1. Introduction
With the increasing demand for livestock products, the feedstuffs are being tightened. Accordingly, numerous cost-effective alternatives concerning feed sources are emerging for the sustainable development of the livestock industry. Many researchers have demonstrated in recent years that unconventional feed ingredients, such as brown rice, corn germ meal, wheat gluten meal and distillers dried grains with solubles, are available as partial substitutes for grain-based concentrates in livestock feeds without adverse effects on animal growth performance [
1,
2]. Moreover, there are multiple benefits of unconventional feed ingredients, with the most notable being their antimicrobial and antioxidant activities [
3].
Mulberry trees (
Morus alba L.) are rapid-growing, long-living, mow-resistant and deciduous plants with high adaptability to climate and soil, which can be used for 20–30 years after planting once and can be mowed 3–4 times annually. The cultivated area of mulberry in China is approximately 106 hectares [
4], and the annual fresh mulberry leaves weight can reach 80–120 tons/hectares in areas with fertile and well-irrigated soil. The principal active components of mulberry leaves, such as flavonoids and polyphenols, have anti-inflammatory, antioxidant, anti-diabetic, hypolipidemic and neuroprotective properties [
5,
6], which have been utilized in Chinese herbal medicine for centuries, such as for the treatment of cough, fever, diabetes and rheumatic diseases [
7,
8]. Research on the use of mulberry leaves for feed was initiated abroad as early as the beginning of the 20th century, and the Food and Agriculture Organization of the United Nations (FAO) has given high priority to the utilization of mulberry resources for the development of livestock [
9]. Related studies have indicated that mulberry leaf powder (MP) can be supplemented as a potential protein source for cattle [
10] and as a fermentable energy and potential protein source for sheep [
2,
11]. The findings of a study concerning rumen and gastrointestinal digestibility in sheep demonstrated that the digestible energy and crude protein values of mulberry leaves were comparable to those of alfalfa hay [
12]. Furthermore, mulberry leaves can be used as a new feed supplement to modulate the antioxidant capacity of laying hens [
13]. China promulgated and implemented “Mulberry leaf powder for feed” (SB/T 0998-2013) in 2013, aiming to actively develop mulberry resources and expand its utilization pathways. Nevertheless, the effect of MP on the immuno-antioxidant properties and meat quality of finishing pigs has been seldom investigated, especially for intestinal health.
In the current study, we aim to evaluate the effect of MP of varying levels on growth performance, serum biochemistry and immuno-antioxidant characteristics, meat antioxidant and lipid profile, with a finishing pig model, especially for intestinal morphology, tight junction expression and microbiotas, which provided a basis for future utilization and optimization of MP in livestock.
2. Materials and Methods
All procedures of the present research were approved by the Institutional Animal Care and Use Committee of China Agricultural University (No. AW10601202–1-2, Beijing, China).
2.1. MP Product
The MP was supplied by the Manborui Biotechnology Co., Ltd. (Jingzhou, Hubei, China), which is primarily obtained from the leaves, buds and some young branches of mulberry trees. The mulberry leaves and shoots with a harvested length of 45–60 cm were laid directly in a dry and ventilated place, shade-dried to a moisture content of approximately 30%, then spread out in the sun or into a drying room to dry quickly, lowering the moisture to 8–12%, and crushed using a feed or straw grinder. The nutritional composition of MP is shown in
Table S1.
2.2. Experimental Design, Animals and Management
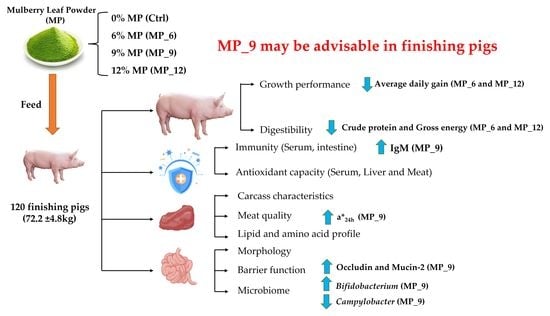
A total of 120 healthy finishing pigs (Duroc × [Landrace × Yorkshire]; 72.2 ± 4.8 kg) were selected for this experiment and divided into 4 treatments, according to randomized complete block design with 3 replicates of 10 pigs each (barrow:gilt = 1:1). The dietary treatments were as follows: (1) Ctrl, corn-soybean meal basal diet; (2) MP_6: basal diet with 6% MP. (3) MP_9: basal diet with 9% MP; (4) MP_12, basal diet with 12%MP. The whole experiment lasted for 33 days and the formulations of experimental diets were shown in
Table S2, which either satisfied or excelled the NRC [
14] of finishing pigs.
The research was conducted at the Fengning animal experimental base of China Agricultural University (Chengde, China). The experiment was carried out in a fully enclosed finishing house, which was thoroughly cleaned and disinfected before the experiment and managed with an all-in/all-out feeding pattern. The finishing house was equipped with intelligent equipment to control the temperature (20 °C), humidity (75%) and ventilation intensity, and the house floor was semi-cement and semi-leakage. The chosen pigs were randomly allocated to 12 pens (2.7 m × 1.8 m × 0.9 m) with the same equipment and conditions per pen, all of which used stainless steel adjustable troughs and nipple-type waterers. The finishing pigs were fed and drank ad libitum during the experiment, and the health status of the finishing pigs was observed daily. Moreover, routine deworming and immunization were necessary, according to the management procedures of the base. The whole experiment was performed in strict accordance with the standards of animal welfare of China Agricultural University.
2.3. Growth Performance
The amounts of feed intake and residual feed for finishing pigs were logged daily on a pen basis to calculate the average daily feed intake (ADFI) and the selected pigs were weighed on D 0, D 20 and D 33 to calculate the average daily gain (ADG), then the feed conversion ratios were calculated (FCR = ADFI/ADG).
2.4. Sampling and Slaughtering
2.4.1. Feed and Fecal Samples
At the beginning, middle and end of the experiment, approximately 1 kg of feed samples were collected for each treatment, then mixed well and stored at −20 °C for analysis.
On the 30th day of the experiment, manure was completely cleaned in the fattening house. From the 31st to the 33rd day, fecal samples (approximately 1 kg) were collected in each replicate, and the contamination should be avoided during the collection process. Finally, the collected fecal samples were well-mixed and stored at −20 °C for further analysis.
2.4.2. Serum Sample
The blood (10 mL in the anterior vena cava) was collected from one finishing pig with near average body weight in each replicate on the morning of the 20th and 33rd days of the experiment. The blood sample was centrifuged at 3000× g for 10 min at 4 °C after standing for 3 h, then collected the serum and stored at −20 °C for examination.
2.4.3. Slaughtering
At the end of the feeding experiment, one pig with near average body weight was picked from each pen, transported to the slaughterhouse (about 1 h) on the next morning after 12 h of fasting and rested for 4 h, then bled to slaughter by electric shock.
After slaughter, the longissimus dorsi muscle of the left carcass and liver were gathered; the intestinal mucous membrane samples from the duodenum, jejunum and ileum at 1/3 of the posterior segment in finishing pigs were washed off with 0.9% saline gently and collected, respectively, then stored at −80 °C. Meanwhile, approximately 2 cm tissues from duodenum, jejunum and ileum at the middle of the segment were collected, respectively, and fixed in 4% paraformaldehyde for examining morphology. Additionally, approximately 10 mL of the cecum and colon digesta were collected, respectively, transferred to the liquid nitrogen tank and later stored at −80 °C. All samples were obtained in duplicate.
2.5. Feed/Fecal Nutrient Composition
The fecal samples were defrosted at 4 °C and subsequently dried at 65 °C within an oven for 72 h. The feed and fecal samples were crushed and sieved through 40 mesh. Dry matter (DM), ash, crude protein (CP) ether extract (EE), calcium (Ca) and phosphorus (P) were determined based on the methods of the Association of Official Analytical Chemists (AOAC) [
15], respectively. Organic matter (OM) was calculated (OM (%) = 1-Ash (DM-basis) × 100%). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined with reference to the method of van Soest et al. [
16] using a filter bag and ANKOM200 fiber analyzer (Ankom, USA). Gross energy (GE) was analyzed using a 6400 automatic isoperibol calorimeter (Parr, USA). Chromium (Cr) level was determined by Z-5000 atomic absorption spectrophotometer (Hitachi, Japan), according to the methodology of Williams et al. [
17], to calculate the apparent total tract digestibility (ATTD) of nutrients with the following equation:
2.6. Serological Analysis and Intestinal Enzyme Activity
Serum immunoglobulins (IgA, IgG, IgM), inflammatory factors interleukin (IL-1β, IL-6, IL-8, IL- 10), tumor necrosis factor-α (TNF-α), gamma-interferon (IFN-γ), D-lactate (D-LA), hormones, glucocorticoid (GC), adrenocorticotropic hormone (ACTH), epinephrine (EPI), epidermal growth factor (EGF) and non-esterified fatty acid (NEFA), as well as intestinal inflammatory factors (IL-1β, IL-4, IL-6, IL-10 and TNF-α) and secretory immunoglobulin A (sIgA), were analyzed using Multiskan Ascent fully automated enzyme marker (Thermo, Waltham, USA) with commercial ELISA test kits. Serum growth hormone, insulin and insulin-like growth factor-1 (IGF-1) levels were determined by the methodology of radioimmunoassay using a DFM-96 radioimmunoassay gamma counter (Zhongcheng Electromechanical Technology, China). The glucose (GLU), total cholesterol (TC), total triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), globulin (GLB), alkaline phosphatase (AKP), lactate dehydrogenase (LDH), uric acid (UA), blood urea nitrogen (BUN) in serum and the superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), catalase (CAT) and malondialdehyde (MDA) in serum and liver were analyzed by a CLS880 fully automatic biochemical analyzer (Zecen Biotech, China). The determination of amylase, lipase, trypsin and chymotrypsin activities in the pancreas, jejunum and ileum of finishing pigs was performed using immunoturbidimetric assay.
All commercial kits were acquired from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China) and all analytical procedures were followed in strict compliance with the manufacturer’s instructions.
2.7. Determination of Carcass Trait and Meat Quality
After slaughtering the pigs, the head, hooves, tail and offal were completely removed and the hot carcass weight was recorded to calculate the slaughter rate. The back fat thickness was measured using a vernier caliper at shoulder fat thickness, the last rib fat thickness, lumbosacral fat thickness, the 6th to 7th rib fat thickness and the 10th rib fat thickness, respectively. Furthermore, the length and width of the longissimus dorsi cross-section were measured to calculate the loin-eye area. The pH values of the longissimus dorsi were recorded at 45 min and 24 h using a 205 pH meter (Testo, Lenzkirch, Germany). The flesh color luminosity (L*), redness (a*) and yellowness (b*) of the samples was determined using a CR-410 colorimeter (Minolta, Osaka, Japan). The longissimus dorsi was scored using official color and marbling standards card (NPPC, Des Moines, IA, USA). Drip loss is the percentage of water reduction after 100 g of meat samples were suspended in a refrigerator at 4 °C for 24 h. The relevant calculation formula is as follows:
Note: LEH, loin-eye length; LEW, loin-eye width; HW, hot carcass weight; LW, live weight; Mm, meat weight; Mh, meat weight after heating.
2.8. Muscle Lipid Profile and Amino Acid
The fatty acid and amino acid concentration assays of meat samples were performed with the assistance of the Ministry of Agricultural Feed Industry Center of China Agricultural University. The meat samples need to be lyophilized for 60 h by lyophilizer and thawed first, then quantification can take place in accordance with the methods of Sukhija and Palmquist [
18]. The detailed procedures were as follows: 0.5 g of the sample was weighed accurately into a hydrolysis tube; supplemented with 4 mL of 10% chloroacetylmethanol solution and 1 mL of undecanoic acid, (1.0 mg/mL) as an internal standard solution; then 1 mL of n-hexane was added and capped tightly; and put into a thermostat water bath at 80 °C for 2 h. After cooling, the sample was supplemented with 7% potassium carbonate 5 mL and shaken evenly and centrifuged at 1000 r/min for 5 min, then the lipid fatty acid of the muscle was determined using 6890 N gas chromatography (Agilent, Santa Clara, CA, USA) after the samples passed through the 0.2 μm filter membrane.
The determination of amino acids in muscle was performed by hydrochloric acid hydrolysis method. Firstly, 0.1 g of dried muscle sample powder was accurately weighed into an ampoule, 10 mL of 6 mol/L hydrochloric acid solution was added, the ampoule on the flame of an alcohol lamp was sealed, then it was hydrolyzed in a thermostat water bath at 110 °C for 24 h. After cooling and mixing, it was filtered and transferred to a 100 mL volumetric flask, then 1 mL of liquid was pipetted into a 10 mL centrifuge tube and evaporated in a thermostat water bath at 60 °C, before being supplemented with 1 L of distilled water to dissolve the residue, and it then continued to evaporate, being repeated twice to remove the hydrochloric acid and repeated twice to remove the hydrochloric acid completely. Later, 1 mL of double-distilled water was added and shaken evenly, the content of amino acid was determined using L-8900 fully automatic amino acid analyzer (Hitachi, Tokyo, Japan) after the samples were filtered through a 0.45 m microporous membrane.
2.9. Intestinal Morphometry
After being fixed in 10% formaldehyde solution for 48 h, sections (5 μm) were washed, excised, and dehydrated in ethanol, transparent in xylene and embedded in paraffin, and the sections were stained with hematoxylin-eosin; the images of the sections were acquired using CX31 light microscopy (Olympus, Tokyo, Japan) combined with true color image analysis software; the multiple typical fields of view (with clear and intact villi) were chosen on each section to measure villi height (VH) and crypt depth (CD), and the ratio of villi height to crypt depth (VH/CD) was calculated.
2.10. RNA Extraction and Real-Time PCR
Total RNA was extracted, respectively, from the liver, muscle, jejunum and ileum of finishing pigs by TRizol (Invitrogen, Carlsbad, USA) kits, as described in the manufacturing instructions and the concentration and quality of RNA were verified by 1% agarose gel electrophoresis. The RNA was reverse transcribed into cDNA with All-in-One First-Strand cDNA Synthesis SuperMix for qPCR kit (QIAGEN, Hilden, Germany). Reverse transcription system: total RNA, 0.5 μg; 5 × TransScript® All-in-one SuperMix for qPCR, 5 μL; gDNA Remover, 0.5 μL; RNase-free water supplemented to 10 μL. Reaction system: 42 °C, 15 min; 85 °C, 5 s. An amount of 90 μL RNase-free water was added after reverse transcription and stored at −20 °C.
Fluorescent quantitative PCR procedures were conducted using the Roche LightCycler
® 480II Real-Time PCR System (Roche, Basel, Switzerland; PCR efficiency: 96~l02%). PCR reaction mixture (10 μL): cDNA, 1 μL; 2× PerfectStart
TMGreen qPCR SuperMix, 5 μL; upstream primer, 0.2 μL; downstream primer, 0.2 μL; RNase-free water, 3.6 μL. Then, it was incubated using a Real-Time PCR 384-well plate (Roche, Basel, Switzerland). Reaction system: pre-denaturation at 95 °C for 5 min; denaturation at 95 °C for 10 s, renaturation at 60 °C for 30 s; extension at 72 °C for 30 s, 40 cycles. The melting curves were analyzed at the end of the PCR cycle to validate the specific generation of the expected PCR products. Each sample was analyzed twice. The primer sequences listed in
Table S3 were designed and synthesized by Beijing Tianyi Huiyuan Biotechnology (Beijing, China). The expression levels of mRNA were standardized using β-actin (housekeeping gene) and the relative expression of mRNA was calculated using the 2
−ΔΔCt method.
2.11. The 16S rRNA Microbial Sequencing
The 16S rRNA microbial sequencing was accomplished with the collaboration of Shanghai Majorbio (China). The samples of the cecum and colon digesta were taken from the −80 °C refrigerator, and the DNA kit (OmegaBio-Tek, Norcross, GA, USA) was used for extracting the total genomic DNA of bacteria. The concentration and purity of DNA were examined by NanoDrop2000 spectrophotometers (Thermo, Waltham, MA, USA). The 16S rRNA gene in the variable region of bacterial V3-V4 was amplified with PCR, in accordance with universal primers 338F (5-ACTCCTACGGGAGGCAGCAG-3) and 806R (5-GGACTACHVGGGTWTCTAAT-3). Then, the amplification products were purified and recovered by Axyprep DNA Gel Extraction kit (Axygen Biosciences, Union, CA, USA) and quantified to homogeneous concentrations by Qubit2.0 fluorometer (Thermo, Waltham, MA, USA). The purified amplified fragments were established as amplified libraries and sequenced on Illumina-HiSeq-PE300 platform (Illumina, San Diego, CA, USA) with paired-end reads of 300 bp. The raw sequences were quality-controlled by Fastp, spliced by FLASH [
19], operational taxonomic unit (OTU) clustered by UPARSE based on the UPARSE algorithm with a 97% similarity threshold [
20,
21], and Taxonomic analysis by RDP classifier based on Bayesian algorithm. Lastly, the identified classifications were aligned using the Silva 16S rRNA database with a 70% confidence threshold [
22]. The Sobs, Shannon, Simpson, Ace, Chao and phylogenetic diversity (PD) indices were analyzed by Mothur to assess microbial α-diversity. Principal co-ordinate analysis (PCoA) and partial least squares discriminant analysis (PLS-DA) based on the Bray–Curtis distance matrix algorithm and analysis of similarity (ANOSIM) were analyzed by R software to evaluate microbial β-diversity; Circos diagrams were plotted by the mapping software Circos to reveal the proportion of dominant species composition in each group and the proportion of distribution of each dominant species in different groups. The relative abundance of microbes was presented as a percentage. The relevant software information is listed below: Fastp (v0.23.2,
https://github.com/OpenGene/fastp, accessed on 7 April 2022); Flash (v1.2.11,
https://ccb.jhu.edu/software/FLASH/index.shtml, accessed on 7 April 2022); UPARSE (v 7.1,
http://drive5.com/uparse/, accessed on 7 April 2022); RDP classifier (v 2.13,
http://rdp.cme.msu.edu/, accessed on 7 April 2022); Silva 16S rRNA database (v138,
http://www.arb-silva.de, accessed on 7 April 2022); Mothur (v1.30.2,
https://mothur.org/wiki/calculators/, accessed on 27 April 2022); Circos (v 0.69.9,
http://circos.ca/ accessed on 21 May 2022). Lefse analysis based on the nonparametric factorial Kruskal–Wallis sum test and Wilcoxon rank sum test with all-against-all multi-group comparison strategy was used to estimate features with significant differences in abundance and to identify taxa with significantly different abundances and to show only taxa with LDA scores larger than 3. The raw microbial dataset has been uploaded on NCBI with the accession number PRJNA890454 and PRJNA890458.
2.12. Statistical Analysis
All data were calculated as individuals, except for growth performance data, which were calculated based on pens. Source data were initially organized by Excel (Microsoft, Redmond, WA, USA). The one-way ANOVA with generalized linear models (GLM) procedure and the Turkey–Kramer post hoc test were performed by SAS 9.2 software (SAS Institute, Cary, NC, USA). The linear and quadratic comparisons were employed to establish the dose effect of MP in finishing pigs. Consideration was assigned to statistical significance when p ≤ 0.05 and a trend with statistical significance was regarded when 0.05 < p ≤ 0.10.
4. Discussion
Mulberry leaves are wealthy in carbohydrates, proteins, fatty acids and fiber. Additionally, the vitamins and mineral elements, especially amino acids are abundant, as well as containing numerous natural active substances, such as sitosterol, quercetin, γ-aminobutyric acid, 1-deoxynojirimycin and mulberry flavonoids [
23], suggesting that mulberry leaves may be suitable as functional feed to enhance performance and health of finishing pigs. Recently, the impact of the epidemic has led to continuously elevated prices of traditional feed ingredients, which has brought concern for many businesses. Accordingly, the application of mulberry leaves as an unconventional feed source for livestock to substitute the traditional expensive feed ingredients (alfalfa, peanut meal, wheat bran) in animal nutrition is gradually gaining attention. In the present study, diets supplemented with 9% MP had no difference on ADG, ADFI and FCR, indicating that dietary supplementation of MP with 9% may have had no negative effect on the growth performance of finishing pigs, which was in agreement with the findings on pigs and cattle by Liu et al. [
24] and Vu et al. [
10]. However, diets supplemented with 6% and 12%, the ADG of finishing pigs decreased, which was in line with Zhu et al. [
25] who suggested that diets supplemented with 15% MP decreased the ADG of finishing pigs in the whole period. The reason may be attributed to the lower amount of essential amino acids (6%) and the high concentration of fiber (12%) in the MP, compared with wheat bran [
26]; the growth-promoting effect of MP_9 is most likely correlated with the positive effect of the increased active substances, such as flavonoids and polyphenolic compounds although this requires further validation. Research has revealed that high-fiber diets have a detrimental effect on energy and nutrient absorption [
27], leading to decreased growth performance in finishing pigs [
28]. Hence, it is necessary to consider lowering the fiber level or enhancing the essential amino acid level in MP to obtain a superior growth performance in finishing pigs.
To further investigate the effect of the dietary supplementation of MP on the growth in finishing pigs, the blood was collected at days 20 and 33 of the experiment and analyzed for immuno-antioxidant capacity and biochemical metabolic indices, as well as hormone levels. Interestingly, in the current study, an increased level of IFN-γ, as well as the decreased content of ALP in MP_12. ALP improves bone-portion and accelerates the deposition of Ca and P in bone tissue [
29]. IFN-γ, as a pro-inflammatory cytokine, is capable of promoting its inflammatory response and adversely affects the organism [
30]. Furthermore, an increased level of IgM and decreased level of GLB in MP_9 was observed. Serum glucose is an essential indicator of the host metabolic conditions [
31]. IgM facilitates the improvement of immune function in finishing pigs. The linearly reduction in GLU levels by MP was probably associated with the MP active substance 1-deoxynojirimycin, which is a potent α-glucosidase inhibitor [
32]. Similarly, for hormones, such as GS and IGF, MP_9 was superior to MP_6 and MP_12, but there was no significant difference compared to the Ctrl, except for ACTH, which may be related to the action of beneficial microbiota in the hindgut. Collectively, diets supplemented with 9% MP had a beneficial effect on the growth of finishing pigs, which may be associated with the function of the active substances in MP, such as mulberry flavonoids and mulberry polysaccharides, and the action of beneficial microbiota, such as
Bifidobacterium and
Lactobacillus, but 12% MP may have the opposite effect. It is currently unclear why HDL levels are elevated in MP_12, which needs further investigation.
Antioxidation is one of the primary functions of MP; therefore, we detected the foremost antioxidant enzymes in serum and liver, as well as mRNA expression, pertaining to antioxidant function in muscle and liver to verify its antioxidant capacity, respectively. T-AOC indicates the total antioxidant capacity of the body, and MDA reveals the degree of tissue lipid peroxidation [
33]. In the current study, diets supplemented with MP at 9% could increase the levels of T-AOC and decrease the content of MDA, compared with MP_6, but there was no difference compared with the Ctrl. For mRNA expression of liver and muscle, there was no adverse effect between treatments and Ctrl for finishing pigs, indicating MP_9 is more powerful than MP_6 and MP_12, regarding antioxidant function. Our findings are comparable to previous results. Liu et al. [
34] reported that diets supplemented with MP at 3%, 6% and 9% in finishing pigs could not impact the antioxidant-related gene expression of muscle with the exception of the MP at 12%. Fan et al. [
35] demonstrated that the antioxidant function in serum and muscle with 5% mulberry leaf powder in the diet of finishing pigs was comparable to the control group. Hence, diets supplemented with 9% MP in finishing pigs might be preferable.
The integrity of the intestinal morphology is crucial for the maintenance of normal intestinal function [
36]. Shrunken intestinal villi or increased crypt depth, as well as the reduced activity of digestive enzymes, indicate a reduction in nutrient absorption in the small intestine, which are detrimental to nutrient digestibility in pigs [
37]. In the current study, diets supplemented with 9% MP had no negative effect on the digestibility of DM, CP and GE, while 6% and 12% had an adverse effect. Likewise, diets supplemented with MP neither affected the activity of digestive enzymes and intestinal morphology nor harmed the intestinal inflammation-related factors, suggesting that the digestive and absorption capability of finishing pigs may not be compromised by the 9% MP. Notably, diets supplemented with MP did not involve microbial diversity (α and β diversity) and composition of the hindgut, only some microbiota will be affected. In the current study, the relative abundance of beneficial microbiota
Bifidobacterium and
Lactobacillus were enriched in MP_9, while MP_6 and MP_12 experienced the contrary, which may well be instrumental in explaining the up-regulated mRNA expression of tight junction protein occludin and Mucin-2. Moreover,
Campylobacter is capable of triggering acute gastroenteritis [
38], which was enriched in the Ctrl but not in the MP group, indicating that some active ingredients in MP are able to suppress the proliferation and growth of Campylobacter, which is beneficial to the healthy status of finishing pigs.
Senegalimassilia is a specialized microorganism that degrades cellulose [
39], which was enriched in MP_12, revealing that high cellulose content of 12% MP led to an elevated abundance of cellulose-degrading microorganisms in the intestine. This also contributes to explaining the reduction in nutrient digestibility and performance in finishing pigs supplemented with 12% MP.
It is well-known that the excessive subcutaneous adipose tissue of fattening pigs is unfavorable for carcass traits [
24]. Our findings revealed that the dietary supplementation of MP with increasing levels showed no effect on the carcass trait of finishing pigs, which is in disagreement with the findings of Liu et al. [
24] who evidenced that diets supplemented with 9% MP could reduce the back fat thickness and increase the loin-eye area, improving the carcass characteristics. Zhu et al. [
25] also revealed that dietary supplementation of 4% MP could ameliorate the carcass trait. Astonishingly, Chen et al. [
40] were concordant with our results that the dietary supplementation of MP exhibited no significant effect on carcass qualities of fattening pigs, and the inconsistency in the results may be attributed to differences in pig species, age and composition of MP, which remains to be further verified.
The meat color L* value (luminosity) represents the level of oxidized myoglobin, the a* value (redness) reflects the concentration and presence of deoxygenated myoglobin in the muscle, and the b* value (yellowness) describes the amount of myoglobin oxidized to high-iron myoglobin [
41]. Therefore, it is necessary for the meat to have more redness. In the current study, diets supplemented with 9% MP was capable of elevating the a*
24h, which was helpful for improving the meat quality. Liu et al. [
24] reported that diets supplemented with 6% and 9% MP could modulate the shear force, modifying the tenderness and resulting in ameliorating the meat quality. The same results were observed in the study of Chen et al. [
40] and Zhang et al. [
42]. Additionally, they assumed that the improvement in meat quality might be involved with quercetin, one of the active substances in mulberry leaf powder, which has the potent water-holding ability and antioxidant properties. Interestingly, there were no differences observed between the treatments and the Ctrl in either muscle lipid profile or amino acid composition, suggesting that diets supplemented with MP do not affect the muscle composition of finishing pigs, which also remained in accordance with the findings that there was no significant difference in the mRNA expression levels of genes related to muscle fiber and lipid metabolism between the MP group and the Ctrl. Information concerning the effect of MP on muscle composition in finishing pigs is limited. Hence, it is necessary to deeply investigate the relationship between the active substance related to MP and meat composition.