Melatonin–Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions
Abstract
:1. Introduction
2. Melatonin in the Gut
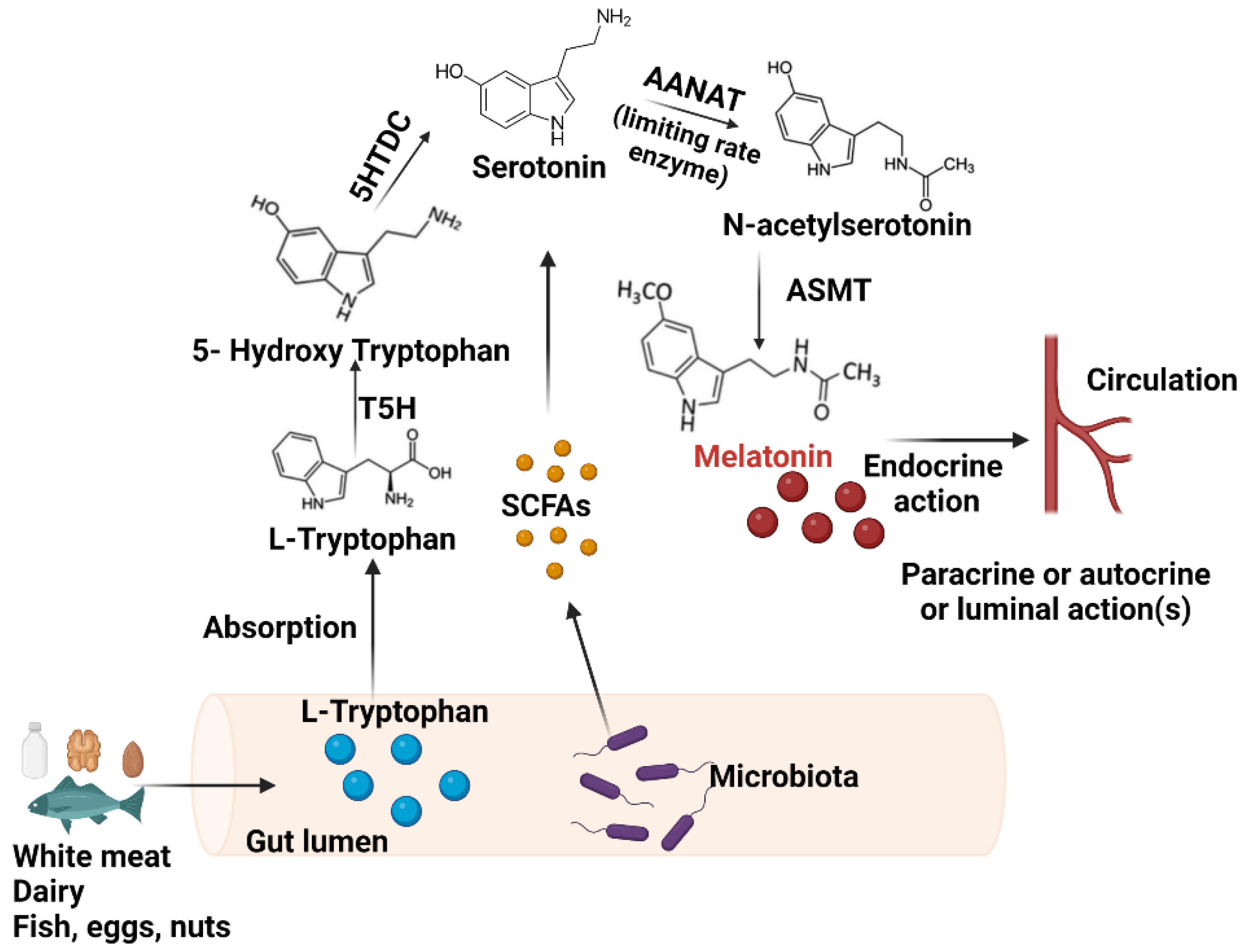
2.1. Synthesis in the GIT
2.2. Receptor Expression in the Gut
3. The Gut Microbiota
4. Melatonin and the Gut Microbiome Interplay
4.1. The Influence of Gut Bacteria on Melatonin
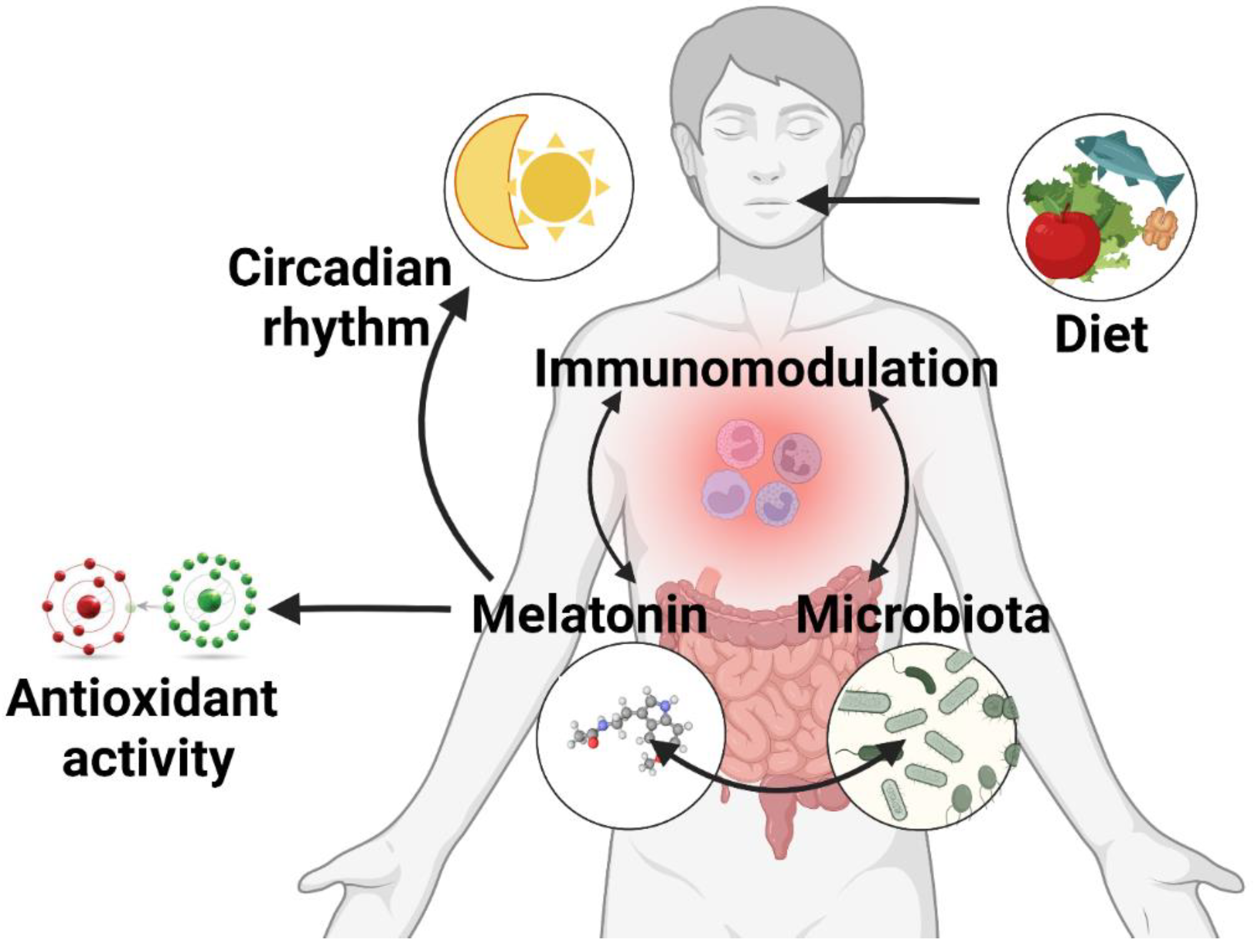
4.2. Melatonin’s Role on the Gut Microbiota
4.2.1. Circadian Rhythm Modulation and the Microbiome
4.2.2. Antioxidative Function of Melatonin in the Gut
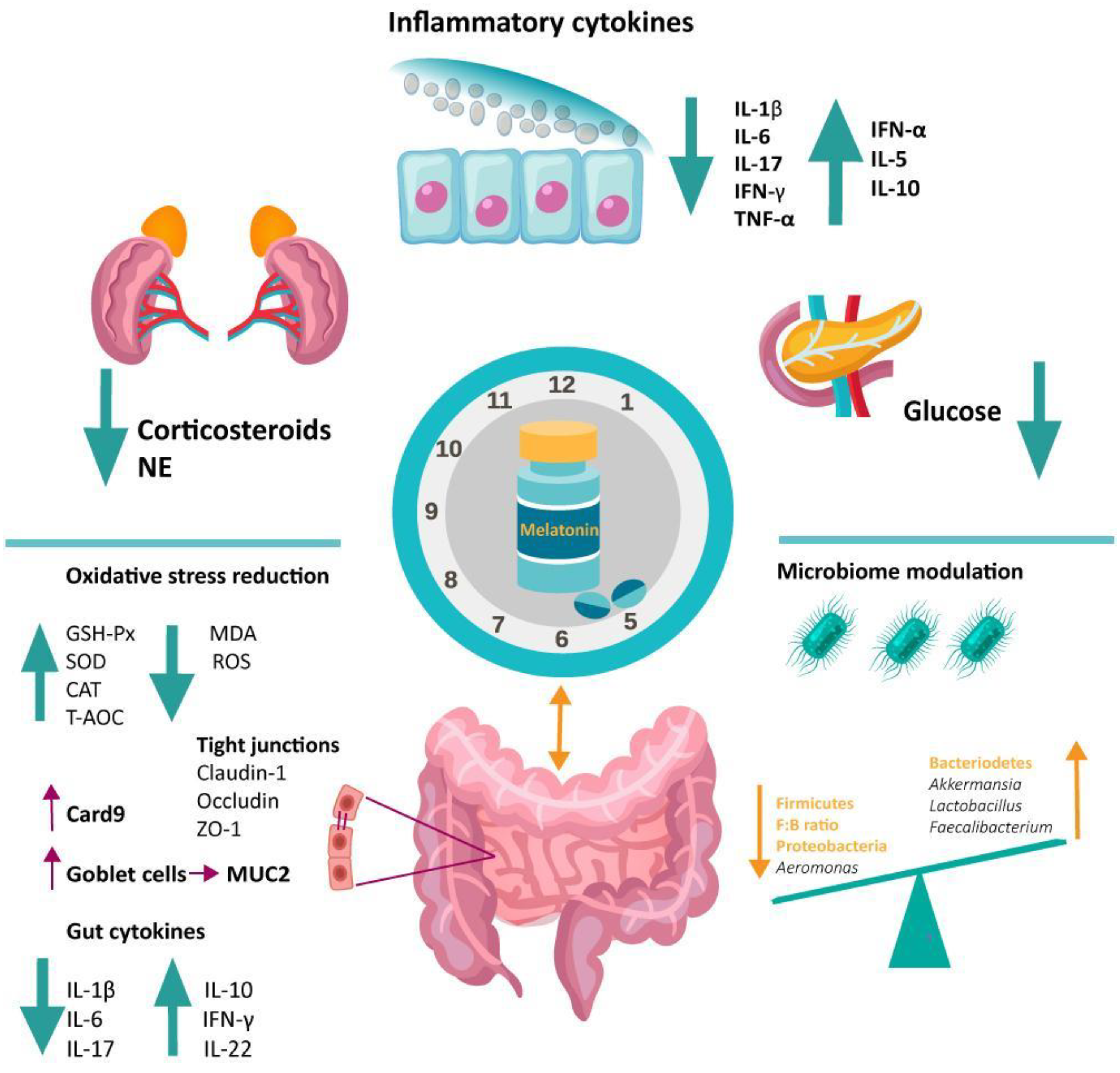
4.2.3. Immunomodulatory Function of Melatonin in the Gut
5. Melatonin Involvement in Dysbiosis-Associated Conditions via Microbiome Modulation
5.1. Melatonin and the Inflammatory Bowel Disease
5.2. Melatonin in Sleep Disturbance-Induced Colitis
5.2.1. Sleep Physiology and Gut Microbiome
5.2.2. Melatonin in Pathologic Sleep-Induced Dysbiosis
5.2.3. Melatonin, Sleep Disturbance, and IBD
5.3. Abnormal Light Exposure and Dysbiosis
5.4. Melatonin and Gut Microbiome Modulation in Other Diseases
5.4.1. Melatonin–Microbiome–Gut–Brain Axis
5.4.2. Melatonin–Microbiome in Obesity
5.5. Melatonin in COVID-19-Associated Dysbiosis
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cipolla-Neto, J.; Amaral, F.G. do Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Gitto, E.; Aversa, S.; Reiter, R.J.; Barberi, I.; Pellegrino, S. Update on the Use of Melatonin in Pediatrics. J. Pineal Res. 2011, 50, 21–28. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenberg, J.; Gögenur, I. A Systematic Review of Peri-Operative Melatonin. Anaesthesia 2014, 69, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Skene, D.J. Melatonin as a Chronobiotic. Sleep Med. Rev. 2005, 9, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never-Ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.-M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Kvetnoy, I.M.; Ingel, I.E.; Kvetnaia, T.V.; Malinovskaya, N.K.; Rapoport, S.I.; Raikhlin, N.T.; Trofimov, A.V.; Yuzhakov, V.V. Gastrointestinal Melatonin: Cellular Identification and Biological Role. Neuro Endocrinol. Lett. 2002, 23, 121–132. [Google Scholar] [PubMed]
- Novais, A.A.; Chuffa, L.G.d.A.; Zuccari, D.A.P.d.C.; Reiter, R.J. Exosomes and Melatonin: Where Their Destinies Intersect. Front. Immunol. 2021, 12, 692022. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Localization, Physiological Significance and Possible Clinical Implication of Gastrointestinal Melatonin. Biol. Signals Recept 2001, 10, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Brown, G.M.; Grota, L.J. Immunohistological Localization of Melatonin in the Rat Digestive System. Experientia 1977, 33, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A.; Niles, L.P.; Pang, S.F.; Pentney, P.J. Diurnal Variation and Binding Characteristics of Melatonin in the Mouse Brain and Gastrointestinal Tissues. Comp. Biochem. Physiol. C Comp. Pharm. Toxicol. 1993, 104, 221–224. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Hacker, R.R.; Brown, G.M.; Bartos, L. Melatonin Concentrations in the Luminal Fluid, Mucosa, and Muscularis of the Bovine and Porcine Gastrointestinal Tract. J. Pineal Res. 1999, 26, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Q.; Fichna, J.; Bashashati, M.; Li, Y.-Y.; Storr, M. Distribution, Function and Physiological Role of Melatonin in the Lower Gut. World J. Gastroenterol. 2011, 17, 3888–3898. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut Melatonin: A Potent Candidate in the Diversified Journey of Melatonin Research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Melatonin-Mediated Colonic Microbiota Metabolite Butyrate Prevents Acute Sleep Deprivation-Induced Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 11894. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.-H.; Choi, Y.J.; Jun, J.-H.; Jung, H.C.; Kim, E.K. Melatonin in the Colon Modulates Intestinal Microbiota in Response to Stress and Sleep Deprivation. Intest. Res. 2020, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P.M. Gene Expression of the Key Enzymes of Melatonin Synthesis in Extrapineal Tissues of the Rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.X.; Pang, S.F. N-Acetyltransferase Activity in the Quail (Coturnix Coturnix Jap) Duodenum. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 112, 251–255. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Brown, G.M. Pinealectomy Reduces Melatonin Levels in the Serum but Not in the Gastrointestinal Tract of Rats. Biol. Signals 1997, 6, 40–44. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Pang, S.F. Melatonin Levels in the Gastrointestinal Tissues of Fish, Amphibians, and a Reptile. Gen. Comp. Endocrinol. 1997, 106, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Huether, G.; Lorf, T.; Ramadori, G.; Schwörer, H. Presence of Melatonin in the Human Hepatobiliary-Gastrointestinal Tract. Life Sci. 2001, 69, 543–551. [Google Scholar] [CrossRef]
- Vician, M.; Zeman, M.; Herichová, I.; Juráni, M.; Blazícek, P.; Matis, P. Melatonin Content in Plasma and Large Intestine of Patients with Colorectal Carcinoma before and after Surgery. J. Pineal Res. 1999, 27, 164–169. [Google Scholar] [CrossRef]
- Pontoire, C.; Bernard, M.; Silvain, C.; Collin, J.P.; Voisin, P. Characterization of Melatonin Binding Sites in Chicken and Human Intestines. Eur. J. Pharm. 1993, 247, 111–118. [Google Scholar] [CrossRef]
- Poon, A.M.; Chow, P.H.; Mak, A.S.; Pang, S.F. Autoradiographic Localization of 2[125I]Iodomelatonin Binding Sites in the Gastrointestinal Tract of Mammals Including Humans and Birds. J. Pineal Res. 1997, 23, 5–14. [Google Scholar] [CrossRef]
- Pal, P.K.; Sarkar, S.; Chattopadhyay, A.; Tan, D.X.; Bandyopadhyay, D. Enterochromaffin Cells as the Source of Melatonin: Key Findings and Functional Relevance in Mammals. Melatonin Res. 2019, 2, 61–82. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Franceschetti, L.; Gianò, M.; Favero, G. A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. Int. J. Mol. Sci. 2022, 23, 3758. [Google Scholar] [CrossRef] [PubMed]
- Söderquist, F.; Hellström, P.M.; Cunningham, J.L. Human Gastroenteropancreatic Expression of Melatonin and Its Receptors MT1 and MT2. PLoS ONE 2015, 10, e0120195. [Google Scholar] [CrossRef] [Green Version]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan. Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikander, A.; Rana, S.V.; Prasad, K.K. Role of Serotonin in Gastrointestinal Motility and Irritable Bowel Syndrome. Clin. Chim. Acta 2009, 403, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Li, Y.; Liu, N.; Ge, Y.; Yang, Y.; Ren, F.; Wu, Z. Tryptophan and the Innate Intestinal Immunity: Crosstalk between Metabolites, Host Innate Immune Cells, and Microbiota. Eur. J. Immunol. 2022, 52, 856–868. [Google Scholar] [CrossRef]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.-Y.; Xu, D.-P.; Li, H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- von Gall, C.; Stehle, J.H.; Weaver, D.R. Mammalian Melatonin Receptors: Molecular Biology and Signal Transduction. Cell Tissue Res. 2002, 309, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Bondi, C.D.; McKeon, R.M.; Bennett, J.M.; Ignatius, P.F.; Brydon, L.; Jockers, R.; Melan, M.A.; Witt-Enderby, P.A. MT1 Melatonin Receptor Internalization Underlies Melatonin-Induced Morphologic Changes in Chinese Hamster Ovary Cells and These Processes Are Dependent on Gi Proteins, MEK 1/2 and Microtubule Modulation. J. Pineal Res. 2008, 44, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.L.; Lai, F.P.L.; Lo, R.K.H.; Voyno-Yasenetskaya, T.A.; Stanbridge, E.J.; Wong, Y.H. Melatonin Mt1 and MT2 Receptors Stimulate C-Jun N-Terminal Kinase via Pertussis Toxin-Sensitive and -Insensitive G Proteins. Cell. Signal. 2002, 14, 249–257. [Google Scholar] [CrossRef]
- Ho, M.K.; Yung, L.Y.; Chan, J.S.; Chan, J.H.; Wong, C.S.; Wong, Y.H. Galpha(14) Links a Variety of G(i)- and G(s)-Coupled Receptors to the Stimulation of Phospholipase C. Br. J. Pharm. 2001, 132, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin Receptors, Heterodimerization, Signal Transduction and Binding Sites: What’s New? Br. J. Pharm. 2008, 154, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.-X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.-X. Involvement of Nuclear Receptor RZR/RORγ in Melatonin-Induced HIF-1α Inactivation in SGC-7901 Human Gastric Cancer Cells. Oncol. Rep. 2015, 34, 2541–2546. [Google Scholar] [CrossRef] [Green Version]
- Winczyk, K.; Pawlikowski, M.; Karasek, M. Melatonin and RZR/ROR Receptor Ligand CGP 52608 Induce Apoptosis in the Murine Colonic Cancer. J. Pineal Res. 2001, 31, 179–182. [Google Scholar] [CrossRef]
- Aydin, M.; Canpolat, S.; Kuloğlu, T.; Yasar, A.; Colakoglu, N.; Kelestimur, H. Effects of Pinealectomy and Exogenous Melatonin on Ghrelin and Peptide YY in Gastrointestinal System and Neuropeptide Y in Hypothalamic Arcuate Nucleus: Immunohistochemical Studies in Male Rats. Regul. Pept. 2008, 146, 197–203. [Google Scholar] [CrossRef]
- Jaworek, J.; Brzozowski, T.; Konturek, S.J. Melatonin as an Organoprotector in the Stomach and the Pancreas. J. Pineal Res. 2005, 38, 73–83. [Google Scholar] [CrossRef]
- Sjöblom, M.; Flemström, G. Melatonin in the Duodenal Lumen Is a Potent Stimulant of Mucosal Bicarbonate Secretion. J. Pineal Res. 2003, 34, 288–293. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, N.; Yang, H. Factors Affecting the Composition of the Gut Microbiota, and Its Modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalog Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Young, V.B.; Schmidt, T.M. Overview of the Gastrointestinal Microbiota. Adv. Exp. Med. Biol. 2008, 635, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and Metabolic Diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlawat, S.; Kumar, P.; Mohan, H.; Goyal, S.; Sharma, K.K. Inflammatory Bowel Disease: Tri-Directional Relationship between Microbiota, Immune System and Intestinal Epithelium. Crit. Rev. Microbiol. 2021, 47, 254–273. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 1489. [Google Scholar] [CrossRef]
- Oka, A.; Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of Gut Microbiota on Neuropsychiatric Disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, C.; Popławski, T.; Blasiak, J.; Chojnacki, J.; Reiter, R.J.; Klupinska, G. Expression of Melatonin Synthesizing Enzymes in Helicobacter Pylori Infected Gastric Mucosa. BioMed Res. Int. 2013, 2013, e845032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.K.; Yang, C.; Song, G.-H.; Wong, J.; Ho, K.-Y. Melatonin Regulation as a Possible Mechanism for Probiotic (VSL#3) in Irritable Bowel Syndrome: A Randomized Double-Blinded Placebo Study. Dig. Dis. Sci. 2015, 60, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, E.; Basili, D.; Falcinelli, S.; Morillas, L.; Carnevali, O.; Capilla, E.; Navarro, I. The Probiotic Lactobacillus Rhamnosus Mimics the Dark-Driven Regulation of Appetite Markers and Melatonin Receptors’ Expression in Zebrafish (Danio Rerio) Larvae: Understanding the Role of the Gut Microbiome. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110634. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of Melatonin in Sleep Deprivation-Induced Intestinal Barrier Dysfunction in Mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Attenuates Microbiota Dysbiosis of Jejunum in Short-Term Sleep Deprived Mice. J. Microbiol. 2020, 58, 588–597. [Google Scholar] [CrossRef]
- Zhang, L.; Guadarrama, L.; Corona-Morales, A.A.; Vega-Gonzalez, A.; Rocha, L.; Escobar, A. Rats Subjected to Extended L-Tryptophan Restriction during Early Postnatal Stage Exhibit Anxious-Depressive Features and Structural Changes. J. Neuropathol. Exp. Neurol. 2006, 65, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-C.; Brown, J.; Gong, M.; Ge, Y.; Zadeh, M.; Li, W.; Croker, B.P.; Michailidis, G.; Garrett, T.J.; Mohamadzadeh, M.; et al. Gut Microbiota Dysbiosis and Altered Tryptophan Catabolism Contribute to Autoimmunity in Lupus-Susceptible Mice. Sci. Transl. Med. 2020, 12, eaax2220. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Arabacı Tamer, S.; Sahin, D.; Bagriacik, F.; Kahraman, M.M.; Onur, N.D.; Cayirli, Y.B.; Cilingir Kaya, Ö.T.; Aksu, B.; Akdeniz, E.; et al. The Effects of Antibiotics and Melatonin on Hepato-Intestinal Inflammation and Gut Microbial Dysbiosis Induced by a Short-Term High-Fat Diet Consumption in Rats. Br. J. Nutr. 2019, 122, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, Q.; Huo, D.; Jiang, S.; Ma, C.; Chang, H.; Chen, K.; Li, C.; Pan, Y.; Zhang, J. Melatonin Regulates the Neurotransmitter Secretion Disorder Induced by Caffeine Through the Microbiota-Gut-Brain Axis in Zebrafish (Danio Rerio). Front. Cell. Dev. Biol. 2021, 9, 678190. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Wu, Q.; Reiter, R.J.; Sun, C. The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolism Through Reducing Escherichia Coli-Derived Lipopolysaccharide. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1643–1667. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Billings, H.J.; Lehman, M.N. The Suprachiasmatic Nucleus: A Clock of Multiple Components. J. Biol. Rhythm. 2003, 18, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.J.; Allen, C.N.; Brown, R.L.; Robinson, D.W. The Light-Activated Signaling Pathway in SCN-Projecting Rat Retinal Ganglion Cells. Eur. J. Neurosci. 2006, 23, 2477–2487. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Weaver, D.R.; Jin, X.; Shearman, L.P.; Pieschl, R.L.; Gribkoff, V.K.; Reppert, S.M. Molecular Dissection of Two Distinct Actions of Melatonin on the Suprachiasmatic Circadian Clock. Neuron 1997, 19, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.E.; Al-Ghoul, W.M.; Gillette, M.U.; Dubocovich, M.L. Activation of MT(2) Melatonin Receptors in Rat Suprachiasmatic Nucleus Phase Advances the Circadian Clock. Am. J. Physiol. Cell. Physiol. 2001, 280, C110–C118. [Google Scholar] [CrossRef]
- Agez, L.; Laurent, V.; Pévet, P.; Masson-Pévet, M.; Gauer, F. Melatonin Affects Nuclear Orphan Receptors MRNA in the Rat Suprachiasmatic Nuclei. Neuroscience 2007, 144, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Pévet, P.; Agez, L.; Bothorel, B.; Saboureau, M.; Gauer, F.; Laurent, V.; Masson-Pévet, M. Melatonin in the Multi-Oscillatory Mammalian Circadian World. Chronobiol. Int. 2006, 23, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, F.J.; Torres-Farfan, C.; Richter, H.G.; Mendez, N.; Campino, C.; Torrealba, F.; Valenzuela, G.J.; Serón-Ferré, M. Clock Gene Expression in Adult Primate Suprachiasmatic Nuclei and Adrenal: Is the Adrenal a Peripheral Clock Responsive to Melatonin? Endocrinology 2008, 149, 1454–1461. [Google Scholar] [CrossRef] [Green Version]
- Zeman, M.; Herichova, I. Melatonin and Clock Genes Expression in the Cardiovascular System. Front. Biosci. 2013, 5, 743–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandu, C.; Liu, T.; Malan, A.; Challet, E.; Pévet, P.; Felder-Schmittbuhl, M.-P. Circadian Clocks in Rat Skin and Dermal Fibroblasts: Differential Effects of Aging, Temperature and Melatonin. Cell. Mol. Life Sci. 2015, 72, 2237–2248. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in Intestinal Epithelium Is Orchestrated by the Circadian Clock and Microbiota Cues Transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Fowler, S.; Hoedt, E.C.; Talley, N.J.; Keely, S.; Burns, G.L. Circadian Rhythms and Melatonin Metabolism in Patients With Disorders of Gut-Brain Interactions. Front. Neurosci. 2022, 16, 825246. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [PubMed]
- Paulose, J.K.; Wright, J.M.; Patel, A.G.; Cassone, V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE 2016, 11, e0146643. [Google Scholar] [CrossRef] [PubMed]
- Heddes, M.; Altaha, B.; Niu, Y.; Reitmeier, S.; Kleigrewe, K.; Haller, D.; Kiessling, S. The Intestinal Clock Drives the Microbiome to Maintain Gastrointestinal Homeostasis. Nat. Commun. 2022, 13, 6068. [Google Scholar] [CrossRef]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Kolbe, I.; Oster, H. Chronodisruption, Metabolic Homeostasis, and the Regulation of Inflammation in Adipose Tissues. Yale J. Biol. Med. 2019, 92, 317–325. [Google Scholar]
- Murakami, M.; Tognini, P. The Circadian Clock as an Essential Molecular Link Between Host Physiology and Microorganisms. Front. Cell. Infect. Microbiol. 2020, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002-20. [Google Scholar] [CrossRef]
- Hardeland, R.; Reiter, R.J.; Poeggeler, B.; Tan, D.X. The Significance of the Metabolism of the Neurohormone Melatonin: Antioxidative Protection and Formation of Bioactive Substances. Neurosci. Biobehav. Rev. 1993, 17, 347–357. [Google Scholar] [CrossRef]
- Hardeland, R.; Balzer, I.; Poeggeler, B.; Fuhrberg, B.; Uría, H.; Behrmann, G.; Wolf, R.; Meyer, T.J.; Reiter, R.J. On the Primary Functions of Melatonin in Evolution: Mediation of Photoperiodic Signals in a Unicell, Photooxidation, and Scavenging of Free Radicals. J. Pineal Res. 1995, 18, 104–111. [Google Scholar] [CrossRef]
- Allegra, M.; Reiter, R.J.; Tan, D.-X.; Gentile, C.; Tesoriere, L.; Livrea, M.A. The Chemistry of Melatonin’s Interaction with Reactive Species. J. Pineal Res. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of Melatonin in the Reduction of Oxidative Stress. A Review. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of Antioxidant Enzymes: A Significant Role for Melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin Induces Gamma-Glutamylcysteine Synthetase Mediated by Activator Protein-1 in Human Vascular Endothelial Cells. Free Radic. Biol. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and Synergistic Antioxidative Actions of Melatonin: Studies with Vitamin E, Vitamin C, Glutathione and Desferrioxamine (Desferoxamine) in Rat Liver Homogenates. J. Pharm. Pharm. 2001, 53, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ahlawat, S.; Mohan, H.; Gill, S.S.; Sharma, K.K. Balancing Reactive Oxygen Species Generation by Rebooting Gut Microbiota. J. Appl. Microbiol. 2022, 132, 4112–4129. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Le Gal, K.; Schmidt, E.E.; Sayin, V.I. Cellular Redox Homeostasis. Antioxidants 2021, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive Oxygen Production Induced by the Gut Microbiota: Pharmacotherapeutic Implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, P.; Li, Q.; Han, Z. Oxidative Stress and Gut Microbiome in Inflammatory Skin Diseases. Front. Cell. Dev. Biol. 2022, 10, 849985. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxid. Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef] [Green Version]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host Mitochondria Influence Gut Microbiome Diversity: A Role for ROS. Sci. Signal. 2019, 12, eaaw3159. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, J.M.; Reiter, R.J. Melatonin-Immune System Relationships. Curr. Top Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Currier, N.L.; Sun, L.Z.; Miller, S.C. Exogenous Melatonin: Quantitative Enhancement in Vivo of Cells Mediating Non-Specific Immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Peña, C.; Rincon, J.; Pedreanez, A.; Viera, N.; Mosquera, J. Chemotactic Effect of Melatonin on Leukocytes. J. Pineal Res. 2007, 43, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Inserra, P.; Zhang, Z.; Ardestani, S.K.; Araghi-Niknam, M.; Liang, B.; Jiang, S.; Shaw, D.; Molitor, M.; Elliott, K.; Watson, R.R. Modulation of Cytokine Production by Dehydroepiandrosterone (DHEA) plus Melatonin (MLT) Supplementation of Old Mice. Proc. Soc. Exp. Biol. Med. 1998, 218, 76–82. [Google Scholar] [CrossRef]
- Jaworek, J.; Szklarczyk, J.; Jaworek, A.K.; Nawrot-Porąbka, K.; Leja-Szpak, A.; Bonior, J.; Kot, M. Protective Effect of Melatonin on Acute Pancreatitis. Int. J. Inflam. 2012, 2012, 173675. [Google Scholar] [CrossRef] [Green Version]
- Veneroso, C.; Tuñón, M.J.; González-Gallego, J.; Collado, P.S. Melatonin Reduces Cardiac Inflammatory Injury Induced by Acute Exercise. J. Pineal Res. 2009, 47, 184–191. [Google Scholar] [CrossRef]
- Lin, X.-J.; Mei, G.-P.; Liu, J.; Li, Y.-L.; Zuo, D.; Liu, S.-J.; Zhao, T.-B.; Lin, M.-T. Therapeutic Effects of Melatonin on Heatstroke-Induced Multiple Organ Dysfunction Syndrome in Rats. J. Pineal Res. 2011, 50, 436–444. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-KappaB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Kwak, M.; Zhang, L.; Lee, P.C.W.; Jin, J.-O. Protective Effect of Melatonin Against Polymicrobial Sepsis Is Mediated by the Anti-Bacterial Effect of Neutrophils. Front. Immunol. 2019, 10, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Wu, X.; Zhang, Q.; Li, Y.; Ye, Y.; Li, P.; Chen, S.; Peng, Y.; Hardeland, R.; Xia, Y. Bacteriostatic Potential of Melatonin: Therapeutic Standing and Mechanistic Insights. Front. Immunol. 2021, 12, 2053. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. BioMed Res. Int. 2018, 2018, e2607679. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin Controls Microbiota in Colitis by Goblet Cell Differentiation and Antimicrobial Peptide Production through Toll-like Receptor 4 Signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, W.; Zhu, M.; Wang, F.; Zhao, X.; Dong, S.; Xu, Y.; Wang, S.; Yang, J.; Wang, K.; Liu, W. Hyaluronic Acid-Melatonin Nanoparticles Improve the Dysregulated Intestinal Barrier, Microbiome and Immune Response in Mice with Dextran Sodium Sulfate-Induced Colitis. J. Biomed Nanotechnol. 2022, 18, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Duplaga, K.; Gosiewski, T.; Kapusta, P.; Sroka-Oleksiak, A.; Wędrychowicz, A.; Pieczarkowski, S.; Ludwig-Słomczyńska, A.H.; Wołkow, P.P.; Fyderek, K. Differences in the Intestinal Microbiome of Healthy Children and Patients with Newly Diagnosed Crohn’s Disease. Sci. Rep. 2019, 9, 18880. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial Imbalance in Inflammatory Bowel Disease Patients at Different Taxonomic Levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Ghozlane, A.; Hu, H.; Li, X.; Xiao, Y.; Li, D.; Yu, G.; Zhang, T. Characteristics of Faecal Microbiota in Paediatric Crohn’s Disease and Their Dynamic Changes During Infliximab Therapy. J. Crohn’s Colitis 2018, 12, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.-X.; Yuan, X.; Cui, Y.-Y.; Liu, J.; Shen, J.; Jin, B.-Y.; Feng, B.-C.; Zhai, Y.-J.; Zheng, M.-Q.; Kou, G.-J.; et al. Melatonin Mitigates Oxazolone-Induced Colitis in Microbiota-Dependent Manner. Front. Immunol. 2021, 12, 783806. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, C.; Huang, J.; Kuai, X.; Shao, X. The Potential Therapeutic Role of Lactobacillus Reuteri for Treatment of Inflammatory Bowel Disease. Am. J. Transl. Res. 2020, 12, 1569–1583. [Google Scholar] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What Role Do Proteobacteria Play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Heidarian, F.; Noormohammadi, Z.; Aghdaei, H.A.; Alebouyeh, M. Relative Abundance of Streptococcus Spp. and Its Association with Disease Activity in Inflammatory Bowel Disease Patients Compared with Controls. Arch. Clin. Infect. Dis. 2017, 12, e57291. [Google Scholar] [CrossRef]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Calvin Coffey, J.; O’Connell, P.R. Desulfovibrio Bacterial Species Are Increased in Ulcerative Colitis. Dis. Colon Rectum 2010, 53, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E.; et al. Diets Enriched with Cranberry Beans Alter the Microbiota and Mitigate Colitis Severity and Associated Inflammation. J. Nutr. Biochem. 2016, 28, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Ho Szeto, C.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus Anaerobius Promotes Colorectal Carcinogenesis and Modulates Tumour Immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Yang, Y.; Yang, S.; Yao, C.; Liu, K.; Cui, S.; Zou, Q.; Sun, H.; Guo, G. Lachnospiraceae Shift in the Microbial Community of Mice Faecal Sample Effects on Water Immersion Restraint Stress. AMB Express 2017, 7, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the Intestinal Barrier: The Involvement of Epithelial Cells and Microbiota—A Mutual Relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Ihara, S.; Hirata, Y.; Serizawa, T.; Suzuki, N.; Sakitani, K.; Kinoshita, H.; Hayakawa, Y.; Nakagawa, H.; Ijichi, H.; Tateishi, K.; et al. TGF-β Signaling in Dendritic Cells Governs Colonic Homeostasis by Controlling Epithelial Differentiation and the Luminal Microbiota. J. Immunol. 2016, 196, 4603–4613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamanara, M.; Rashidian, A.; Mehr, S.E.; Dehpour, A.-R.; Shirkohi, R.; Akbarian, R.; Abdollahi, A.; Rezayat, S.-M. Melatonin Ameliorates TNBS-Induced Colitis in Rats through the Melatonin Receptors: Involvement of TLR4/MyD88/NF-ΚB Signalling Pathway. Inflammopharmacology 2019, 27, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.S.Y.; Coller, J.K.; Prestidge, C.A.; Bowen, J.M. Investigation of TLR4 Antagonists for Prevention of Intestinal Inflammation. Inflammation 2022, 1–12. [Google Scholar] [CrossRef]
- Niu, S.; Jing, M.; Wen, J.; Wei, S.; Li, H.; Li, X.; Ma, X.; Zhao, Y. Jatrorrhizine Alleviates DSS-Induced Ulcerative Colitis by Regulating the Intestinal Barrier Function and Inhibiting TLR4/MyD88/NF-ΚB Signaling Pathway. Evid. Based Complement Altern. Med. 2022, 2022, 3498310. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, P.; Xu, Y.; Feng, G.; Wang, Q.; He, X.; Song, Y.; Liu, P.; Chen, J. Trypsin Inhibitor LH011 Inhibited DSS-Induced Mice Colitis via Alleviating Inflammation and Oxidative Stress. Front. Pharmacol. 2022, 13, 986510. [Google Scholar] [CrossRef] [PubMed]
- Akcan, A.; Kucuk, C.; Sozuer, E.; Esel, D.; Akyildiz, H.; Akgun, H.; Muhtaroglu, S.; Aritas, Y. Melatonin Reduces Bacterial Translocation and Apoptosis in Trinitrobenzene Sulphonic Acid-Induced Colitis of Rats. World J. Gastroenterol. 2008, 14, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, K.R.; Halliday, M.I.; Barclay, G.R.; Milne, L.; Brown, D.; Stephens, S.; Maxwell, R.J.; Rowlands, B.J. Significance of Systemic Endotoxaemia in Inflammatory Bowel Disease. Gut 1995, 36, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, C.; Wisniewska-Jarosinska, M.; Walecka-Kapica, E.; Klupinska, G.; Jaworek, J.; Chojnacki, J. Evaluation of Melatonin Effectiveness in the Adjuvant Treatment of Ulcerative Colitis. J. Physiol. Pharm. 2011, 62, 327–334. [Google Scholar]
- Shahrokh, S.; Qobadighadikolaei, R.; Abbasinazari, M.; Haghazali, M.; Aghdaei, H.A.; Abdi, S.; Balaii, H.; Khanzadeh-Moghaddam, N.; Zali, M.R. Efficacy and Safety of Melatonin as an Adjunctive Therapy on Clinical, Biochemical, and Quality of Life in Patients with Ulcerative Colitis. Iran. J. Pharm. Res. 2021, 20, 197–205. [Google Scholar] [CrossRef]
- Rakhimova, O.Y.; Rakhimova, O.Y. Use of melatonin in combined treatment for inflammatory bowel diseases. Ter. Arkhiv 2010, 82, 64–68. [Google Scholar]
- Chojnacki, C.; Błasiak, J.; Fichna, J.; Chojnacki, J.; Popławski, T. Evaluation of Melatonin Secretion and Metabolism Exponents in Patients with Ulcerative and Lymphocytic Colitis. Molecules 2018, 23, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacki, C.; Wiśniewska-Jarosińska, M.; Kulig, G.; Majsterek, I.; Reiter, R.J.; Chojnacki, J. Evaluation of Enterochromaffin Cells and Melatonin Secretion Exponents in Ulcerative Colitis. World J. Gastroenterol. 2013, 19, 3602–3607. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The Metabolic Consequences of Sleep Deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef]
- Nagai, M.; Hoshide, S.; Kario, K. Sleep Duration as a Risk Factor for Cardiovascular Disease- a Review of the Recent Literature. Curr. Cardiol. Rev. 2010, 6, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bishir, M.; Bhat, A.; Essa, M.M.; Ekpo, O.; Ihunwo, A.O.; Veeraraghavan, V.P.; Mohan, S.K.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; et al. Sleep Deprivation and Neurological Disorders. Biomed Res. Int. 2020, 2020, 5764017. [Google Scholar] [CrossRef]
- Ibarra-Coronado, E.G.; Pantaleón-Martínez, A.M.; Velazquéz-Moctezuma, J.; Prospéro-García, O.; Méndez-Díaz, M.; Pérez-Tapia, M.; Pavón, L.; Morales-Montor, J. The Bidirectional Relationship between Sleep and Immunity against Infections. J. Immunol. Res. 2015, 2015, 678164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, S.; Hemarajata, P.; Versalovic, J. The Human Gut Microbiome and Body Metabolism: Implications for Obesity and Diabetes. Clin. Chem. 2013, 59, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Tremlett, H.; Bauer, K.C.; Appel-Cresswell, S.; Finlay, B.B.; Waubant, E. The Gut Microbiome in Human Neurological Disease: A Review. Ann. Neurol. 2017, 81, 369–382. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut Microbiota and Glucometabolic Alterations in Response to Recurrent Partial Sleep Deprivation in Normal-Weight Young Individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Bai, L.; Goel, N.; Bailey, A.; Jang, C.J.; Bushman, F.D.; Meerlo, P.; Dinges, D.F.; Sehgal, A. Human and Rat Gut Microbiome Composition Is Maintained Following Sleep Restriction. Proc. Natl. Acad. Sci. USA 2017, 114, E1564–E1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zou, J.; Xu, H.; Huang, W.; Zhang, X.; Wei, Z.; Li, X.; Liu, Y.; Zou, J.; Liu, F.; et al. Effects of Chronic Intermittent Hypoxia and Chronic Sleep Fragmentation on Gut Microbiome, Serum Metabolome, Liver and Adipose Tissue Morphology. Front. Endocrinol. 2022, 13, 820939. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Prevents the Dysbiosis of Intestinal Microbiota in Sleep-Restricted Mice by Improving Oxidative Stress and Inhibiting Inflammation. Saudi J. Gastroenterol. 2022, 28, 209–217. [Google Scholar] [CrossRef]
- Mezzatesta, M.L.; Gona, F.; Stefani, S. Enterobacter Cloacae Complex: Clinical Impact and Emerging Antibiotic Resistance. Future Microbiol. 2012, 7, 887–902. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut Bacteria Akkermansia Is Associated with Reduced Risk of Obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef]
- Salín-Pascual, R.J.; Ortega-Soto, H.; Huerto-Delgadillo, L.; Camacho-Arroyo, I.; Roldán-Roldán, G.; Tamarkin, L. The Effect of Total Sleep Deprivation on Plasma Melatonin and Cortisol in Healthy Human Volunteers. Sleep 1988, 11, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Redwine, L.; Hauger, R.L.; Gillin, J.C.; Irwin, M. Effects of Sleep and Sleep Deprivation on Interleukin-6, Growth Hormone, Cortisol, and Melatonin Levels in Humans1. J. Clin. Endocrinol. Metab. 2000, 85, 3597–3603. [Google Scholar] [CrossRef] [Green Version]
- Honma, A.; Revell, V.L.; Gunn, P.J.; Davies, S.K.; Middleton, B.; Raynaud, F.I.; Skene, D.J. Effect of Acute Total Sleep Deprivation on Plasma Melatonin, Cortisol and Metabolite Rhythms in Females. Eur. J. Neurosci. 2020, 51, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Ameliorates Corticosterone-Mediated Oxidative Stress-Induced Colitis in Sleep-Deprived Mice Involving Gut Microbiota. Oxid. Med. Cell. Longev. 2021, 2021, 9981480. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, B.; Yang, L.; Yang, Z. Molecular and Physiological Roles of the Adaptor Protein CARD9 in Immunity. Cell. Death Dis. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.-S.; Chung, S.-H.; Lee, S.-K.; Kim, J.-H.; Kim, J.-B.; Kim, T.-K.; Kim, D.-S.; Baik, H.-W. Melatonin Improves Experimental Colitis with Sleep Deprivation. Int. J. Mol. Med. 2015, 35, 979–986. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. The Role of Aeromonas-Goblet Cell Interactions in Melatonin-Mediated Improvements in Sleep Deprivation-Induced Colitis. Oxid. Med. Cell. Longev. 2022, 2022, 8133310. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Long, M.D.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Sleep Disturbance and Risk of Active Disease in Patients with Crohn’s Disease and Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2013, 11, 965–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbaran, Z.; Keefer, L.; Farhadi, A.; Stepanski, E.; Sedghi, S.; Keshavarzian, A. Impact of Sleep Disturbances in Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2007, 22, 1748–1753. [Google Scholar] [CrossRef]
- Tang, Y.; Preuss, F.; Turek, F.W.; Jakate, S.; Keshavarzian, A. Sleep Deprivation Worsens Inflammation and Delays Recovery in a Mouse Model of Colitis. Sleep Med. 2009, 10, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Brainard, J.; Gobel, M.; Scott, B.; Koeppen, M.; Eckle, T. Health Implications of Disrupted Circadian Rhythms and the Potential for Daylight as Therapy. Anesthesiology 2015, 122, 1170–1175. [Google Scholar] [CrossRef] [Green Version]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian Rhythm Disruption and Mental Health. Transl. Psychiatry 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The Biological Impacts of Artificial Light at Night: The Research Challenge. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiculescu, S.E.; Le Duc, D.; Roșca, A.E.; Zeca, V.; Chiţimuș, D.M.; Arsene, A.L.; Drăgoi, C.M.; Nicolae, A.C.; Zăgrean, L.; Schöneberg, T.; et al. Behavioral and Molecular Effects of Prenatal Continuous Light Exposure in the Adult Rat. Brain Res. 2016, 1650, 51–59. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian Disorganization Alters Intestinal Microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol. Clin. Exp. Res. 2016, 40, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Deaver, J.A.; Eum, S.Y.; Toborek, M. Circadian Disruption Changes Gut Microbiome Taxa and Functional Gene Composition. Front. Microbiol. 2018, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Chu, W.; Zhai, J.; Xu, J.; Li, S.; Li, W.; Chen, Z.-J.; Du, Y. Continuous Light-Induced PCOS-Like Changes in Reproduction, Metabolism, and Gut Microbiota in Sprague-Dawley Rats. Front. Microbiol. 2020, 10, 3145. [Google Scholar] [CrossRef]
- Malik, I.; Batra, T.; Das, S.; Kumar, V. Light at Night Affects Gut Microbial Community and Negatively Impacts Host Physiology in Diurnal Animals: Evidence from Captive Zebra Finches. Microbiol. Res. 2020, 241, 126597. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The Neuroprotective Role of Melatonin in Neurological Disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, T.; Cao, M.; Yuan, C.; Reiter, R.J.; Zhao, Z.; Zhao, Y.; Chen, L.; Fan, W.; Wang, X.; et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022, 13, 900132. [Google Scholar] [CrossRef]
- Jing, Y.; Yang, D.; Bai, F.; Zhang, C.; Qin, C.; Li, D.; Wang, L.; Yang, M.; Chen, Z.; Li, J. Melatonin Treatment Alleviates Spinal Cord Injury-Induced Gut Dysbiosis in Mice. J. Neurotrauma 2019, 36, 2646–2664. [Google Scholar] [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of Gut Microbiota in People with Obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms Underlying the Resistance to Diet-Induced Obesity in Germ-Free Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin Reprogramming of Gut Microbiota Improves Lipid Dysmetabolism in High-Fat Diet-Fed Mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin Prevents Obesity through Modulation of Gut Microbiota in Mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-K.; Yue, H.-Y.; Cai, J.; Zhai, Y.-J.; Peng, J.-H.; Hui, J.-F.; Hou, D.-Y.; Li, W.-P.; Yang, J.-S. COVID-19 and the Digestive System: A Comprehensive Review. World J. Clin. Cases 2021, 9, 3796–3813. [Google Scholar] [CrossRef]
- Delavari, A.; Asgari, S.; Alimohamadi, Y.; Vosoogh-Moghaddam, A.; Sadeghi, A.; Shahrousvand, S.; Zakeri, A.; Moradzadeh, R.; Akbarpour, S. XsGastrointestinal Symptoms Are Associated with a Lower Risk of Hospitalization and Mortality and Outcomes in COVID-19. BMC Gastroenterol. 2022, 22, 119. [Google Scholar] [CrossRef]
- van der Lelie, D.; Taghavi, S. COVID-19 and the Gut Microbiome: More than a Gut Feeling. mSystems 2020, 5, e00453-20. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wu, X.; Wen, W.; Lan, P. Gut Microbiome Alterations in COVID-19. Genom. Proteom. Bioinform. 2021, 19, 679–688. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in Microbiota of Patients with COVID-19: Potential Mechanisms and Therapeutic Interventions. Signal Transduct. Target. 2022, 7, 143. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stawinski, P.; Dziadkowiec, K.N.; Marcus, A. COVID-19-Induced Colitis: A Novel Relationship During Troubling Times. Cureus 2021, 13, e15870. [Google Scholar] [CrossRef] [PubMed]
- Rutigliani, M.; Bozzo, M.; Barberis, A.; Greppi, M.; Anelli, E.; Castellaro, L.; Bonsignore, A.; Azzinnaro, A.; Pesce, S.; Filauro, M.; et al. Case Report: A Peculiar Case of Inflammatory Colitis After SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 849140. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Alqusairi, R.; Adams, A.; Paul, M.; Kothari, N.; Peters, S.; DeBenedet, A.T. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am. J. Gastroenterol. 2020, 115, 942–946. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a Potential Adjuvant Treatment. Life Sci 2020, 250, 117583. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Abreu-Gonzalez, P.; Marik, P.E.; Dominguez-Rodriguez, A. Therapeutic Algorithm for Use of Melatonin in Patients With COVID-19. Front. Med. 2020, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Yayıcı Köken, Ö.; Gültutan, P.; Güngören, M.S.; Bayhan, G.İ.; Yılmaz, D.; Gürkaş, E.; Özyürek, H.; Çıtak Kurt, A.N. Impact of COVID-19 on Serum Melatonin Levels and Sleep Parameters in Children. Turk. J. Med. Sci. 2021, 51, 1640–1646. [Google Scholar] [CrossRef]
- Camp, O.G.; Bai, D.; Gonullu, D.C.; Nayak, N.; Abu-Soud, H.M. Melatonin Interferes with COVID-19 at Several Distinct ROS-Related Steps. J. Inorg. Biochem. 2021, 223, 111546. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.E.G.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-Blind Clinical Trial. Arch. Med. Res. 2022, 53, 79–85. [Google Scholar] [CrossRef]
- Hasan, Z.T.; Atrakji, D.M.Q.Y.M.A.A.; Mehuaiden, D.A.K. The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients. Int. J. Infect. Dis. 2022, 114, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Heydari, K.; Mehravaran, H.; Saeedi, M.; Alizadeh-Navaei, R.; Hedayatizadeh-Omran, A.; Shamshirian, A. Melatonin Effects on Sleep Quality and Outcomes of COVID-19 Patients: An Open-Label, Randomized, Controlled Trial. J. Med. Virol. 2022, 94, 263–271. [Google Scholar] [CrossRef] [PubMed]

| Preclinical Studies of MT’s Microbiota-Mediated Anti-Colitic Effect | ||||
| Study | Experimental Model of Colitis | MT Administration | Outcomes of MT Treatment—Related to Microbiota | Other Outcomes |
| [134] | 5% DSS in water in ICR mice aged 8 weeks | 0.2 mg/L MT in water, for one week | ↑Firmicutes/Bacteroidetes ratio ↑Coprococcus_1, Ruminococcaceae UCG-014 | ↑overall antioxidant capability |
| [135] | 2.5% (w/v) DSS in the drinking water for 6 days, in 8–9-week-old male WT/TLR4 KO BALB/c mice | 10 mg/kg/day MT i.p. for 8 days | ↑richness and diversity ↑Firmicutes (trend) ↓Proteobacteria (including Salmonella and Escherichia coli), Bacteroidetes (trend) ↑Ruminococcaceae | ↓DAI ↑goblet cells, Reg3β ↓IL-1β, IL-17 (only in WT mice) |
| [136] | DSS | Amphiphilic conjugate of hyaluronic acid and MT | Restores the ratio of Firmicutes/Bacteroidetes ↑richness and diversity ↑Lactobacillus ↓Bacteroides, Blautia, Streptococcus | Improvement of the colitis symptoms Alleviation of the damaged intestinal barrier Inhibition of the colonic inflammation |
| [137] | 4.5 mL/g of 3% oxazolone solution injected in the colon, in male C57BL/6 mice aged 6–8 weeks | 50 mg/kg of MT daily for one week before induction of colitis | ↑Verrucomicrobiota, Actinobacteria ↑Bifidobacterium ↓Desulfovibrio, Lachnospiraceae, Peptococcaceae | ↑colon length ↓body weight loss ↓pathology score ↑occludin and ZO-1 ↓TNF-α, IL-1β, IL-5, IL-13, CD11b + Ly6G+ neutrophils |
| Clinical Studies of MT in IBD | ||||
| Study | Design | Participants | MT Administration | Treatment Outcomes |
| [138] | Comparative study | 40 patients with UC/CD | 30 days | ↓inflammatory gut infiltration Improved intestinal ultrastructure |
| [139] | RCT | 60 patients with left-sided UC (38 women and 22 men, aged 26–49 years) | Mesalazine in daily doses 2 × 1.0 g and MT 5 mg daily at bedtime (group I) or placebo (group II) | ↑remission rate ↓MCDAI prevention of CRP increases and hemoglobin reduction |
| Taxonomic Level | Microorganism | Effect of MT Administration | Role of Microorganism in IBD |
|---|---|---|---|
| Phylum | Firmicutes/Bacteroidetes (ratio) | Increase | Index of intestinal homeostasis; reduced in IBD |
| Proteobacteria | Decrease | Role in IBD pathogenesis | |
| Family | Ruminococcaceae | Increase | SCFA-producing; decreased in IBD |
| Peptococcaceae | Decrease | Intestinal inflammation | |
| Lachnospiraceae | Decrease | Increased in stress-induced gut dysbiosis | |
| Genus | Coprococcus | Increase | SCFA-producing; decreased in IBD |
| Bifidobacterium | Increase | Probiotic used in IBD | |
| Lactobacillus | Increase | Probiotic used in IBD | |
| Streptococcus | Decrease | Associated with disease activity in IBD | |
| Desulfovibrio | Decrease | Associated with UC |
| Experimental Model | Gut Microbiome Changes | Local Effects | Systemic Effects | Reference |
|---|---|---|---|---|
| ICR mice—experimental colitis (5% DSS) for 6 days; 3 days of SD; MT (i.p. 10 mg/kg for 3 days) | ↓gross rectal bleeding ↓colon inflammation ↑iNOS, Wnt5a | ↓weight loss ↑survival ↓inflammatory cytokines (IL-1β, IL-6, IL-17, TNF-α, INF-γ) | [181] | |
| CD1 mice—3 days of SD; MT (i.p. 20 mg/kg or 40 mg/kg for 3 days) | Phylum: ↓Firmicutes: Bacteroidetes ratio Family: ↓Strepococcaceae, Lachnospiraceae, Gammaproteobacteria, Moraxellaceae ↑Akkermansia, Bacteroides, Faecalibacterium ↓Aeromonas | ↑goblet cells (MUC2) ↑enterocyte proliferation ↑claudin-1, occludin, ZO-1 ↑GSH-Px, SOD, CAT, T-AOC ↓MDA ↓NF-κB pathway ↓autophagy (ATG5, Beclin1) | ↓NE, IL-1β, IL-6, TNF-α ↑IL-5, IL-10, IFN-γ | [76] |
| CD1 mice—3 days of SD; MT (i.p. 20 mg/kg or 40 mg/kg for 3 days) | Phylum: ↓Firmicutes: Bacteroidetes ratio Family: ↑Prevotellaceae, Bacteroidaceae ↓Moraxellaceae, Aeromonadaceae, Rikenellaceae, Ruminococcaceae, Gammaproteobacteria Genus: ↓Aeromonas | ↓ROS ↓IL-17 ↑IL-22 | ↓Cort | [77] |
| C57BL/6 mice—10 days of partial SD (6 h during light cycle); MT (i.p. 10 mg/kg for 10 days) | Family: ↓Erysipelotrichaceae Species: ↑Akkermansia muciniphila, Lactobacillus murinus ↓Bacteroides massiliensis, Enterobacter cloacae, Enterobacter asburia | [21] | ||
| CD1 mice—3 days of SD; MT (i.p. 20 mg/kg for 3 days) w/o FMT | Phylum: ↓Firmicutes:Bacteroidetes ratio ↓Proteobacteria Family: ↑Prevotellaceae | ↑goblet cells (MUC2) ↑claudin-1, ZO-1 ↑CARD9 ↓IL-17 ↓ROS ↓HSP90 ↑HSP70, P23 ↓STAT3/AP-1/NF-kB | ↓ DAI ↓Cort, GR | [182] |
| ICR mice—3 days of SD; MT (i.p. 20 mg/kg for 3 days) w/o AB cocktail, 40 mM butyrate, FMT | Phylum: ↓Firmicutes:Bacteroidetes ratio ↓Proteobacteria ↑Verrucomicrobia Genus: ↑Faecalibacterium-↑butyrate (↑MCT1) | ↓IL-1β , IL-6, TNF-α, IL-17 ↑IL-10, IFN-γ ↓NF-kB/NLRP3 loop ↓HDAC3-↑p-GSK-3β/β-catenin/HIF-1α ↑ CARD9 | [20] | |
| C57BL/6J mice—JL induction; MT in drinking water (0.4 mg/mL); AB for 10 days | Family: ↓Enterobacteriales Species: ↑Akkermansia muciniphila ↓Escherichia coli | ↓LPCAT3, FATP4, NPC1L1, CD36 (associated with ileal lipid intake) ↓ fat accumulation in eWAT | [84] | |
| ICR mice—3 days of SD; MT (i.p. 20 mg/kg for 3 days), FMT, w/o 108 CFU (Aeromonas veronii), LPS (i.p., 2 mg/kg) w/o TAK-242 (TLR4 inhibitor) | Phylum: ↓Firmicutes: Bacteroidetes ratio Species: ↓Aeromonas veronii | ↓colon shortening ↓fecal occult blood ↓intestinal permeability ↑goblet cells (MUC2, Villin, Tff3) ↓ROS ↓IL-1β, TNF-α, IL-17 ↑IL-10, IFN-γ ↓NF-kB/NLRP3 ↓TLR4/MyD88/GSK-3β/β-catenin/NF-κB loop | ↓weight loss ↓DAI | [183] |
| CD1 mice—28 days of SR (4 h/day); MT (10−5 mol/L in drinking water) | Phylum: ↓Firmicutes: Bacteroidetes ratio Genus: ↑Lactobacillus ↓Helicobacter, Clostridium | ↓ IL-6, TNF-α ↑ IL-10, IFN-γ ↑GSH-Px, SOD, CAT, T-AOC ↓MDA | ↓NE, Cort, Glucose | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iesanu, M.I.; Zahiu, C.D.M.; Dogaru, I.-A.; Chitimus, D.M.; Pircalabioru, G.G.; Voiculescu, S.E.; Isac, S.; Galos, F.; Pavel, B.; O’Mahony, S.M.; et al. Melatonin–Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions. Antioxidants 2022, 11, 2244. https://doi.org/10.3390/antiox11112244
Iesanu MI, Zahiu CDM, Dogaru I-A, Chitimus DM, Pircalabioru GG, Voiculescu SE, Isac S, Galos F, Pavel B, O’Mahony SM, et al. Melatonin–Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions. Antioxidants. 2022; 11(11):2244. https://doi.org/10.3390/antiox11112244
Chicago/Turabian StyleIesanu, Mara Ioana, Carmen Denise Mihaela Zahiu, Ioana-Alexandra Dogaru, Diana Maria Chitimus, Gratiela Gradisteanu Pircalabioru, Suzana Elena Voiculescu, Sebastian Isac, Felicia Galos, Bogdan Pavel, Siobhain M. O’Mahony, and et al. 2022. "Melatonin–Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions" Antioxidants 11, no. 11: 2244. https://doi.org/10.3390/antiox11112244
APA StyleIesanu, M. I., Zahiu, C. D. M., Dogaru, I.-A., Chitimus, D. M., Pircalabioru, G. G., Voiculescu, S. E., Isac, S., Galos, F., Pavel, B., O’Mahony, S. M., & Zagrean, A.-M. (2022). Melatonin–Microbiome Two-Sided Interaction in Dysbiosis-Associated Conditions. Antioxidants, 11(11), 2244. https://doi.org/10.3390/antiox11112244









