Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts
Abstract
1. Introduction
2. Materials and Methods
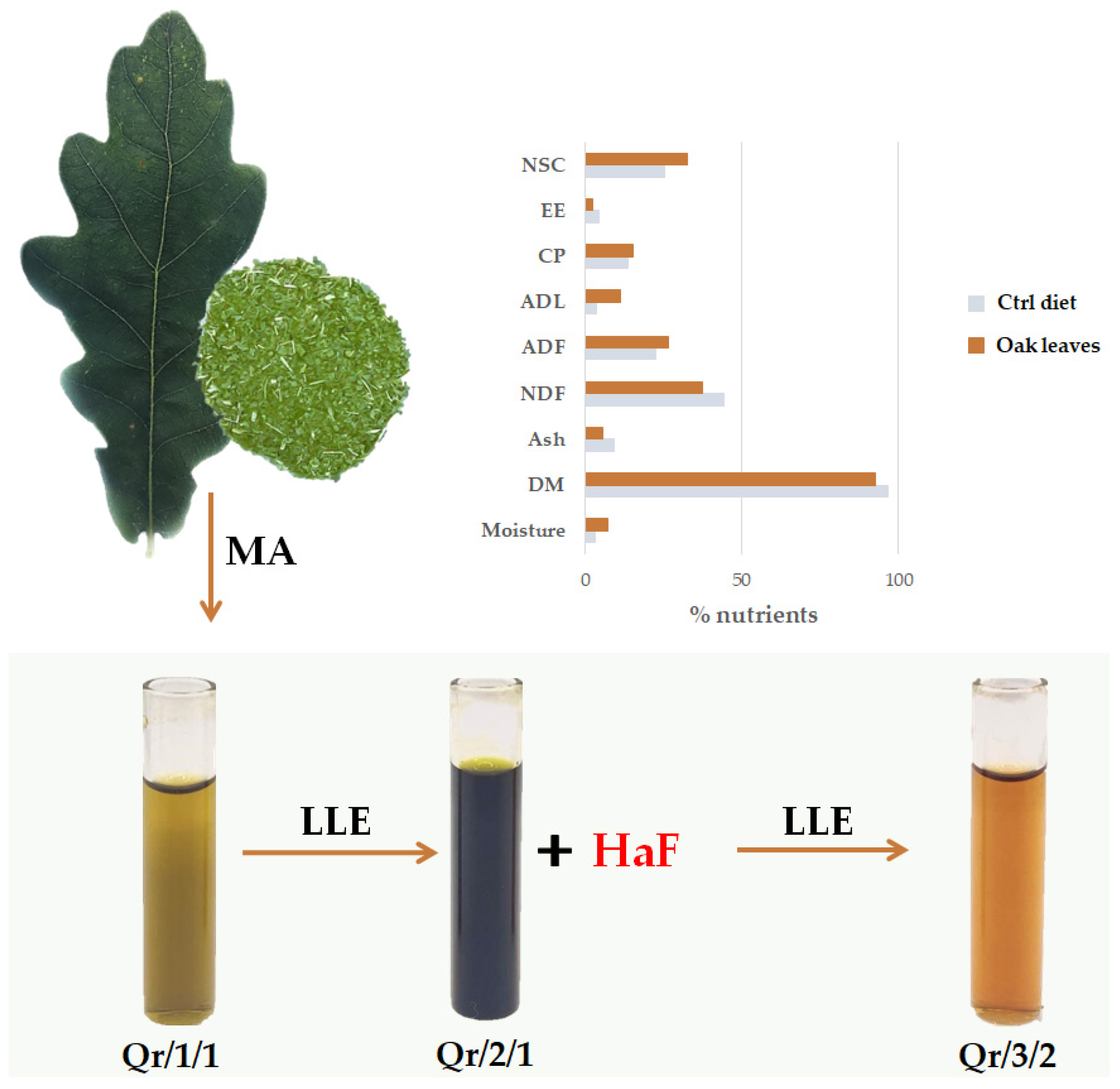
2.1. Plant Collection, Fractionation and Evaluation of Leaf Chemical Composition
2.2. UHPLC-HRMS and MS/MS Parameters and UV-Vis Analyses
2.3. Radical Scavenging Capacity: DPPH and ABTS Tests
2.4. Determination of Potassium Ferricyanide Reducing Power (PFRAP)
2.5. Determination of Total Phenol Content
2.6. Determination of Total Flavonoid Content
2.7. Determination of Total Condensed Tannins
2.8. In Vitro Fermentation
2.9. Fermentation End Products Assessment
2.10. Data Processing and Statistical Analysis
3. Results
3.1. Oak Leaf Nutritional Value for Livestock Feed
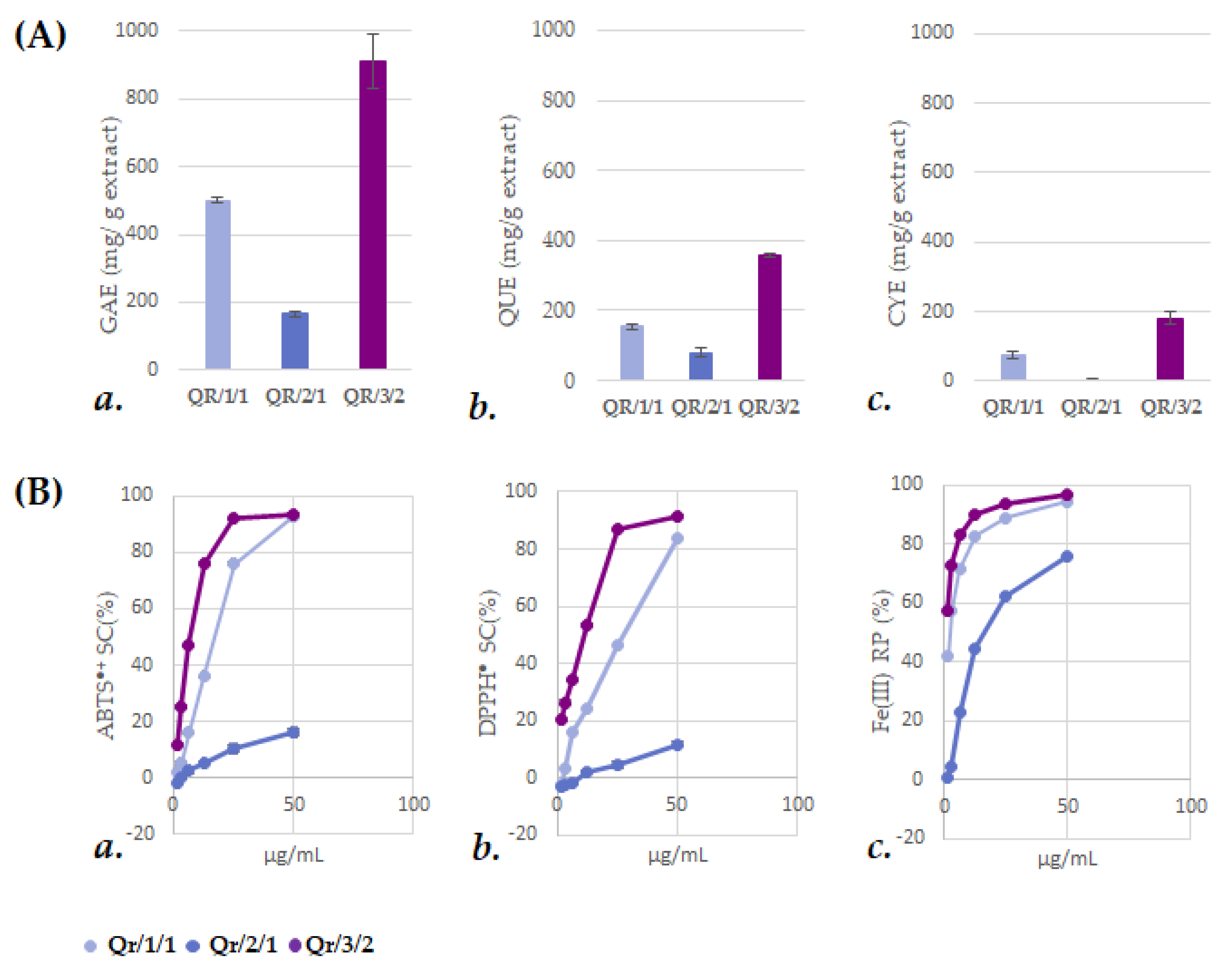
3.2. The Fractionation of an Alcoholic Oak Leaf Extract Showed up Antioxidant Polyphenols
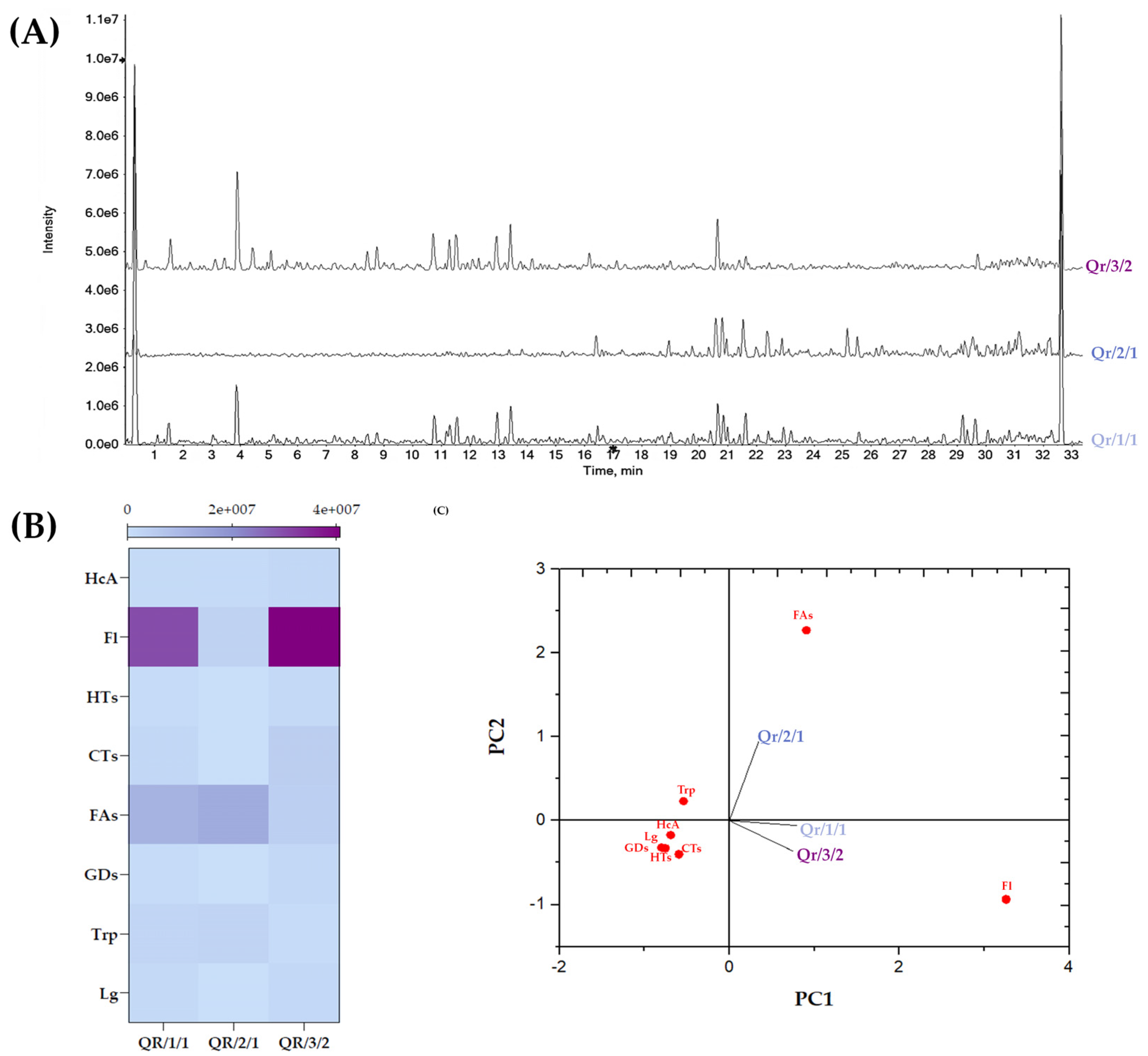
3.3. Chemical Insights into the Qr/1/1 Extract and Its Qr/2/1 and Qr/3/2 Fractions
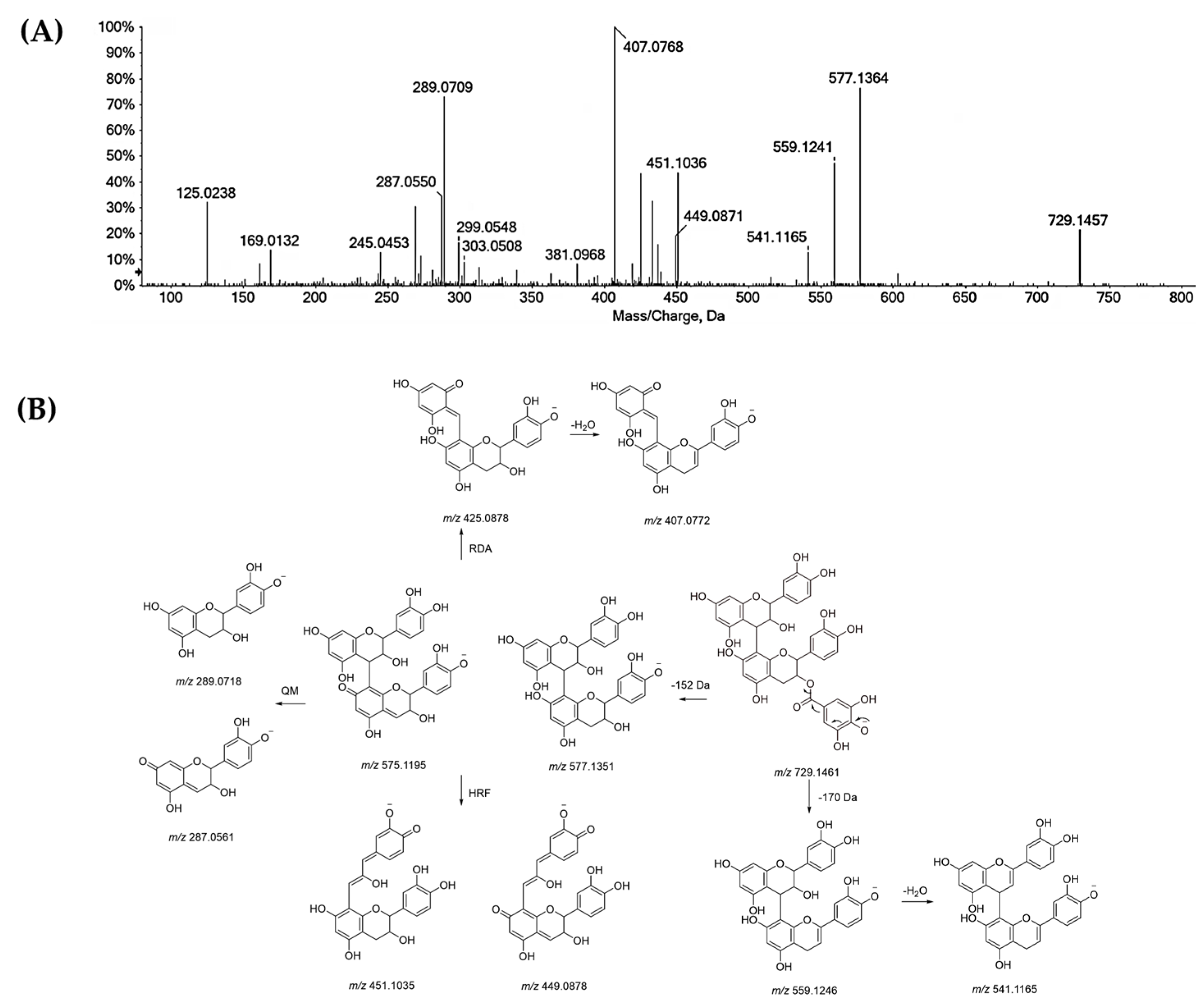
3.3.1. Tannins in Oak Leaf, beyond Other Low-Molecular Weight Compounds
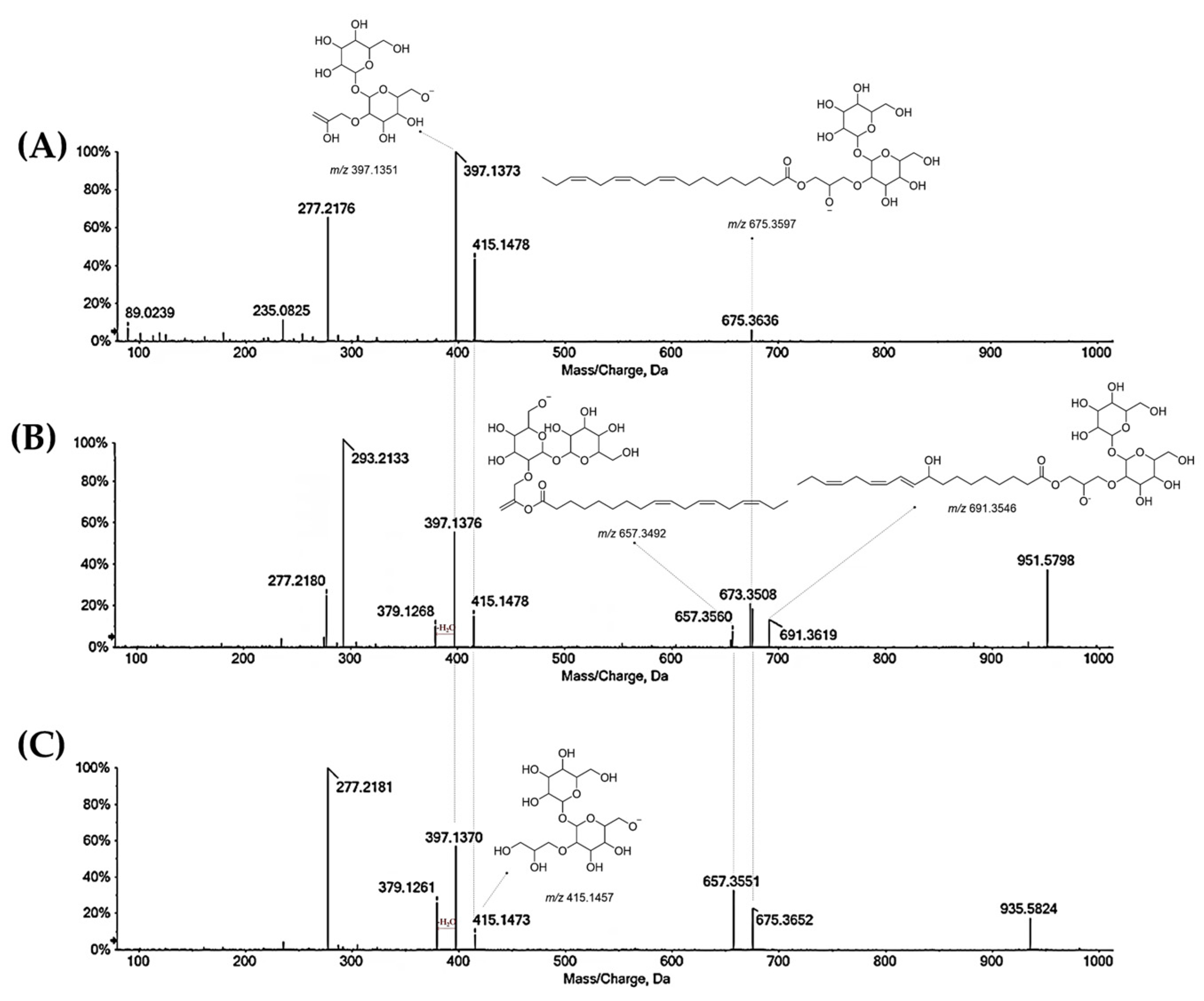
3.3.2. Flavonoids in Oak Leaf
3.3.3. Fatty Acids in Oak Leaf
3.4. Effects of Oak Leaf Alcoholic Extract and Its Fractions on In Vitro Rumen Fermentation
3.5. Influence of Oak Leaf Alcoholic Extract and Its Fractions on Fermentation End Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; European Commission: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal Uses, Phytochemistry, and Pharmacological Activities of Quercus Species. Evid. Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Renda, G. The polyphenolic profile of Oak (Quercus) species: A phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Custódio, L.; Patarra, J.; Alberício, F.; Neng, N.R.; Nogueira, J.M.F.; Romano, A. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind. Crop. Prod. 2013, 45, 114–120. [Google Scholar] [CrossRef]
- Vinha, A.F.; Costa, A.S.G.; Barreira, J.C.M.; Pacheco, R.; Oliveira, M.; Beatriz, P.P. Chemical and antioxidant profiles of acorn tissues from Quercus spp.: Potential as new industrial raw materials. Ind. Crop. Prod. 2016, 94, 143–151. [Google Scholar] [CrossRef]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Nevares, I.; del Alamo-Sanza, M. New Materials for the Aging of Wines and Beverages: Evaluation and Comparison. In Food Packaging and Preservation; Grumezescu, M.A., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 375–407. [Google Scholar]
- Broda, M. Natural Compounds for Wood Protection against Fungi-A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Elansary, S.K.; Ekiert, M.A.Y.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafarasi, M.A.; El-Ansary, D.O.; El-Abedin, T.Z.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Koseki, J.; Matsumoto, T.; Matsubara, Y.; Tsuchiya, K.; Mizuhara, Y.; Sekiguchi, K.; Nishimura, H.; Watanabe, J.; Kaneko, A.; Hattori, T.; et al. Inhibition of Rat 5α-Reductase Activity and Testosterone-Induced Sebum Synthesis in Hamster Sebocytes by an Extract of Quercus acutissima cortex. Evid. Based Complement. Altern. Med. 2015, 2015, 846–853. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. Assessment Report on Quercus robur L., Quercus petraea (Matt.) Liebl., Quercus pubescens Willd., Cortex; European Medicines Agency: London, UK, 2010; EMA/HMPC/3206/2009.
- EMEA Committee for Veterinary Medicinal Products. Quercus Cortex; Summary Report EMEA/MRL/282/97-Final 1997; EMEA: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Almeida, I.F.; Amaral, M.H.; Costa, P.C.; Bahia, M.F.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Pereira, T.M.; Amaral, M.H.; Costa, C.C.; et al. Oak leaf extract as topical antioxidant: Free radical scavenging and iron chelating activities and in vivo skin irritation potential. BioFactors 2008, 33, 267–279. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. UHPLC-QToF-MS characterization of bioactive metabolites from Quercus robur L. grown in South Africa for antioxidant and antidiabetic properties. Arab. J. Chem. 2020, 14, 102970. [Google Scholar] [CrossRef]
- Kumar, R.; Vaithiyanathan, S. Occurrence, nutritional significance and effect on animal productivity of tannins in tree leaves. Anim. Feed Sci. Technol. 1990, 30, 21–38. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Cimmino, R.; Barone, C.M.A.; Claps, S.; Varricchio, E.; Rufrano, D.; Caroprese, M.; Albenzio, M.; De Paolo, P.; Campanile, G.; Neglia, G. Effects of dietary supplementation with polyphenols on meat quality in Saanen goat kids. BMC Vet. Res. 2018, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Reduction in Methane Emissions from Ruminants by Plant Secondary Metabolites: Effects of Polyphenols and Saponins. In Sustainable Improvement of Animal Production and Health; Odongo, N.E., Garcia, M., Viljoen, G.J., Eds.; Food and Agriculture Organization: Rome, Italy, 2010; pp. 151–157. [Google Scholar]
- Tedeschi, L.O.; Ramírez-Restrepo, C.A.; Muir, J.P. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 2014, 8, 1095–1105. [Google Scholar] [CrossRef]
- Bursal, E.; Boga, R. Polyphenols analysed by UHPLC-ESI-MS/MS and antioxidant activities of molasses, acorn and leaves of oak (Quercus robur subsp. pedunculiflora). Prog. Nutr. 2018, 20, 167–175. [Google Scholar]
- Popović, B.M.; Štajner, D.; Ždero, R.; Orlović, S.; Galić, Z. Antioxidant Characterization of Oak Extracts Combining Spectrophotometric Assays and Chemometrics. Sci. World J. 2013, 2013, 134656. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 1–4. [Google Scholar] [CrossRef]
- Focant, M.; Froidmont, E.; Archambeau, Q.; Dang Van, Q.C.; Larondelle, Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. J. Dairy Sci. 2018, 102, 1144–1159. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Pacifico, S. UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet. Molecules 2022, 27, 2217. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005; p. 24. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and no starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Galasso, S.; Fiorentino, A.; Kretschmer, N.; Pan, S.P.; Nocera, P.; Lettieri, A.; Bauer, R.; Monaco, P. Influence of harvest season on chemical composition and bioactivity of wild rue plant hydroalcoholic extracts. Food Chem. Toxicol. 2016, 90, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Nocera, P.; Lettieri, A.; Bauer, R.; Monaco, P. Winter wild fennel leaves as a source of anti-inflammatory and antioxidant polyphenols. Arab. J. Chem. 2018, 11, 513–524. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Pacifico, S. UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols. Antioxidants 2021, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Raso, G.; Todaro, M. Silage of Prickly Pears (Opuntia spp.) Juice By-Products. Animals 2020, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- EC Council. Regulation 882/2004 on Official controls performed to ensure verification of compliance with feed and food law, animal health and animal welfare rules. Off. J. Eur. Union 2004, L191/1, 1–52. [Google Scholar]
- Pelagalli, A.; Musco, N.; Trotta, N.; Cutrignelli, M.I.; Di Francia, A.; Infascelli, F.; Tudisco, R.; Lombardi, P.; Vastolo, A.; Calabrò, S. Chemical characterisation and in vitro gas production kinetics of eight faba bean varieties. Animals 2020, 10, 398. [Google Scholar] [CrossRef]
- Calabrò, S.; Musco, N.; Roberti, F.; Vastolo, A.; Coppola, M.; Esposito, L.; Cutrignelli, M.I. Fermentability characteristics of different Saccharomyces cerevisiae cell wall using cat faeces as inoculum. Ital. J. Anim. Sci. 2020, 19, 186–193. [Google Scholar] [CrossRef]
- Groot, J.C.J.; Cone, J.W.; William, B.A.; Debersaque, F.M.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feedstuff. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Voigt, C.; Mosenthin, R.; Verstegen, M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. J. Anim. Sci. 2001, 73, 313–322. [Google Scholar] [CrossRef]
- Ünlü, H.B.; Özkan, Ç.Ö.; Kamalak, A. Potential nutritive value of some tree leaves commonly used for small ruminant in the Aegean region of Turkey. Progr. Nutr. 2022, 24, 1–9. [Google Scholar]
- Ky, I.; Le Floch, A.; Zeng, L.; Pechamat, L.; Jourdes, M.; Teissedre, P.-L. Tannins. In Encyclopedia of Food and Health; Caballero, B., Finglas, M.P., Toldra, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 247–255. [Google Scholar]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2017, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rush, M.D.; Rue, E.A.; Wong, A.M.; Kowalski, P.; Glinski, J.A.; van Breemen, R.B. Rapid Determination of Procyanidins Using MALDI-ToF/ToF Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 11355–11361. [Google Scholar] [CrossRef]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl flavonol glycosides and more in marketed green teas: An intrinsic value beyond much-lauded catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef]
- Nidiry, E.S.J. Tentative detection of some alkyl coumarates and alkyl ferulates in Ipomoea carnea subsp. fistulosa by HRESIMS and comparison of these compounds among Convolvulaceae plants. Phcog. Commn. 2013, 3, 12–15. [Google Scholar]
- He, D.; Simoneit, B.R.T.; Jara, B.; Jaffé, R. Gas chromatography mass spectrometry-based profiling of alkyl coumarates and ferulates in two species of cattail (Typha domingensis P., and Typha latifolia L.). Phytochem. Lett. 2015, 13, 91–98. [Google Scholar] [CrossRef]
- Dey, S.; Deepak, M.; Setty, M.; D’Souza, P.; Agarwal, A.; Sangli, G.K. Bioactive caffeic acid esters from Glycyrrhiza glabra. Nat. Prod. Res. 2009, 23, 1657–1663. [Google Scholar] [CrossRef]
- Coniglio, D.; Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Lipidomics of the Edible Brown Alga Wakame (Undaria pinnatifida) by Liquid Chromatography Coupled to Electrospray Ionization and Tandem Mass Spectrometry. Molecules 2021, 26, 4480. [Google Scholar] [CrossRef]
- Guella, G.; Frassanito, R.; Mancini, I. A new solution for an old problem: The regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1982–1994. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhou, H.; Khalil, M.B.; Seebun, D.; Bennett, S.A.L.; Figeys, D. Lyso-form fragment ions facilitate the determination of stereospecificity of diacyl glycerophospholipids. Rapid Commun. Mass Spectrom. 2010, 25, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.J.; Pecio, Ł.; Kowalczyk, M.; Kontek, R.; Gajek, G.; Stopinsek, L.; Mirt, I.; Oleszek, W.; Stochmal, A. Triterpenoid Components from Oak Heartwood (Quercus robur) and Their Potential Health Benefits. J. Agric. Food Chem. 2017, 65, 4611–4623. [Google Scholar] [CrossRef] [PubMed]
- Gammacurta, M.; Waffo-Teguo, P.; Winstel, D.; Dubourdieu, D.; Marchal, A. Isolation of Taste-Active Triterpenoids from Quercus robur: Sensory Assessment and Identification in Wines and Spirit. J. Nat. Prod. 2020, 83, 1611–1622. [Google Scholar] [CrossRef]
- Arramon, G.; Saucier, C.; Colombani, D.; Glories, Y. Identification of triterpene saponins in Quercus robur L. and Q. petraea Liebl. Heartwood by LC-ESI/MS and NMR. Phytochem Anal. 2002, 13, 305–310. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Kilic, U.; Boga, M.; Guven, I. Chemical Composition and Nutritive Value of Oak (Quercus robur) Nut and Leaves. J. Appl. Anim. Res. 2010, 38, 101–104. [Google Scholar] [CrossRef]
- Müslüm, Ö.R.Ü.N.; Sibel, E. Determination of In Vitro True Digestibility and Relative Feed Values of Alternative Roughage Sources. Yuzuncu Yil Univ. J. Agric. Sci. 2022, 32, 576–583. [Google Scholar]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.; Costa, P.C.; Bahia, M.F. Protective effect of Castanea sativa and Quercus robur leaf extracts against oxygen and nitrogen reactive species. J. Photochem. Photobiol. B Biol. 2008, 91, 87–95. [Google Scholar] [CrossRef]
- Kim, J.J.; Ghimire, B.K.; Shin, H.C.; Lee, K.J.; Song, S.K.; Chung, S.Y.; Yoon, T.S.; Lee, Y.J.; Kim, E.H.; Chung, I.M. Comparison of phenolic compounds content in indeciduous Quercus species. J. Med. Plant Res. 2012, 6, 5228–5239. [Google Scholar]
- Moharram, F.A.; Marzouk, M.S.; El Dib, R.A.M.; El-Shenawy, S.M.; Abdel-Rahman, R.F.; Ibrahim, R.R. Hepatoprotective, Gastroprotective, Antioxidant Activity and Phenolic Constituents of Quercus robur Leaves. J. Pharm. Sci. Res 2015, 7, 1055–1065. [Google Scholar]
- Sánchez-Burgos, J.A.; Ramírez-Mares, M.V.; Larrosa, M.M.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Medina-Torres, L.; Rocha-Guzmán, N.E. Antioxidant, antimicrobial, antitopoisomerase and gastroprotective effect of herbal infusions from four Quercus species. Ind. Crop. Prod. 2013, 42, 57–62. [Google Scholar] [CrossRef]
- Andrenšek, S.; Simonovska, B.; Vovk, I.; Fyhrquist, P.; Vuorela, H.; Vuorela, P. Antimicrobial and antioxidative enrichment of oak (Quercus robur) bark by rotation planar extraction using ExtraChrom®. Int. J. Food Microbiol. 2004, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Uddin, G.; Rauf, A. Phytochemical Screening, Antimicrobial and Antioxidant Activities of Aerial Parts of Quercus robur L. J. Med. Plant Res. 2012, 1, 1–4. [Google Scholar]
- Salminen, J.-P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal Variation in the Content of Hydrolyzable Tannins, Flavonoid Glycosides, and Proanthocyanidins in Oak Leaves. J. Chem. Ecol. 2004, 30, 1693–1711. [Google Scholar] [CrossRef]
- Tikkanen, O.-P.; Julkunen-Tiitto, R. Phenological variation as protection against defoliating insects: The case of Quercus robur and Operophtera brumata. Oecologia 2003, 136, 244–251. [Google Scholar] [CrossRef]
- Formato, M.; Scharenberg, F.; Pacifico, S.; Zidorn, C. Seasonal variations in phenolic natural products in Fagus sylvatica (European beech) leaves. Phytochemistry 2022, 203, 113385. [Google Scholar] [CrossRef]
- Mo, Q.G.; Zhou, G.; Zhu, W.D.; Ge, L.L.; Wang, Y.W. Coumaroyl and feruloyl flavonoid glycosides from the male flowers of Ginkgo biloba L. and their inhibitory activity against α-glucosidase. Nat. Prod. Res. 2022, 36, 4371–4378. [Google Scholar] [CrossRef]
- Kim, N.; Park, S.; Nhiem, N.X.; Song, J.H.; Ko, H.J.; Kim, S.H. Cycloartane-type triterpenoid derivatives and a flavonoid glycoside from the burs of Castanea crenata. Phytochemistry 2019, 158, 135–141. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the p-coumaroyl moiety in the antioxidant and cytoprotective effects of flavonoid glycosides: Comparison of astragalin and tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, S.S. Peracylated glucosyl kaempferols from Pasania dodonfifolia leaf. Nat. Prod. Commun. 2015, 10, 1373–1375. [Google Scholar] [PubMed]
- Doce, R.R.; Belenguer, A.; Toral, P.G.; Hervás, G.; Frutos, P. Effect of the administration of young leaves of Quercus pyrenaicaon rumen fermentation in relation to oak tannin toxicosis in cattle. J Anim. Physiol. Anim. Nutr. 2011, 97, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootecn. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Martínez, T.F.; McAllister, T.A.; Wang, Y.; Reuter, T. Effects of tannic acid and quebracho tannins on in vitro ruminal fermentation of wheat and corn grain. J. Sci. Food Agric. 2006, 86, 1244–1256. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. Biomed. Res. Int. 2013, 2013, 349129. [Google Scholar] [CrossRef]
- Bae, H.D.; McAllister, T.A.; Yanke, L.J.; Cheng, K.J.; Muir, A.D. Effect of condensed tannins on endoglucanase activity and filter paper digestion by Fibrobacter succinogenes. Appl. Environ. Microbiol. 1993, 59, 2132–2138. [Google Scholar] [CrossRef]
- Kamalak, A.; Canbolat, O.; Ozay, O.; Aktas, S. Nutritive value of oak (Quercus spp.) leaves. Small Rum. Res. 2004, 53, 161–165. [Google Scholar] [CrossRef]
- Rajkumar, K.; Bhar, R.; Kannan, A.; Jadhav, R.V.; Singh, B.; Mal, A.G. Effect of replacing oat fodder with fresh and chopped oak leaves on in vitro rumen fermentation, digestibility and metabolizable energy. Vet. World 2015, 8, 1021–1026. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane productionin vitro. J. Sci. Food Agric. 2012, 93, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Balcells, J.; Aris, A.; Serrano, A.; Seradj, A.R.; Crespo, J.; Devant, M. Effects of an extract of plant flavonoids (Bioflavex) on rumen fermentation and performance in heifers fed high-concentrate diets. J. Anim. Sci. 2012, 90, 4975–4984. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Greenhalgh, J.F.D.; Morgan, C.A. Digestion. In Animal Nutrion; Pearson Education Limited: London, UK, 2011; pp. 156–191. [Google Scholar]
- Koli, P.; Singh, S.; Bhadoria, B.K.; Agarwal, M.; Lata, S.; Ren, Y. Sequential Extraction of Proanthocyanidin Fractions from Ficus Species and Their Effects on Rumen Enzyme Activities In Vitro. Molecules 2022, 27, 5153. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koli, P.; Bhadoria, B.K.; Agarwal, M.; Lata, S.; Ren, Y.; Du, X. Proanthocyanidins Modulate Rumen Enzyme Activities and protein utilization in vitro. Molecules 2022, 27, 5870. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Stochmal, A.; Sadowinska, A.; Salem, A.Z.M.; Kowalczyk, D.; Zobonik, P.; Szumacher-Strabel, M. Effects of Two Sources of Tannins (Quercus L. and Vaccinium vitis idaea L.) on Rumen Microbial Fermentation: An in vitro Study. Ital. J. Anim. Sci. 2014, 13, 3133–3139. [Google Scholar] [CrossRef]
- Van, Q.C.D.; Gardin, C.; Mignolet, É.; Froidmont, É.; Focant, M.; Larondelle, Y. In vitro effects of hop pellets and oak extracts in combination on ruminal fermentation parameters. Biotechnol. Agron. Soc. Environ. 2018, 22, 75–83. [Google Scholar] [CrossRef]
- Carreño, D.; Hervás, G.; Toral, P.G.; Belenguer, A.; Frutos, P. Ability of different types and doses of tannin extracts to modulate in vitro ruminal biohydrogenation in sheep. Anim. Feed Sci. Technol. 2015, 202, 42–51. [Google Scholar] [CrossRef]
- Jalc, D.; Certik, M.; Kundrikova, K.; Namestkova, P. Effect of unsaturated C18 fatty acids (oleic, linoleic and α-linolenic acids) on ruminal fermentation and production of fatty acids isomers in artificial rumen. Vet. Med. 2008, 52, 87–94. [Google Scholar] [CrossRef]


Condensed tannins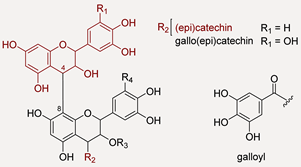 | Hydrolysable tannins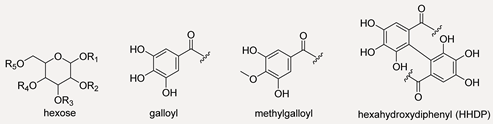 | ||||
| Condensed Tannins | |||||
| Peaks | R1, R2 and R3 | (M − H)− Found m/z | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) |
| 5 | R1 = OH; R2 = R3 = R4 = H (I) | 593.1318 | 2.9 | 18 | 593.1299; 467.09992; 425.0862; 407.0767; 381.0983; 339.0861; 289.0706; 245.0804; 205.0496; 177.0187; 125.0244 |
| 6 | R1 = R4 = OH; R2 = R3 = H | 609.1265 | 2.5 | 18 | 609.1232; 441.0825; 423.0716; 305.0656; 297.0387; 219.0641; 177.0190; 165.0179; 125.0245 |
| 7 | R1 = OH; R2 = R3 = R4 = H (II) | 593.1322 | 3.6 | 18 | 425.0854; 407.0759; 381.0958; 339.0841; 289.0697; 245.0799; 205.0488; 177.0179; 125.0238 |
| 12 | R1 = R2 = R3 = R4 = H (I) | 577.1349 | −0.4 | 18 | 577.1368; 451.1026; 425.0861; 407.0769; 381.0964; 339.0865; 289.0711; 273.0399; 245.0813; 205.0501; 161.0243; 125.0245 |
| 14 | R1 = R2 = R3=R4 = H (II) | 577.1365 | 2.3 | 18 | 577.1359; 425.0888; 407.0765; 381.0964; 339.0859; 289.0707; 273.0395; 245.0810; 205.0513; 161.0237; 125.0243 |
| 18 | R1 = H = R3 = R4 = H; R2=(epi)catechin (I) | 865.1971 | −1.7 | 27 | 865.1992; 847.1872; 739.1667; 713.1516; 695.1415; 677.1283; 587.1193; 577.1353; 575.1204; 525.0818; 451.1037; 449.0848; 425.0876; 413.0874; 407.0763; 405.0610; 341.0652; 289.0706; 287.0550; 243.0291; 161.0242; 125.0243 |
| 19 | R1 = H = R3 = R4 = H; R2 = (epi)catechin (II) | 865.1971 | −1.7 | 27 | 865.1985; 847.1869; 739.1663; 713.1510; 695.1411; 587.1189; 577.1348; 451.1032; 449.0859; 425.0872; 407.0760; 405.0603; 363.0500; 299.0547; 289.0703; 287.0549; 245.0443; 243.0287; 161.0241; 125.0241 |
| 22 | R1 = OH; R2 = R3 = R4 = H (III) | 593.1318 | 2.9 | 18 | 593.1314; 509.1305; 467.0954; 425.0898; 407.0754; 339.0873; 289.0700; 273.0359; 245.0810; 205.0491; 177.0186; 137.0241; 125.0242 |
| 28 | R1 = H = R3 = R4 = H; R2 = (epi)catechin (II) | 865.1967 | −2.1 | 27 | 865.1992; 847.1919; 739.1710; 713.1501; 695.1418; 613.1355; 587.1197; 577.1356; 575.1180; 543.0945; 525.0847; 451.1023; 425.0862; 413.0893; 407.0763, 299.0550; 289.0704; 287.0541; 243.0287; 161.0237; 125.0245 |
| 29 | R1 = R2 = H = R4 = H; R3 = galloyl | 729.1457 | −0.6 | 23 | 729.1457; 577.1364; 559.1241; 541.1165; 451.1036; 407.0768; 381.0968; 299.0548; 289.0709; 287.0550; 269.0434; 245.0453; 169.0132; 125.0238 |
| 31 | R1 = R2 = R3 = R4 = H (II) | 577.1363 | 2.0 | 18 | 577.1335; 451.1021; 425.0863; 407.0761; 381.0957; 289.0700; 287.0548; 245.0805; 161.0238; 125.0238. |
| Hydrolyzable Tannins | |||||
| Peaks | R1, R2 and R3 | (M − H)− Found m/z | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) |
| 9 | R1 = R2 = HHDP; R3 = R4 = HHDP; R5 = OH | 783.0702 | 2.0 | 23 | 783.0693; 481.0625; 300.9975; 275.0180 |
| 23 | R1 = R2 = galloyl; R3 = R4 = HHDP; R5 = OH | 785.0870 | 3.4 | 22 | 785.0872; 633.0690; 615.0645; 483.0784; 419.0601; 300.9974; 275.0174; 249.0390 |
| 25 | R1 = R2 = galloyl; R3 = R4 = R5 = H | 467.0843 | 2.5 | 11 | 467.0817; 449.0747; 423.0920; 374.7814; 315.0709; 313.0545; 241.0324; 169.0127; 152.0116; 125.0252; 124.0151; 109.0290 |
| 27 | R1 = galloyl; R2 = methylgalloyl; R3 = R4 = H; R5 = OH | 497.0951 | 2.9 | 11 | 497.0907; 465.0688; 345.0813; 313.0569; 297.0230; 225.0407; 183.0288; 169.0127; 124.060 |
| 30 | R1 = R2 = R3 = Gallic acid;R4 = H; R5 = OH | 635.0885 | −0.8 | 16 | 465.0670; 313.0549; 169.0131 |
| 32 | R1 = galloyl; R2 = R3 = HHDP;R4 = R5 = HHDP | 467.0375 [M − 2H]2− | nc | 19 | 391.0292; 300.9985; 275.0195; 169.1045 |
| 37 | R1 = R2 = R3 = galloyl; R4 = R5 = HHDP | 468.0438 [M − 2H]2− | nc | 27 | 300.9974; 299.9866; 275.0192; 273.0025; 169.0133; 125.0235 |
Flavonol Skeleton and Main Substituent Residues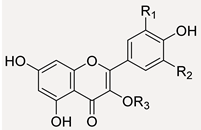 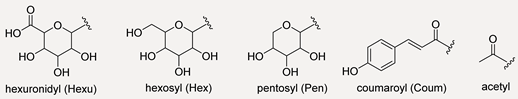 | |||||
| Kaempferol Derivatives | |||||
| Peaks | R1, R2 and R3 | [M−H]− Found m/z | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) |
| 47 | R1 = R2 = H; R3 = Hex (I) | 447.0926 | −1.5 | 12 | 447.0922; 285.0389; 284.0314; 255.0285; 227.0339 |
| 48 | R1 = R2 = H; R3 = Hex (II) | 447.0919 | −3.1 | 12 | 447.0947; 285.0397; 284.0320; 255.0295; 227.0341 |
| 49 | R1 = R2 = H; R3 = Pen-Hex | 593.1498 | 2.9 | 13 | 593.1504; 384.9863; 340.9965; 285.0381; 284.0308; 255.0272 |
| 53 | R1 = R2 = H; R3 = (Acetyl)-Hex (I) | 489.1058 | 4.0 | 13 | 489.1043; 285.0393; 284.0312; 255.0284; 227.0331 |
| 58 | R1 = R2 = H; R3 = (Acetyl)-Hex (I) | 489.1035 | −0.7 | 13 | 489.1020; 285.0394; 284.0315; 255.0290; 227.0336 |
| 62 | R1 = R2 = H; R3 = p-Coum-Hex (I) | 593.1323 | 3.8 | 18 | 593.1322; 447.0945; 307.0825; 285.0395; 284.0317; 255.0288 |
| 63 | R1 = R2 = R3 = H | 285.0393 | −4.1 | 11 | 285.0391; 229.0480; 110.9081 |
| 68 | R1 = R2 = H; R3 = p-Coum-Hex (II) | 593.1336 | 0.1 | 18 | 593.1318; 447.0932; 285.0387; 284.0309 |
| 72 | R1 = R2 = H; R3 = (Acetyl)-p-Coum-Hex | 635.1401 | −0.6 | 19 | 635.1411; 489.1021; 285.0390; 284.0309; 257.0443; 255.0283 |
| 73 | R1 = R2 = H; R3 = di-p-Coum-Hex | 739.1654 | −2.0 | 24 | 739.1664; 593.1335; 575.1186; 453.1176; 307.0787; 285.0388; 284.0315; 145.0284 |
| 74 | R1 = R2 = H; R3 = p-Coum-di-(Acetyl)-Hex (I) | 677.1492 | −2.9 | 20 | 677.1545; 531.1173; 285.0402; 284.0325; 283.0266 |
| 75 | R1 = R2 = H; R3 = p-Coum-di-(Acetyl)-Hex (II) | 677.1426 | 2.1 | 20 | 677.1579; 617.1397; 531.1201; 285.0406; 284.0326; 283.0230; 255.0296 |
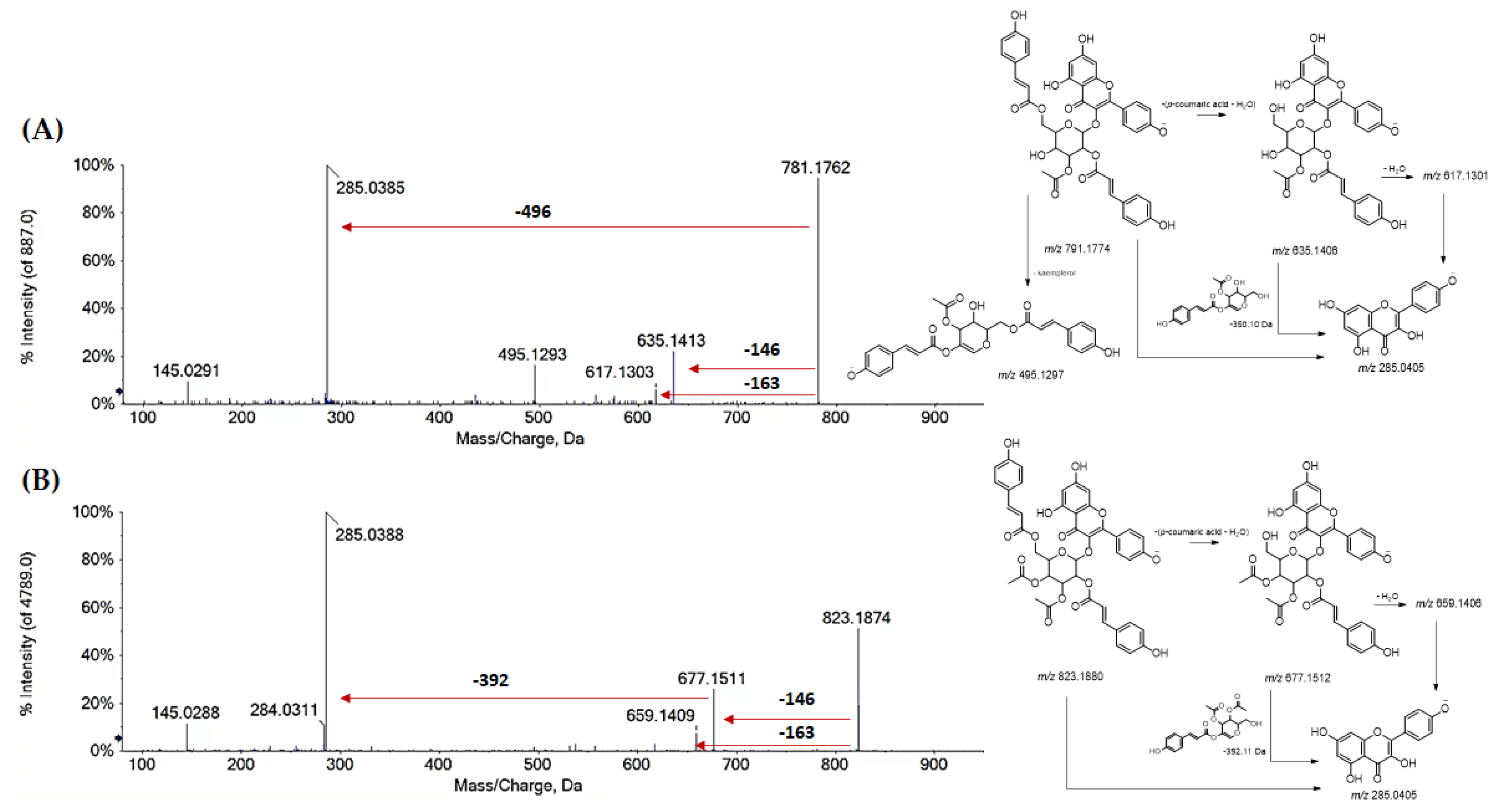
| 78 | R1 = R2 = H; R3 = (Acetyl)-di-p-Coum-Hex (I) | 781.1759 | −1.9 | 25 | 781.1770; 635.1395; 617.1257; 575.1170; 495.1284: 285.0390; 284.0307; 145.0280 |
| 79 | R1 = R2 = H; R3 = (Acetyl)-di-p-Coum-Hex (I) | 781.1767 | −0.8 | 25 | 781.1762; 635.1413; 617.1303; 495.1293; 285.0385; 284.0300; 145.0291 |
| 81 | R1 = R2 = H; R3 = di-(Acetyl)-di-p-Coum-Hex (I) | 823.1863 | −2.0 | 26 | 823.1906; 677.1527; 659.1425; 285.0399; 284.0315; 145.0286 |
| 82 | R1 = R2 = H; R3 = di-(Acetyl)-di-p-Coum Hex (II) | 823.1865 | −1.8 | 26 | 823.1874; 677.1511; 659.1409; 285.0388; 284.0311; 145.0288 |
| Quercetin Derivatives | |||||
| Peaks | R1, R2 and R3 | [M − H]− Found m/z | Error | RDB | MS/MS Fragment Ions (m/z) |
| 35 | R1 = OH; R2 = H; R3 = di-Hex | 625.1431 | 3.3 | 13 | 625.1411; 445.0739; 301.0339; 300.0254; 271.0230; 178.9979 |
| 38 | R1 = OH; R2 = H; R3 = Pen-Hex (I) | 595.1307 | 0.4 | 13 | 595.1329; 301.0348; 300.0274; 271.0238; 255.0286 |
| 40 | R1 = OH; R2 = H; R3 = Hex (I) | 463.0872 | −2.2 | 12 | 463.0866; 301.0343; 300.0269; 271.0238; 255.0286 |
| 41 | R1 = OH; R2 = H; R3 = Hexu | 477.0661 | −2.9 | 13 | 301.0345; 178.9977; 151.0037 |
| 42 | R1 = OH; R2 = H; R3 = Hex (II) | 463.0869 | −2.8 | 12 | 463.0860; 301.0341; 300.0264; 271.0235; 255.0283 |
| 43 | R1 = OH; R2 = H; R3 = Pen-Hex (II) | 595.1302 | −0.4 | 13 | 595.1317; 301.0341; 300.0268; 271.0236; 255.0291 |
| 46 | R1 = OH; R2 = H; R3 = Pen | 433.0769 | −1.7 | 12 | 433.0784; 301.0331; 300.0259; 271.0229; 255.0277 |
| 54 | R1 = OH; R2 = R3 = H | 301.0356 | 0.7 | 11 | 301.0373; 245.0430; 178.9976; 151.0031; 121.0292; 107.0141 |
| 56 | R1 = OH; R2 = H; R3 = p-Coum-Pen-Hex | 741.1704 | −3.7 | 10 | 741.1679; 695.3644; 595.1309; 485.2925; 301.0341; 300.0255; 271.0240 |
| 60 | R1 = OH; R2 = H; R3 = p-Coum-Hex (I) | 609.1254 | 0.7 | 18 | 609.1250; 463.0877; 358.9632; 327.2136; 301.0332; 300.0258; 271.0235 |
| 61 | R1 = OH; R2 = H; R3 = p-Coum-Hex (II) | 609.1277 | 4.5 | 18 | 609.1248; 463.0888; 327.2172; 301.0340; 300.0262; 271.0249 |
| Isorhamnetin Derivatives | |||||
| Peaks | R1, R2 and R3 | (M − H)− Found m/z | Error | RDB | MS/MS Fragment Ions (m/z) |
| 50 | R1 = OCH3; R2 = H; R3 = Pen-Hex | 623.1608 | −1.5 | 13 | 623.1629; 315.0504; 314.0421; 300.0268; 299.0157 |
| 51 | R1 = OCH3; R2 = H; R3 = Hex (I) | 477.1039 | 0.1 | 12 | 477.1036; 315.0486; 314.0423; 300.0264; 299.0174; 285.0389; 271.0235; 257.0442 |
| 52 | R1 = OCH3; R2 = H; R3 = Hex (II) | 477.1054 | 3.2 | 12 | 477.1032; 315.0488; 314.0417; 300.0271; 299.0188; 285.0402; 271.0233; 257.0445 |
| 64 | R1 = OCH3; R2 = H; R3 = p-Coum-Hex | 623.1429 | 3.6 | 18 | 623.1394; 477.1020; 315.0492; 314.0409; 307.0797; 300.0256; 299.0185 |
| 69 | R1 = OCH3; R2 = H; R3 = H | 315.0495 | −4.8 | 11 | 315.0544; 300.0273; 271.0241; 135.0087 |
| 76 | R1 = OCH3; R2 = H; R3 = di-p-Coum-Hex | 769.1789 | 1.9 | 24 | 769.1740; 623.1384; 605.1287; 453.1174; 315.0490; 314.0444; 307.0795; 300.0247; 145.0284 |
| Myricetin Derivatives | |||||
| Peaks | R1, R2 and R3 | (M − H)− Found m/z | Error | RDB | MS/MS Fragment Ions (m/z) |
| 33 | R1 = OH; R2 = OH; R3 = Pen-Hex | 611.1269 | 2.5 | 13 | 611.1266; 317.0290; 316.0212; 271.0240 |
| 34 | R1 = OH; R2 = OH; R3 = Hex (I) | 479.0826 | −1.1 | 12 | 479.0824; 317.0299; 316.0212; 287.0176; 271.0242; 178.9969 |
| 36 | R1 = OH; R2 = OH; R3 = Hex (II) | 479.0825 | −1.3 | 12 | 479.0818; 317.0288; 316.0207; 287.0170; 271.0231 |
| (A) | (B) | |||
| Control Diet | Qr/1/1 | 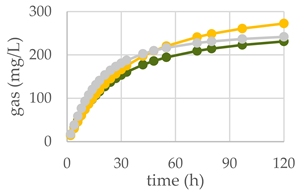 | ||
| Parameter | 50 mg | 200 mg | ||
| OMD (%) | 74.9 | 65.9 *** | 73.8 NS | |
| OMCV (mL/g) | 244 | 220 *** | 224 ** | |
| Tmax (h) | 3.11 | 1.77 * | 3.86 NS | |
| Rmax (mL/h) | 10.6 | 8.58 * | 8.07 *** | |
| Control Diet | Qr/2/1 | |||
| 50 mg | 200 mg | 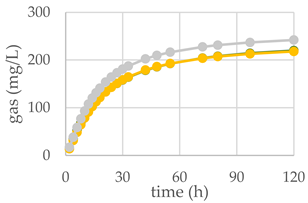 | ||
| OMD (%) | 74.9 | 68.5 *** | 72.1 * | |
| OMCV (mL/g) | 244 | 210 *** | 184 *** | |
| Tmax (h) | 3.11 | 1.78 * | 3.41 NS | |
| Rmax (mL/h) | 10.6 | 9.35 NS | 8.77 *** | |
| Control Diet | Qr/3/2 | |||
| 50 mg | 200 mg |  | ||
| OMD (%) | 74.9 | 69.0 *** | 65.5 *** | |
| OMCV (mL/g) | 244 | 215 *** | 212 ** | |
| Tmax (h) | 3.11 | 3.16 NS | 2.12 * | |
| Rmax (mL/h) | 10.6 | 8.55 * | 8.83 *** | |
| (A) | (B) | |||
| Control Diet | Qr/1/1 | 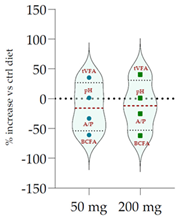 | ||
| Parameter | 50 mg | 200 mg | ||
| pH | 6.20 | 6.29 NS | 6.27 NS | |
| Total VFA (mmol/L) | 57.20 | 77.5 *** | 80.5 *** | |
| BCFA (% VFA) | 7.42 | 2.90 *** | 2.82 *** | |
| A/P | 4.27 | 2.86 *** | 3.20 ** | |
| Control Diet | Qr/2/1 | |||
| 50 mg | 200 mg | 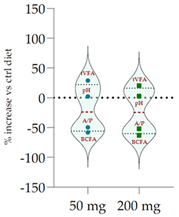 | ||
| pH | 6.20 | 6.33 * | 6.37 ** | |
| Total VFA (mmol/L) | 57.20 | 73.7 *** | 68.9 ** | |
| BCFA (% VFA) | 7.42 | 3.11 *** | 2.76 *** | |
| A/P | 4.27 | 2.15 *** | 2.06 *** | |
| Control Diet | Qr/3/2 | |||
| 50 mg | 200 mg | 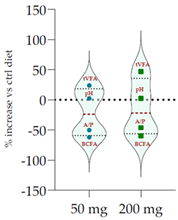 | ||
| pH | 6.20 | 6.35 * | 6.34 * | |
| Total VFA (mmol/L) | 57.20 | 70.8 *** | 84.0 *** | |
| BCFA (% VFA) | 7.42 | 2.82 *** | 3.00 *** | |
| A/P | 4.27 | 2.13 *** | 2.30 *** | |
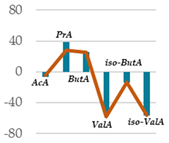
| Control Diet | Qr/1/1 | Qr/2/1 | Qr/3/2 | MSE | ||||
| (% VFA) | 50 mg | 200 mg | 50 mg | 200 mg | 50 mg | 200 mg | ||
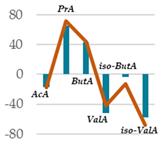
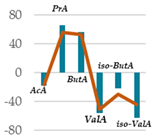
| AcA | 64.7 | 60.0 * | 61.8 NS | 53.8 *** | 53.3 *** | 53.1 *** | 53.9 *** | 0.77 |
| PrA | 15.1 | 21.0 *** | 19.3 ** | 25.0 *** | 25.9 *** | 25.0 *** | 23.5 *** | 0.31 |
| ButA | 11.9 | 15.0 * | 14.9 * | 17.0 ** | 17.1 ** | 18.6 ** | 18.2 ** | 0.65 |
| ValA | 3.98 | 1.78 *** | 1.66 *** | 1.91 *** | 2.32 *** | 1.76 *** | 1.96 *** | 0.006 |
| iso-ButA | 0.99 | 0.85 * | 0.85 * | 0.96 NS | 0.86 * | 0.77 ** | 0.69 ** | 0.0009 |
| iso-ValA | 3.25 | 1.40 *** | 1.42 *** | 1.38 *** | 1.04 *** | 1.21 *** | 1.82 ** | 0.03 |
 |  |  | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts. Antioxidants 2022, 11, 2366. https://doi.org/10.3390/antiox11122366
Formato M, Vastolo A, Piccolella S, Calabrò S, Cutrignelli MI, Zidorn C, Pacifico S. Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts. Antioxidants. 2022; 11(12):2366. https://doi.org/10.3390/antiox11122366
Chicago/Turabian StyleFormato, Marialuisa, Alessandro Vastolo, Simona Piccolella, Serena Calabrò, Monica Isabella Cutrignelli, Christian Zidorn, and Severina Pacifico. 2022. "Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts" Antioxidants 11, no. 12: 2366. https://doi.org/10.3390/antiox11122366
APA StyleFormato, M., Vastolo, A., Piccolella, S., Calabrò, S., Cutrignelli, M. I., Zidorn, C., & Pacifico, S. (2022). Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts. Antioxidants, 11(12), 2366. https://doi.org/10.3390/antiox11122366











