The Effect Citrox BCL on Legionella pneumophila Mechanisms of Biofilm Formation, Oxidative Stress and Virulence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of Minimum Inhibitory and Minimum Bactericidal Concentration
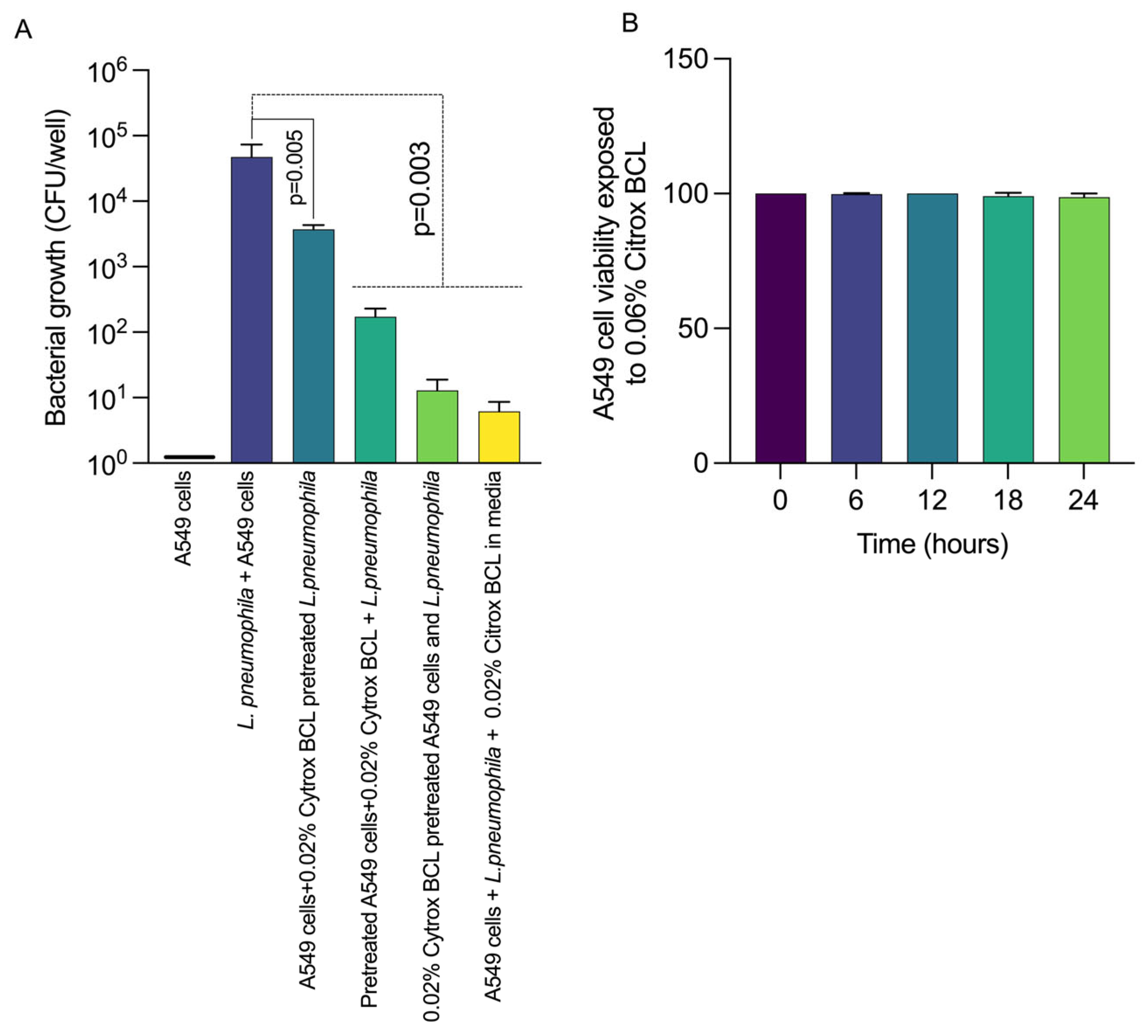
2.2. Infection and Cytotoxicity Assay
2.3. c-di-GMP Assay
2.4. Biofilm Microtiter Plate Assay
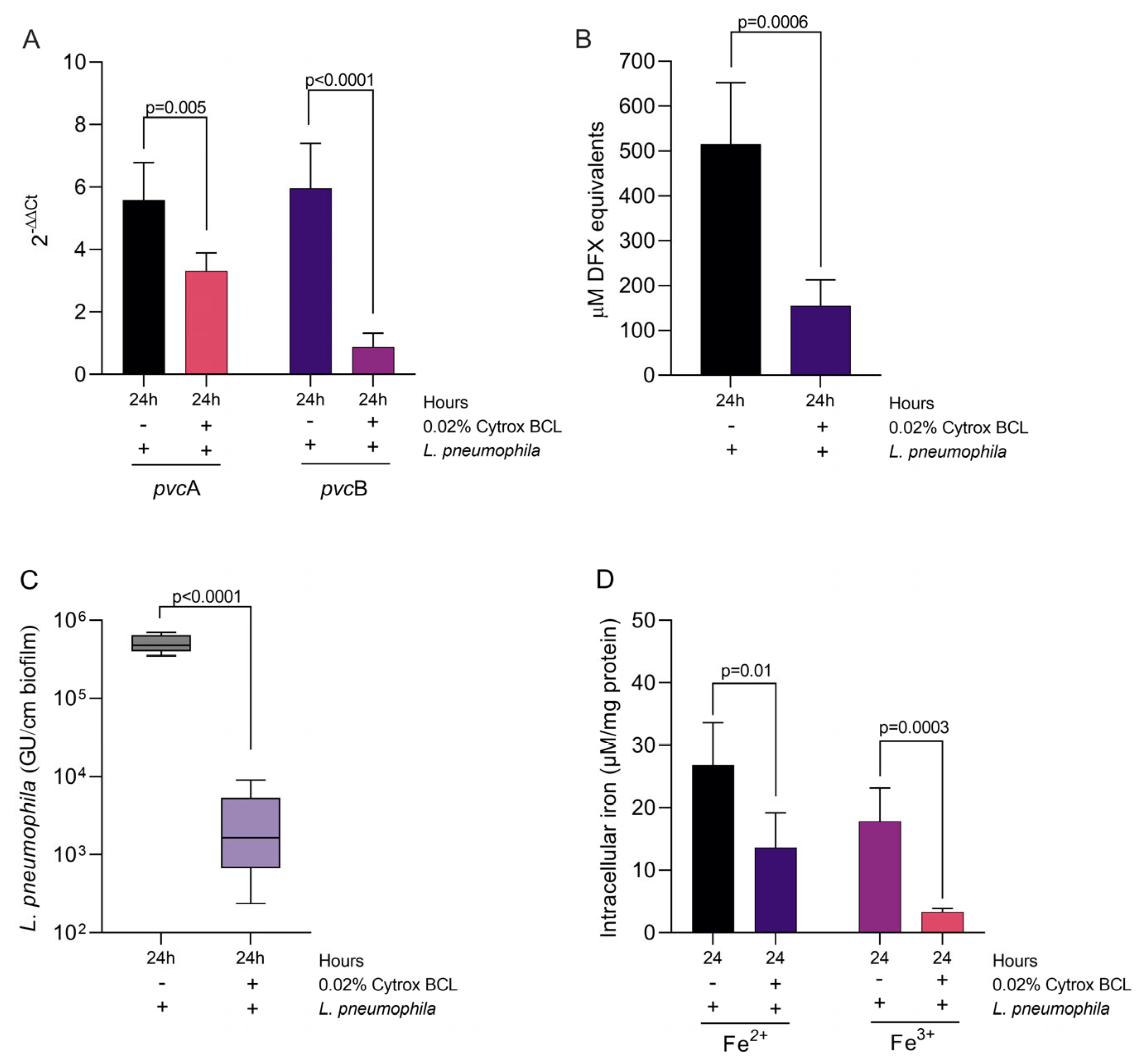
2.5. RNA Extraction and qRT-PCR
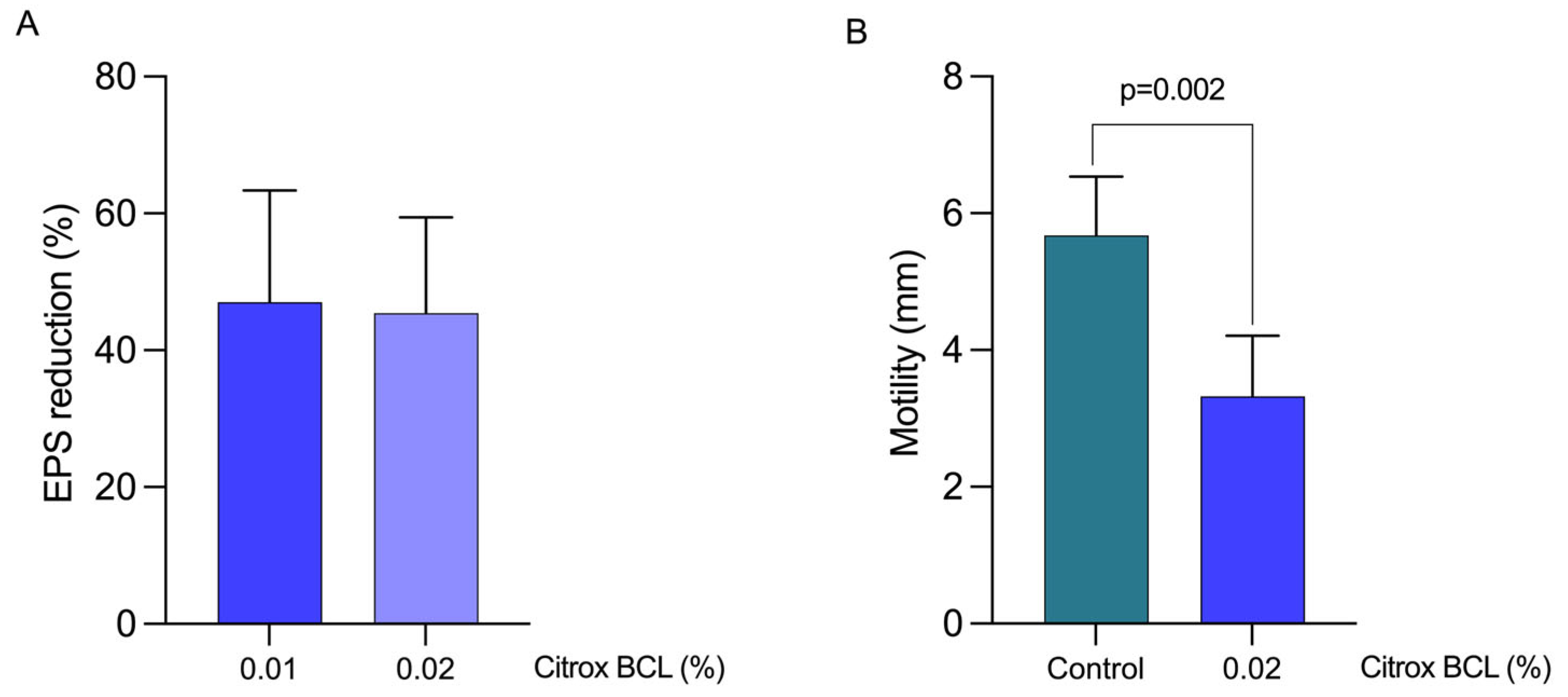
2.6. Exopolysaccharide (EPS) Measurement and Motility Assay
2.7. Intracellular ROS in L. pneumophila SG68703154 Infected A549 Cells
2.8. Siderophore and Intracellular Iron Measurement
3. Results
3.1. Citrox BCL Effect on L. pneumophilia Growth, Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.2. Citrox BCL Reduces L. pneumophila Infection of A549 Cells
3.3. Citrox BCL Mediates Biofilm Formation and tetB and tetC Biofilm Related Gene Expression during Infection
3.4. Impact on EPS Production and Bacterial Motility
3.5. Impact on Iron Sequestration and Siderophore Production
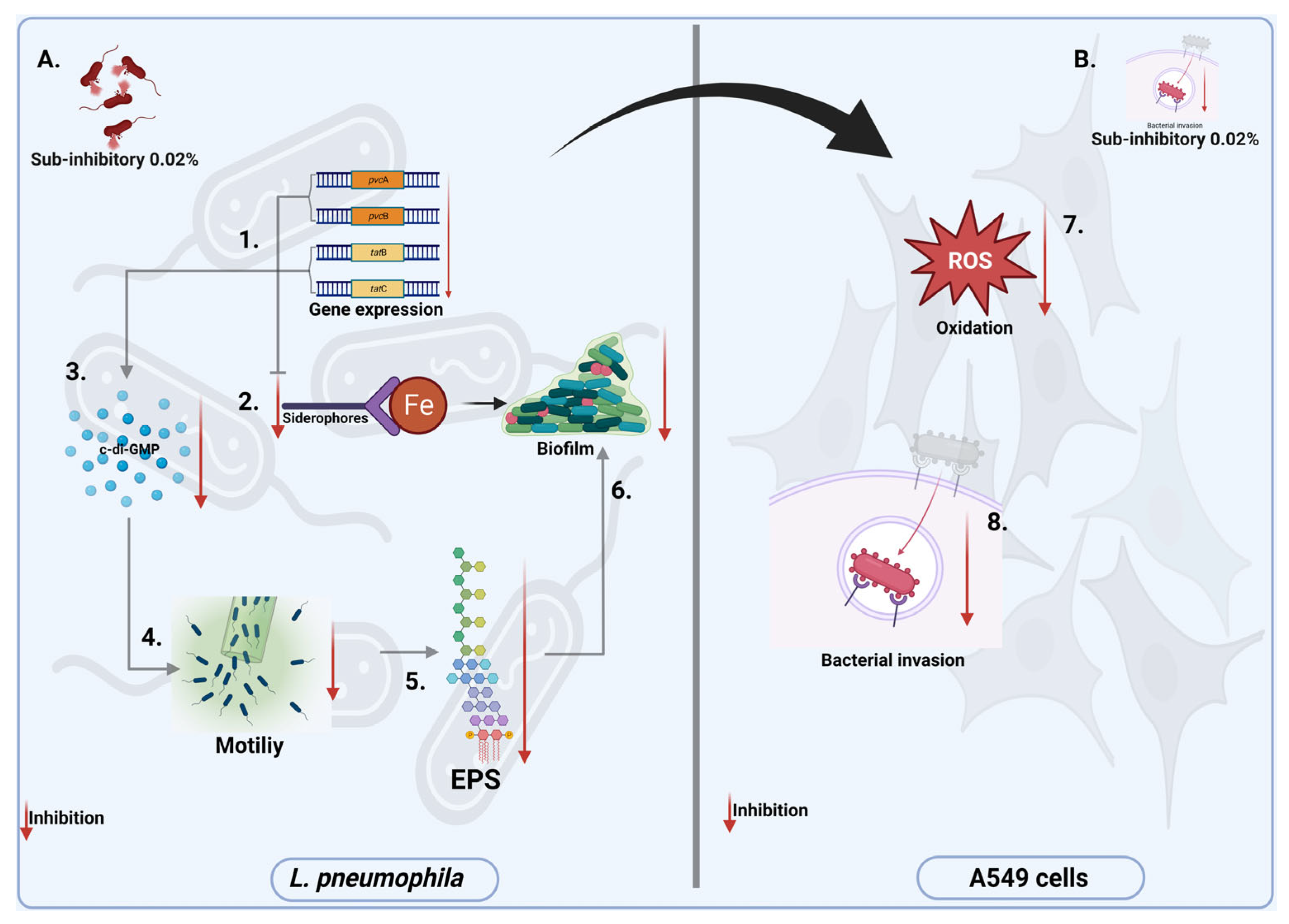
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mraz, A.L.; Weir, M.H. Knowledge to Predict Pathogens: Legionella pneumophila Lifecycle Critical Review Part I Uptake into Host Cells. Water 2018, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Jomehzadeh, N.; Moosavian, M.; Saki, M.; Rashno, M. Legionella and legionnaires’ disease: An overview. J. Acute Dis. 2019, 8, 221. [Google Scholar]
- Boamah, D.K.; Zhou, G.; Ensminger, A.W.; O’Connor, T.J. From Many Hosts, One Accidental Pathogen: The Diverse Protozoan Hosts of Legionella. Front. Cell. Infect. Microbiol. 2017, 7, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palusińska-Szysz, M.; Jurak, M.; Gisch, N.; Waldow, F.; Zehethofer, N.; Nehls, C.; Schwudke, D.; Koper, P.; Mazur, A. The human LL-37 peptide exerts antimicrobial activity against Legionella micdadei interacting with membrane phospholipids. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2022, 1867, 159138. [Google Scholar] [CrossRef] [PubMed]
- Crépin, A.; Jégou, J.-F.; André, S.; Ecale, F.; Croitoru, A.; Cantereau, A.; Berjeaud, J.-M.; Ladram, A.; Verdon, J. In vitro and intracellular activities of frog skin temporins against Legionella pneumophila and its eukaryotic hosts. Sci. Rep. 2020, 10, 3978. [Google Scholar] [CrossRef] [Green Version]
- Falkinham, J.O. Living with Legionella and Other Waterborne Pathogens. Microorganisms 2020, 8, 2026. [Google Scholar] [CrossRef]
- Khweek, A.; Amer, A. Biofilm, a Cozy Structure for Legionella pneumophila Growth and Persistence in the Environment. In Bacterial Biofilms; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Abu Khweek, A.; Amer, A.O. Factors Mediating Environmental Biofilm Formation by Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 8, 38. [Google Scholar] [CrossRef]
- Chauhan, D.; Shames, S.R. Pathogenicity and Virulence of Legionella: Intracellular replication and host response. Virulence 2021, 12, 1122–1144. [Google Scholar] [CrossRef]
- Hooper, S.J.; Lewis, M.A.O.; Wilson, M.J.; Williams, D.W. Antimicrobial activity of Citrox® bioflavonoid preparations against oral microorganisms. Br. Dent. J. 2011, 210, E22-E22. [Google Scholar] [CrossRef] [Green Version]
- Yehia, H.M.; Al-Masoud, A.H.; Elkhadragy, M.F.; Korany, S.M.; Nada, H.M.S.; Albaridi, N.A.; Alzahrani, A.A.; Al-Dagal, M.M. Improving the Quality and Safety of Fresh Camel Meat Contaminated with Campylobacter jejuni Using Citrox, Chitosan, and Vacuum Packaging to Extend Shelf Life. Animals 2021, 11, 1152. [Google Scholar] [CrossRef]
- Balta, I.; Linton, M.; Pinkerton, L.; Kelly, C.; Stef, L.; Pet, I.; Stef, D.; Criste, A.; Gundogdu, O.; Corcionivoschi, N. The effect of natural antimicrobials against Campylobacter spp. and its similarities to Salmonella spp, Listeria spp., Escherichia coli, Vibrio spp., Clostridium spp. and Staphylococcus spp. Food Control 2021, 121, 107745. [Google Scholar] [CrossRef]
- Balta, I.; Linton, M.; Pinkerton, L.; Kelly, C.; Ward, P.; Stef, L.; Pet, I.; Horablaga, A.; Gundogdu, O.; Corcionivoschi, N. The effect of natural antimicrobials on the Campylobacter coli T6SS+/− during in vitro infection assays and on their ability to adhere to chicken skin and carcasses. Int. J. Food Microbiol. 2021, 338, 108998. [Google Scholar] [CrossRef]
- Pinkerton, L.; Linton, M.; Kelly, C.; Ward, P.; Gradisteanu Pircalabioru, G.; Pet, I.; Stef, L.; Sima, F.; Adamov, T.; Gundogdu, O.; et al. Attenuation of Vibrio parahaemolyticus Virulence Factors by a Mixture of Natural Antimicrobials. Microorganisms 2019, 7, 679. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, O.; Turasay, B. Removing Legionella pneumophila and biofilms from water supply systems using plant essential oils. J. Water Sanit. Hyg. Dev. 2017, 7, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Gelmini, F.; Testa, C.; Angioletti, S.; Beretta, G. Essential Oils in the Environmental Bacteria Control: The Legionella Pneumophila case. J. Complement. Med. Altern. Healthc. 2017, 2. [Google Scholar] [CrossRef]
- Bićanić, L.; Mežnarić, S.; Gobin, I. Antimicrobial activity of the volatile phase of essential oils and their constituents on. Sanit. Inženirstvo Int. J. Sanit. Eng. Res. 2020, 14, 54–61. [Google Scholar] [CrossRef]
- Balta, I.; Marcu, A.; Linton, M.; Kelly, C.; Stef, L.; Pet, I.; Ward, P.; Pircalabioru, G.G.; Chifiriuc, C.; Gundogdu, O.; et al. The in vitro and in vivo anti-virulent effect of organic acid mixtures against Eimeria tenella and Eimeria bovis. Sci. Rep. 2021, 11, 16202. [Google Scholar] [CrossRef]
- Sima, F.; Stratakos, A.C.; Ward, P.; Linton, M.; Kelly, C.; Pinkerton, L.; Stef, L.; Gundogdu, O.; Lazar, V.; Corcionivoschi, N. A Novel Natural Antimicrobial Can Reduce the in vitro and in vivo Pathogenicity of T6SS Positive Campylobacter jejuni and Campylobacter coli Chicken Isolates. Front. Microbiol. 2018, 9, 2139. [Google Scholar] [CrossRef] [Green Version]
- Balta, I.; Stef, L.; Butucel, E.; Gradisteanu Pircalabioru, G.; Venig, A.; Ward, P.; Deshaies, M.; Pet, I.; Stef, D.; Koyun, O.Y.; et al. The Antioxidant Effect of Natural Antimicrobials in Shrimp Primary Intestinal Cells Infected with Nematopsis messor. Antioxidants (Basel) 2022, 11, 974. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Liu, J.; Chen, Y.; Zhou, X.; Ru, Y.; Zhu, J.; Liu, W. cAMP and c-di-GMP synergistically support biofilm maintenance through the direct interaction of their effectors. Nat. Commun. 2022, 13, 1493. [Google Scholar] [CrossRef]
- De Buck, E.; Lebeau, I.; Maes, L.; Geukens, N.; Meyen, E.; Van Mellaert, L.; Anné, J.; Lammertyn, E. A putative twin-arginine translocation pathway in Legionella pneumophila. Biochem. Biophys. Res. Commun. 2004, 317, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Mou, Q.; Leung, P.H.M. Differential expression of virulence genes in Legionella pneumophila growing in Acanthamoeba and human monocytes. Virulence 2018, 9, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portier, E.; Bertaux, J.; Labanowski, J.; Hechard, Y. Iron Availability Modulates the Persistence of Legionella pneumophila in Complex Biofilms. Microbes Environ. 2016, 31, 387–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratakos, A.C.; Linton, M.; Ward, P.; Campbell, M.; Kelly, C.; Pinkerton, L. The antimicrobial effect of a commercial mixture of natural antimicrobials against Escherichia coli O157:H7. Foodborne Pathog. Dis. 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, J.; Ünal, C.M.; Thiem, S.; Grimpe, L.; Goldmann, T.; Gaßler, N.; Richter, M.; Shevchuk, O.; Steinert, M. PilY1 Promotes Legionella pneumophila Infection of Human Lung Tissue Explants and Contributes to Bacterial Adhesion, Host Cell Invasion, and Twitching Motility. Front. Cell. Infect. Microbiol. 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Xu, X.; Wang, Y.; Shen, X.; Chen, Z.; Yang, J. Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A549 human lung carcinoma cells. Infect. Immun. 2008, 76, 4405–4413. [Google Scholar] [CrossRef] [Green Version]
- Starkenburg, S.R.; Casey, J.M.; Cianciotto, N.P. Siderophore activity among members of the Legionella genus. Curr. Microbiol. 2004, 49, 203–207. [Google Scholar] [CrossRef]
- Payne, S.M. Detection, isolation, and characterization of siderophores. Methods Enzymol. 1994, 235, 329–344. [Google Scholar] [CrossRef]
- Liles, M.R.; Scheel, T.A.; Cianciotto, N.P. Discovery of a nonclassical siderophore, legiobactin, produced by strains of Legionella pneumophila. J. Bacteriol. 2000, 182, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Andrews, K.J.; Jeon, B. Enhanced Biofilm Formation by Ferrous and Ferric Iron Through Oxidative Stress in Campylobacter jejuni. Front. Microbiol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef]
- Chang, C.W.; Chang, W.L.; Chang, S.T.; Cheng, S.S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286. [Google Scholar] [CrossRef]
- Laird, K.; Kurzbach, E.; Score, J.; Tejpal, J.; Chi Tangyie, G.; Phillips, C. Reduction of Legionella spp. in water and in soil by a citrus plant extract vapor. Appl. Environ. Microbiol. 2014, 80, 6031–6036. [Google Scholar] [CrossRef] [Green Version]
- Tamayo, R.; Pratt, J.T.; Camilli, A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 2007, 61, 131–148. [Google Scholar] [CrossRef] [Green Version]
- De Buck, E.; Maes, L.; Meyen, E.; Van Mellaert, L.; Geukens, N.; Anne, J.; Lammertyn, E. Legionella pneumophila Philadelphia-1 tatB and tatC affect intracellular replication and biofilm formation. Biochem. Biophys. Res. Commun. 2005, 331, 1413–1420. [Google Scholar] [CrossRef]
- Fernandez, N.L.; Waters, C.M. Cyclic di-GMP Increases Catalase Production and Hydrogen Peroxide Tolerance in Vibrio cholerae. Appl. Environ. Microbiol. 2019, 85, e01043-19. [Google Scholar] [CrossRef] [Green Version]
- Oglesby-Sherrouse, A.G.; Djapgne, L.; Nguyen, A.T.; Vasil, A.I.; Vasil, M.L. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014, 70, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Mraz, A.L.; Weir, M.H. Knowledge to Predict Pathogens: Legionella pneumophila Lifecycle Systematic Review Part II Growth within and Egress from a Host Cell. Microorganisms 2022, 10, 141. [Google Scholar] [CrossRef]
- Appelt, S.; Heuner, K. The Flagellar Regulon of Legionella—A Review. Front. Cell. Infect. Microbiol. 2017, 7, 454. [Google Scholar] [CrossRef]
- Personnic, N.; Striednig, B.; Hilbi, H. Quorum sensing controls persistence, resuscitation, and virulence of Legionella subpopulations in biofilms. ISME J. 2021, 15, 196–210. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butucel, E.; Balta, I.; McCleery, D.; Popescu, C.A.; Iancu, T.; Pet, I.; Marcu, A.; Horablaga, N.-M.; Stef, L.; Corcionivoschi, N. The Effect Citrox BCL on Legionella pneumophila Mechanisms of Biofilm Formation, Oxidative Stress and Virulence. Antioxidants 2022, 11, 2186. https://doi.org/10.3390/antiox11112186
Butucel E, Balta I, McCleery D, Popescu CA, Iancu T, Pet I, Marcu A, Horablaga N-M, Stef L, Corcionivoschi N. The Effect Citrox BCL on Legionella pneumophila Mechanisms of Biofilm Formation, Oxidative Stress and Virulence. Antioxidants. 2022; 11(11):2186. https://doi.org/10.3390/antiox11112186
Chicago/Turabian StyleButucel, Eugenia, Igori Balta, David McCleery, Cosmin Alin Popescu, Tiberiu Iancu, Ioan Pet, Adela Marcu, Nicolae-Marinel Horablaga, Lavinia Stef, and Nicolae Corcionivoschi. 2022. "The Effect Citrox BCL on Legionella pneumophila Mechanisms of Biofilm Formation, Oxidative Stress and Virulence" Antioxidants 11, no. 11: 2186. https://doi.org/10.3390/antiox11112186
APA StyleButucel, E., Balta, I., McCleery, D., Popescu, C. A., Iancu, T., Pet, I., Marcu, A., Horablaga, N.-M., Stef, L., & Corcionivoschi, N. (2022). The Effect Citrox BCL on Legionella pneumophila Mechanisms of Biofilm Formation, Oxidative Stress and Virulence. Antioxidants, 11(11), 2186. https://doi.org/10.3390/antiox11112186









