Abstract
An imbalance between oxidants and antioxidants in the body can lead to oxidative stress, which is one of the major causes of neurodegenerative diseases. The gut microbiota contains trillions of beneficial bacteria that play an important role in maintaining redox homeostasis. In the last decade, the microbiota–gut–brain axis has emerged as a new field that has revolutionized the study of the pathology, diagnosis, and treatment of neurodegenerative diseases. Indeed, a growing number of studies have found that communication between the brain and the gut microbiota can be accomplished through the endocrine, immune, and nervous systems. Importantly, dysregulation of the gut microbiota has been strongly associated with the development of oxidative stress-mediated neurodegenerative diseases. Therefore, a deeper understanding of the relationship between the gut microbiota and redox homeostasis will help explain the pathogenesis of neurodegenerative diseases from a new perspective and provide a theoretical basis for proposing new therapeutic strategies for neurodegenerative diseases. In this review, we will describe the role of oxidative stress and the gut microbiota in neurodegenerative diseases and the underlying mechanisms by which the gut microbiota affects redox homeostasis in the brain, leading to neurodegenerative diseases. In addition, we will discuss the potential applications of maintaining redox homeostasis by modulating the gut microbiota to treat neurodegenerative diseases, which could open the door for new therapeutic approaches to combat neurodegenerative diseases.
1. Introduction
Progressive neuronal necrosis and degeneration are hallmarks of neurodegenerative diseases (NDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple sclerosis (MS), which are caused by neurotoxic etiological agents in the brain and surrounding organs. Because of the diverse and complex pathological symptoms, uncertain pathogenesis, restricted clinical examination, difficulty in making an early diagnosis, and lack of treatment options, NDs have imposed significant societal and economic burdens [1,2,3,4]. The current treatment of NDs mainly focuses on relieving symptoms, and no effective cure is currently available. Therefore, the search for novel and effective strategies to treat NDs remains a priority.
As a result of normal metabolic processes, reactive oxygen species (ROS) and reactive nitrogen species (RNS), being free radicals, are neutralized by endogenous antioxidants in cells and tissues to maintain redox homeostasis [5,6]. This redox homeostasis is disrupted under certain pathophysiological conditions, such as injury, inflammation, genetic mutations, and ischemia/reperfusion, causing oxidative stress, which is associated with a variety of progressive NDs [5,7]. The central nervous system (CNS) can form large amounts of free radicals due to its high oxygen demand and metabolism of neurotransmitters [8,9]. Notably, nerve cells are particularly vulnerable to damage because of the abundance of free radicals and relatively weak antioxidant defenses compared with other organs [10,11]. In addition, oxidative stress causes mitochondrial dysfunction, which is unable to meet the high energy requirements for normal biochemical and physiological functions of neuronal cells, thus leading to neuronal cell death [12]. Thus, maintenance of redox homeostasis is essential for neuronal survival and function.
The gut microbiota comprises the microorganisms that exist in different ecological niches of the gut, including bacteria, fungi, viruses, and protozoa [13]. The gut microbiota significantly affects multiple aspects of host physiology, including the immune system, anti-infection, nutritional metabolism, and nervous system [14,15]. Recently, the gut microbiota has been shown to play an essential role in various biological and physiological processes in the brain, such as glial cell activation, myelination, and neurogenesis [16]. In addition, dysbiosis of the gut microbiota is strongly associated with gastrointestinal diseases, anxiety, depression, metabolic disorders, as well as NDs [17,18,19,20]. These things considered, probiotic strains, such as Bifidobacterium and Lactobacillus, can produce potential antioxidants, vitamins, and bioactive molecules to maintain redox homeostasis, thereby preventing oxidative stress-related diseases [21,22]. Importantly, the gut microbiota is involved in the communication between the gut and the brain through neurotransmitters and various metabolites [23,24]. The current evidence strongly suggests that the gut microbiota can influence the brain aging process and the initiation and progression of NDs, making the gut–brain crosstalk a promising and exciting research area in neuroscience [16]. It is, therefore, of interest to find novel therapeutic targets and strategies from the perspective of the gut microbiota and redox homeostasis. In this review, we will describe the roles of oxidative stress and the gut microbiota in NDs, as well as the underlying mechanisms by which the gut microbiota affects redox homeostasis. In addition, the potential applications of maintaining redox homeostasis by shaping the gut microbiota to treat NDs will also be discussed.
2. Oxidative Stress and NDs
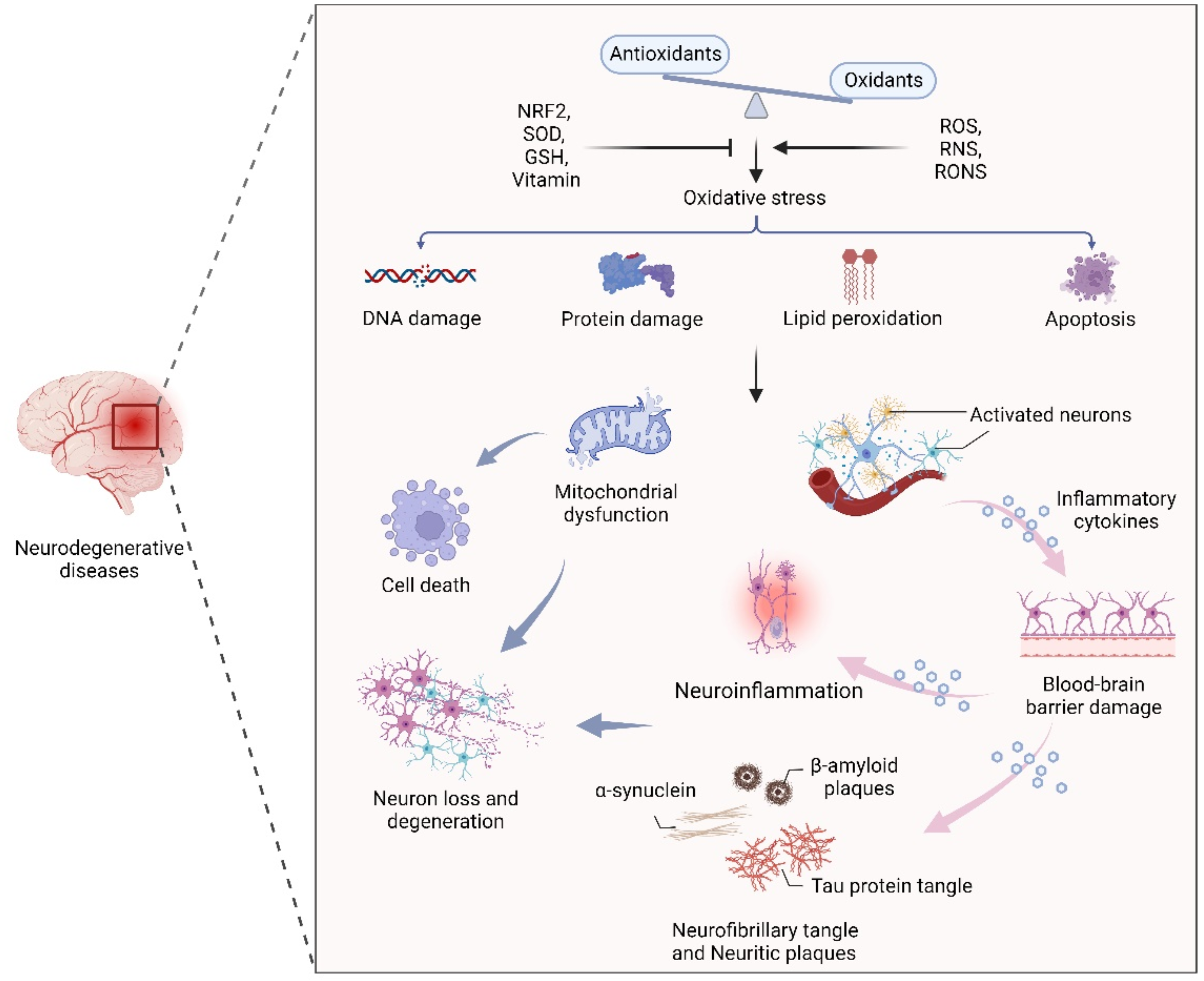
Oxidative stress, the result of an imbalance in the relative abundance of reactive ROS and antioxidants, can create a detrimental state that leads to cellular damage and dysfunction. Increased oxidative stress is able to damage cell membranes, alter protein structure and function, and cause DNA damage [25,26]. Therefore, maintenance of redox homeostasis is essential for cell biological function. As mentioned earlier, the CNS is particularly sensitive to oxidative stress due to its high metabolic rate, relative antioxidant scarcity, and unique structural features [27,28]. In addition, due to the presence of high levels of metal ions and polyunsaturated fatty acids, neuronal cells are more prone to oxidative stress, leading to cell damage and a series of NDs-related events through mitochondrial dysfunction, inflammation, and neuronal death [28,29,30]. In fact, an oxidative stress-induced imbalance in redox homeostasis is still a central component of the pathogenesis of several NDs, such as AD, PD, and MS. The common features among these NDs are ineffective antioxidant defense systems, imbalances of redox homeostasis, mitochondrial dysfunction, neuroinflammation, neuronal loss and degeneration (Figure 1).
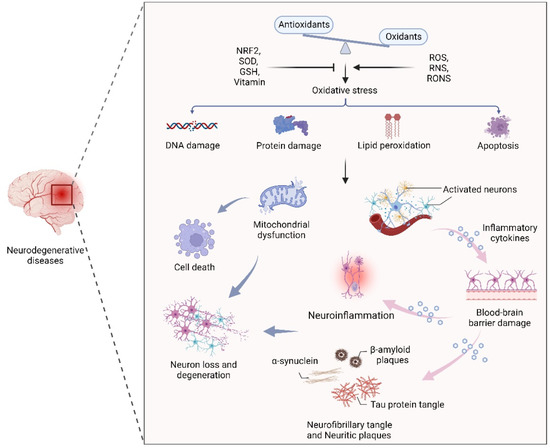
Figure 1.
The effect of oxidative stress in neurodegenerative diseases. An oxidant/antioxidant imbalance leads to oxidative stress, which causes DNA and protein damage, lipid peroxidation, and apoptosis. Dysfunctional mitochondria and activated neurons secrete inflammatory cytokines that cross the blood–brain barrier, leading to inflammation, α-synuclein, β-aggregation, and neuronal plaque accumulation in neurons, leading to neuron loss and degeneration.
Oxidative stress and disruption of cerebral redox homeostasis frequently occur in human NDs. For instance, in the pathology of AD, amyloid β (Aβ) and tau protein aggregates can interact with metal ions and maintain normal cellular signaling [31,32]. Furthermore, previous studies have shown that the high levels of zinc in the neocortical and hippocampal regions of AD patients suggest the vital role of zinc in the maintenance of redox homeostasis in the affected brain regions [33,34]. Notably, accumulated Aβ-induced oxidative stress can inhibit the activity of complex IV, leading to ATP depletion and mitochondrial dysfunction [35,36]. It has been demonstrated that the abnormal aggregation of α-synuclein (α-syn), mitochondrial dysfunction, and excessive oxidative stress are closely related to dopaminergic neuron death during PD progression [37,38,39]. As the main pathogenic factor of HD, soluble and aggregated mutant Htt (mHtt) protein with cytotoxicity induces apoptosis through oxidative stress, resulting in continuous degeneration of neurons [40,41,42]. Interestingly, in patients with NDs, oxidative stress biomarkers such as malondialdehyde and 8-hydroxyguanosine are elevated, and the gene superoxide dismutase 1 (SOD1), which plays an important role in oxidative stress defense mechanisms, is also frequently mutated [43,44].
Despite the advanced understanding of the mechanisms described above, a wide gap remains between this knowledge and the availability of effective therapies. Given that the imbalance of redox homeostasis is one of the key factors in the pathogenesis of NDs, numerous studies have been conducted on the treatment of NDs using various types of antioxidants (Table 1). Overall, most of the clinical trial results of NDs have shown favorable therapeutic effects, especially the alterations of pathological markers and improvements in neurological function, suggesting that antioxidants have great therapeutic potential for NDs. However, more efforts are required to explore novel therapeutic strategies and approaches to achieve broad therapeutic applicability and functional recovery of the nervous system.

Table 1.
Antioxidants with therapeutic effects on neurodegenerative diseases.
3. Gut Microbiota, Oxidative Stress, and Neurodegeneration
The human gastrointestinal tract is the largest immune organ and harbors complex and dynamic microbiota [56,57]. Gut microbiota stability can be impacted by several variables, including genetics, lifestyle, nutrition, medications, illness, and age, which in turn have a significant impact on the regulation of metabolism, homeostasis, immunological response, and other processes [58,59]. Therefore, an imbalance in the representation of the gut microbiota may contribute to various diseases, from inflammatory bowel disease to obesity and diabetes, as well as several common NDs, such as AD, PD, and MS (Table 2). In addition, growing numbers of studies have demonstrated that the gut microbiota alters the oxidative/antioxidant balance in the CNS and causes neurodegeneration [60,61,62,63].

Table 2.
Alterations in the gut microbiota composition in various neurodegenerative diseases.
3.1. Gut–Brain Axis under Physiological Conditions
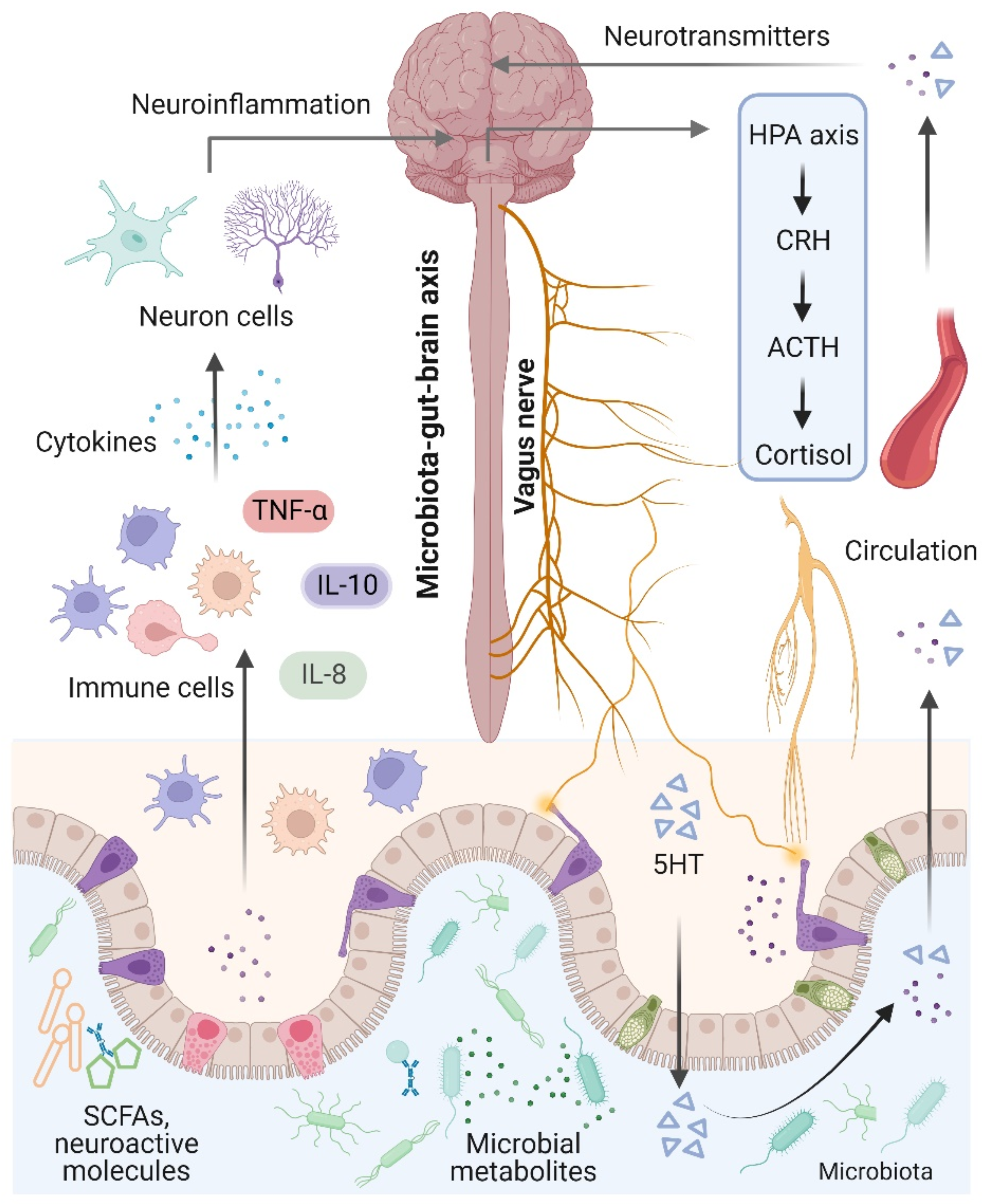
The brain and the gut microbiota are strictly intertwined and communicate in a variety of ways, including the production of bacterial metabolites, neurotransmitters, and cytokines [74] (Figure 2). Notably, the term “microbiota–gut–brain axis” refers to an interaction between the brain and the gut microbiota that involves four major routes of communication [75,76]. The first route of communication involves the vagus nerve, which connects the enteric nerve system and the brain stem. Recent research indicates that the gut microbiota influences host behaviors such as anxiety, feeding, and depression by activating vagal neurons and altering neurotransmitters such as γ-aminobutyric acid (GABA) and oxytocin in the brain [77,78]. The second important mode that directly or indirectly affects brain activity involves serotonin, which is mainly produced by gut enterochromaffin cells and modulates a variety of physiological processes. Interestingly, increased levels of serotonin and serotonin precursors alleviated depression in a mouse model of depression after treatment with the probiotic Bifidobacterium [79]. Thirdly, the gut microbiota plays an essential role in microglial activation and neuroinflammation. For instance, Luck and colleagues demonstrated that germ-free mice carry more immature microglia than conventional mice, and Bifidobacterium spp. can activate microglia through transcriptional activation [80]. In addition, alterations in microglial function were also observed in NDs and other behaviors, suggesting that the gut microbiota mediates effects on NDs through microglia [81]. Notably, the gut microbiota plays a vital role in energy harvest and neuroinflammation, and alterations in the gut–brain vagal pathway may promote obesity. It has been demonstrated that a diet-induced shift in the gut microbiome may disrupt vagal gut–brain communication resulting in microglia activation, increased gut inflammation, and body fat accumulation [82,83]. Finally, the gut microbiota communicates by transferring chemical signals directly to the brain. A previous study indicated that short-chain fatty acids (SCFAs) derived from the fermentation of the gut microbiota had been shown to modulate neuroplasticity in the CNS and improve depressive behavior in mice [62].
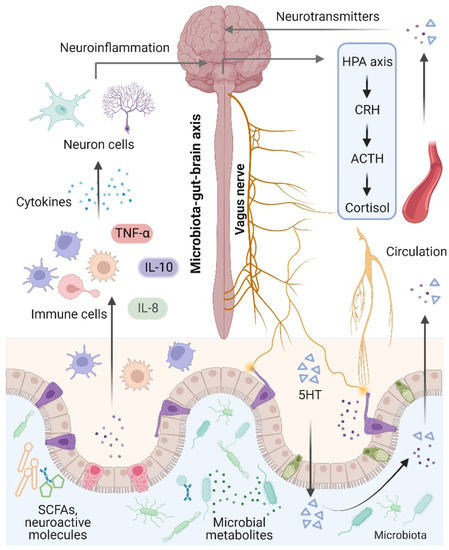
Figure 2.
Microbiota–gut–brain axis. The brain and gut communicate through neural, metabolic, endocrine, and immunological pathways. The brain influences gut health through the vagus nerve, the hypothalamic–pituitary–adrenal (HPA) axis, and systemic circulation. Signals from the gut, including short-chain fatty acids (SCFAs), neurotransmitters, and amino acids, modulate brain function via neuronal cells, the immune system, and endocrine mechanisms.
3.2. Gut Microbiota-Mediated Oxidative Stress and Neurodegeneration
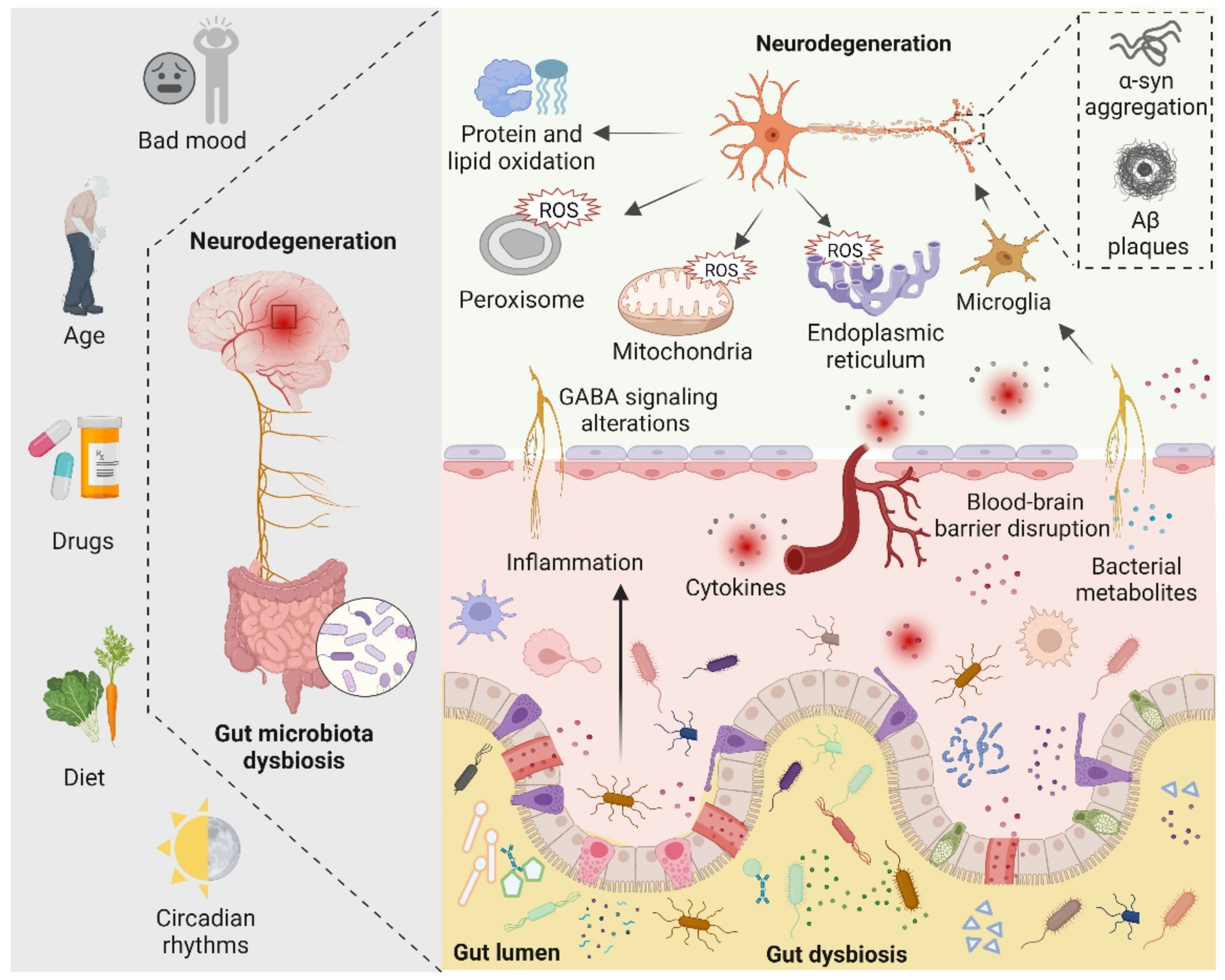
There are four main symbiotic bacteria that are parasitic in the human gut, namely Actinobacteria, Bacteroidetes, Proteobacteria, and Firmicutes. Among them, Firmicutes accounts for the largest proportion, including Streptococcus, Lactobacillus, and Mycoplasma [81]. Recent studies have found that, in the presence of the microbiota, the intestinal epithelium cells produce physiological levels of oxidative stress that affect the composition and function of the gut microbiota. Such alterations in gut microbiota increase the alterations of biomacromolecules reaching the systemic circulation and CNS by directly affecting the permeability of the intestine [84]. Indeed, the gut microbiota can alter cellular oxidative stress status by regulating mitochondrial activity [85]. In addition, gut Lactobacilli, Bifidobacterium, and Streptococcus can produce nitric oxide (NO) in various ways in the gut [86,87]. It is now generally accepted that NO in nanomolar concentrations is neuroprotective, whereas higher concentrations of NO may result in oxidative stress, which is closely related to axonal degeneration, neuroinflammation, and NDs [81]. In addition, certain pathogenic bacteria such as Salmonella and Escherichia coli are able to produce hydrogen sulfide (H2S) in the gut by degrading sulfur-containing amino acids. Furthermore, increased levels of H2S alter various host metabolic activities, such as increased lactate, decreased oxygen consumption, decreased ATP production, and elevated levels of proinflammatory compounds, which have been linked to neuroinflammation [88,89,90]. The role of gut microbiota in neurodegeneration is shown in Figure 3.
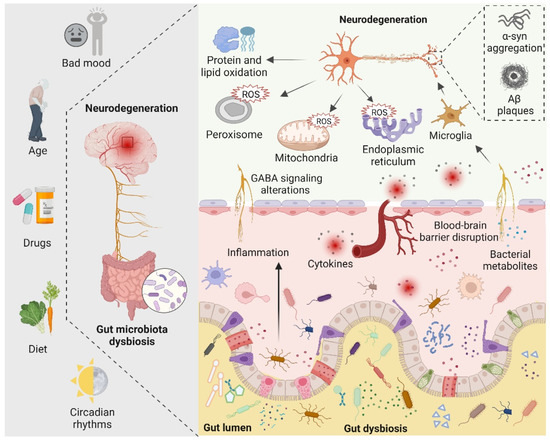
Figure 3.
The role of the gut microbiota in neurodegeneration is depicted schematically. Bad mood, increasing age, drugs, dietary changes, and circadian rhythms can disrupt gut microbiota homeostasis. When gut dysbiosis occurs, beneficial bacteria in the gut are transformed into pathogenic bacteria, producing a large number of harmful metabolites and proinflammatory molecules, resulting in increased blood–brain barrier permeability and peripheral inflammatory responses, thereby aggravating oxidative stress in the brain. At the same time, dysbiosis can induce bad mood. Increased levels of ROS in neuronal mitochondria, endoplasmic reticulum, and peroxisomes, increased protein and lipid oxidation, and accumulation of neurotoxic proteins lead to neurodegeneration.
In-depth studies on the pathogenesis of NDs, including AD, PD, and MS, have mainly focused on the misfolding and aggregation of proteins in neurons. In addition, oxidative stress has also been considered to be closely related to the occurrence and development of NDs, but the exact underlying mechanisms remain unclear. Numerous studies have demonstrated the close association of microbiota-mediated oxidative stress with neurodegeneration. Here, we summarize recent links between the gut microbiota, oxidative stress, and NDs, with a focus on AD, PD, and MS.
3.2.1. Alzheimer’s Disease
AD is the most common ND worldwide with an insidious onset and progressive development [4,91]. It is characterized by progressive impairment of cognition and episodic memory, culminating in the development of dementia [92]. Specific histopathological hallmarks in the brain associated with AD include Aβ plaques, neurofibrillary tangles (NFTs), hyperphosphorylation of tau proteins (tau tangles), and neuronal loss [93,94]. Oxidative stress has been suggested to play an essential role in AD etiology prior to plaque formation, leading to mitochondrial dysfunction in neurons and synapses, as well as Aβ protein production [95,96]. Previous studies have shown that oxidative stress plays a pivotal role in the development of AD. For instance, it has been demonstrated that aggregated Aβ protein stimulates microglia to produce ROS through positive feedback on Aβ plaque deposition [91]. In addition, tau protein aggregation in neurons leads to reduced NADH-ubiquitin reductase activity, leading to oxidative stress and mitochondrial dysfunction [97]. Interestingly, ROS can affect the activity of stress kinases, such as the phosphorylation-c-Jun N-terminal kinase 1 (p-JNK) pathway, which is associated with neuronal cell death due to tau hyperphosphorylation and accumulation of Aβ [98]. There is ample evidence that the oxidation of nucleic acid species in the AD brain is dominated by the mitochondrial genome, and lipid peroxidation results in the production of certain cytotoxic agents, such as 4-hydroxyalkenals [99,100,101].
Recent studies have shown that the gut microbiota plays a significant role in the pathogenesis of AD [102]. Dysregulation of the gut microbiota leads to oxidative stress, inflammation, disruption of the blood–brain barrier, activation of the immune system, neurofibrillary tangles, and Aβ plaques followed by neurodegeneration [21,103]. There are numerous bacteria in the human gut that play a vital role in the etiology of AD, including Staphylococcus aureus, Escherichia coli, Salmonella, Mycobacterium, Klebsiella pneumoniae, and Streptococcus, which promote the production and aggregation of the Aβ protein in the enteric nervous system [104,105]. Interestingly, in the APPSWE/PS1ΔE9 transgenic mouse model of AD chronically treated with broad-spectrum combination antibiotics, the gut microbiome of transgenic mice shifted toward proinflammatory bacteria, with a decrease in amyloid plaque deposition and neuroinflammation [66,106]. Additionally, microbial amyloid protein, produced by coccus-shaped bacteria, is able to activate the innate immune system and triggers responses by Toll-like receptors (TLRs) and cluster of differentiation 14 (CD14), resulting in inadequate recognition of misfolded Aβ and decreased Aβ clearance, followed by the production of cytokines leading to intestinal disturbances [107]. Notably, age-related reductions in gut microbial diversity are also implicated in AD. It has been demonstrated that with growing age, there is an increase in Proteobacteria and a decrease in Bifidobacterium spp., which results in interference in lipid metabolism and a failure to maintain hippocampal plasticity as well as memory functions [108,109].
Another possible connecting link between the gut microbiota and microbiota-mediated cerebral amyloid accumulation involves a cross-seeding mechanism of microbial amyloid (i.e., promoting misfolded aggregation of amyloid from one protein to another) in a manner similar to the reproduction of prions [107,110,111]. Notably, distinct amyloid conformations interact with cellular targets to produce various toxicities, which may explain the different AD phenotypes [112]. Given the multiple roles of gut microbiota dysbiosis in the pathogenesis of AD, modulation of AD through dietary and gut microbiota interventions may be potential therapeutic strategies, which will be discussed in detail later.
3.2.2. Parkinson’s Disease
PD, the second most common ND after AD, is a long-term neurological disorder that causes both motor and non-motor symptoms [113,114,115]. Motor symptoms include resting tremors, akinesia, muscular rigidity, postural instability, and gait abnormalities [116,117,118]. Non-motor symptoms include anxiety, depression, autonomic dysfunction, cognitive decline, and sleep disturbances [119,120]. The hallmarks of PD are loss of dopaminergic (DA) neurons and abnormal accumulation of α-syn within the cytoplasm of nerve cells called Levy bodies [121,122,123,124]. Notably, oxidative stress, mitochondrial dysfunction, dopamine metabolism, abnormal protein aggregation, and the gut microbiota are associated with the pathogenesis of PD [125,126,127]. As one of the main pathogenic factors of PD, oxidative stress has been linked to α-syn protein aggregation and degeneration in DA neurons [98,121,128]. For instance, analysis of the postmortem brain tissue in PD showed that oxidative stress degenerates DA neurons, reduces the levels of glutathione (GSH), increases the levels of oxidative stress markers, and stimulates lipid, DNA, and RNA oxidation [129,130]. Additionally, Tong and colleagues have demonstrated that oxidative stress in DA neurons can activate the p38 mitogen-activated protein kinase pathway, ultimately leading to neuronal apoptosis [131]. Interestingly, in the 6-hydroxydopamine-induced PD model in mice, Antrodia camphorata polysaccharide reduced ROS by increasing the expression and activity of antioxidant enzymes, ultimately attenuating the damage of DA neurons in the substantia nigra and improving motor performance [132].
Notably, PD patients often present with gastrointestinal dysfunction, which suggests that the imbalance of the gut microbiota is one of the causes of triggering or aggravating PD [133,134]. Indeed, gut inflammation, early accumulation of α-syn, increased intestinal permeability, and constipation problems are common in PD patients, again demonstrating the critical role of the gut microbiota in PD [81,135]. It has been demonstrated that the disruption of gut microbiota leads to oxidative stress through overstimulation of the immune system, which in turn activates intestinal neurons and intestinal glia cells, leading to increased misfolding and accumulation of α-syn in the CNS [136,137]. In addition, coming to the role of the gut microbiota, toxins and microbial products produced by certain pathogenic bacteria are able to cause mitochondrial dysfunction in intestinal cells and the CNS, which is directly associated with PD pathogenesis [138]. In line with this, it is proposed that the pathogenic bacterium E. coli can produce an amyloid protein called curli, which promotes the accumulation of α-syn protein in the brain and causes motor defects in mice [139]. Conversely, when treated with gut-restricted amyloid inhibitors, these mice showed significant improvements in constipation and motor function, suggesting the role of the gut microbiota in the etiology of PD symptoms [140]. Interestingly, when the gut microbiota from PD patients was transplanted into a germ-free α-syn overexpressed mouse model, a similar pattern of physical injury to PD patients was observed, suggesting the vital role of the gut microbiota in PD. As discussed above, decreased production of hydrogen (H2) by the gut microbiota has been proposed as one of the essential factors in PD [141]. According to a recent study, 50% H2 saturated water was successful in preventing nigrostriatal degeneration in PD rats and reducing the oxidative stress markers in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine (MPTP) mouse model [142]. Taken together, these observations suggest a critical role for gut microbiota in PD, and intervention through gut microbiota is expected to provide promising strategies for the prevention and treatment of PD.
3.2.3. Multiple Sclerosis
MS is an immune-mediated chronic inflammatory and central nervous system demyelinating disease with a complex and unclear pathogenesis [143]. It has been established that the pathogenesis of MS involves both genetic and environmental factors. The most extensively accepted hypothesis is that autoreactive B and T cells cause axonal and myelin damage, as well as neurodegeneration [144,145]. The major neuropathological hallmarks of MS pathology are inflammation and degeneration of both white matter and gray matter [146]. However, the development of MS may be influenced by a combination of internal and external factors, ultimately leading to immune dysregulation.
Growing evidence suggests that the imbalance of redox homeostasis plays a vital role in the pathogenesis of MS. For instance, it has been demonstrated that the excessive generation of ROS, mitochondrial dysfunction, and impairment of antioxidant defense systems play important roles in the pathogenesis of MS [147]. Notably, ROS has been shown to be a mediator of axonal injury and demyelination in both MS patients and animal models of MS. In addition, oxidative stress mediates mitochondrial dysfunction in MS patients and leads to CNS energy failure in MS-susceptible individuals [148,149]. Furthermore, recent studies have demonstrated that the gut microbiota has a significant impact on MS and can be influenced by external factors [150]. For instance, Cosorich et al. have demonstrated that T helper 17 (TH17) cells, key players in MS, originate in the gut and that increased TH17 cell frequency is associated with specific alterations of the gut microbiota in MS patients [151]. Interestingly, transplantation of the MS microbiota in a mouse model resulted in an increased incidence of autoimmune encephalomyelitis, leading to an exacerbation of MS symptoms [152,153]. Additionally, diets have been shown to affect the balance of the gut microbiota and indirectly influence the development of MS [154]. Moreover, dietary studies in MS patients suggest that dietary interventions supplemented with vitamin D in a low-calorie diet have a positive effect on alleviating chronic inflammatory symptoms in MS [155]. Recently, intermittent fasting was introduced into the treatment of MS due to its availability of abundant gut microbiota as well as the secretion of glutathione and leptin [156]. All these studies have shown that modification of the gut microbiota can be considered a promising therapeutic strategy for MS.
4. Gut Microbiota in Neuroprotection
The complex gut microbiota and microbiota–host interactions may directly and indirectly affect the oxidative state of the CNS by producing numerous metabolites such as absorbable vitamins, SCFAs, polyphenols, diffusible antioxidants, and oxidant gases [60]. Notably, the gut microbiota is also able to optimize dietary energy harvest, influence the permeability of the blood–brain barrier and the intestinal barrier, modulate the immune response, and prevent the extensive colonization of pathogens [157,158]. As a component of the parasympathetic nervous system, the vagus nerve can sense intestinal metabolites, communicate with the CNS, and integrate into the central autonomic network to generate specific responses. For instance, under stressful conditions, the vagus nerve can be suppressed, with deleterious effects on the gastrointestinal tract and microbes, such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) due to dysbiosis [157]. Furthermore, the beneficial gut microbiota produces a large number of CNS neurotransmitters such as serotonin, dopamine, and γ-aminobutyric acid, which modulate enteric nervous system (ENS) activity and may be associated with their respective levels within the CNS depending on gut permeability and the blood–brain barrier [10].
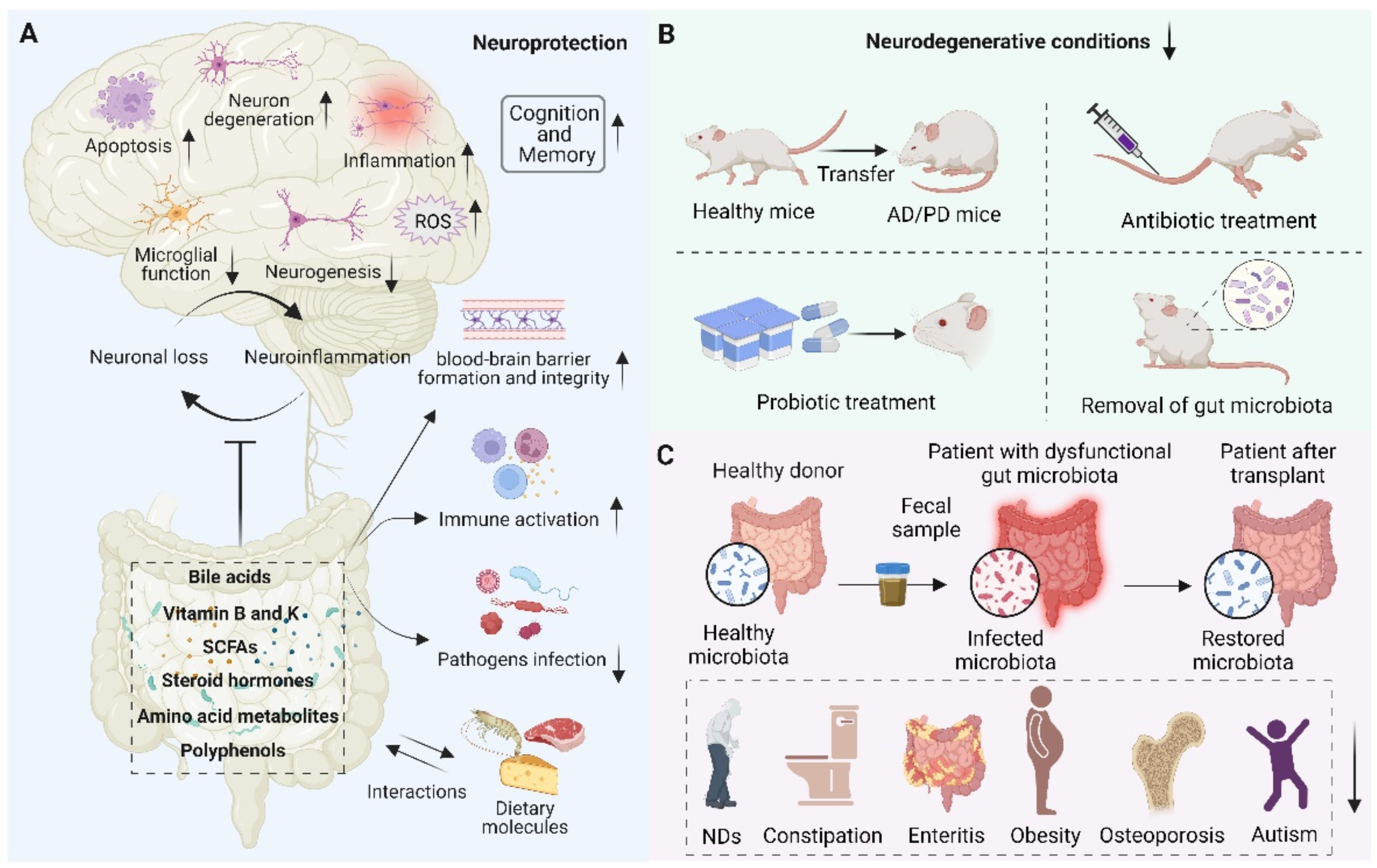
With our increasing understanding of the essential role of gut microbiota and oxidative stress in NDs, there is a growing need to develop therapies based on antioxidant strategies to treat NDs. Although antioxidants have a potent therapeutic effect on certain diseases, the therapeutic effects of antioxidants for NDs are still limited and require deep mechanistic understanding [159]. As discussed above, the dichotomous role of gut microbiota has been observed. On the one hand, the gut microbiota is closely related to the underlying pathogenesis of neurodegeneration. On the other hand, the gut microbiota and its metabolites modulate numerous NDs-related pathways, suggesting their potential therapeutic role in neuroprotection (Figure 4). Therefore, maintaining neuronal health by modulating gut microbiota homeostasis holds great promise. Here, we describe the role of metabolites from the gut microbiota as well as the antioxidative and anti-inflammatory probiotics in neuroprotection.
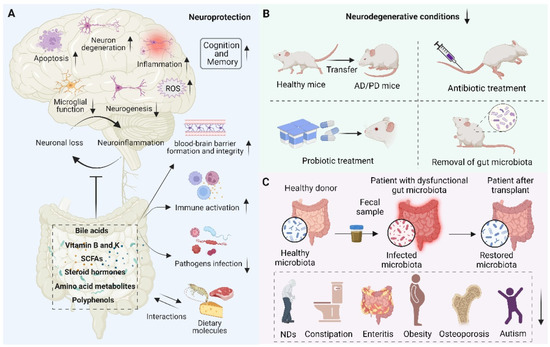
Figure 4.
Schematic representation of the role of the gut microbiota in neuroprotection. (A) Beneficial metabolites such as bile acids, vitamins, short-chain fatty acids (SCFAs), steroid hormones, amino acid metabolites, polyphenols, etc., released by the gut microbiota and interacting with dietary molecules, can promote blood–brain barrier formation and integrity, reduce inflammation, reduce oxidative stress, reduce neuronal apoptosis, activate immune response, prevent pathogen infection, and thus play an important role in neuroprotection. (B) Fecal microbial transfer (FMT), antibiotic and probiotic treatment, and removal of gut microbiota were found to decrease neurodegenerative conditions and reduce the pathophysiology of NDs. (C) The scheme of transferring the gut microbiota from a healthy donor to a patient with dysfunctional gut microbiota and restoring the microbiota, thereby improving human health, such as treating NDs, constipation, enteritis, obesity, osteoporosis, and autism.
4.1. Interactions of Gut Microbiota with Host and Dietary Molecules
Many host molecules, such as bile acids and steroid hormones, are produced via multistep biosynthetic pathways and can interact with the gut microbiota to have beneficial or deleterious effects on the host system. Bile acids are produced in the liver and released in the gut, mainly associated with the dissolution of lipids and fat-soluble vitamins. They also play a vital role in the physiological and pathological processes of the CNS [160]. Bile acids such as taurine deoxycholic acid (TUDCA) and ursodeoxycholic acid (UDCA) have been shown to be neuroprotective without cytotoxicity [161,162]. Moreover, Cuevas et al. revealed that pretreatment with TUDCA protected against dopaminergic neuronal damage, attenuated protein oxidation and autophagy, and also prevented α-syn aggregation [163]. Likewise, UDCA was found to convey neuroprotection in drosophila and mammalian models of charged multivesicular body protein 2B (CHMP2B) Intron 5 (CHMP2BIntron5) induced frontotemporal dementia (FTD) [164]. Indeed, the gut microbiota plays a vital role in the conversion of primary bile acids to secondary bile acids and can alter their solubility, nuclear receptor binding, and blood circulation [165]. Notably, altering bile acid levels and properties by modulating the gut microbiota may have neurodegenerative and neuroprotective effects. For example, alterations in secondary bile acid levels have been found in human and mouse models of a variety of NDs such as AD, PD, and MS [166,167,168]. Furthermore, a previous study indicated that TUDCA might exert neuroprotective effects by inhibiting inflammatory responses and oxidative stress in microglia [169]. However, extensive research is still required to elucidate the specific mechanisms and potential roles of the gut microbiota in manipulating bile acids. Other common host molecules are steroid hormones, which are crucial for brain physiology and function [170]. Through degradation and activation pathways, the gut microbiota can modulate the level of steroid hormones [171]. Moreover, it has been demonstrated that both androgen and estrogen secretions are affected by the gut microbiota. Actually, most gut bacteria are able to metabolize estrogen and can also promote estrogen to undergo oxidation–reduction reactions [172,173]. Interestingly, estrogen influenced by the gut microbiota is neuroprotective and shows anti-inflammatory and differentiated effects in nerve cells [174,175]. Accordingly, an altered gut microbiota leads to low levels of estrogen, which leads to neuroinflammation and neurodegeneration [176,177].
Additionally, dietary molecules such as amino acids, dietary fibers, and polyphenols are also inextricably linked to the gut microbiota. Dietary amino acids are normally metabolized by the gut microbiota, and the resulting dietary amino acids affect the CNS [178,179]. Norepinephrine can be produced by the gut microbiota in the millimolar range, and it protects neurons from H2O2-induced death by increasing the supply of GSH from astrocytes [180]. In addition, indole propionic acid, an indole derivative, is a product of tryptophan metabolism by the gut microbiota and acts as an antioxidant that reduces neuroinflammation and attenuates AD pathology [181,182]. Notably, as a branch of tryptophan metabolism, the disturbance of the kynurenine (KYN) pathway was found to affect memory, anxiety, and stress-related behavior and promote inflammatory responses and neurotoxicity, suggesting the neuroprotective and anti-inflammatory role of KYN in NDs [183,184]. Furthermore, as one of the metabolites of arginine, agmatine is involved in the major processes of synaptic plasticity and memory formation and has therapeutic effects on a variety of NDs [185,186]. Meanwhile, agmatine has been shown to stimulate the NRF2 signaling pathway to reduce the production of ROS and protect neuronal cells from oxidative stress-induced damage [187,188]. In addition, undigested dietary fiber in the body is converted into SCFAs through anaerobic fermentation of gut microbiota, which can not only provide energy but also affect the development and function of the CNS directly or indirectly [189]. It has been reported that SCFAs maintain redox homeostasis in the brain via regulating microglia homeostasis, thus attenuating neuroinflammation in AD and PD [190]. Interestingly, transplantation of fecal microbiota from wild-type mice into the mouse model of PD, combined with butyrate treatment, significantly improved PD symptoms [191]. Butyrate has also been shown to affect the neuroinflammation and the cellular oxidative status of astrocytes [192]. Indeed, fecal transplantation is seeing increased use for the clinical management of neurodegenerative diseases and a number of clinical trials have been undertaken [193,194]. In summary, SCFAs obtained from dietary fiber have great therapeutic potential for NDs. Another group of bioactive molecules in plants is the polyphenols, which can be classified into phenolic acids, flavonoids, and tannins. Due to their special structure, they are able to scavenge free radicals and have been widely applied as antioxidants in the treatment of NDs [195]. Many dietary polyphenols have been shown to be actively converted by the gut microbiota to phenolic acids such as 3-hydroxybenzoic acid and 3-(3-hydroxyphenyl) propionic acid, which inhibit A aggregation and the progression of AD [196]. Similarly, proanthocyanidins (PA) can attenuate oxidative stress in dopaminergic neurons by inhibiting p38, ERK, and JNK signaling pathways, which may provide a new perspective for PD therapy [197]. Taken together, the above studies suggest the therapeutic potential of the interactions of gut microbiota with host and dietary molecules for NDs.
4.2. Vitamins from Gut Microbiota in Neuroprotection
Because the human body lacks the biosynthetic capacity for most vitamins, they must be exogenously supplied to meet demand. Although vitamins are present in various foods, inadequate food intake and poor dietary habits can still cause vitamin deficiencies [198]. Notably, the gut microbiota is a rich source of vitamins, especially vitamins B and K, which are required by both the host and certain gut microbiota [199,200]. In the gut, lactic acid bacteria, Bacillus subtilis, and E. coli produce vitamin B2 (riboflavin). B6 (pyridoxine, pyridoxamine, and pyridoxal) is produced by pyridoxine, pyridoxamine, and pyridoxal, and vitamin K is produced by Escherichia coli, Propionibacterium, and Eubacterium [198,200]. Although dietary vitamins are absorbed primarily from the small intestine, vitamins derived from gut microbiota are taken up in the distal colon and perform various important functions in the body, particularly in neuroprotection. For instance, it has been demonstrated that vitamin K deficiency is strongly associated with the pathogenesis of AD and that increasing the intake of dietary vitamin K is helpful in improving memory function in elderly patients [81]. Additionally, vitamin K2 exerts potent antioxidant properties by inhibiting the activation of the P38 signaling pathway, ROS generation, and the activity of caspase-1, thereby restoring mitochondrial membrane potential, demonstrating its potential for PD treatment [201]. Interestingly, supplementation of B vitamins such as B6, B9, and B12 can slow the shrinkage of specific brain regions associated with cognitive decline in AD [202]. The above studies suggest that vitamins B and K play an important role in improving neuronal health. However, further constructive research is required to demonstrate the neuroprotective potential of vitamins produced by the gut microbiota.
4.3. The Effect of Probiotics in Neuroprotection
Probiotics are non-pathogenic living microorganisms known for their beneficial effects on health; they include Lactobacillus, Streptococcus, Propionibacterium, and Bifidobacterium [203,204,205]. Notably, when the gut microbiota is perturbated leading to certain diseases, probiotic treatment can restore the gut microbiota and ensure the normal functions of the body, which indicates the vital role of probiotics in health [206]. As the most common probiotics, Lactobacillus and Bifidobacterium can alter the composition and quantity of gut microbiota, improve intestinal barrier function, regulate mood and cranial nerve status, and confer resilience to stress [77,207,208,209,210]. Growing evidence indicates the positive effect of probiotics on redox homeostasis in NDs. Probiotic strains dominated by Bifidobacterium and Lactobacillus are able to combat excess free radicals in the form of ROS in the body by producing antioxidants, vitamins, and other bioactive molecules, thereby preventing oxidative stress-related diseases, especially NDs [22,211].
Research on probiotics in NDs in recent years has focused on their antioxidant effects. Probiotics affect brain function and the progression of NDs via their ability to modulate ROS-producing enzymes, chelate metal ions, activate antioxidant pathways, and produce antioxidant metabolites [212,213]. For instance, it has been shown that probiotic consumption has a positive effect on cognitive function and certain metabolic statuses in AD patients [214]. In addition, Akbari et al. have revealed that L. plantarum can reduce malondialdehyde and stimulate the activity of SOD and GPX, thereby scavenging hydroxyl radicals in mice with d-galactose-induced oxidative stress [214]. Interestingly, in addition to inhibiting ROS production by NETs to exert antioxidant effects, L. rhamnosus was found to have antidepressant and antianxiety properties, possibly related to neuroactive substances of bacterial origin [215]. Notably, the gut microbiota can exert antioxidant activity through their anti-inflammatory effects, which is considered to improve the symptoms of a wide range of disorders [216,217]. Wu et al. have revealed that an important strain of Lactobacillus, L. fermentum, could prevent ROS formation by stimulating the production of IL-10 [218]. In addition, the combination of L. mucosae AN1 and L. fermentum SNR1, with strong antioxidant activity, can decrease the level of proinflammatory cytokines, increase the level of anti-inflammatory cytokines, and inhibit related mediators in the gut, thereby alleviating oxidative stress, inhibiting the activation of inflammation-related pathways and maintaining redox homeostasis [217,219]. Similarly, a previous study indicated that a significant reduction in the abundance of anti-inflammatory bacteria with an increase in the abundance of proinflammatory bacteria is possibly associated with cognitive impairment in AD patients [68]. Taken together, the above studies suggest that probiotics may serve as a potential therapeutic intervention for NDs.
5. Shaping the Gut Microbiota to Maintain Redox Homeostasis for NDs Treatment
Given the close interactions between oxidative stress and the gut microbiota, targeting the gut microbiota and redox homeostasis may represent potential therapeutic strategies for treating NDs. Actually, numerous studies and clinical trials have comprehensively revealed the positive role of gut microbiota in the treatment of NDs. For instance, sirtuin-1 (SIRT1), a probiotic bacterial protein, has been shown to have neuroprotective effects, and alterations in the expression and activity of SIRT1 protein are closely associated with Aβ and tau accumulation in the cerebral cortex in both animal models of AD and human AD patients. When treated with probiotic supplements, the SIRT1 pathway is activated to exert antioxidant effects [81,220,221]. In addition, prolonged diet supplementation with a Lactobacillus strain upregulates the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, thereby preventing age-associated cognitive decline [222]. Similarly, Lactobacillus paracasei PS23 supplements can prevent aging-related neurological damage and ameliorate cognitive dysfunction, possibly by increasing the activity of antioxidant enzymes in the hippocampus and modulating microbiota–gut–brain axis communication [223]. Indeed, Bifidobacterium bifidum ATCC 29,521 exerts a beneficial effect on murine gut microbiota and redox homeostasis and could be a potential bioresource antioxidant in effective functional foods [224].
Notably, growing evidence indicates the efficacy of this novel therapeutic strategy for treating or alleviating NDs. A previous study indicated that probiotic mixtures consisting of S. thermophilus, L. plantarum, B. breve, and other probiotics in the treatment of early AD in transgenic mice could improve cognitive dysfunction and reduce brain damage by inhibiting Aβ plaque formation and altering gut microbiota [211]. Moreover, Bifidobacteria and Lactobacilli strains have been shown to produce vitamins and bioactive molecules as antioxidants, which play a major role in the pathology of PD [225]. Furthermore, it has been demonstrated that various species of Lactobacillus and Bifidibacterium are able to prevent the progression of the experimental autoimmune encephalomyelitis (EAE) animal model of MS and improve clinical symptoms [226,227,228]. Indeed, clinical trials indicate that Streptococcus spp., Lactobacillus spp., and Bifidobacterium spp. supplementation is able to reverse MS-induced variations in the gut microbiota composition in MS subjects [229]. The current methods of intestinal flora operation, including cecal fistula, have a broad application prospect. Notably, a previous study shows that 3D-printed cecal fistula implantation, which, through the body wall and into the cecum of rats to obtain long-term access to the gut microbiome, is an effective procedure that allows long-term and minimally invasive access to the gut microbiome [230]. Taken together, modification of the gut microbiota while maintaining redox homeostasis could be a promising therapeutic strategy for NDs in the future.
6. Conclusions and Perspectives
Overall, growing lines of evidence support the close link between redox homeostasis and the gut microbiota in the development of NDs. In this regard, probiotic supplements have a positive effect on redox imbalance and damaged gut microbiota, effectively exerting neuroprotective effects. Therefore, shaping the gut microbiota to maintain redox homeostasis appears to be a novel and effective therapeutic strategy for the treatment of NDs.
However, the gut microbiota is a vast and diverse reservoir of microorganisms, and more investigation is required to examine and characterize the role of the gut microbiota, as well as the interactions between oxidative stress and the microbiota–gut–brain axis. As high-throughput sequencing technologies continue to evolve, as well as multi-omics approaches to machine learning, they can help unravel the intricate networks of interactions involved. For instance, mass spectrometry-based metabolomics, single-cell RNA sequencing, and spatial transcriptomics should enable the identification of associated metabolic pathways and untangle the connections involved in the microbiota–gut–brain axis, contributing to the development of precision medicine. Bioinformatics tools will be essential, to mine the resulting data, enabling drugs to be designed that target the specific gut microbiota that mediate oxidative stress and the production of harmful metabolites, and exploring the neuroprotective and neurodegenerative roles of the gut microbial metabolites. Accordingly, further studies will be conducted to reveal how gut microbiota-mediated oxidative stress contributes to the prevalence of NDs and reveal novel therapeutic strategies to combat such conditions.
Author Contributions
Conceptualization, J.X., W.Z. and Y.W.; investigation, J.X. and W.Z.; writing—original draft preparation, Y.W.; writing—review and editing, Y.W., Z.Z., B.L., B.H., L.L. and E.C.N.; visualization, Y.W. and Z.Z.; supervision, J.X. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82130082, 52007087, 82102738, and 82103168), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21004 and ZYGD22007).
Acknowledgments
The authors acknowledge BioRender (www.biorender.com, accessed on 25 September 2022). Figures in this review were created with the BioRender platform.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Alzheimer’s disease |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| CD14 | Cluster of differentiation 14 |
| DA | Dopaminergic |
| ENS | Enteric nervous system |
| EAE | Experimental autoimmune encephalomyelitis |
| FTD | Frontotemporal dementia |
| GSH | Glutathione |
| GABA | γ-aminobutyric acid |
| H2 | Hydrogen |
| HD | Huntington’s disease |
| HPA | Hypothalamic–pituitary–adrenal |
| H2S | Hydrogen sulfide |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| KYN | Kynurenine |
| MS | Multiple sclerosis |
| MPTP | 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine |
| NDs | Neurodegenerative diseases |
| NO | Nitric oxide |
| NFTs | Neurofibrillary tangles |
| PA | Proanthocyanidins |
| PD | Parkinson’s disease |
| p-JNK | phosphorylation-c-Jun N-terminal kinase |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| SOD1 | Superoxide dismutase 1 |
| SCFAs | Short-chain fatty acids |
| SIRT1 | Sirtuin-1 |
| TLRs | Toll-like receptors |
| TH17 | T helper 17 |
| TUDCA | Taurine deoxycholic acid |
References
- Chen-Plotkin, A.S. Unbiased approaches to biomarker discovery in neurodegenerative diseases. Neuron 2014, 84, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kim, S.; Han, M.H.; Lee, S.B. Epigenetic Changes in Neurodegenerative Diseases. Mol. Cells 2016, 39, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative Diseases—Is Metabolic Deficiency the Root Cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, R.; Yang, Z.; Wen, Q.; Cao, X.; Zhao, N.; Yan, J. Protective Effects of Polysaccharides in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 917629. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxid. Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Siraki, A.G.; O’Brien, P.J. Prooxidant activity of free radicals derived from phenol-containing neurotransmitters. Toxicology 2002, 177, 81–90. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 2017, 168, 928–943.e911. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Alsegiani, A.S.; Shah, Z.A. The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regen. Res. 2022, 17, 2407–2412. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Soheili, M.; Alinaghipour, A.; Salami, M. Good bacteria, oxidative stress and neurological disorders: Possible therapeutical considerations. Life Sci. 2022, 301, 120605. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Aubry, C.; Cortes-Perez, N.G.; de Moreno de LeBlanc, A.; Vergnolle, N.; Langella, P.; Azevedo, V.; Chatel, J.M.; Miyoshi, A.; Bermúdez-Humarán, L.G. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: An update. FEMS Microbiol. Lett. 2013, 344, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Li, B.; Jiang, J.; Assaraf, Y.G.; Xiao, H.; Chen, Z.S.; Huang, C. Surmounting cancer drug resistance: New insights from the perspective of N(6)-methyladenosine RNA modification. Drug Resist. Updates 2020, 53, 100720. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Selivanov, V.A.; Votyakova, T.V.; Pivtoraiko, V.N.; Zeak, J.; Sukhomlin, T.; Trucco, M.; Roca, J.; Cascante, M. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput. Biol. 2011, 7, e1001115. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimer’s Dis. JAD 2001, 3, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.P.; Fagét, K.Y. Zinc takes the center stage: Its paradoxical role in Alzheimer’s disease. Brain Res. Brain Res. Rev. 2003, 41, 44–56. [Google Scholar] [CrossRef]
- Huang, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I.; Rogers, J.T. Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann. N. Y. Acad. Sci. 2004, 1012, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Mandemakers, W.; Morais, V.A.; De Strooper, B. A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J. Cell Sci. 2007, 120, 1707–1716. [Google Scholar] [CrossRef]
- Ren, X.; Butterfield, D.A. Fidelity of the PINK1 knockout rat to oxidative stress and other characteristics of Parkinson disease. Free Radic. Biol. Med. 2021, 163, 88–101. [Google Scholar] [CrossRef]
- Reijonen, S.; Putkonen, N.; Nørremølle, A.; Lindholm, D.; Korhonen, L. Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp. Cell Res. 2008, 314, 950–960. [Google Scholar] [CrossRef]
- Ayala-Peña, S. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington’s disease pathogenesis. Free Radic. Biol. Med. 2013, 62, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pelegrí, C.; Duran-Vilaregut, J.; del Valle, J.; Crespo-Biel, N.; Ferrer, I.; Pallàs, M.; Camins, A.; Vilaplana, J. Cell cycle activation in striatal neurons from Huntington’s disease patients and rats treated with 3-nitropropionic acid. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2008, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bai, Z.; Qin, X.; Cheng, Y. Aberrations in Oxidative Stress Markers in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 1712323. [Google Scholar] [CrossRef] [PubMed]
- Pansarasa, O.; Bordoni, M.; Diamanti, L.; Sproviero, D.; Gagliardi, S.; Cereda, C. SOD1 in Amyotrophic Lateral Sclerosis: “Ambivalent” Behavior Connected to the Disease. Int. J. Mol. Sci. 2018, 19, 1345. [Google Scholar] [CrossRef]
- Chen, H.Q.; Jin, Z.Y.; Wang, X.J.; Xu, X.M.; Deng, L.; Zhao, J.W. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci. Lett. 2008, 448, 175–179. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Conte, V.; Uryu, K.; Fujimoto, S.; Yao, Y.; Rokach, J.; Longhi, L.; Trojanowski, J.Q.; Lee, V.M.; McIntosh, T.K.; Praticò, D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J. Neurochem. 2004, 90, 758–764. [Google Scholar] [CrossRef]
- Cuadrado, A.; Moreno-Murciano, P.; Pedraza-Chaverri, J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert Opin. Ther. Targets 2009, 13, 319–329. [Google Scholar] [CrossRef]
- Nakashima, H.; Ishihara, T.; Yokota, O.; Terada, S.; Trojanowski, J.Q.; Lee, V.M.; Kuroda, S. Effects of alpha-tocopherol on an animal model of tauopathies. Free Radic. Biol. Med. 2004, 37, 176–186. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Kutscher, H.L.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.A.; Prasad, P.N.; Mahajan, S.D. Neuroprotective effects of a biodegradable poly(lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: A potential nanotherapy for Alzheimer’s disease? J. Drug Target. 2018, 26, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Morris, V.B.; Labhasetwar, V.; Ghorpade, A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis 2013, 4, e903. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release Off. J. Control. Release Soc. 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, Z.Y.; Sun, C.S.; Wang, C.Y.; Jiang, T.Y.; Wang, S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials 2010, 31, 908–915. [Google Scholar] [CrossRef]
- Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles. Free Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef]
- Kraehenbuhl, J.P.; Neutra, M.R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol. Rev. 1992, 72, 853–879. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Ho, Y.S.; Chang, R.C. Linking circadian rhythms to microbiome-gut-brain axis in aging-associated neurodegenerative diseases. Ageing Res. Rev. 2022, 78, 101620. [Google Scholar] [CrossRef]
- Zhuang, L.; Chen, H.; Zhang, S.; Zhuang, J.; Li, Q.; Feng, Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genom. Proteom. Bioinform. 2019, 17, 13–25. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid. Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Banerjee, N.; Liang, Y.; Du, X.; Boor, P.J.; Hoffman, K.L.; Khan, M.F. Aberrant Gut Microbiome Contributes to Intestinal Oxidative Stress, Barrier Dysfunction, Inflammation and Systemic Autoimmune Responses in MRL/lpr Mice. Front. Immunol. 2021, 12, 651191. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Cilia, R.; Piatti, M.; Cereda, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cassani, E.; Bonvegna, S.; Ferrarese, C.; Zecchinelli, A.L.; et al. Does Gut Microbiota Influence the Course of Parkinson’s Disease? A 3-Year Prospective Exploratory Study in de novo Patients. J. Park. Dis. 2021, 11, 159–170. [Google Scholar] [CrossRef]
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Mogna, L.; De Marchi, F.; Amoruso, A.; Pane, M.; Aloisio, I.; Cionci, N.B.; Gaggìa, F.; Lucenti, A.; Bersano, E.; et al. Potential Role of Gut Microbiota in ALS Pathogenesis and Possible Novel Therapeutic Strategies. J. Clin. Gastroenterol. 2018, 52, S68–S70. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Zhu, F.; Arnold, D.; Bar-Or, A.; Bernstein, C.N.; Bonner, C.; Forbes, J.D.; Graham, M.; Hart, J.; Knox, N.C.; et al. The gut microbiota in pediatric multiple sclerosis and demyelinating syndromes. Ann. Clin. Transl. Neurol. 2021, 8, 2252–2269. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef]
- Natale, G.; Ryskalin, L.; Morucci, G.; Lazzeri, G.; Frati, A.; Fornai, F. The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease. Life 2021, 11, 732. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259.e246. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Luck, B.; Engevik, M.A.; Ganesh, B.P.; Lackey, E.P.; Lin, T.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Sillitoe, R.V.; et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 2020, 10, 7737. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.C.; Cooper, E.M.; DiLorenzo, P.M.; O’Loughlin, L.J.; Konkel, M.E.; Peters, J.H.; Hajnal, A.; Sen, T.; Lee, S.H.; de La Serre, C.B.; et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. 2017, 77, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Cho, E.H.; Klitzman, B.; Nichols, S.P.; Wisniewski, N.A.; Villa, M.M.; Durand, H.K.; Jiang, S.; Midani, F.S.; Nimmagadda, S.N.; et al. Antibiotic-induced changes in the microbiota disrupt redox dynamics in the gut. eLife 2018, 7, e35987. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Plotnikov, E.Y.; Silachev, D.N.; Zorova, L.D.; Pevzner, I.B.; Zorov, S.D.; Babenko, V.A.; Jankauskas, S.S.; Popkov, V.A.; Savina, P.S. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochemistry. Biokhimiia 2014, 79, 1017–1031. [Google Scholar] [CrossRef]
- Migeotte, I.; Communi, D.; Parmentier, M. Formyl peptide receptors: A promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006, 17, 501–519. [Google Scholar] [CrossRef]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar] [CrossRef]
- Leschelle, X.; Goubern, M.; Andriamihaja, M.; Blottière, H.M.; Couplan, E.; Gonzalez-Barroso, M.D.; Petit, C.; Pagniez, A.; Chaumontet, C.; Mignotte, B.; et al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim. Et Biophys. Acta 2005, 1725, 201–212. [Google Scholar] [CrossRef]
- Beaumont, M.; Andriamihaja, M.; Lan, A.; Khodorova, N.; Audebert, M.; Blouin, J.M.; Grauso, M.; Lancha, L.; Benetti, P.H.; Benamouzig, R.; et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic. Biol. Med. 2016, 93, 155–164. [Google Scholar] [CrossRef]
- Donertas Ayaz, B.; Zubcevic, J. Gut microbiota and neuroinflammation in pathogenesis of hypertension: A potential role for hydrogen sulfide. Pharm. Res 2020, 153, 104677. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Outeiro, T.F.; Koss, D.J.; Erskine, D.; Walker, L.; Kurzawa-Akanbi, M.; Burn, D.; Donaghy, P.; Morris, C.; Taylor, J.P.; Thomas, A.; et al. Dementia with Lewy bodies: An update and outlook. Mol. Neurodegener. 2019, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Barthélemy, N.R.; Mawuenyega, K.G.; Patterson, B.W.; Gordon, B.A.; Jockel-Balsarotti, J.; Sullivan, M.; Crisp, M.J.; Kasten, T.; Kirmess, K.M.; et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 2018, 97, 1284–1298.e1287. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.R. Linking Oxidative Stress and Proteinopathy in Alzheimer’s Disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Potapova, E.V.; Dremin, V.V.; Dunaev, A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life 2020, 10, 101. [Google Scholar] [CrossRef]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimer’s Dis. JAD 2010, 20 (Suppl. S2), S357–S367. [Google Scholar] [CrossRef]
- Hong, W.K.; Han, E.H.; Kim, D.G.; Ahn, J.Y.; Park, J.S.; Han, B.G. Amyloid-beta-peptide reduces the expression level of mitochondrial cytochrome oxidase subunits. Neurochem. Res. 2007, 32, 1483–1488. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Pan, H.; Zheng, W.; Feng, C.; Wang, Y.; Deng, Z.; Wang, L.; Luo, J.; Chen, S. Lost region in amyloid precursor protein (APP) through TALEN-mediated genome editing alters mitochondrial morphology. Sci. Rep. 2016, 6, 22244. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 491, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hadidi Zavareh, A.H.; Haji Khani, R.; Pakpour, B.; Soheili, M.; Salami, M. Probiotic treatment differentially affects the behavioral and electrophysiological aspects in ethanol exposed animals. Iran. J. Basic Med. Sci. 2020, 23, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Di Mauro, M.; Di Mauro, M.; Luca, A. Gut Microbiota in Alzheimer’s Disease, Depression, and Type 2 Diabetes Mellitus: The Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 4730539. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.T.; Hsieh, Y.T.; Wang, S.Y.; Chen, M.J. Improving effect of a probiotic mixture on memory and learning abilities in d-galactose-treated aging mice. J. Dairy Sci. 2019, 102, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients With Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Yin, Y.N.; Yu, Q.F.; Fu, N.; Liu, X.W.; Lu, F.G. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J. Gastroenterol. 2010, 16, 3394–3401. [Google Scholar] [CrossRef]
- Bo, T.B.; Wen, J.; Zhao, Y.C.; Tian, S.J.; Zhang, X.Y.; Wang, D.H. Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food. J. Steroid Biochem. Mol. Biol. 2020, 198, 105602. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Brandner, S. Invited Review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2020, 46, 522–545. [Google Scholar] [CrossRef]
- Morales, R.; Moreno-Gonzalez, I.; Soto, C. Cross-seeding of misfolded proteins: Implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog. 2013, 9, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Diamond, M.I. Prion-like mechanisms in neurodegenerative diseases. Nat. Rev. Neurosci. 2010, 11, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Ferrarin, M.; Carpinella, I.; Rabuffetti, M.; Calabrese, E.; Mazzoleni, P.; Nemni, R. Locomotor disorders in patients at early stages of Parkinson’s disease: A quantitative analysis. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 1224–1227. [Google Scholar] [CrossRef]
- Lu, G.; Wang, Y.; Shi, Y.; Zhang, Z.; Huang, C.; He, W.; Wang, C.; Shen, H.M. Autophagy in health and disease: From molecular mechanisms to therapeutic target. MedComm 2022, 3, e150. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, A.; Crook, N.; Gasparrini, A.J.; Dantas, G. Multiscale Evolutionary Dynamics of Host-Associated Microbiomes. Cell 2018, 172, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef]
- Fyfe, I. Movement disorders: Comparison of cognitive impairment in Parkinson disease and essential tremor. Nat. Rev. Neurol. 2017, 13, 260. [Google Scholar] [CrossRef]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R.; et al. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007, 150, 963–976. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Menozzi, E.; Macnaughtan, J.; Schapira, A.H.V. The gut-brain axis and Parkinson disease: Clinical and pathogenetic relevance. Ann. Med. 2021, 53, 611–625. [Google Scholar] [CrossRef]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 1996, 47, S161–S170. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, X.; Meng, X.; Lu, L.; Mai, D.; Qu, S. Simvastatin Inhibits Activation of NADPH Oxidase/p38 MAPK Pathway and Enhances Expression of Antioxidant Protein in Parkinson Disease Models. Front. Mol. Neurosci. 2018, 11, 165. [Google Scholar] [CrossRef]
- Han, C.; Shen, H.; Yang, Y.; Sheng, Y.; Wang, J.; Li, W.; Zhou, X.; Guo, L.; Zhai, L.; Guan, Q. Antrodia camphorata polysaccharide resists 6-OHDA-induced dopaminergic neuronal damage by inhibiting ROS-NLRP3 activation. Brain Behav. 2020, 10, e01824. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Clin. Neurosci. 1998, 5, 136–146. [Google Scholar] [PubMed]
- Lubomski, M.; Davis, R.L.; Sue, C.M. The gut microbiota: A novel therapeutic target in Parkinson’s disease? Park. Relat. Disord. 2019, 66, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.Y.; Diwakarla, S.; Pustovit, R.V.; McQuade, R.M.; Di Natale, M.; Ermine, C.M.; Parish, C.L.; Finkelstein, D.I.; Furness, J.B. Investigation of nerve pathways mediating colorectal dysfunction in Parkinson’s disease model produced by lesion of nigrostriatal dopaminergic neurons. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2020, 32, e13893. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Dodiya, H.B.; Engen, P.A.; Forsyth, C.B.; Huschens, A.M.; Shaikh, M.; Voigt, R.M.; Naqib, A.; Green, S.J.; Kordower, J.H.; et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut 2019, 68, 829–843. [Google Scholar] [CrossRef]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Grassi, D.; Howard, S.; Zhou, M.; Diaz-Perez, N.; Urban, N.T.; Guerrero-Given, D.; Kamasawa, N.; Volpicelli-Daley, L.A.; LoGrasso, P.; Lasmézas, C.I. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E2634–E2643. [Google Scholar] [CrossRef]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W.; et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 2020, 9, e53111. [Google Scholar] [CrossRef]
- Yang, X.; Qian, Y.; Xu, S.; Song, Y.; Xiao, Q. Longitudinal Analysis of Fecal Microbiome and Pathologic Processes in a Rotenone Induced Mice Model of Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 441. [Google Scholar] [CrossRef]
- Ostojic, S.M. Inadequate Production of H(2) by Gut Microbiota and Parkinson Disease. Trends Endocrinol. Metab. TEM 2018, 29, 286–288. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Derfuss, T.; Linington, C.; Hohlfeld, R.; Meinl, E. Axo-glial antigens as targets in multiple sclerosis: Implications for axonal and grey matter injury. J. Mol. Med. 2010, 88, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Greer, J.M. Immunology of multiple sclerosis. Curr. Allergy Asthma Rep. 2007, 7, 285–292. [Google Scholar] [CrossRef]
- Tommasin, S.; Giannì, C.; De Giglio, L.; Pantano, P. Neuroimaging Techniques to Assess Inflammation in Multiple Sclerosis. Neuroscience 2019, 403, 4–16. [Google Scholar] [CrossRef]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Kynurenines in the Pathogenesis of Multiple Sclerosis: Therapeutic Perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Tobore, T.O. Oxidative/Nitroxidative Stress and Multiple Sclerosis. J. Mol. Neurosci. MN 2021, 71, 506–514. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The Gut Microbiota in Multiple Sclerosis: An Overview of Clinical Trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal T(H)17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Amato, M.P.; Derfuss, T.; Hemmer, B.; Liblau, R.; Montalban, X.; Soelberg Sørensen, P.; Miller, D.H. Environmental modifiable risk factors for multiple sclerosis: Report from the 2016 ECTRIMS focused workshop. Mult. Scler. 2018, 24, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R.; Larocca, M.; Trotta, V.; Mennella, I.; Vitaglione, P.; Ettorre, M.; Graverini, A.; De Santis, A.; Di Monte, E.; et al. Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: A pilot study. Exp. Biol. Med. 2016, 241, 620–635. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.K.Y. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem. Neurosci. 2017, 8, 1438–1447. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection-A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Sola, S.; Nan, Z.; Castro, R.E.; Ribeiro, P.S.; Low, W.C.; Steer, C.J. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc. Natl. Acad. Sci. USA 2003, 100, 6087–6092. [Google Scholar] [CrossRef]
- Parry, G.J.; Rodrigues, C.M.; Aranha, M.M.; Hilbert, S.J.; Davey, C.; Kelkar, P.; Low, W.C.; Steer, C.J. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 2010, 33, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Burks, S.; Raymick, J.; Robinson, B.; Gómez-Crisóstomo, N.P.; Escudero-Lourdes, C.; Lopez, A.G.G.; Chigurupati, S.; Hanig, J.; Ferguson, S.A.; et al. Tauroursodeoxycholic acid (TUDCA) is neuroprotective in a chronic mouse model of Parkinson’s disease. Nutr. Neurosci. 2022, 25, 1374–1391. [Google Scholar] [CrossRef] [PubMed]
- West, R.J.H.; Ugbode, C.; Fort-Aznar, L.; Sweeney, S.T. Neuroprotective activity of ursodeoxycholic acid in CHMP2B(Intron5) models of frontotemporal dementia. Neurobiol. Dis. 2020, 144, 105047. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review on Bile Acids: Effects of the Gut Microbiome, Interactions with Dietary Fiber, and Alterations in the Bioaccessibility of Bioactive Compounds. J. Agric. Food Chem. 2019, 67, 9124–9138. [Google Scholar] [CrossRef] [PubMed]
- Nho, K.; Kueider-Paisley, A.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; Kastenmüller, G.; et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Hertel, J.; Harms, A.C.; Heinken, A.; Baldini, F.; Thinnes, C.C.; Glaab, E.; Vasco, D.A.; Pietzner, M.; Stewart, I.D.; Wareham, N.J.; et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep. 2019, 29, 1767–1777.e1768. [Google Scholar] [CrossRef]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Investig. 2020, 130, 3467–3482. [Google Scholar] [CrossRef]
- Yanguas-Casás, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramírez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J. Cell. Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef]
- Diotel, N.; Charlier, T.D.; Lefebvre d’Hellencourt, C.; Couret, D.; Trudeau, V.L.; Nicolau, J.C.; Meilhac, O.; Kah, O.; Pellegrini, E. Steroid Transport, Local Synthesis, and Signaling within the Brain: Roles in Neurogenesis, Neuroprotection, and Sexual Behaviors. Front. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef]
- Gloux, K.; Berteau, O.; El Oumami, H.; Béguet, F.; Leclerc, M.; Doré, J. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4539–4546. [Google Scholar] [CrossRef]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Rankin, K.A.; Mei, F.; Kim, K.; Shen, Y.A.; Mayoral, S.R.; Desponts, C.; Lorrain, D.S.; Green, A.J.; Baranzini, S.E.; Chan, J.R.; et al. Selective Estrogen Receptor Modulators Enhance CNS Remyelination Independent of Estrogen Receptors. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef]
- Fontana, A.; Panebianco, C.; Picchianti-Diamanti, A.; Laganà, B.; Cavalieri, D.; Potenza, A.; Pracella, R.; Binda, E.; Copetti, M.; Pazienza, V. Gut Microbiota Profiles Differ among Individuals Depending on Their Region of Origin: An Italian Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 4065. [Google Scholar] [CrossRef]
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B.M.; Hamase, K.; Waldor, M.K. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 2016, 1, 16125. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Negoro, R.; Kadoi, H.; Motegi, T.; Shibagaki, F.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Noradrenaline protects neurons against H(2) O(2) -induced death by increasing the supply of glutathione from astrocytes via β(3) -adrenoceptor stimulation. J. Neurosci. Res. 2021, 99, 621–637. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front. Neurosci. 2018, 12, 216. [Google Scholar] [CrossRef]
- Yanovsky, I.; Finkin-Groner, E.; Zaikin, A.; Lerman, L.; Shalom, H.; Zeeli, S.; Weill, T.; Ginsburg, I.; Nudelman, A.; Weinstock, M. Carbamate derivatives of indolines as cholinesterase inhibitors and antioxidants for the treatment of Alzheimer’s disease. J. Med. Chem. 2012, 55, 10700–10715. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, N.; Fujiwara, S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids 2020, 52, 181–197. [Google Scholar] [CrossRef]
- Barua, S.; Kim, J.Y.; Kim, J.Y.; Kim, J.H.; Lee, J.E. Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors. Neurochem. Res. 2019, 44, 735–750. [Google Scholar] [CrossRef]
- Chai, J.; Luo, L.; Hou, F.; Fan, X.; Yu, J.; Ma, W.; Tang, W.; Yang, X.; Zhu, J.; Kang, W.; et al. Agmatine Reduces Lipopolysaccharide-Mediated Oxidant Response via Activating PI3K/Akt Pathway and Up-Regulating Nrf2 and HO-1 Expression in Macrophages. PLoS ONE 2016, 11, e0163634. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Hong, S.; Park, Y.M.; Lee, W.T.; Park, K.A.; Lee, J.E. Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res. 2011, 1373, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef]
- Yang, T.; Rodriguez, V.; Malphurs, W.L.; Schmidt, J.T.; Ahmari, N.; Sumners, C.; Martyniuk, C.J.; Zubcevic, J. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol. Rep. 2018, 6, e13732. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, F.; Zhang, S.; Xin, R.; Sun, Y. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Park. Dis. 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef]
- Ma, J.; Gao, S.S.; Yang, H.J.; Wang, M.; Cheng, B.F.; Feng, Z.W.; Wang, L. Neuroprotective Effects of Proanthocyanidins, Natural Flavonoids Derived From Plants, on Rotenone-Induced Oxidative Stress and Apoptotic Cell Death in Human Neuroblastoma SH-SY5Y Cells. Front. Neurosci. 2018, 12, 369. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Hill, M.J. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 1997, 6 (Suppl. S1), S43–S45. [Google Scholar] [CrossRef]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol. Nutr. Food Res. 2020, 64, e2000426. [Google Scholar] [CrossRef]
- Yu, Y.X.; Li, Y.P.; Gao, F.; Hu, Q.S.; Zhang, Y.; Chen, D.; Wang, G.H. Vitamin K2 suppresses rotenone-induced microglial activation in vitro. Acta Pharmacol. Sin. 2016, 37, 1178–1189. [Google Scholar] [CrossRef]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, F.; Bonnassie, S.; Rabah, H.; Marchand, P.; Blanc, P.; Jeantet, R.; Jan, G. Review: Adaptation of Beneficial Propionibacteria, Lactobacilli, and Bifidobacteria Improves Tolerance Toward Technological and Digestive Stresses. Front. Microbiol. 2019, 10, 841. [Google Scholar] [CrossRef]
- Verna, E.C.; Lucak, S. Use of probiotics in gastrointestinal disorders: What to recommend? Ther. Adv. Gastroenterol. 2010, 3, 307–319. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Masry, S.S.; El-Dougdoug, N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019, 10, 337–350. [Google Scholar] [CrossRef]
- Hütt, P.; Shchepetova, J.; Lõivukene, K.; Kullisaar, T.; Mikelsaar, M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J. Appl. Microbiol. 2006, 100, 1324–1332. [Google Scholar] [CrossRef]
- Partrick, K.A.; Rosenhauer, A.M.; Auger, J.; Arnold, A.R.; Ronczkowski, N.M.; Jackson, L.M.; Lord, M.N.; Abdulla, S.M.; Chassaing, B.; Huhman, K.L. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 2021, 11, 3763. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef]
- Arora, K.; Green, M.; Prakash, S. The Microbiome and Alzheimer’s Disease: Potential and Limitations of Prebiotic, Synbiotic, and Probiotic Formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Poluektova, E.; Yunes, R.; Danilenko, V. The Putative Antidepressant Mechanisms of Probiotic Bacteria: Relevant Genes and Proteins. Nutrients 2021, 13, 1591. [Google Scholar] [CrossRef]
- de Moreno de Leblanc, A.; Del Carmen, S.; Zurita-Turk, M.; Santos Rocha, C.; van de Guchte, M.; Azevedo, V.; Miyoshi, A.; Leblanc, J.G. Importance of IL-10 modulation by probiotic microorganisms in gastrointestinal inflammatory diseases. ISRN Gastroenterol. 2011, 2011, 892971. [Google Scholar] [CrossRef] [PubMed]
- Ayyanna, R.; Ankaiah, D.; Arul, V. Anti-inflammatory and Antioxidant Properties of Probiotic Bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar Albino Rats. Front. Microbiol. 2018, 9, 3063. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Tan, F.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus fermentum CQPC07 attenuates obesity, inflammation and dyslipidemia by modulating the antioxidant capacity and lipid metabolism in high-fat diet induced obese mice. J. Inflamm. 2021, 18, 5. [Google Scholar] [CrossRef]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef]
- Julien, C.; Tremblay, C.; Emond, V.; Lebbadi, M.; Salem, N., Jr.; Bennett, D.A.; Calon, F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009, 68, 48–58. [Google Scholar] [CrossRef]
- Khalili, L.; Alipour, B.; Asghari Jafarabadi, M.; Hassanalilou, T.; Mesgari Abbasi, M.; Faraji, I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: A randomized controlled trial. Diabetol. Metab. Syndr. 2019, 11, 5. [Google Scholar] [CrossRef]
- Corpuz, H.M.; Ichikawa, S.; Arimura, M.; Mihara, T.; Kumagai, T.; Mitani, T.; Nakamura, S.; Katayama, S. Long-Term Diet Supplementation with Lactobacillus paracasei K71 Prevents Age-Related Cognitive Decline in Senescence-Accelerated Mouse Prone 8. Nutrients 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Chen, L.H.; Wang, M.F.; Hsu, C.C.; Chan, C.H.; Li, J.X.; Huang, H.Y. Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice. Nutrients 2018, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Xu, H.B.; Xu, F.; Zeng, Z.L.; Wei, H. Efficacy of oral Bifidobacterium bifidum ATCC 29521 on microflora and antioxidant in mice. Can. J. Microbiol. 2016, 62, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative activity of probiotics. Arch. Med. Sci. AMS 2021, 17, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.E.; Nam, J.H.; Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ezendam, J.; de Klerk, A.; Gremmer, E.R.; van Loveren, H. Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clin. Exp. Immunol. 2008, 154, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Minaya, D.M.; Weinstein, N.L.; Czaja, K. Development of a 3D-Printed High Temperature Resin Cecal Fistula Implant for Long-Term and Minimally Invasive Access to the Gut Microbiome. Nutrients 2021, 13, 4515. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).