Structural Effects on the Antioxidant Properties of Amino Acid Betaxanthins
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Obtaining of Betalamic Acid
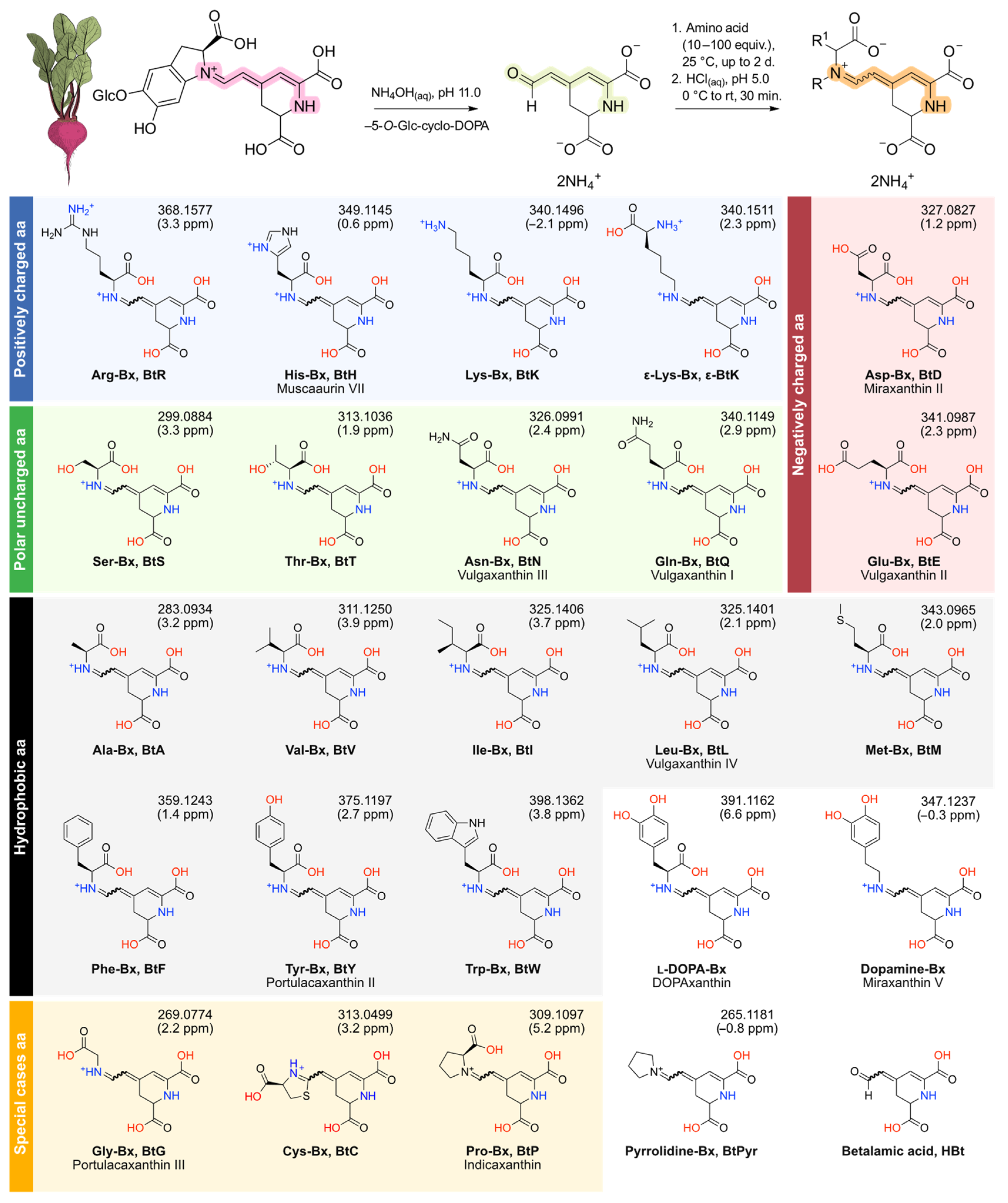
2.3. Semisynthesis of Betaxanthins
2.4. High-Performance Liquid Chromatography Coupled to Mass Spectrometry
2.5. Nuclear Magnetic Resonance Spectroscopy
2.6. Trolox Equivalent Antioxidant Capacity
2.6.1. ABTS Assay
2.6.2. FRAP Method
2.7. Cyclic Voltammetry
2.8. Computational Methods
3. Results
3.1. Semisynthesis of Betaxanthins
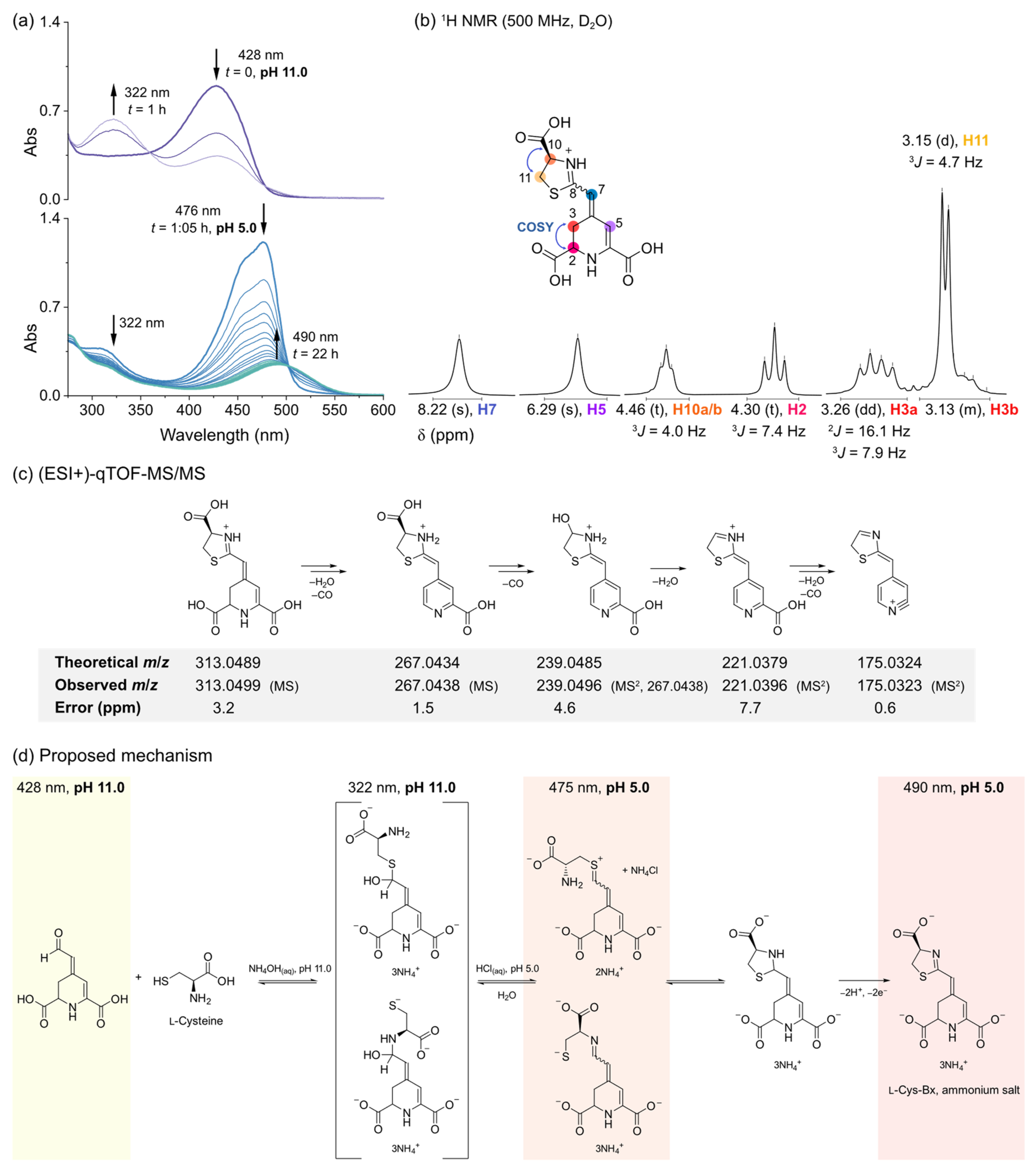
3.1.1. The Elusive Betaxanthin of l-Cysteine
3.1.2. The Two Betaxanthins of l-Lysine
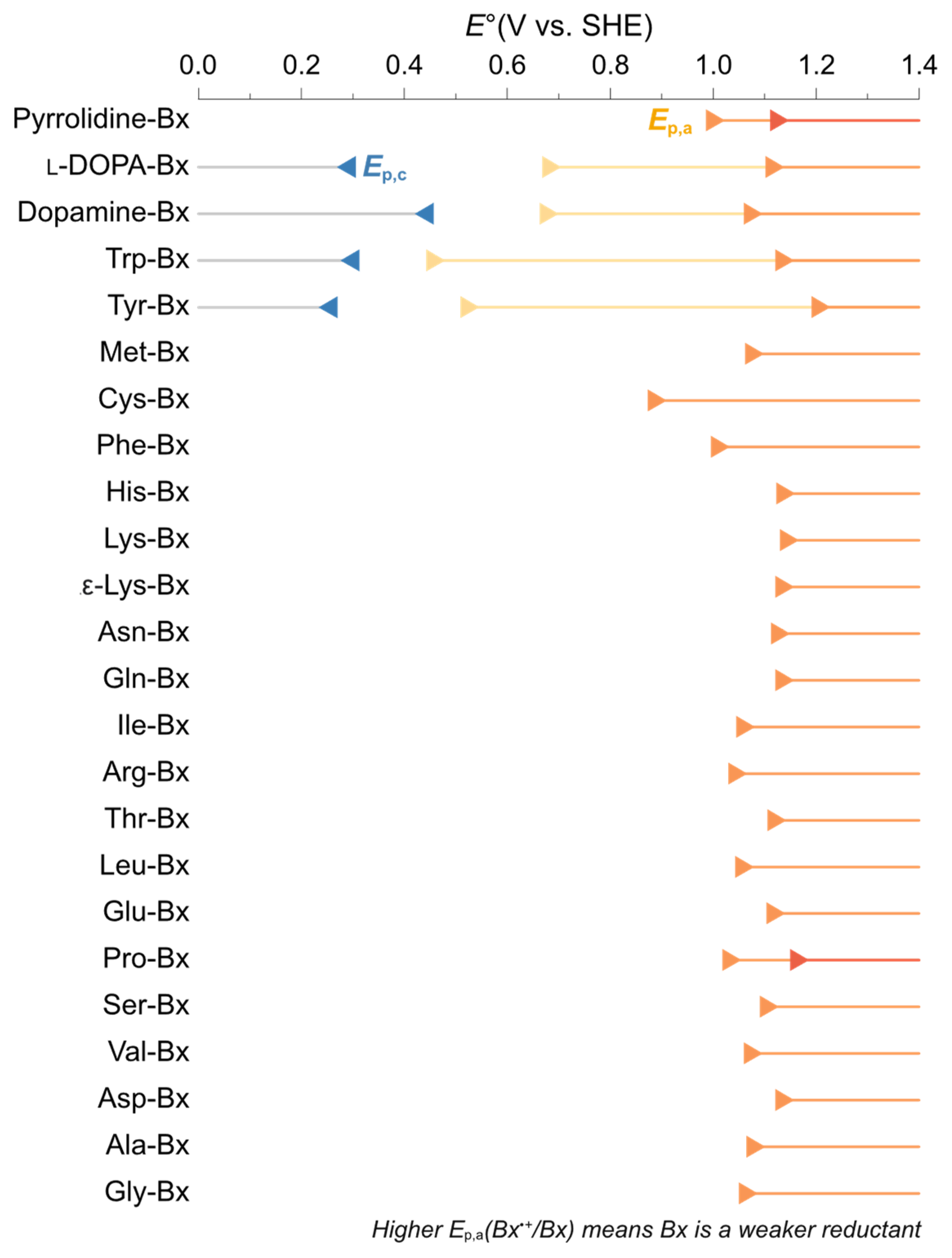
3.2. Antioxidant Capacity and Redox Potentials of Amino Acid Betaxanthins
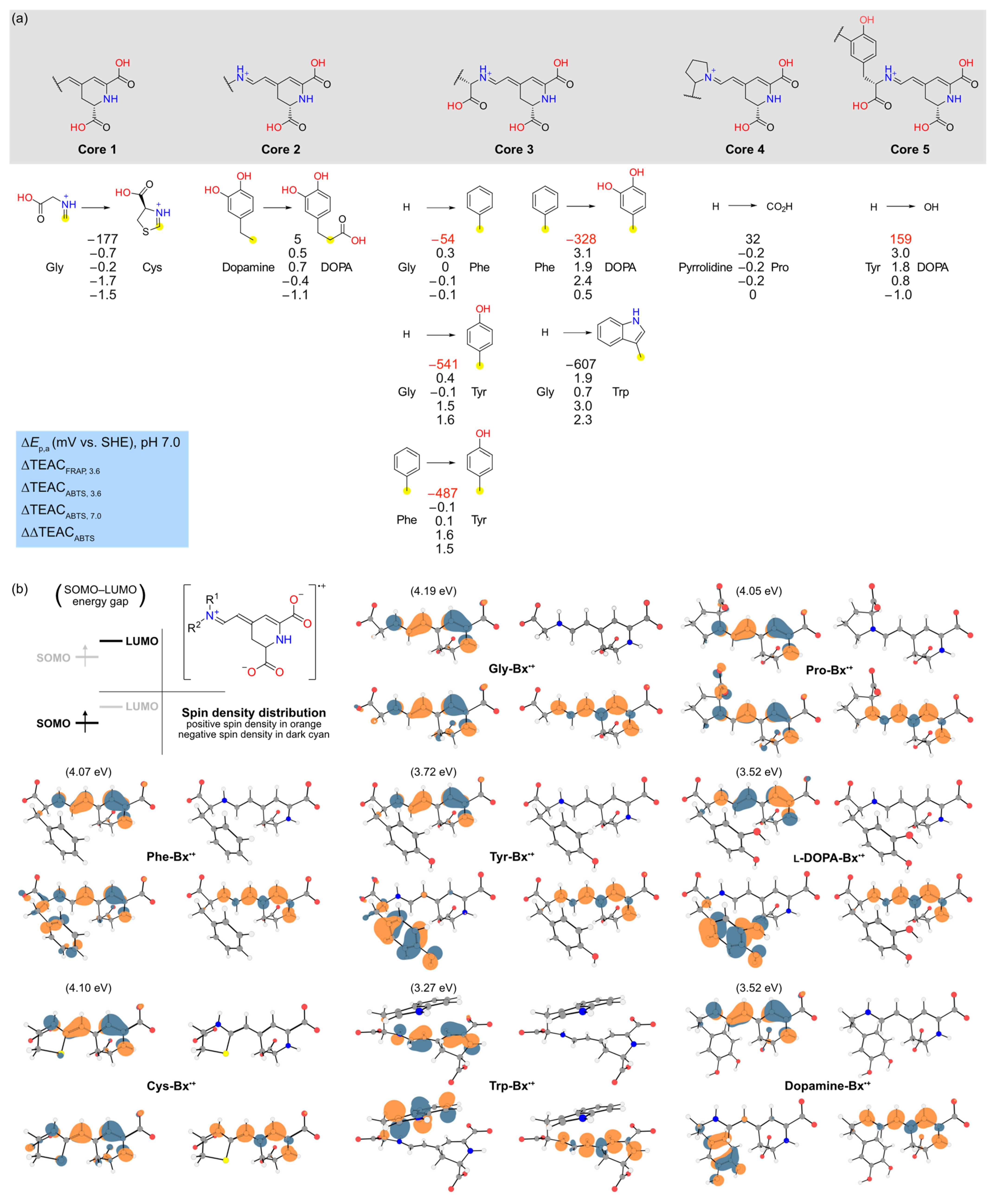
3.3. Insight on the Structural Features of Betaxanthins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maggert, K.A. Stress: An evolutionary mutagen. Proc. Natl. Acad. Sci. USA 2019, 116, 17616–17618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granold, M.; Hajieva, P.; Tosa, M.I.; Irimie, F.D.; Moosmann, B. Modern diversification of the amino acid repertoire driven by oxygen. Proc. Natl. Acad. Sci. USA 2018, 115, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, K.M.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Functions of flavonoid and betalain pigments in abiotic stress tolerance in plants. In Annual Plant Reviews Online; Roberts, J.A., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2018; Volume 1, pp. 21–62. [Google Scholar]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Lichman, B.R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 2021, 38, 103–129. [Google Scholar] [CrossRef]
- Nadot, S.; Carrive, L. The colourful life of flowers. Bot. Lett. 2020, 168, 120–130. [Google Scholar] [CrossRef]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Guo, R.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Lodge, D.J.; Padamsee, M.; Matheny, P.B.; Aime, M.C.; Cantrell, S.A.; Boertmann, D.; Kovalenko, A.; Vizzini, A.; Dentinger, B.T.M.; Kirk, P.M.; et al. Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Divers. 2014, 64, 1–99. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, F.; Schliemann, W. Pigments of fly agaric (Amanita muscaria). Z Naturforsch. C J. Biosci. 2007, 62, 779–785. [Google Scholar] [CrossRef]
- Gill, M. Pigments of fungi (Macromycetes). Nat. Prod. Rep. 1994, 11, 67–90. [Google Scholar] [CrossRef]
- Contreras-Llano, L.E.; Guerrero-Rubio, M.A.; Lozada-Ramirez, J.D.; Garcia-Carmona, F.; Gandia-Herrero, F. First Betalain-Producing Bacteria Break the Exclusive Presence of the Pigments in the Plant Kingdom. mBio 2019, 10, e00345-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Bastos, E.L.; Schliemann, W. Betalains as Antioxidants. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–44. [Google Scholar]
- Kusznierewicz, B.; Mroz, M.; Koss-Mikolajczyk, I.; Namiesnik, J. Comparative evaluation of different methods for determining phytochemicals and antioxidant activity in products containing betalains—Verification of beetroot samples. Food Chem. 2021, 362, 130132. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globosa L. and Bougainvillea sp. by HPLC-DAD-ESI-MSn. Anal. Bioanal. Chem. 2007, 387, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Stintzing, F.C.; Carle, R. Identification of betalains from petioles of differently colored Swiss chard (Beta vulgaris L. ssp cicla L. Alef. Cv. Bright lights) by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2004, 52, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef]
- Schliemann, W.; Cai, Y.; Degenkolb, T.; Schmidt, J.; Corke, H. Betalains of Celosia argentea. Phytochemistry 2001, 58, 159–165. [Google Scholar] [CrossRef]
- Hempel, J.; Bohm, H. Betaxanthin pattern of hairy roots from Beta vulgaris var lutea and its alteration by feeding of amino acids. Phytochemistry 1997, 44, 847–852. [Google Scholar] [CrossRef]
- Trezzini, G.F.; Zryd, J.P. Characterization of some natural and semi-synthetic betaxanthins. Phytochemistry 1991, 30, 1901–1903. [Google Scholar] [CrossRef]
- Musso, H. The pigments of fly agaric, Amanita muscaria. Tetrahedron 1979, 35, 2843–2853. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Piattelli, M.; Sciuto, S. A new betaxanthin from Glottiphyllum longum. Phytochemistry 1973, 12, 2293–2294. [Google Scholar] [CrossRef]
- Piattelli, M.; Minale, L.; Nicolaus, R.A. Pigments of centrospermae-V. Betaxanthins from Mirabilis jalapa L. Phytochemistry 1965, 4, 817–823. [Google Scholar] [CrossRef]
- Piattelli, M.; Minale, L.; Prota, G. Pigments of centrospermae-III. Betaxanthins from Beta vulgaris L. Phytochemistry 1965, 4, 121–125. [Google Scholar] [CrossRef]
- Schliemann, W.; Kobayashi, N.; Strack, D. The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiol. 1999, 119, 1217–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preczenhak, A.P.; Orsi, B.; Lima, G.P.P.; Tezotto-Uliana, J.V.; Minatel, I.O.; Kluge, R.A. Cysteine enhances the content of betalains and polyphenols in fresh-cut red beet. Food Chem. 2019, 286, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.M.M.; Goncalves, L.C.P.; Machado, C.O.; Esteves, L.C.; Stevani, C.V.; Oliveira, C.C.; Dorr, F.A.; Pinto, E.; Adachi, F.M.M.; Hotta, C.T.; et al. Reannotation of Fly Amanita l-DOPA Dioxygenase Gene Enables Its Cloning and Heterologous Expression. ACS Omega 2022, 7, 16070–16079. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Rubio, M.A.; Escribano, J.; Garcia-Carmona, F.; Gandia-Herrero, F. Light Emission in Betalains: From Fluorescent Flowers to Biotechnological Applications. Trends Plant Sci. 2020, 25, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Polturak, G.; Aharoni, A. Advances and future directions in betalain metabolic engineering. New Phytol. 2019, 224, 1472–1478. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, H.; Ozeki, Y.; Sasaki, N. In Vitro Synthesis of Betaxanthins Using Recombinant DOPA 4,5-Dioxygenase and Evaluation of Their Radical-Scavenging Activities. J. Agric. Food Chem. 2010, 58, 12504–12509. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, X.; Li, S.; Zhang, X.; Yu, S.; Zhao, G.R. Metabolic Engineering of Escherichia coli for de Novo Production of Betaxanthins. J. Agric. Food Chem. 2020, 68, 8370–8380. [Google Scholar] [CrossRef]
- Cabanes, J.; Gandía-Herrero, F.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Fluorescent bioinspired protein labeling with betalamic acid. Derivatization and characterization of novel protein-betaxanthins. Dye. Pigment 2016, 133, 458–466. [Google Scholar] [CrossRef]
- Kugler, F.; Graneis, S.; Stintzing, F.C.; Carle, R. Studies on betaxanthin profiles of vegetables and fruits from the Chenopodiaceae and Cactaceae. Z. Naturforsch. C J. Biosci. 2007, 62, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Rubio, M.A.; López-Llorca, R.; Henarejos-Escudero, P.; García-Carmona, F.; Gandía-Herrero, F. Scaled-up biotechnological production of individual betalains in a microbial system. Microb. Biotechnol. 2019, 12, 993–1002. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Rubio, M.A.; Hernandez-Garcia, S.; Escribano, J.; Jimenez-Atienzar, M.; Cabanes, J.; Garcia-Carmona, F.; Gandia-Herrero, F. Betalain health-promoting effects after ingestion in Caenorhabditis elegans are mediated by DAF-16/FOXO and SKN-1/Nrf2 transcription factors. Food Chem. 2020, 330, 127228. [Google Scholar] [CrossRef]
- Pioli, R.M.; Mattioli, R.R.; Esteves, L.C.; Dochev, S.; Bastos, E.L. Comparison of the effect of N-methyl and N-aryl groups on the hydrolytic stability and electronic properties of betalain dyes. Dye. Pigment. 2020, 183, 108609. [Google Scholar] [CrossRef]
- Girod, P.A.; Zryd, J.P. Secondary metabolism in cultured red beet (Beta vulgaris L.) cells: Differential regulation of betaxanthin and betacyanin biosynthesis. Plant Cell Tissue Organ Cult. 1991, 25, 1–12. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Fernandes, D.L.A.; Paun, C.; Pavliuk, M.V.; Fernandes, A.B.; Bastos, E.L.; Sa, J. Green microfluidic synthesis of monodisperse silver nanoparticles via genetic algorithm optimization. RSC Adv. 2016, 6, 95693–95697. [Google Scholar] [CrossRef]
- Hussain, J.; Rea, C. Computationally efficient algorithm to identify matched molecular pairs (MMPs) in large data sets. J. Chem. Inf. Model. 2010, 50, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Kugler, F.; Carle, R.; Conrad, J. First C-13-NMR assignments of betaxanthins. Helvetica Chim. Acta 2006, 89, 1008–1016. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Conrad, J.; Klaiber, I.; Beifuss, U.; Carle, R. Structural investigations on betacyanin pigments by LC NMR and 2D NMR spectroscopy. Phytochemistry 2004, 65, 415–422. [Google Scholar] [CrossRef]
- Kumorkiewicz-Jamro, A.; Wiergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S.A. Multi-colored shades of betalains: Recent advances in betacyanin chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 2010, 232, 449–460. [Google Scholar] [CrossRef]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. The Role of Phenolic Hydroxy Groups in the Free Radical Scavenging Activity of Betalains. J. Nat. Prod. 2009, 72, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2017, 95, 197–206. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Medda, L.; Salis, A.; Magner, E. Specific ion effects on the electrochemical properties of cytochrome c. Phys. Chem. Chem. Phys. 2012, 14, 2875–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brotzel, F.; Mayr, H. Nucleophilicities of amino acids and peptides. Org. Biomol. Chem. 2007, 5, 3814–3820. [Google Scholar] [CrossRef]
- Brotzel, F.; Chu, Y.C.; Mayr, H. Nucleophilicities of Primary and Secondary Amines in Water. J. Org. Chem. 2007, 72, 3679–3688. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Fazzi, R.B.; Esteves, L.C.; Machado, C.O.; Dorr, F.A.; Pinto, E.; Hattori, Y.; Sa, J.; da Costa Ferreira, A.M.; Bastos, E.L. A bioinspired nitrone precursor to a stabilized nitroxide radical. Free Radic. Biol. Med. 2021, 168, 110–116. [Google Scholar] [CrossRef]
- Freitas-Dorr, B.C.; Machado, C.O.; Pinheiro, A.C.; Fernandes, A.B.; Dorr, F.A.; Pinto, E.; Lopes-Ferreira, M.; Abdellah, M.; Sa, J.; Russo, L.C.; et al. A metal-free blue chromophore derived from plant pigments. Sci. Adv. 2020, 6, eaaz0421. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.C.B.; Mariz, I.D.A.; Macoas, E.M.S.; Tonelli, R.R.; Martinho, J.M.G.; Quina, F.H.; Bastos, E.L. Bioinspired water-soluble two-photon fluorophores. Dye. Pigment. 2018, 150, 105–111. [Google Scholar] [CrossRef]
- Esteves, L.C.; Pinheiro, A.C.; Pioli, R.M.; Penna, T.C.; Baader, W.J.; Correra, T.C.; Bastos, E.L. Revisiting the Mechanism of Hydrolysis of Betanin. Photochem. Photobiol. 2018, 94, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Guimond, N.; Mayer, P.; Trauner, D. Development of an Iron(II)-Catalyzed Aerobic Catechol Cleavage and Biomimetic Synthesis of Betanidin. Chem. Eur. J. 2014, 20, 9519–9523. [Google Scholar] [CrossRef] [PubMed]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation: Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Johnson, J.E.; Morales, N.M.; Gorczyca, A.M.; Dolliver, D.D.; McAllister, M.A. Mechanisms of acid-catalyzed Z/E isomerization of imines. J. Org. Chem. 2001, 66, 7979–7985. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Xie, G.-R.; Chen, H.-J. Comprehensive Betalain Profiling of Djulis (Chenopodium formosanum) Cultivars Using HPLC-Q-Orbitrap High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 15699–15715. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Sergeeva, N.N.; Frutos, M.J.; Marshall, L.J.; Boesch, C. Novel approach for purification of major betalains using flash chromatography and comparison of radical scavenging and antioxidant activities. Food Chem. 2022, 385, 132632. [Google Scholar] [CrossRef]
- Terzo, S.; Attanzio, A.; Calvi, P.; Mule, F.; Tesoriere, L.; Allegra, M.; Amato, A. Indicaxanthin from Opuntia ficus-indica Fruit Ameliorates Glucose Dysmetabolism and Counteracts Insulin Resistance in High-Fat-Diet-Fed Mice. Antioxidants 2021, 11, 80. [Google Scholar] [CrossRef]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; D’Anneo, A.; Frazzitta, A.; Restivo, I.; Livrea, M.A.; Attanzio, A.; Tesoriere, L. The Phytochemical Indicaxanthin Synergistically Enhances Cisplatin-Induced Apoptosis in HeLa Cells via Oxidative Stress-Dependent p53/p21(waf1) Axis. Biomolecules 2020, 10, 994. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, K.; Kuusi, T. Stability Properties of Golden Beet and Red Beet Pigments—Influence of pH, Temperature, and Some Stabilizers. Z. Lebensm. -Unters. Und-Forsch. 1978, 166, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Doba, T.; Burton, G.W.; Ingold, K.U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim. Biophys. Acta Lipids Lipid Metabol. 1985, 835, 298–303. [Google Scholar] [CrossRef]
- Foti, M.C.; Amorati, R. Non-phenolic radical-trapping antioxidants. J. Pham. Pharmacol. 2009, 61, 1435–1448. [Google Scholar] [CrossRef]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarstrom, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Gandia-Herrero, F.; Escribano, J.; Garcia-Carmona, F. Purification and antiradical properties of the structural unit of betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Basilio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry Behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins-Nature’s Bold, Beautiful, and Health-Promoting Colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [Green Version]
- Carfora, V.; Cavallo, C.; Catellani, P.; Del Giudice, T.; Cicia, G. Why Do Consumers Intend to Purchase Natural Food? Integrating Theory of Planned Behavior, Value-Belief-Norm Theory, and Trust. Nutrients 2021, 13, 1904. [Google Scholar] [CrossRef]
- Lourenco, S.C.; Moldao-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coultate, T.; Blackburn, R.S. Food colorants: Their past, present and future. Color. Technol. 2018, 134, 165–186. [Google Scholar] [CrossRef]
- Fernandes, A.B.; Pavliuk, M.V.; Paun, C.; Carvalho, A.C.; Nomura, C.S.; Lewin, E.; Lindblad, R.; Camargo, P.H.C.; Sa, J.; Bastos, E.L. Recoverable and Reusable Polymer Microbead-Supported Metal Nanocatalysts for Redox Chemical Transformations. ACS Appl. Nano Mater. 2020, 3, 1722–1730. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Fernandes, A.B.; Abdellah, M.; Fernandes, D.L.A.; Machado, C.O.; Rocha, I.; Hattori, Y.; Paun, C.; Bastos, E.L.; Sa, J. Nano-hybrid plasmonic photocatalyst for hydrogen production at 20% efficiency. Sci. Rep. 2017, 7, 8670. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, L.C.P.; Lopes, N.B.; Augusto, F.A.; Pioli, R.M.; Machado, C.O.; Freitas-Dorr, B.C.; Suffredini, H.B.; Bastos, E.L. Phenolic betalain as antioxidants: Meta means more. Pure Appl. Chem. 2020, 92, 243–253. [Google Scholar] [CrossRef]
- Nakashima, K.K.; Bastos, E.L. Rationale on the High Radical Scavenging Capacity of Betalains. Antioxidants 2019, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, D.S. Electronic properties of amino acid side chains: Quantum mechanics calculation of substituent effects. BMC Chem. Biol. 2005, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Betancourt, C.; Cejudo-Bastante, M.J.; Heredia, F.J.; Hurtado, N. Pigment composition and antioxidant capacity of betacyanins and betaxanthins fractions of Opuntia dillenii (Ker Gawl) Haw cactus fruit. Food Res. Int. 2017, 101, 173–179. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, L.C.; Machado, C.O.; Gonçalves, L.C.P.; Cavalcante, V.F.; Obeid, G.; Correra, T.C.; Bastos, E.L. Structural Effects on the Antioxidant Properties of Amino Acid Betaxanthins. Antioxidants 2022, 11, 2259. https://doi.org/10.3390/antiox11112259
Esteves LC, Machado CO, Gonçalves LCP, Cavalcante VF, Obeid G, Correra TC, Bastos EL. Structural Effects on the Antioxidant Properties of Amino Acid Betaxanthins. Antioxidants. 2022; 11(11):2259. https://doi.org/10.3390/antiox11112259
Chicago/Turabian StyleEsteves, Larissa Cerrato, Caroline Oliveira Machado, Letícia Christina Pires Gonçalves, Victor Fernandes Cavalcante, Guilherme Obeid, Thiago Carita Correra, and Erick Leite Bastos. 2022. "Structural Effects on the Antioxidant Properties of Amino Acid Betaxanthins" Antioxidants 11, no. 11: 2259. https://doi.org/10.3390/antiox11112259
APA StyleEsteves, L. C., Machado, C. O., Gonçalves, L. C. P., Cavalcante, V. F., Obeid, G., Correra, T. C., & Bastos, E. L. (2022). Structural Effects on the Antioxidant Properties of Amino Acid Betaxanthins. Antioxidants, 11(11), 2259. https://doi.org/10.3390/antiox11112259







