Hand-Portable Miniaturized Liquid Chromatography for the Determination of Chlorogenic Acids in Dietary Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Chromatographic Conditions
2.3. Analysis of the Real Samples
3. Results
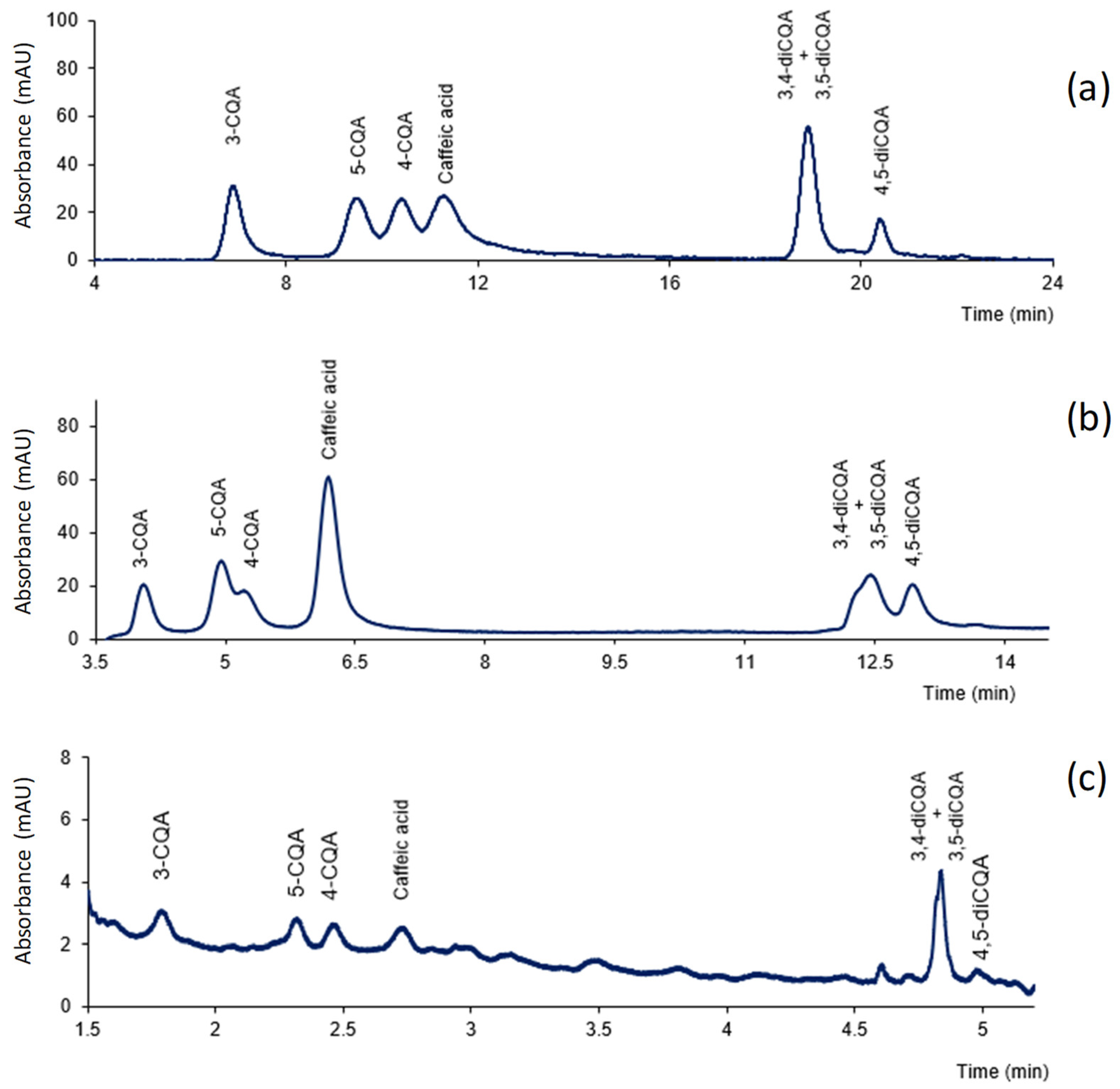
3.1. Chromatographic Conditions
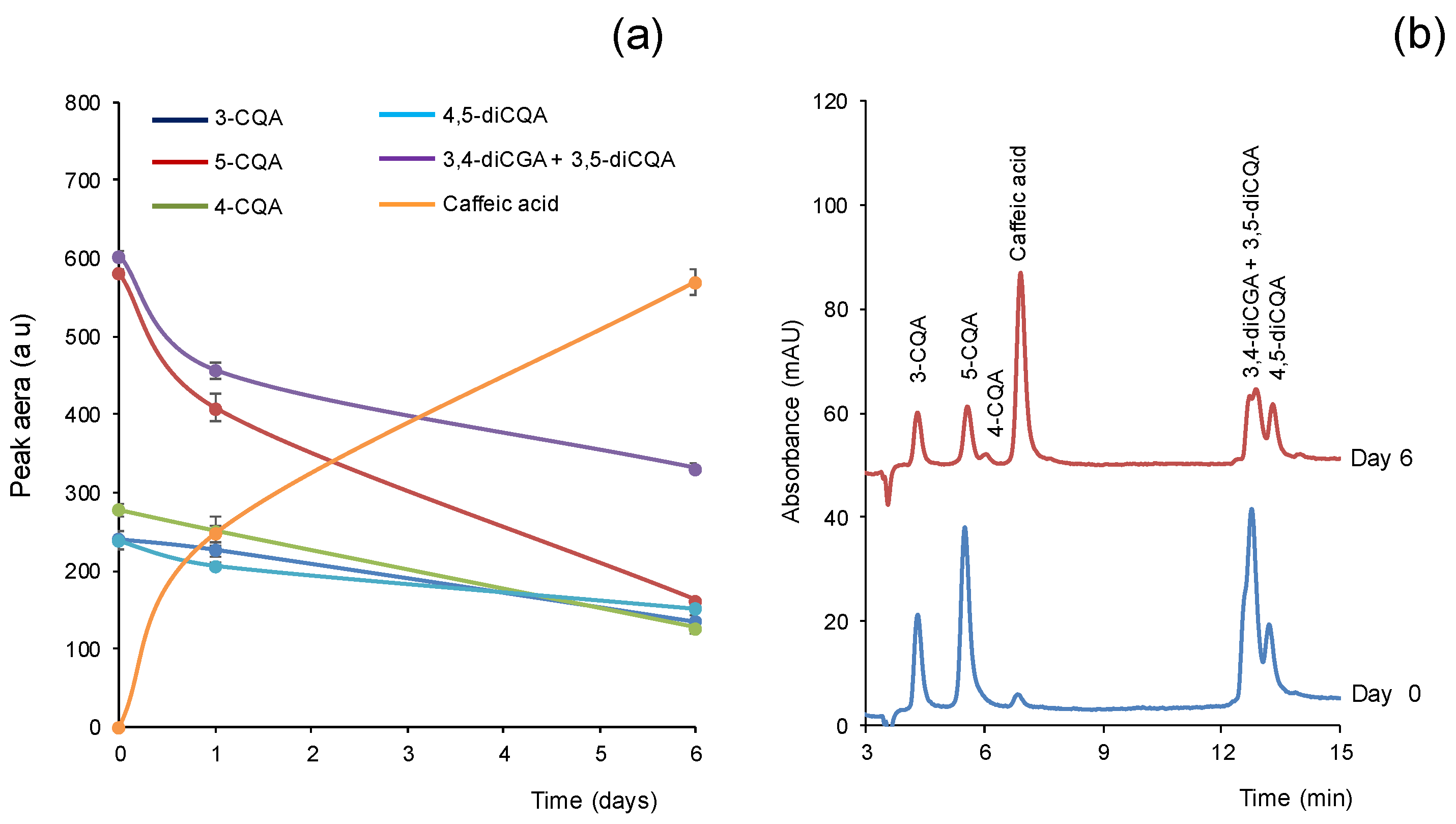
3.2. Stability of CGAs
3.3. Analytical Performance
3.4. Extraction Recoveries and Stability of the Extracts
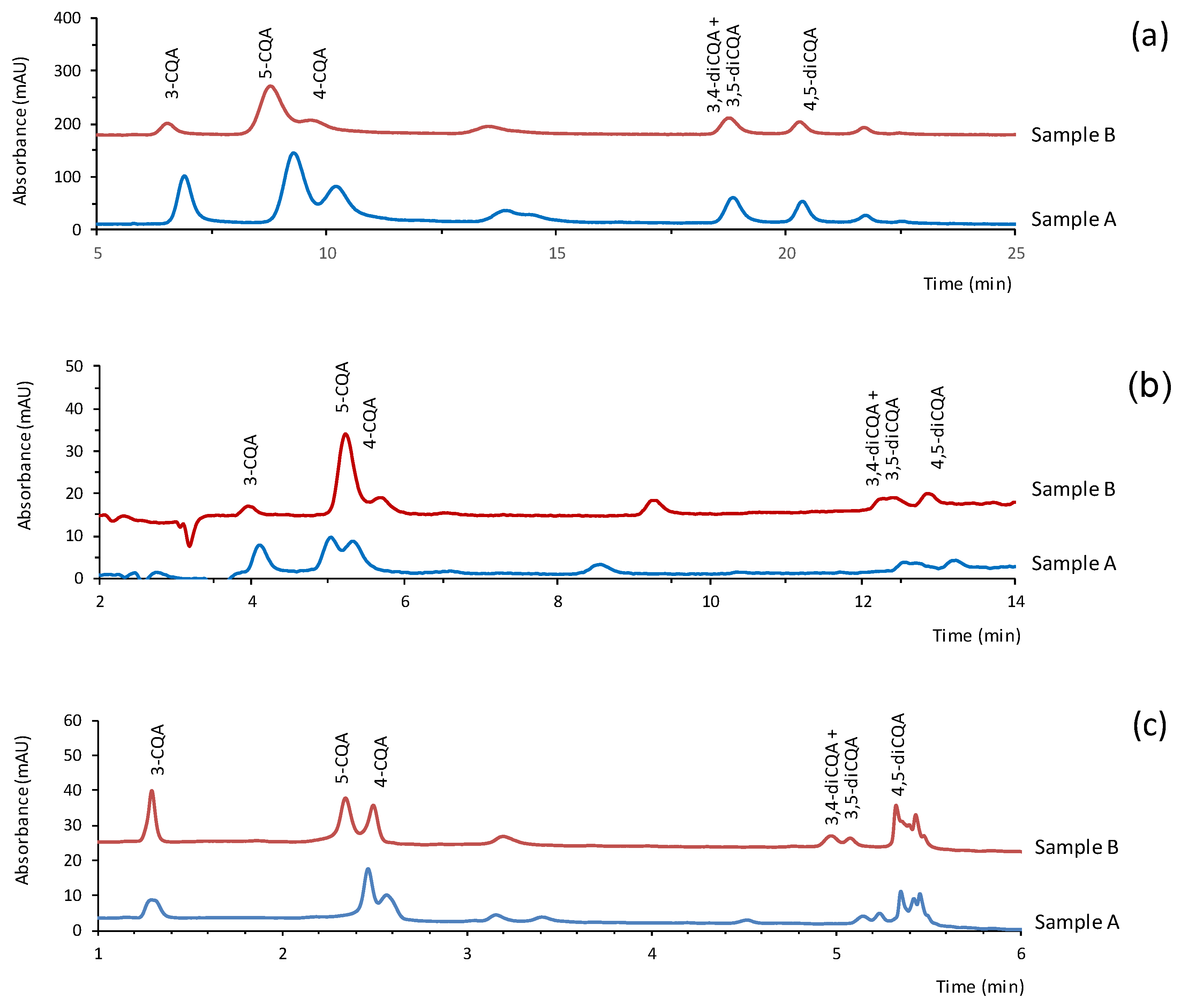
3.5. Application to the Quantification of CGAs in Dietary Supplements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; Filho, M.J.; da Silva, L.C.; Constant, L.S.; Filho, J.T.; Wagner, R.; Godoy, H.T. Chlorogenic and caffeic acids in 64 fruits consumed in Brazil. Food. Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Stanisz, E.; De Peña, M.P. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. LWT-Food Sci. Technol. 2016, 73, 243–250. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Sudeep, H.V.; Venkatesh, B.J.; Prasad, K.S. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HTC-116 cells. Food Sci. Hum. Well. 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Z.; Tian, Z.; Sun, J.; Li, Y.; Fan, X. Validation of spectrophotometric determination of chlorogenic acid in fermentation broth and fruits. Food Chem. 2019, 278, 170–177. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Różyło, R.; Świeca, M.; Krzykowski, A.; Rudy, S. Ground green coffee beans as a functional food supplement—Preliminary study. LWT-Food Sci. Technol. 2015, 63, 691–699. [Google Scholar] [CrossRef]
- Eussen, S.R.B.M.; Verhagen, H.; Klungel, O.H.; Garssen, J.; van Loveren, H.; van Kranen, H.J.; Rompelberg, C.J.M. Functional foods and dietary supplements: Products at the interface between pharma and nutrition. Eur. J. Pharmacol. 2011, 668, 52–59. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- EC (European Commission). DIRECTIVE 2002/46/EC of the European Parliament and of the Council of 10 June 2002, on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Commun. 2002, L 183, 51–57. [Google Scholar]
- Sarma, N.; Giancaspro, G.; Venema, J. Dietary supplements quality analysis tools from the United States Pharmacopeia. Drug Test. Anal. 2016, 8, 418–423. [Google Scholar] [CrossRef]
- Prakash, O.; Baskaran, R.; Kudachikar, V.B. Characterization, quantification of free, esterified and bound phenolics in Kainth (Pyrus pashia Buch.-Ham. Ex. D. Don) fruit pulp by UPLC-ESI-HRMS/MS and evaluation of their antioxidant activity. Food Chem. 2019, 299, 125114. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Fields, C.; Liang, N.; Kitts, D.; Erickson, A. Performance review of a fast HPLC-UV method for the quantification of chlorogenic acids in green coffee bean extracts. Talanta 2016, 154, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Adhikari, K.; Choi, K.S.; Lee, J. Analysis of caffeine, chlorogenic acid, trigonelline, and volatile compounds in cold brew coffee using high-performance liquid chromatography and solid-phase microextraction-gas chromatography-mass spectrometry. Foods 2020, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Ayseli, M.T.; Kelebe, H.; Selli, S. Elucidation of aroma-active compounds and chlorogenic acids of Turkish coffee brewed from medium and dark roasted Coffea arabica beans. Food Chem. 2021, 338, 127821. [Google Scholar] [CrossRef]
- Nemzer, B.; Abshiru, N.; Al-Taher, F. Identification of phytochemical compounds in Coffea arabica whole coffee cherries and their extracts by LC-MS/MS. J. Agric. Food Chem. 2021, 69, 3430–3438. [Google Scholar] [CrossRef]
- Pedan, V.; Stamm, E.; Do, T.; Holinger, M.; Reich, E. HPTLC fingerprint profile analysis of coffee polyphenols during different roast trials. J. Food Compos. Anal. 2020, 94, 103610. [Google Scholar] [CrossRef]
- Munteanu, I.-G.; Apetrei, C. Electrochemical determination of chlorogenic acid in dietary supplements using voltammetric sensors based on screen-printed carbon electrode modified with graphene and gold nanoparticles. Int. J. Mol. Sci. 2021, 22, 8897. [Google Scholar] [CrossRef]
- Fanali, S. An overview to nano-scale analytical techniques: Nano-liquid chromatography and capillary electrochromatography. Electrophoresis 2017, 38, 1822–1829. [Google Scholar] [CrossRef]
- Mejía-Carmona, K.; da Silva Burato, J.S.; Basolli Borsatto, J.V.; de Toffoli, A.L.; Lanças, F.M. Miniaturization of liquid chromatography coupled to mass spectrometry-1. Current trends on miniature LC columns. Trends Anal. Chem. 2020, 122, 115735. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Innovations in extractive phases for in-tube solid-phase microextraction coupled to miniaturized liquid chromatography: A critical review. Molecules 2020, 25, 2460. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; Herráez-Hernández, R.; Verdú-Andrés, J.; Campíns-Falcó, P. Quantitative analysis of terpenic compounds in microsamples of resins by capillary liquid chromatography. Molecules 2019, 24, 4068. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Donati, E.; Touloupakis, E.; Aturki, Z. Miniaturized separation techniques as analytical methods to ensure quality and safety of dietary supplements. Trends Anal. Chem. 2018, 103, 156–183. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Campíns-Falcó, P.; Herráez-Hernández, R. Capillary liquid chromatography for the determination of terpenes in botanical dietary supplements. Pharmaceuticals 2021, 14, 580. [Google Scholar] [CrossRef]
- Soto, C.; Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Determination of caffeine in dietary supplements by miniaturized portable liquid chromatography. J. Chromatogr. A 2022, 1664, 462770. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Zhang, L.; He, Y.; Chi, Y.; Chen, G. A simple capillary electrophoresis with electrochemical detection method for determination of the hydrolysis rate constant of chlorogenic acid. Talanta 2009, 77, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Siebert, M.; Berger, R.G.; Pfeiffer, F. Hydrolysis of chlorogenic acid in apple juice using a p-coumaryl esterase of Rhizoctonia solani. J. Sci. Food Agric. 2019, 99, 6644–6648. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3394–3402. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Ramalakshmi, K.; Rao, J.L.M. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 2012, 130, 184–188. [Google Scholar] [CrossRef]
| System | Compound | Linearity, y = ax + b (n = 10) | LOD (µg mL−1) | Precision *, (n = 3) RSD (%) | |||
|---|---|---|---|---|---|---|---|
| Concentration Range (µg mL−1) | a ± sa | b ± sb | R2 | ||||
| CapLC | 3-CQA | 0.2–2.0 | 1180 ± 20 | −80 ± 20 | 0.998 | 0.05 | 6 |
| 5-CQA | 0.2–2.0 | 1160 ± 30 | −110 ± 30 | 0.996 | 0.05 | 2 | |
| 4-CQA | 0.3–2.0 | 1200 ± 20 | −200 ± 30 | 0.998 | 0.1 | 5 | |
| 3,4-diCQA | 0.3–2.0 | 650 ± 40 | −90 ± 40 | 0.990 | 0.1 | 6 | |
| 3,5-diCQA | 0.3–2.0 | 670 ± 30 | −20 ± 30 | 0.990 | 0.1 | 4 | |
| 4,5-diCQA | 0.3–2.0 | 560 ± 20 | −60 ± 20 | 0.993 | 0.1 | 8 | |
| Caffeic acid | 0.3–2.0 | 2300 ± 100 | −600 ± 100 | 0.993 | 0.1 | 8 | |
| anoLC | 3-CQA | 0.01–0.1 | 643 ± 11 | 4416 | 0.998 | 0.005 | 2 |
| 5-CQA | 0.01–0.1 | 1151 ± 17 | 2 ± 1 | 0.9990 | 0.005 | 5 | |
| 4-CQA | 0.025–0.2 | 650 ± 9 | −9 ± 1 | 0.9990 | 0.010 | 5 | |
| 3,4-diCQA | 0.010–0.1 | 479 ± 8 | 2 ± 1 | 0.998 | 0.005 | 5 | |
| 3,5-diCQA | 0.01–0.1 | 483 ± 9 | 2 ± 1 | 0.998 | 0.005 | 5 | |
| 4,5-diCQA | 0.01–0.1 | 576 ± 11 | 1 ± 1 | 0.998 | 0.005 | 1 | |
| Caffeic acid | 0.01–0.5 | 1920 ± 5 | −40 ± 10 | 0.992 | 0.005 | 1 | |
| Portable LC | 3-CQA | 30–80 | 0.134 ± 0.007 | −2.1 ± 0.3 | 0.98 | 10 | 1 |
| 5-CQA | 30–80 | 0.108 ± 0.003 | −1.1 ± 0.1 | 0.994 | 5 | 1 | |
| 4-CQA | 20–80 | 0.106 ± 0.003 | −0.28 ± 0.08 | 0.994 | 5 | 2 | |
| 3,4-diCQA | 20–80 | 0.161 ± 0.007 | −0.64 ± 0.17 | 0.993 | 5 | 2 | |
| 3,5-diCQA | 20–80 | 0.169 ± 0.006 | −0.43 ± 0.15 | 0.995 | 5 | 2 | |
| 4,5-diCQA | 20–100 | 0.204 ± 0.007 | −0.71 ± 0.17 | 0.990 | 5 | 1 | |
| Caffeic acid | 20–80 | 0.313 ± 0.010 | −4.8 ± 0.5 | 0.993 | 5 | 2 | |
| Compound | Mean Recovery (n = 6) (%) | Precision, RSD (%) | |
|---|---|---|---|
| Intra-Day (n = 3) | Inter-Day (n = 6) | ||
| 3-CQA | 87 | 3 | 6 |
| 5-CQA | 85 | 5 | 8 |
| 4-CQA | 90 | 2 | 6 |
| 3,4-diCQA | 87 | 1 | 1 |
| 3,5-diCQA | 89 | 3 | 2 |
| 4,5-diCQA | 89 | 3 | 2 |
| Caffeic acid | 85 | 3 | 7 |
| Sample | Compound | Amount Found in the Sample (mg g−1) | |||||
|---|---|---|---|---|---|---|---|
| CapLC | NanoLC | Portable LC | |||||
| Dilution | Mass | Dilution | Mass | Dilution | Mass | ||
| A | 3-CQA | 1:300 | 21.0 ± 0.3 | 1:500 | 25.6 ± 0.3 | Not applicable | 21.0 ± 0.4 |
| 5-CQA | 33 ± 3 | 23.6 ± 0.4 | 43 ± 1 | ||||
| 4-CQA | 23 ± 2 | 37 ± 1 | 27 ± 1 | ||||
| 3,4-diCQA + 3,5-diCGA | 9.3 ± 0.5 | 11 ± 1 | 9 ± 1 | ||||
| 4,5-diCQA | 12.0 ± 3.0 | 13 ± 2 | 14 ± 1 | ||||
| Total: | 98 | 110 | 114 | ||||
| B | 3-CQA | 1:300 | 12.2 ± 0.8 | 1:2000 | 18.6 ± 0.4 | Not applicable | 17 ± 2 |
| 5-CQA | 78 ± 2 | 91 ± 2 | 101 ± 8 | ||||
| 4-CQA | 20 ± 2 | 23 ± 2 | 20 ± 1 | ||||
| 3,4-diCQA + 3,5-diCGA | 33 ± 2 | 35 ± 2 | 27 ± 1 | ||||
| 4,5-diCQA | 28 ± 2 | 27 ± 4 | 20 ± 1 | ||||
| Total: | 171 | 195 | 185 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, C.; Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Campíns-Falcó, P.; Herráez-Hernández, R. Hand-Portable Miniaturized Liquid Chromatography for the Determination of Chlorogenic Acids in Dietary Supplements. Antioxidants 2022, 11, 2408. https://doi.org/10.3390/antiox11122408
Soto C, Ponce-Rodríguez HD, Verdú-Andrés J, Campíns-Falcó P, Herráez-Hernández R. Hand-Portable Miniaturized Liquid Chromatography for the Determination of Chlorogenic Acids in Dietary Supplements. Antioxidants. 2022; 11(12):2408. https://doi.org/10.3390/antiox11122408
Chicago/Turabian StyleSoto, Camila, Henry Daniel Ponce-Rodríguez, Jorge Verdú-Andrés, Pilar Campíns-Falcó, and Rosa Herráez-Hernández. 2022. "Hand-Portable Miniaturized Liquid Chromatography for the Determination of Chlorogenic Acids in Dietary Supplements" Antioxidants 11, no. 12: 2408. https://doi.org/10.3390/antiox11122408
APA StyleSoto, C., Ponce-Rodríguez, H. D., Verdú-Andrés, J., Campíns-Falcó, P., & Herráez-Hernández, R. (2022). Hand-Portable Miniaturized Liquid Chromatography for the Determination of Chlorogenic Acids in Dietary Supplements. Antioxidants, 11(12), 2408. https://doi.org/10.3390/antiox11122408







