Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
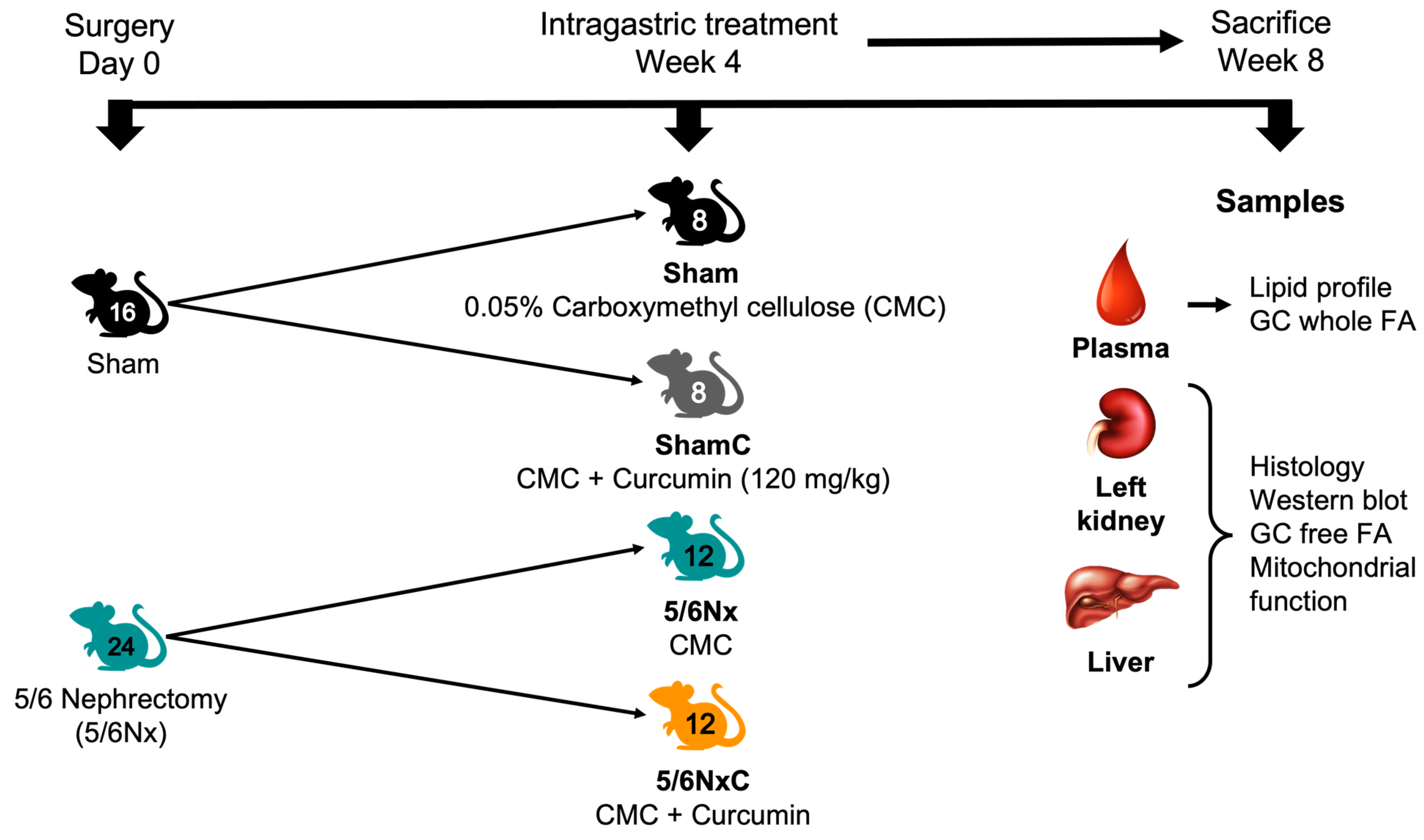
2.2. Experimental Protocol
2.3. General Parameters
2.4. Plasma Lipid Profile
2.5. Gas Chromatography
2.6. Histology
2.7. Western Blot
2.8. Mitochondrial Isolation
2.9. Mitochondrial β-Oxidation Oxygen Consumption and Membrane Potential (ΔΨm)
2.10. Statistics
3. Results
3.1. General Parameters
3.2. Kidney and Liver Damage Markers
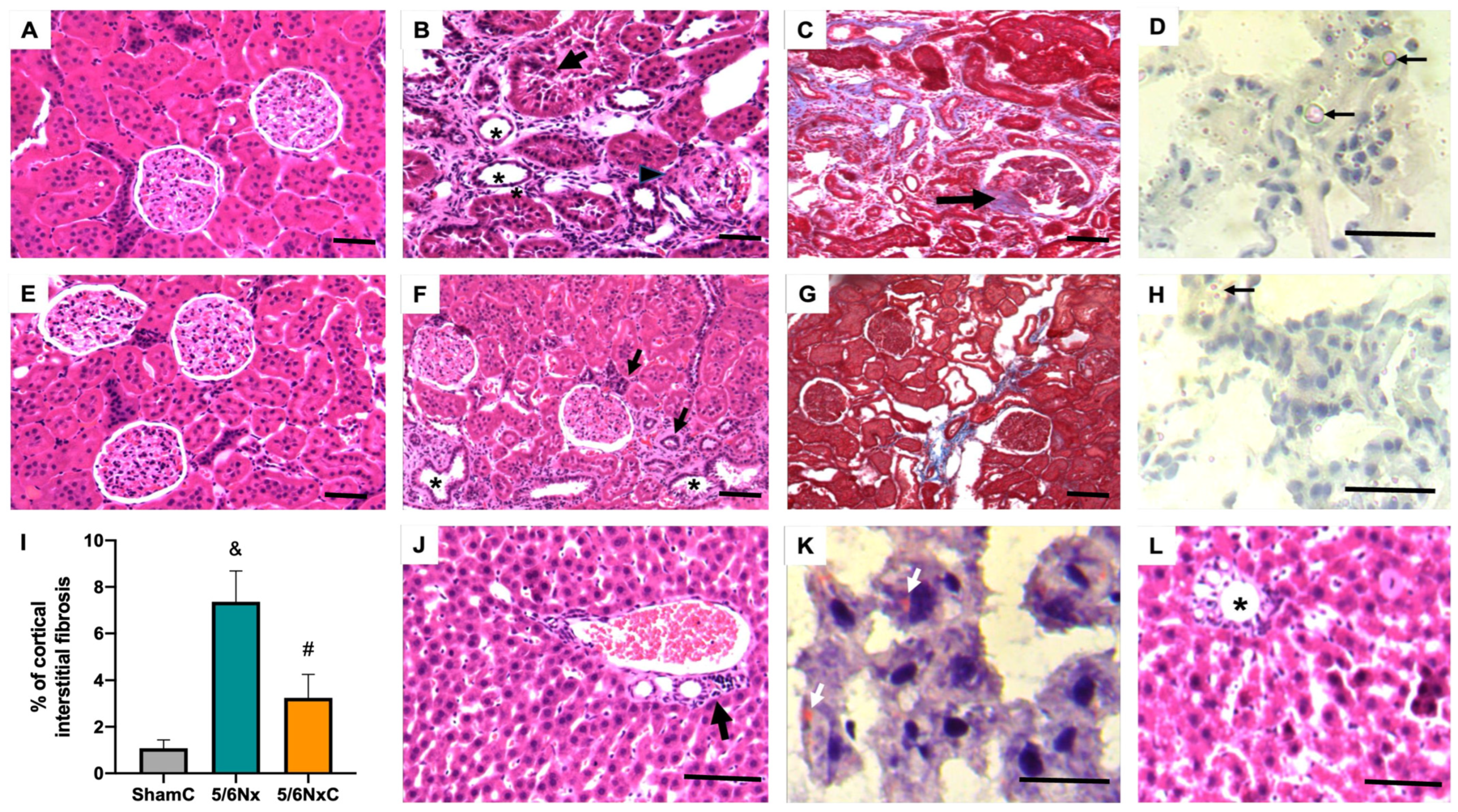
3.3. Kidney and Liver Histology
3.4. Plasma Lipid Profile
Plasma Fatty Acids Profile
3.5. Kidney Lipid Metabolism
3.5.1. The Kidney Free Fatty Acid Profile
3.5.2. Renal Levels of Proteins Involved in Lipid Synthesis
3.5.3. Kidney Mitochondrial β-Oxidation
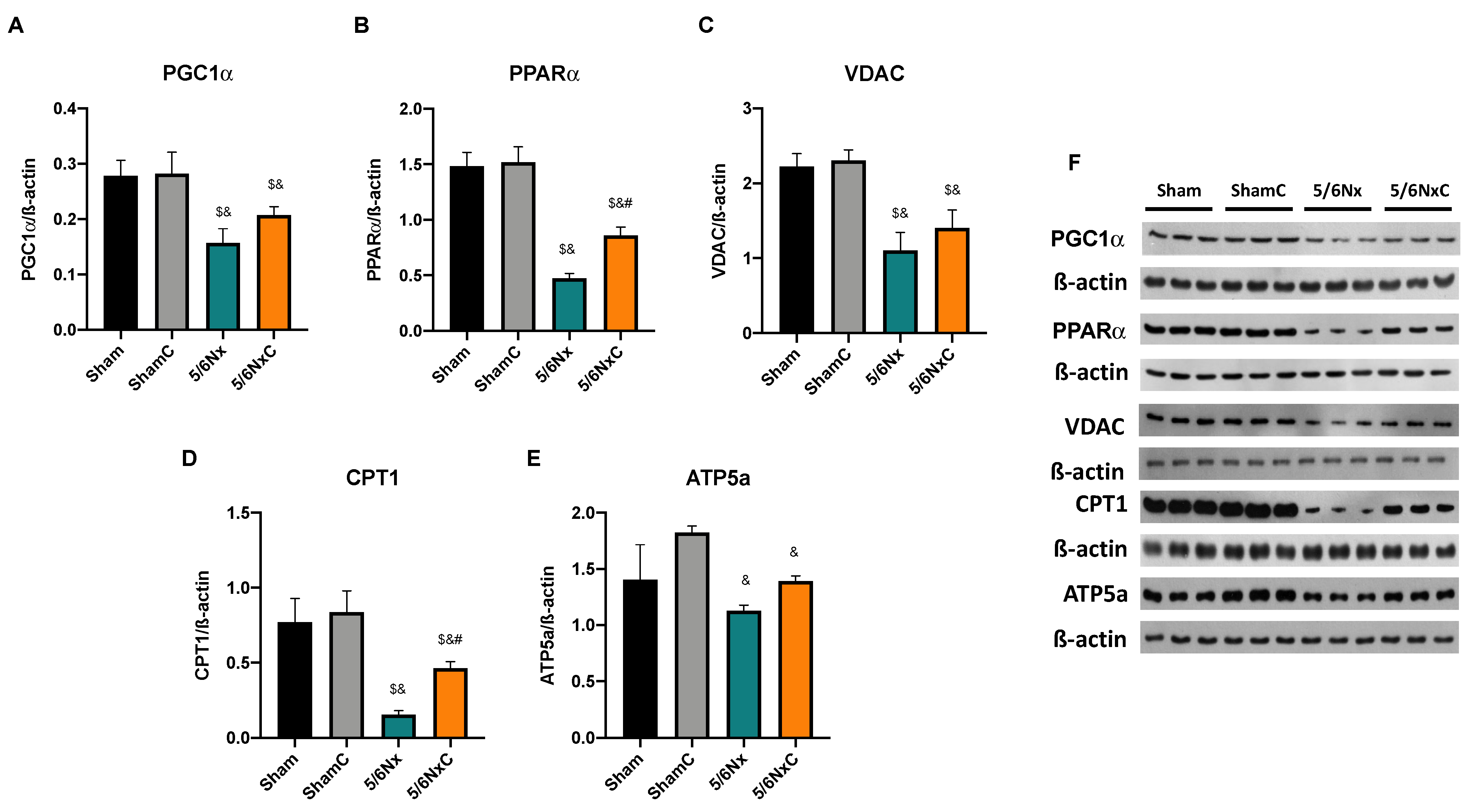
3.5.4. Renal Levels of Proteins Involved in Biogenesis and FA Transport in Mitochondria
3.6. Liver Lipid Metabolism
3.6.1. Liver Free Fatty Acid Profile
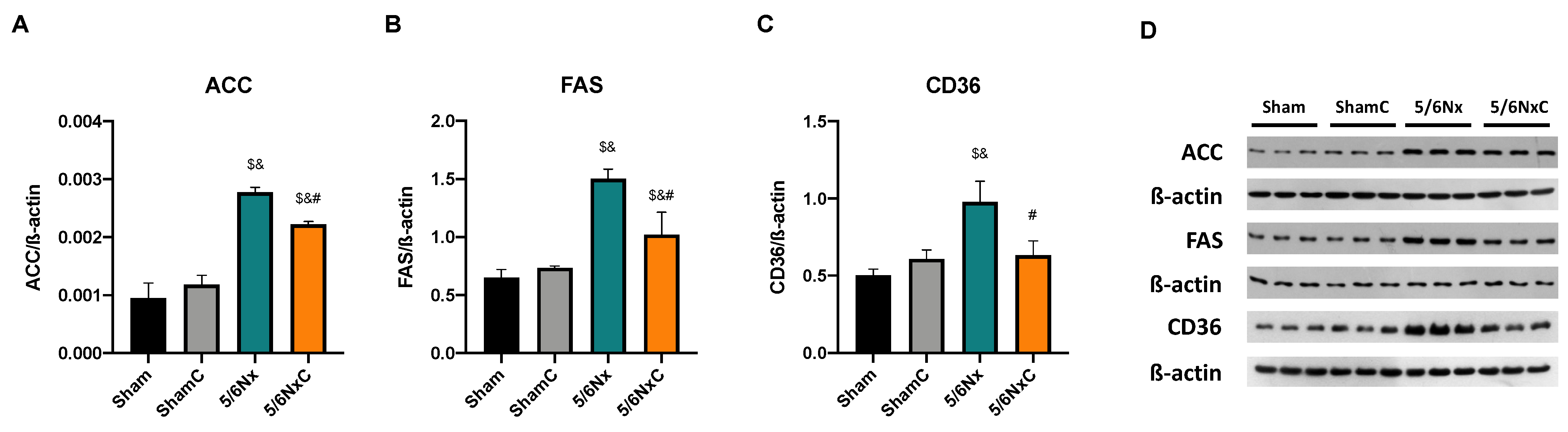
3.6.2. Hepatic Levels of Proteins Involved in Lipid Synthesis and Lipogenesis
3.6.3. Liver Mitochondrial β-Oxidation
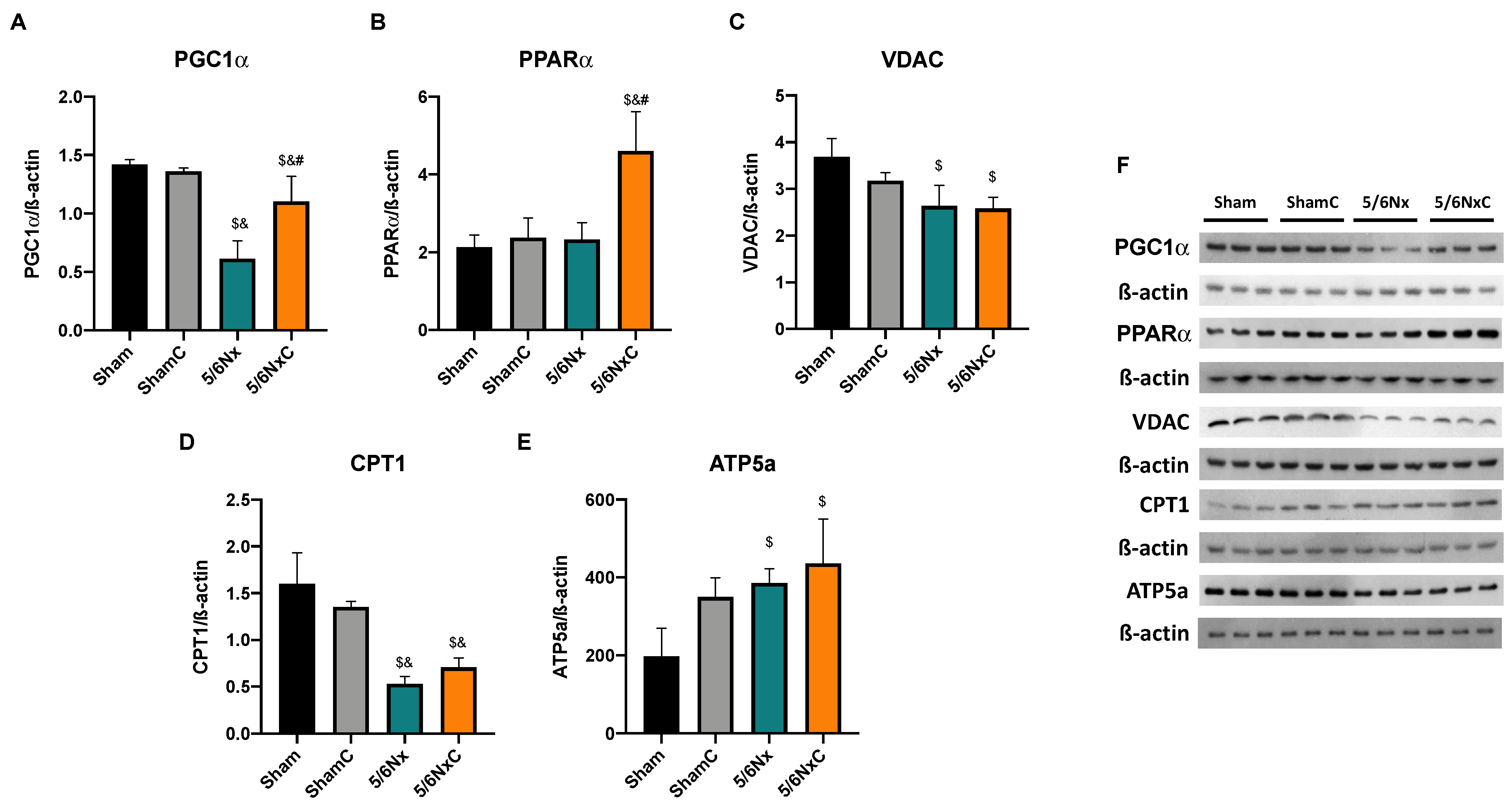
3.6.4. Hepatic Levels of Proteins Involved in Biogenesis and FA Transport in Mitochondria
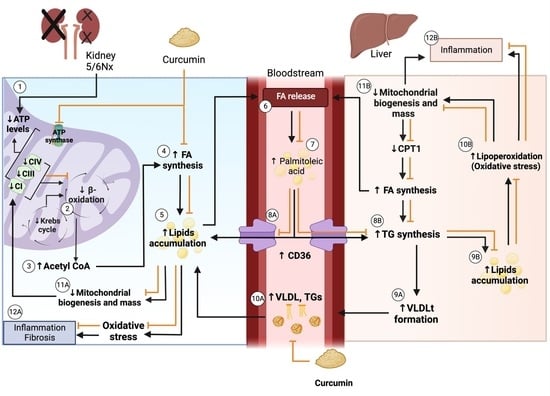
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, J.-C.; Zhang, L.-X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Mancini, G.B.J.; Hegele, R.A.; Leiter, L.A. Dyslipidemia. Can. J. Diabetes 2018, 42 (Suppl. S1), S178–S185. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, D.R. Lipoprotein Metabolism. Am. J. Kidney Dis. 1993, 22, 90–97. [Google Scholar] [CrossRef]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar] [PubMed]
- Barter, P. Lipoprotein Metabolism and CKD: Overview. Clin. Exp. Nephrol. 2014, 18, 243–246. [Google Scholar] [CrossRef]
- Vaziri, N.D. Role of Dyslipidemia in Impairment of Energy Metabolism, Oxidative Stress, Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Clin. Exp. Nephrol. 2014, 18, 265–268. [Google Scholar] [CrossRef]
- Hager, M.R.; Narla, A.D.; Tannock, L.R. Dyslipidemia in Patients with Chronic Kidney Disease. Rev. Endocr. Metab. Disord. 2017, 18, 29–40. [Google Scholar] [CrossRef]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef]
- Tan, R.-Z.; Zhong, X.; Li, J.-C.; Zhang, Y.-W.; Yan, Y.; Liao, Y.; Wen, D.; Diao, H.; Wang, L.; Shen, H.-C. An Optimized 5/6 Nephrectomy Mouse Model Based on Unilateral Kidney Ligation and Its Application in Renal Fibrosis Research. Ren. Fail. 2019, 41, 555–566. [Google Scholar] [CrossRef]
- Tapia, E.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; Pedraza-Chaverri, J. Curcumin Reverses Glomerular Hemodynamic Alterations and Oxidant Stress in 5/6 Nephrectomized Rats. Phytomedicine 2013, 20, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; García-Niño, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin Induces Nrf2 Nuclear Translocation and Prevents Glomerular Hypertension, Hyperfiltration, Oxidant Stress, and the Decrease in Antioxidant Enzymes in 5/6 Nephrectomized Rats. Oxid Med. Cell Longev. 2012, 2012, 269039. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Lipotoxicity and Impaired High Density Lipoprotein-Mediated Reverse Cholesterol Transport in Chronic Kidney Disease. J. Ren. Nutr. 2010, 20, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Norris, K.; Vaziri, N.D. Dysregulation of Hepatic Fatty Acid Metabolism in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2013, 28, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Kim, C.H.; Dang, B.; Zhan, C.-D.; Liang, K. Downregulation of Hepatic Acyl-CoA:Diglycerol Acyltransferase in Chronic Renal Failure. Am. J. Physiol. Ren. Physiol. 2004, 287, F90–F94. [Google Scholar] [CrossRef] [PubMed]
- Gava, A.L.; Freitas, F.P.; Balarini, C.M.; Vasquez, E.C.; Meyrelles, S.S. Effects of 5/6 Nephrectomy on Renal Function and Blood Pressure in Mice. Int. J. Physiol. Pathophysiol. Pharmacol. 2012, 4, 167–173. [Google Scholar]
- Correa, F.; Buelna-Chontal, M.; Hernández-Reséndiz, S.; García-Niño, W.R.; Roldán, F.J.; Soto, V.; Silva-Palacios, A.; Amador, A.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Curcumin Maintains Cardiac and Mitochondrial Function in Chronic Kidney Disease. Free Radic. Biol. Med. 2013, 61, 119–129. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Rojas-Morales, P.; Avila-Rojas, S.H.; León-Contreras, J.C.; Hernández-Pando, R.; Jiménez-Uribe, A.P.; Prieto-Carrasco, R.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Temporal Alterations in Mitochondrial β-Oxidation and Oxidative Stress Aggravate Chronic Kidney Disease Development in 5/6 Nephrectomy Induced Renal Damage. Int. J. Mol. Sci. 2020, 21, 6512. [Google Scholar] [CrossRef]
- Bolli, P. Treatment of Dyslipidemia: The Problem of Reaching the Goal. Atherosclerosis 2014, 236, 142–143. [Google Scholar] [CrossRef][Green Version]
- Okopień, B.; Buldak, L.; Bołdys, A. Fibrates in the Management of Atherogenic Dyslipidemia. Expert Rev. Cardiovasc. Ther. 2017, 15, 913–921. [Google Scholar] [CrossRef]
- Zingg, J.-M.; Hasan, S.T.; Meydani, M. Molecular Mechanisms of Hypolipidemic Effects of Curcumin. Biofactors 2013, 39, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a Potential Candidate for Treating Hyperlipidemia: A Review of Cellular and Metabolic Mechanisms. J. Cell Physiol. 2018, 233, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin Alleviates Oxidative Stress, Inflammation, and Renal Fibrosis in Remnant Kidney through the Nrf2-Keap1 Pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, H.; Razmovski-Naumovski, V.; Chang, D.; Nammi, S. Chronic Treatment of Curcumin Improves Hepatic Lipid Metabolism and Alleviates the Renal Damage in Adenine-Induced Chronic Kidney Disease in Sprague-Dawley Rats. BMC Nephrol. 2019, 20, 431. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morales-Martínez, A.; Zamorano-Carrillo, A.; Montes, S.; El-Hafidi, M.; Sánchez-Mendoza, A.; Soria-Castro, E.; Martínez-Lazcano, J.C.; Martínez-Gopar, P.E.; Ríos, C.; Pérez-Severiano, F. Rich Fatty Acids Diet of Fish and Olive Oils Modifies Membrane Properties in Striatal Rat Synaptosomes. Nutr. Neurosci. 2021, 24, 1–12. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Avila-Rojas, S.H.; Tapia, E.; Rojas-Morales, P.; León-Contreras, J.C.; Martínez-Klimova, E.; Hernández-Pando, R.; Sánchez- Lozada, L.G.; Pedraza-Chaverri, J. Chronic Impairment of Mitochondrial Bioenergetics and β-Oxidation Promotes Experimental AKI-to-CKD Transition Induced by Folic Acid. Free Radic. Biol. Med. 2020, 154, 18–32. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Reyes-Fermín, L.M.; Briones-Herrera, A.; Tapia, E.; León-Contreras, J.C.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J. Protective Effects of N-Acetyl-Cysteine in Mitochondria Bioenergetics, Oxidative Stress, Dynamics and S-Glutathionylation Alterations in Acute Kidney Damage Induced by Folic Acid. Free Radic Biol. Med. 2019, 130, 379–396. [Google Scholar] [CrossRef]
- Sekine, T.; Endou, H. Solute Transport, Energy Consumption, and Production in the Kidney. In Seldin and Giebisch’s The Kidney; Elsevier: Amsterdam, The Netherlands, 2013; pp. 143–175. [Google Scholar]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial Energetics in the Kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin Ameliorates Kidney Function and Oxidative Stress in Experimental Chronic Kidney Disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin Prevents Mitochondrial Dynamics Disturbances in Early 5/6 Nephrectomy: Relation to Oxidative Stress and Mitochondrial Bioenergetics. BioFactors 2017, 43, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Sánchez-Lozada, L.G.; García-Niño, W.R.; García, E.; Cerecedo, A.; García-Arroyo, F.E.; Osorio, H.; Arellano, A.; Cristóbal-García, M.; Loredo, M.L.; et al. Curcumin Prevents Maleate-Induced Nephrotoxicity: Relation to Hemodynamic Alterations, Oxidative Stress, Mitochondrial Oxygen Consumption and Activity of Respiratory Complex I. Free Radic. Res. 2014, 48, 1342–1354. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, S.; Correa, F.; García-Niño, W.R.; Buelna-Chontal, M.; Roldán, F.J.; Ramírez-Camacho, I.; Delgado-Toral, C.; Carbó, R.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Cardioprotection by Curcumin Post-Treatment in Rats with Established Chronic Kidney Disease. Cardiovasc. Drugs Ther. 2015, 29, 111–120. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin Ameliorates CKD-Induced Mitochondrial Dysfunction and Oxidative Stress through Inhibiting GSK-3β Activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef]
- Harris, R.B. Leptin–Much More than a Satiety Signal. Annu. Rev. Nutr. 2000, 20, 45–75. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointestin. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef]
- Nejati-Koshki, K.; Akbarzadeh, A.; Pourhassan-Moghaddam, M. Curcumin Inhibits Leptin Gene Expression and Secretion in Breast Cancer Cells by Estrogen Receptors. Cancer Cell Int. 2014, 14, 66. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, S.; Chen, A. Curcumin Eliminates Leptin’s Effects on Hepatic Stellate Cell Activation via Interrupting Leptin Signaling. Endocrinology 2009, 150, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.; Siliart, B.; Dumon, H. Liver Lipid Metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Moradi, H.; Vaziri, N.D. Lipid Disorders Associated with Chronic Kidney Disease and Nephrotic Syndrome. In Endocrine Disorders in Kidney Disease; Springer: Cham, Switzerland, 2019; pp. 153–169. [Google Scholar]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective Effect of the Antioxidant Curcumin: Recent Findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, T.H.; Olson, J.L.; Renke, H.G.; Venkatachalam, M.A.; Brenner, B.M. Hyperfiltration in Remnant Nephrons: A Potentially Adverse Response to Renal Ablation. J. Am. Soc. Nephrol. 2001, 12, 1315–1325. [Google Scholar] [CrossRef]
- Brenner, B.M. Nephron Adaptation to Renal Injury or Ablation. Am. J. Physiol. 1985, 249, F324–F337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Liu, Z.; Tang, D.; Chen, X.; Chen, Y.; Zhou, R.; Chen, S.; Niu, H. Role of Mitochondrial Dysfunction in Renal Fibrosis Promoted by Hypochlorite-Modified Albumin in a Remnant Kidney Model and Protective Effects of Antioxidant Peptide SS-31. Eur. J. Pharmacol. 2017, 804, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, L.V.; Tamirisa, A.; Kennedy, D.J.; Haller, S.T.; Budnyy, G.; Shapiro, J.I.; Malhotra, D. Mitochondrial Impairment in the Five-Sixth Nephrectomy Model of Chronic Renal Failure: Proteomic Approach. BMC Nephrol. 2013, 14, 209–230. [Google Scholar] [CrossRef]
- Hui, Y.; Lu, M.; Han, Y.; Zhou, H.; Liu, W.; Li, L.; Jin, R. Resveratrol Improves Mitochondrial Function in the Remnant Kidney from 5/6 Nephrectomized Rats. Acta Histochem. 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial Dysfunction in Diabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Tapia, E.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J. Mitochondrial Bioenergetics, Redox State, Dynamics and Turnover Alterations in Renal Mass Reduction Models of Chronic Kidney Diseases and Their Possible Implications in the Progression of This Illness. Pharmacol. Res. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Martínez-Klimova, E.; Aparicio-Trejo, O.E.; Gómez-Sierra, T.; Jiménez-Uribe, A.P.; Bellido, B.; Pedraza-Chaverri, J. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in the Promotion of Fibrosis in Obstructive Nephropathy Induced by Unilateral Ureteral Obstruction. BioFactors 2020, 46, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, J.; Purkerson, M.L.; Klahr, S. Effect of Unilateral Ureteral Obstruction on Metabolism of Renal Lipids in the Rat. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1983, 14, F254–F262. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective Fatty Acid Oxidation in Renal Tubular Epithelial Cells Has a Key Role in Kidney Fibrosis Development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Aranda-Rivera, A.K.; Osorio-Alonso, H.; Martínez-Klimova, E.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Extracellular Vesicles in Redox Signaling and Metabolic Regulation in Chronic Kidney Disease. Antioxidants 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Noh, M.R.; Kim, J.; Padanilam, B.J. Defective Mitochondrial Fatty Acid Oxidation and Lipotoxicity in Kidney Diseases. Front. Med. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Tapia, E.; Sánchez-Lozada, L.G.; García-Arroyo, F.E.; Amador-Martínez, I.; Orozco-Ibarra, M.; Fernández-Valverde, F.; Pedraza-Chaverri, J. Sulforaphane Protects against Unilateral Ureteral Obstruction-Induced Renal Damage in Rats by Alleviating Mitochondrial and Lipid Metabolism Impairment. Antioxidants 2022, 11, 1854. [Google Scholar] [CrossRef]
- Szeto, H.H. Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 2856–2865. [Google Scholar] [CrossRef]
- Jiménez-Uribe, A.P.; Hernández-Cruz, E.Y.; Ramírez-Magaña, K.J.; Pedraza-Chaverri, J. Involvement of Tricarboxylic Acid Cycle Metabolites in Kidney Diseases. Biomolecules 2021, 11, 1259. [Google Scholar] [CrossRef]
- Hallan, S.; Afkarian, M.; Zelnick, L.R.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-Regulation of the Citric Acid (TCA) Cycle in Non-Diabetic CKD Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef]
- Stallons, L.J.; Whitaker, R.M.; Schnellmann, R.G. Suppressed Mitochondrial Biogenesis in Folic Acid-Induced Acute Kidney Injury and Early Fibrosis. Toxicol. Lett. 2014, 224, 326–332. [Google Scholar] [CrossRef]
- Stadler, K.; Goldberg, I.J.; Susztak, K. The Evolving Understanding of the Contribution of Lipid Metabolism to Diabetic Kidney Disease. Curr. Diab. Rep. 2015, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and Fatty Acids in Chronic Kidney Disease: Molecular and Metabolic Alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Hauck, A.K.; Bernlohr, D.A. Oxidative Stress and Lipotoxicity. J. Lipid Res. 2016, 57, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid Signaling and Lipotoxicity in Metaflammation: Indications for Metabolic Disease Pathogenesis and Treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Ibdah, J. Role of Mitochondria in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of Reactive Oxygen Species Generation by Mitochondria Oxidizing Different Substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef]





| Parameter | Groups | |||
|---|---|---|---|---|
| Sham | ShamC | 5/6Nx | 5/6NxC | |
| Water consumption (mL/day) | 38.42 ± 11.67 | 62.50 ± 5.93 | 68.50 ± 7.50 $ | 72.17 ± 4.80 $& |
| Food consumption (g/day) | 25.92 ± 2.11 | 26.50 ± 1.60 | 23.17 ± 1.80 $& | 25.25 ± 1.96 # |
| Body weight (g) | 367.30 ± 35.55 | 397.40 ± 21.00 | 316.00 ± 24.49 $& | 360.40 ± 30.43 $&# |
| Parameter | Groups | |||
|---|---|---|---|---|
| Sham | ShamC | 5/6Nx | 5/6NxC | |
| Triglycerides (mg/dL) | 66.44 ± 21.32 | 56.75 ± 9.824 | 84.63 ± 20.66 & | 63.62 ± 9.43 # |
| Cholesterol (mg/dL) | 43.77 ± 4.574 | 42.43 ± 6.867 | 87.28 ± 14.99 $& | 79.54 ± 13.19 $& |
| HDLc (mg/dL) | 30.48 ± 4.523 | 29.09 ± 3.555 | 61.2 ± 9.517 $& | 59.62 ± 10.55 $& |
| LDLc (mg/dL) | 11.16 ± 2.363 | 11.44 ± 3.863 | 23.65 ± 5.918 $& | 22.58 ± 6.578 $& |
| VLDLc (mg/dL) | 2.122 ± 1.91 | 2.334 ± 1.98 | 4.267 ± 3.805 | 5.263 ± 7.382 |
| VLDLt (mg/dL) | 11.36 ± 5.377 | 11.35 ± 1.963 | 16.92 ± 4.133 & | 12.72 ± 3.886 # |
| Fatty Acids (mol/L) | Groups | |||
|---|---|---|---|---|
| Sham | ShamC | 5/6Nx | 5/6NxC | |
| Lauric acid (C12) | 0.005 ± 0.009 | 0.006 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 & |
| Miristic acid (C14) | 0.004 ± 0.002 | 0.004 ± 0.002 | 0.006 ± 0.003 | 0.004 ± 0.001 |
| Palmitic acid (C16) | 0.119 ± 0.039 | 0.113 ± 0.011 | 0.193 ± 0.046 & | 0.169 ± 0.023 $& |
| Palmitoleic acid (C16:1n-7) | 0.002 ± 0.001 | 0.004 ± 0.002 | 0.012 ± 0.002 $& | 0.006 ± 0.002 $# |
| Estearic acid (C18) | 0.057 ± 0.014 | 0.052 ± 0.006 | 0.093 ± 0.019 $& | 0.088 ± 0.018 $& |
| Oleic acid (C18:1n-9) | 0.054 ± 0.028 | 0.050 ± 0.010 | 0.091 ± 0.036 & | 0.076 ± 0.019 |
| Linoleic acid (C18:2n-6) | 0.083 ± 0.034 | 0.079 ± 0.015 | 0.160 ± 0.037 $& | 0.125 ± 0.022 & |
| α-Linolenic acid (C18:3n-3) | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.004 ± 0.002 | 0.002 ± 0.001 |
| γ-Linolenic acid (C18:3n-6) | 0.001 ± 0.0004 | 0.002 ± 0.0003 | 0.002 ± 0.001 | 0.002 ± 0.0003 |
| Dihomo-gamma-linolenic acid (C20:3n-6) | 0.001 ± 0.0007 | 0.002 ± 0.0009 | 0.002 ± 0.0009 | 0.002 ± 0.0002 |
| Arachidonic acid (C20) | 0.068 ± 0.012 | 0.049 ± 0.008 | 0.133 ± 0.029 $& | 0.099 ± 0.029 & |
| Saturated fatty acids | 0.210 ± 0.058 | 0.180 ± 0.022 | 0.299 ± 0.068 $& | 0.255 ± 0.039 & |
| Unsaturated fatty acids | 0.063 ± 0.029 | 0.051 ± 0.013 | 0.115 ± 0.031 $& | 0.075 ± 0.011 # |
| Polyunsaturated fatty acids | 0.093 ± 0.038 | 0.087 ± 0.013 | 0.169 ± 0.040 $& | 0.129 ± 0.023 |
| Total | 0.419 ± 0.151 | 0.378 ± 0.056 | 0.680 ± 0.182 $& | 0.552 ± 0.082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceja-Galicia, Z.A.; García-Arroyo, F.E.; Aparicio-Trejo, O.E.; El-Hafidi, M.; Gonzaga-Sánchez, G.; León-Contreras, J.C.; Hernández-Pando, R.; Guevara-Cruz, M.; Tovar, A.R.; Rojas-Morales, P.; et al. Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver. Antioxidants 2022, 11, 2195. https://doi.org/10.3390/antiox11112195
Ceja-Galicia ZA, García-Arroyo FE, Aparicio-Trejo OE, El-Hafidi M, Gonzaga-Sánchez G, León-Contreras JC, Hernández-Pando R, Guevara-Cruz M, Tovar AR, Rojas-Morales P, et al. Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver. Antioxidants. 2022; 11(11):2195. https://doi.org/10.3390/antiox11112195
Chicago/Turabian StyleCeja-Galicia, Zeltzin Alejandra, Fernando Enrique García-Arroyo, Omar Emiliano Aparicio-Trejo, Mohammed El-Hafidi, Guillermo Gonzaga-Sánchez, Juan Carlos León-Contreras, Rogelio Hernández-Pando, Martha Guevara-Cruz, Armando R. Tovar, Pedro Rojas-Morales, and et al. 2022. "Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver" Antioxidants 11, no. 11: 2195. https://doi.org/10.3390/antiox11112195
APA StyleCeja-Galicia, Z. A., García-Arroyo, F. E., Aparicio-Trejo, O. E., El-Hafidi, M., Gonzaga-Sánchez, G., León-Contreras, J. C., Hernández-Pando, R., Guevara-Cruz, M., Tovar, A. R., Rojas-Morales, P., Aranda-Rivera, A. K., Sánchez-Lozada, L. G., Tapia, E., & Pedraza-Chaverri, J. (2022). Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver. Antioxidants, 11(11), 2195. https://doi.org/10.3390/antiox11112195