 b |
| 25 | 18.62 ± 2.31 b | 11.04 ± 1.51 b | 12.26 ± 1.63 b | 1.69 ± 0.47 a |
| 50 | 16.22 ± 1.73 ab | 5.70 ± 1.01 a | 6.19 ± 1.49 a | 0.95 ± 0.36 a |
| 75 | 13.47 ± 1.69 a | 3.08 ± 0.90 a | 4.72 ± 1.01 a | 0.51 ± 0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Wang, M.; Liu, Y.; Zhu, Z.; Fahad, S.; Qayyum, A.; Zhu, G. Vanadium Stress Alters Sweet Potato (Ipomoea batatas L.) Growth, ROS Accumulation, Antioxidant Defense System, Stomatal Traits, and Vanadium Uptake. Antioxidants 2022, 11, 2407. https://doi.org/10.3390/antiox11122407
Kumar S, Wang M, Liu Y, Zhu Z, Fahad S, Qayyum A, Zhu G. Vanadium Stress Alters Sweet Potato (Ipomoea batatas L.) Growth, ROS Accumulation, Antioxidant Defense System, Stomatal Traits, and Vanadium Uptake. Antioxidants. 2022; 11(12):2407. https://doi.org/10.3390/antiox11122407
Chicago/Turabian StyleKumar, Sunjeet, Mengzhao Wang, Yonghua Liu, Zhixin Zhu, Shah Fahad, Abdul Qayyum, and Guopeng Zhu. 2022. "Vanadium Stress Alters Sweet Potato (Ipomoea batatas L.) Growth, ROS Accumulation, Antioxidant Defense System, Stomatal Traits, and Vanadium Uptake" Antioxidants 11, no. 12: 2407. https://doi.org/10.3390/antiox11122407
APA StyleKumar, S., Wang, M., Liu, Y., Zhu, Z., Fahad, S., Qayyum, A., & Zhu, G. (2022). Vanadium Stress Alters Sweet Potato (Ipomoea batatas L.) Growth, ROS Accumulation, Antioxidant Defense System, Stomatal Traits, and Vanadium Uptake. Antioxidants, 11(12), 2407. https://doi.org/10.3390/antiox11122407








