Transcriptome and Metabolome Analysis Revealed That Exogenous Spermidine-Modulated Flavone Enhances the Heat Tolerance of Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Measurement of the Physiological and Biochemical Parameters
2.3. Determination of Antioxidant Enzyme Activity
2.4. Metabolite Measurement and Quantification
2.5. RNA Extraction and Quality Testing
2.6. RNA-Seq Data Analysis
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Effect of Exogenous Spermidine on the Growth and MDA and Chlorophyll Contents of Lettuce under High-Temperature Stress
3.2. Effect of Spermidine on the Antioxidant Enzyme Activity of Lettuce under High-Temperature Stress
3.3. Transcriptome Data Quality Analysis
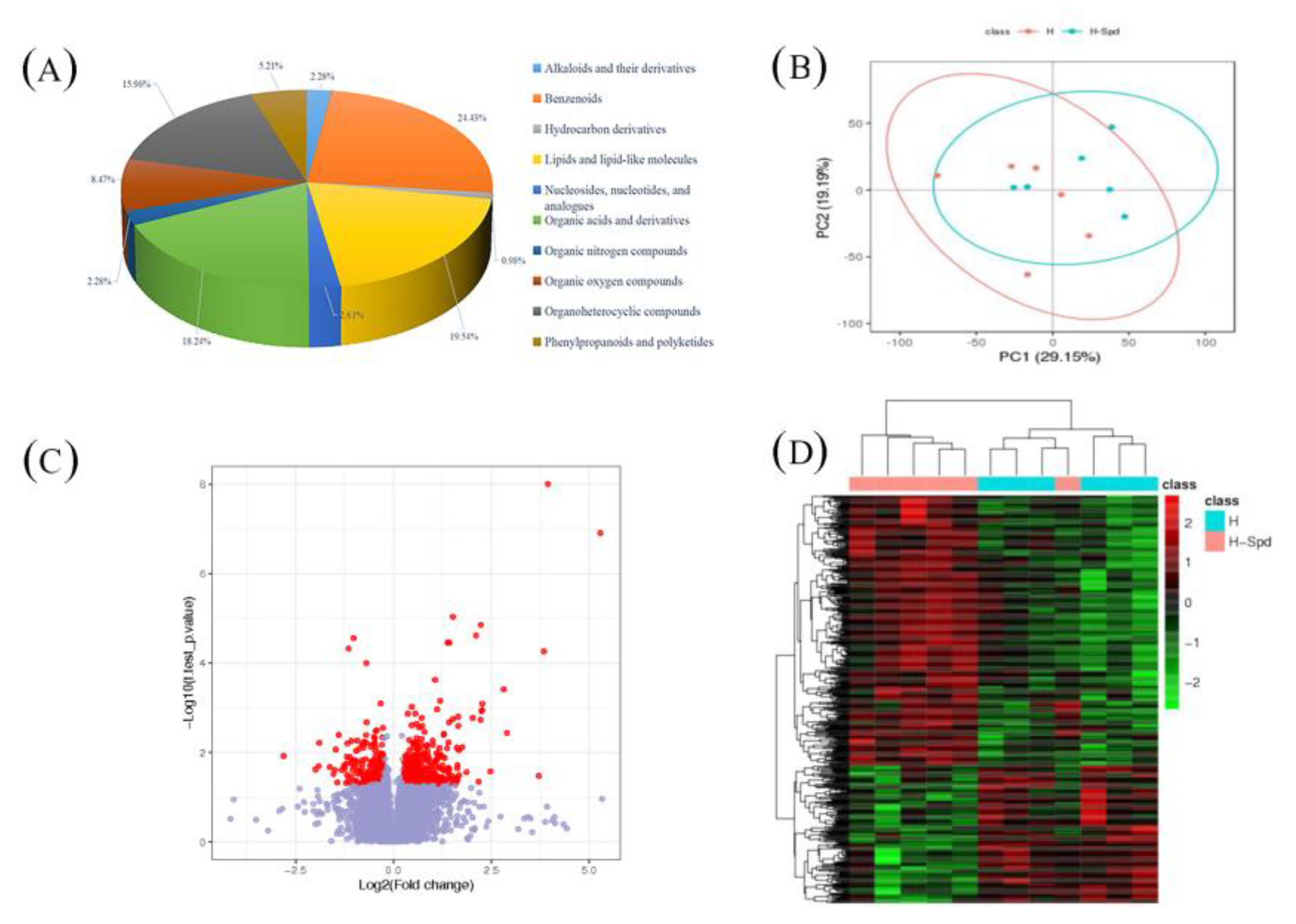
3.4. Metabolomics Assay
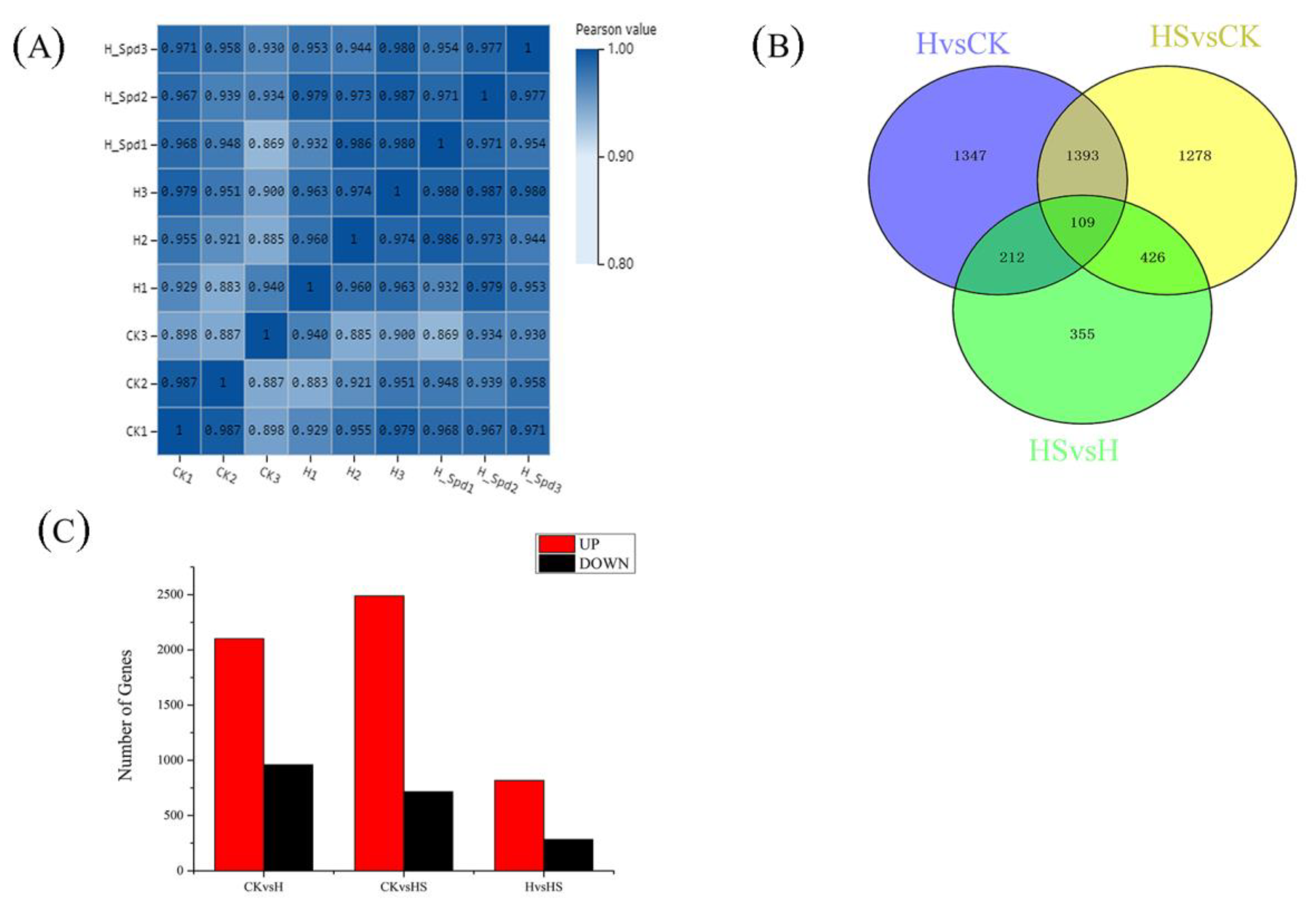
3.5. Analysis of DEGs
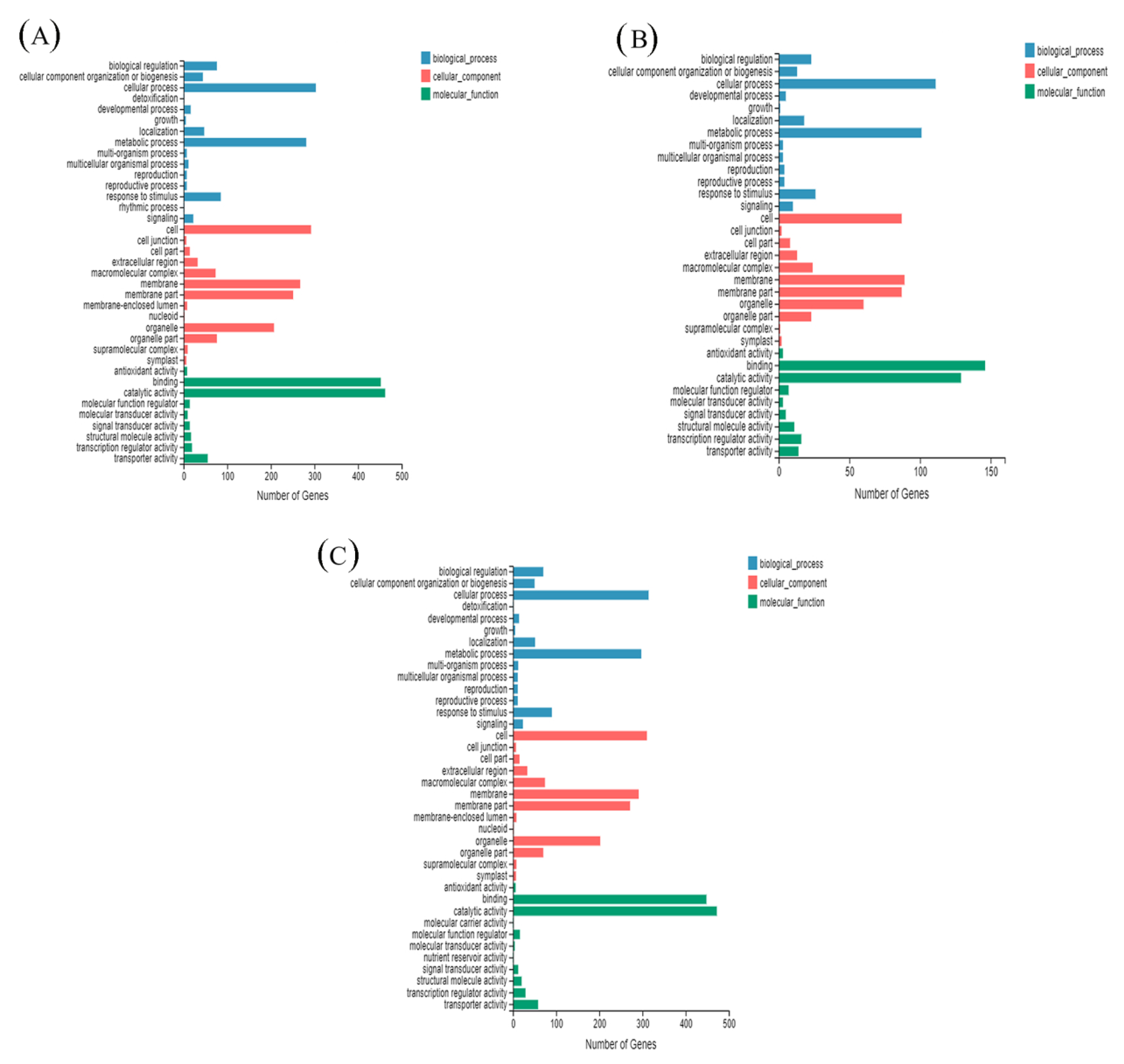
3.6. GO Enrichment Analysis
3.7. KEGG Enrichment Analysis
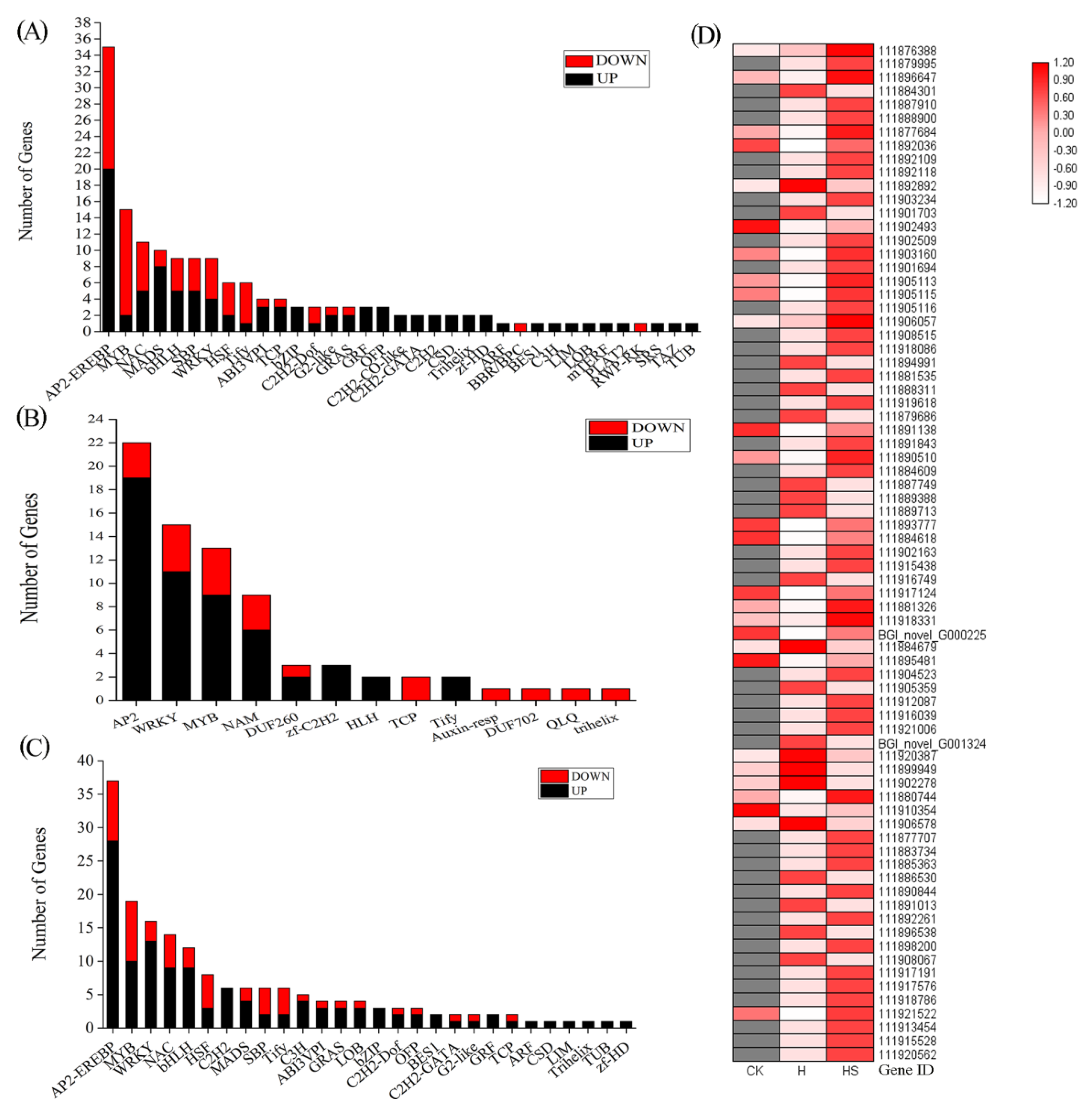
3.8. Transcription Factors
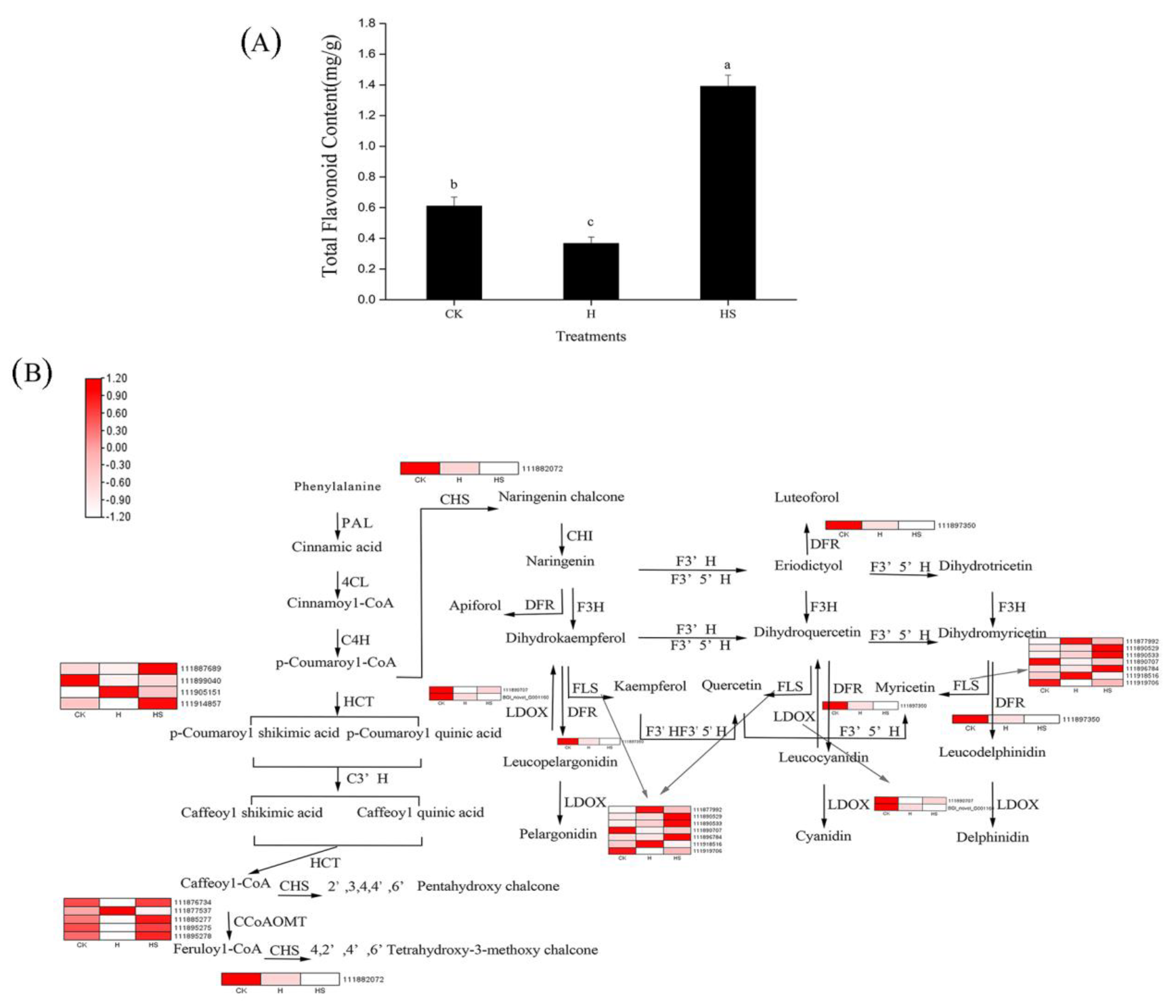
3.9. Spermidine Regulates the Metabolism of Flavonoids under High-Temperature Stress
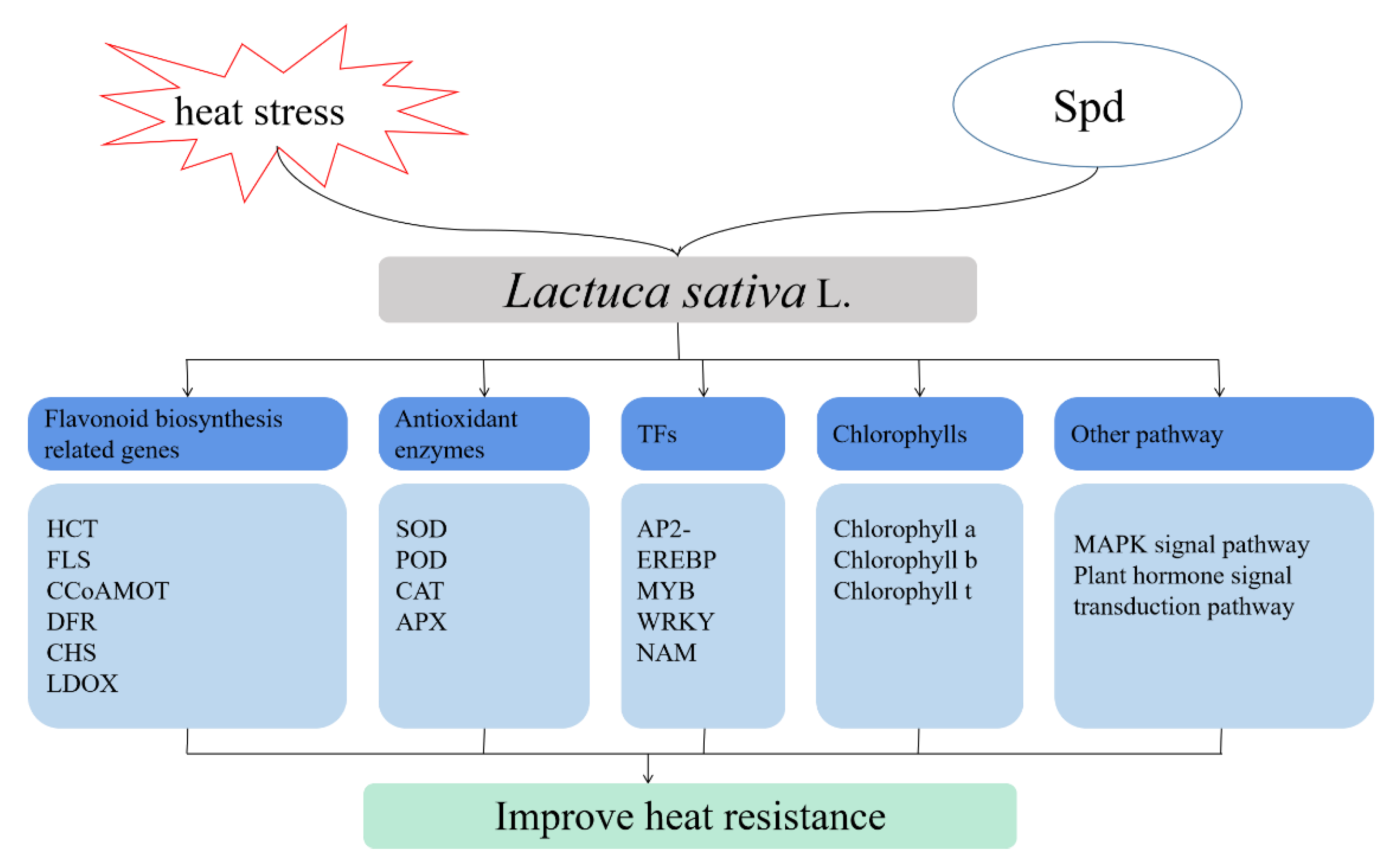
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Org, W.M.; Strachan, P. Intergovernmental Panel on Climate Change (IPCC). United Nations 2007, 114, 48–56. [Google Scholar] [CrossRef]
- Porter, J.R. Rising temperatures are likely to reduce crop yields. Nature 2005, 436, 174. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. Crop Stress Its Manag. Perspect. Strateg. 2012, 8, 261–315. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1704–1721. [Google Scholar] [CrossRef]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Su, G.X.; Zhang, W.H.; Liu, Y.L. Involvement of hydrogen peroxide generated by polyamine oxidative degradation in the development of lateral roots in Soybean. J. Integr. Plant Biol. 2006, 48, 426–432. [Google Scholar] [CrossRef]
- Alcazar, R.; Bitrian, M.; Bartels, D.; Koncz, C.; Altabella, T.; Tiburcio, A.F. Polyamine metabolic canalization in response to drought stress in Arabidopsis and the resurrection plant Craterostigma plantagineum. Plant Signal. Behav. 2011, 6, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Y.; Wang, Z.M.; Kong, F.N.; Zhang, M.J.; Zhou, S.L. Roles of carbohydrate supply and ethylene, polyamines in maize kernel set. J. Integr. Plant Biol. 2011, 53, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R.; Tebartz, F.; Scherer, G.F. Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 2011, 181, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, R.; Qin, G.; Chen, Z.; Gu, H.; Qu, L.J. Over-expression of WOX1 leads to defects in meristem development and polyamine homeostasis in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Alet, A.I.; Sanchez, D.H.; Cuevas, J.C.; Marina, M.; Carrasco, P.; Altabella, T.; Tiburcio, A.F.; Ruiz, O.A. New insights into the role of spermine in Arabidopsis thaliana under long-term salt stress. Plant Sci. 2012, 182, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine catabolism: Target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 2012, 42, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef]
- Sang, Q.Q.; Shu, S.; Shan, X.; Guo, S.R.; Sun, J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ. J. Plant Physiol. 2016, 63, 645–655. [Google Scholar] [CrossRef]
- Sun, J.; Lu, N.; Xu, H.; Maruo, T.; Guo, S. Root zone cooling and exogenous spermidine root-pretreatment promoting Lactuca sativa L. growth and photosynthesis in the high-temperature season. Front. Plant Sci. 2016, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Asthir, B. Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul. 2009, 60, 13–25. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, T.; Amombo, E.; Wang, G.; Xie, Y.; Fu, J. The alleviation of heat damage to photosystem II and enzymatic antioxidants by exogenous spermidine in tall fescue. Front. Plant Sci. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2013, 73, 31–44. [Google Scholar] [CrossRef]
- Sagor, G.H.; Berberich, T.; Takahashi, Y.; Niitsu, M.; Kusano, T. The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res. 2013, 22, 595–605. [Google Scholar] [CrossRef]
- Hao, J.H.; Zhang, L.L.; Li, P.P.; Sun, Y.C.; Li, J.K.; Qin, X.X.; Wang, L.; Qi, Z.Y.; Xiao, S.; Han, Y.Y.; et al. Quantitative proteomics analysis of lettuce (Lactuca sativa L.) reveals molecular basis-associated auxin and photosynthesis with bolting induced by high temperature. Int. J. Mol. Sci. 2018, 19, 2967. [Google Scholar] [CrossRef]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought-induced impacts on malting quality. Front. Plant Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Li, W.; Li, W.F.; Yang, S.J.; Ma, Z.H.; Zhou, Q.; Mao, J.; Han, S.Y.; Chen, B.H. Transcriptome and metabolite conjoint analysis reveals that exogenous methyl jasmonate regulates monoterpene synthesis in grape berry skin. J. Agric. Food Chem. 2020, 68, 5270–5281. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Effects of exogenous spermidine on antioxidants and glyoxalase system of lettuce seedlings under high temperature. Plant Signal. Behav. 2002, 15, 1824697. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Patron, M.; Altabella, T.; Tiburcio, A.F. Abscisic acid modulates polyamine metabolism under water stress in Arabidopsis thaliana. Physiol. Plant. 2006, 128, 448–455. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, W.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine improves the sucrose metabolism of lettuce to resist high-temperature stress. Plant Growth Regul. 2022, 96, 497–509. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H. Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Zahng, Y.P.; Xiang, J.; Wu, H.; Chen, H.Z.; Zhang, Y.K.; Zhu, D.F. Effects of chilling tolerance induced by spermidine pretreatment on antioxidative activity, endogenous hormones and ultrastructure of indica-japonica hybrid rice seedlings. J. Integr. Agric. 2016, 15, 295–308. [Google Scholar] [CrossRef]
- Saravi, H.B.; Gholami, A.; Pirdashti, H.; Firouzabadi, M.B.; Asghari, H.; Yaghoubian, Y. Improvement of salt tolerance in Stevia rebaudiana by co-application of endophytic fungi and exogenous spermidine. Ind. Crops Prod. 2022, 177, 114443. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
- Xie, B.D.; Wang, H.T. Effects of light spectrum and photoperiod on contents of flavonoid and terpene in leaves of Ginkgo biloba. L. J. Nanjing. Univ. 2006, 30, 51–54. [Google Scholar]
- Xu, M.; Dong, J.; Wang, H.; Huang, L. Complementary action of jasmonic acid on salicylic acid in mediating fungal elicitor-induced flavonol glycoside accumulation of Ginkgo biloba cells. Plant Cell Environ. 2009, 32, 960–967. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.P.; Zhang, L.; Yan, P.; Ahammed, G.J.; Han, W.Y. Methyl salicylate enhances flavonoid biosynthesis in tea leaves by stimulating the phenylpropanoid pathway. Molecules 2019, 24, 362. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Z.; Xing, F.; Wang, N.Q.; Peng, L.Z.; Chun, C.P.; Cao, L.; Ling, L.L.; Jiang, C.L. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotechnol. Biotechnol. Equip. 2014, 28, 192–198. [Google Scholar] [CrossRef]
- Tuteja, N. Integrated calcium signaling in plants. In Signaling in Plants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–49. [Google Scholar] [CrossRef]
- Xu, Z.C.; Wang, M.; Ren, T.T.; Li, K.; Li, K.Y.; Marowa, P.; Zhang, C.S. Comparative transcriptome analysis reveals the molecular mechanism of salt tolerance in Apocynum venetum. Plant Physiol. Biochem. 2021, 167, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Min, Z.; Wu, J.R.; Liu, B.C.; Xu, X.L.; Fang, Y.L.; Ju, Y.L. Physiological and transcriptomic analysis of Cabernet Sauvginon (Vitis vinifera L.) reveals the alleviating effect of exogenous strigolactones on the response of grapevine to drought stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef]
- Rao, S.; Gou, Y.Y.; Yu, T.; Cong, X.; Gui, J.Y.; Zhu, Z.Z.; Zhang, W.W.; Liao, Y.L.; Ye, J.B.; Cheng, S.Y.; et al. Effects of selenate on Se, flavonoid, and glucosinolate in broccoli florets by combined transcriptome and metabolome analyses. Food Res. Int. 2021, 146, 110463. [Google Scholar] [CrossRef]
- Ahmad, P.; Bhardwaj, R.; Tuteja, N. Plant signaling under abiotic stress environment. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 297–323. [Google Scholar]
- Ciarmiello, L.F.; Woodrow, P.; Fuggi, A.; Pontecorvo, G.; Carillo, P. Plant genes for abiotic stress. InTech 2011, 13, 283–308. [Google Scholar] [CrossRef]
- Gong, M.; Chen, S.N.; Song, Y.Q.; Li, Z.G. Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Aust. J. Plant Physiol. 1997, 24, 371–379. [Google Scholar] [CrossRef]
- Dat, J.; Foyer, C.; Scott, I. Change in salicylic acid and antioxidants during induced thermo tolerance in mustard seedlings. Plant Physiol. 1998, 118, 1455–1461. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Schumaker, K.; Zhu, J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004, 55, 225–236. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Shao, H.; Tang, X. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front. Plant Sci. 2016, 7, 67. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
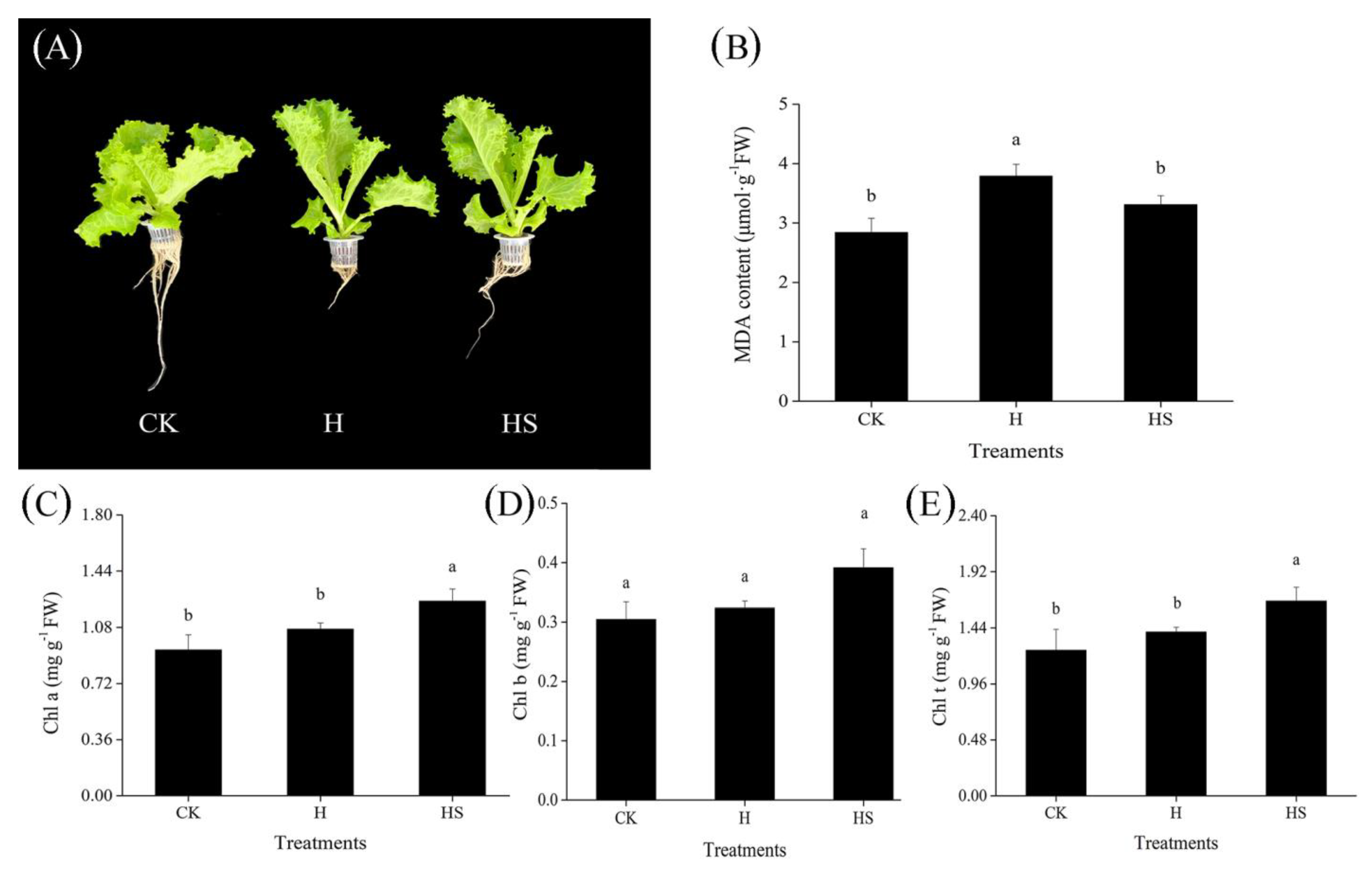


| Treatment | Temperature (Day/Night) | Type of Spraying |
|---|---|---|
| CK | 22 °C/17 °C | distilled water |
| H | 35 °C/30 °C | distilled water |
| HS | 35 °C/30 °C | 1 mmol·L−1 Spd |
| Treatments | Fresh Weight (g) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Root Length (cm) | Dry Weight (g) | Plant Height (cm) | Root-Shoot Ratio | Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|
| CK | 38.60 ± 1.46 a | 22.78 ± 2.90 a | 12.28 ± 0.68 a | 25.83 ± 2.25 a | 1.01 ± 0.23 a | 15.33 ± 2.52 a | 1.71 ± 0.25 a | 1.01 ± 0.23 a |
| H | 19.01 ± 0.93 c | 7.66 ± 0.91 b | 11.04 ± 1.26 a | 9.61 ± 1.57 c | 0.32 ± 0.02 c | 16.57 ± 0.40 a | 0.58 ± 0.10 b | 0.32 ± 0.02 c |
| HS | 24.32 ± 1.54 b | 10.98 ± 0.85 b | 11.22 ± 0.24 a | 14.14 ± 1.36 b | 0.61 ± 0.04 b | 17.50 ± 1.00 a | 0.81 ± 0.03 b | 0.61 ± 0.04 b |
| Group | R2 | Q2 |
|---|---|---|
| H vs. CK | 0.992 | 0.735 |
| HS vs. H | 0.952 | 0.105 |
| HS vs. CK | 0.997 | 0.811 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Hao, J.; Fan, S.; Liu, C.; Han, Y. Transcriptome and Metabolome Analysis Revealed That Exogenous Spermidine-Modulated Flavone Enhances the Heat Tolerance of Lettuce. Antioxidants 2022, 11, 2332. https://doi.org/10.3390/antiox11122332
Sun W, Hao J, Fan S, Liu C, Han Y. Transcriptome and Metabolome Analysis Revealed That Exogenous Spermidine-Modulated Flavone Enhances the Heat Tolerance of Lettuce. Antioxidants. 2022; 11(12):2332. https://doi.org/10.3390/antiox11122332
Chicago/Turabian StyleSun, Wenjing, Jinghong Hao, Shuangxi Fan, Chaojie Liu, and Yingyan Han. 2022. "Transcriptome and Metabolome Analysis Revealed That Exogenous Spermidine-Modulated Flavone Enhances the Heat Tolerance of Lettuce" Antioxidants 11, no. 12: 2332. https://doi.org/10.3390/antiox11122332
APA StyleSun, W., Hao, J., Fan, S., Liu, C., & Han, Y. (2022). Transcriptome and Metabolome Analysis Revealed That Exogenous Spermidine-Modulated Flavone Enhances the Heat Tolerance of Lettuce. Antioxidants, 11(12), 2332. https://doi.org/10.3390/antiox11122332






