Involvement of Mitochondrial Dysfunction in the Inflammatory Response in Human Mesothelial Cells from Peritoneal Dialysis Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture and Stimulation
2.3. Mitochondrial Membrane Potential Assessment
2.4. Determination of COX-2 and IL-8 mRNA Expression
2.5. PGE2 and IL-8 Assays
2.6. Immunofluorescence Detection of p65
2.7. Statistical Analysis
3. Results
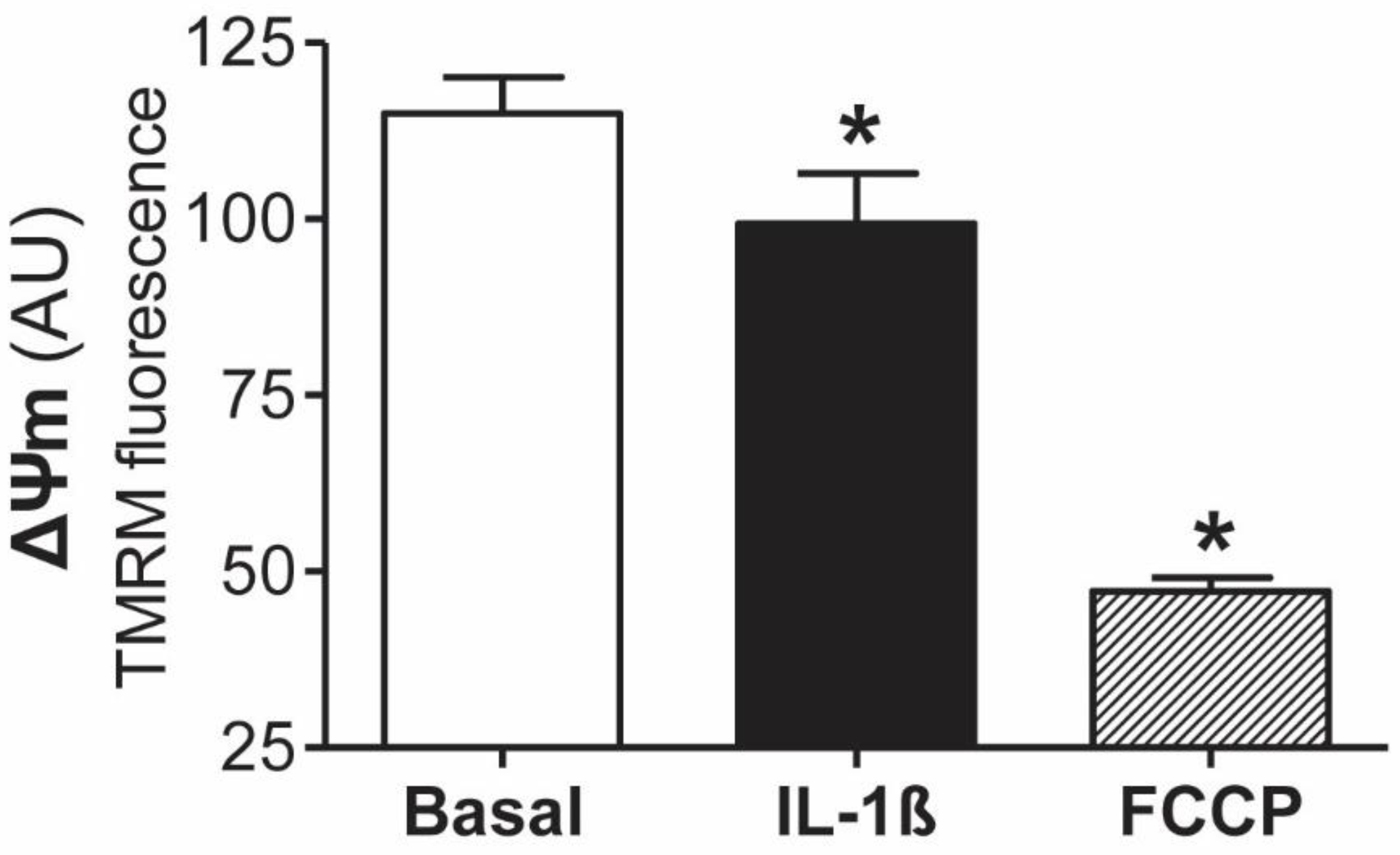
3.1. Involvement of Mitochondrial Dysfunction in the Inflammatory Response of Human Mesothelial Cells
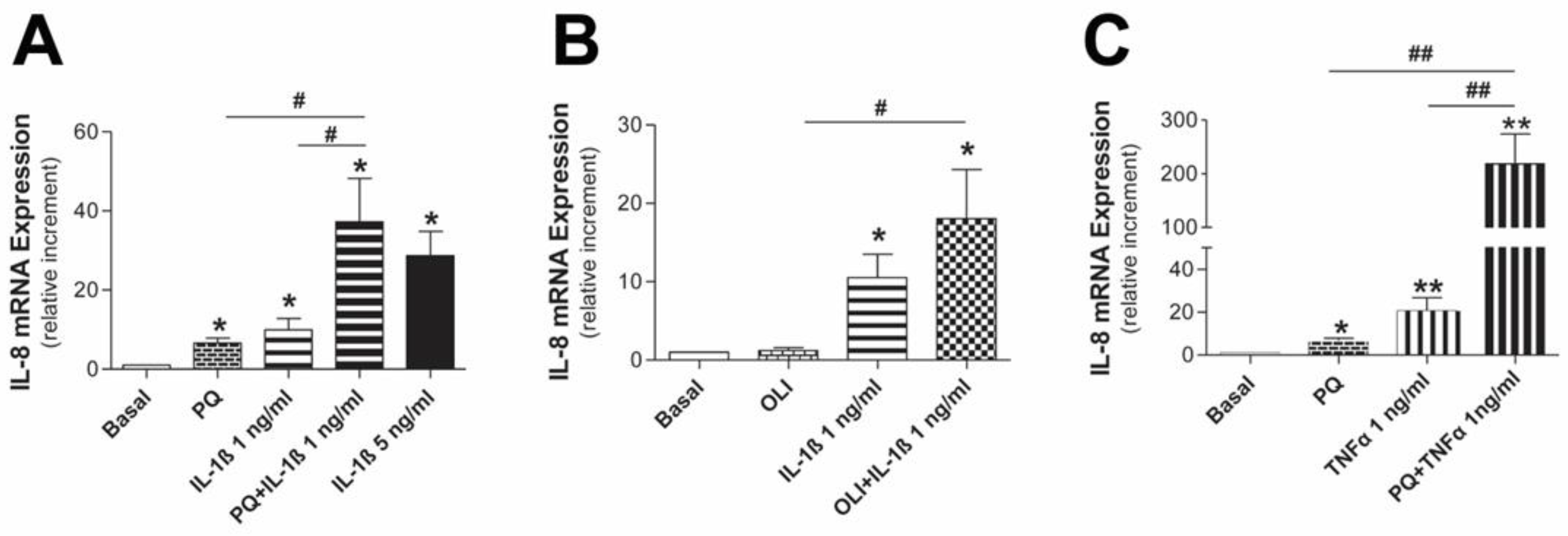
3.2. Mitochondrial Dysfunction Synergizes with Inflammatory Cytokines to Aggravate the Inflammatory Response in Human Mesothelial Cells
3.3. Mediators Involved in the Aggravation of the Inflammatory Response by the Impairment of Mitochondrial Function in Human Mesothelial Cells
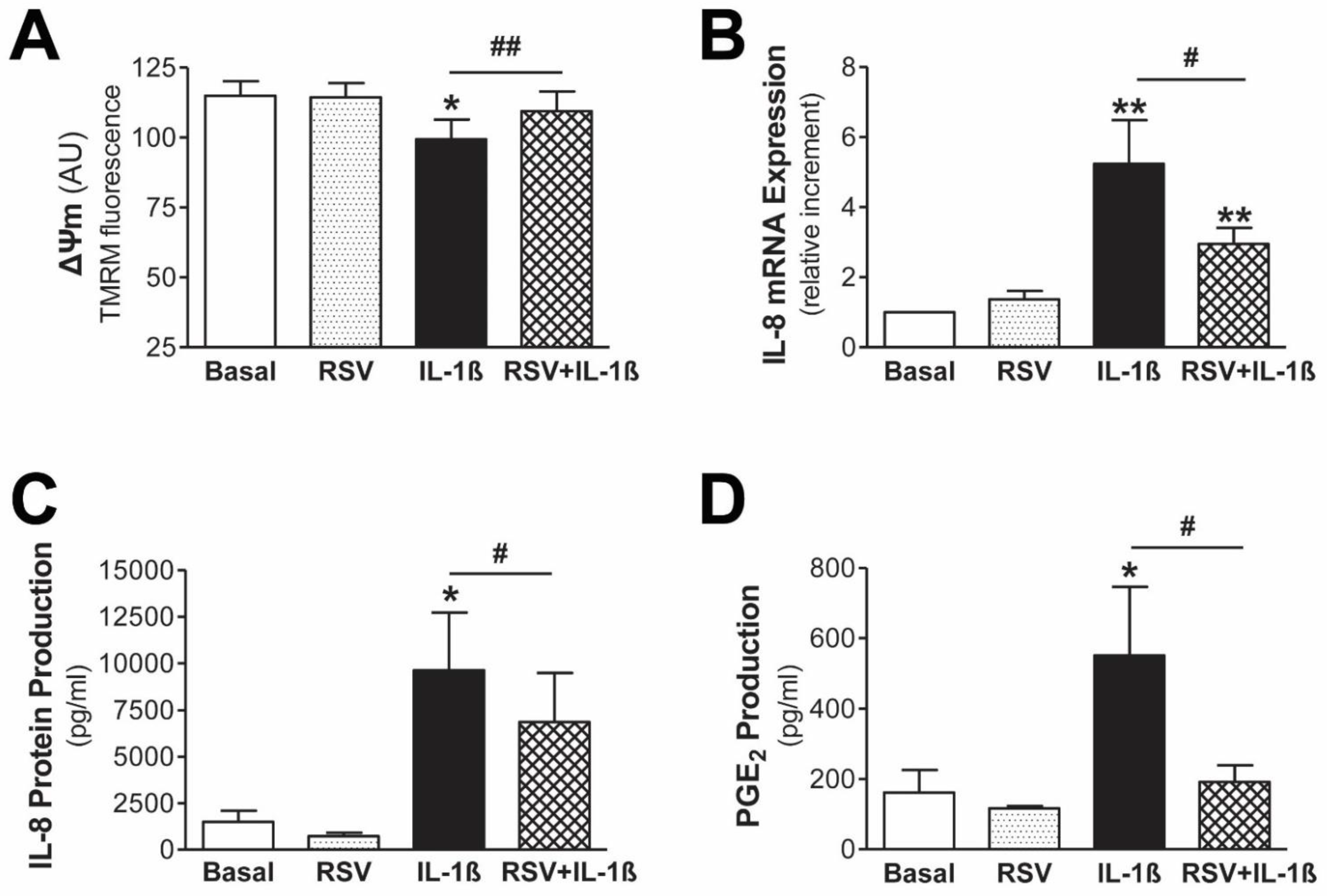
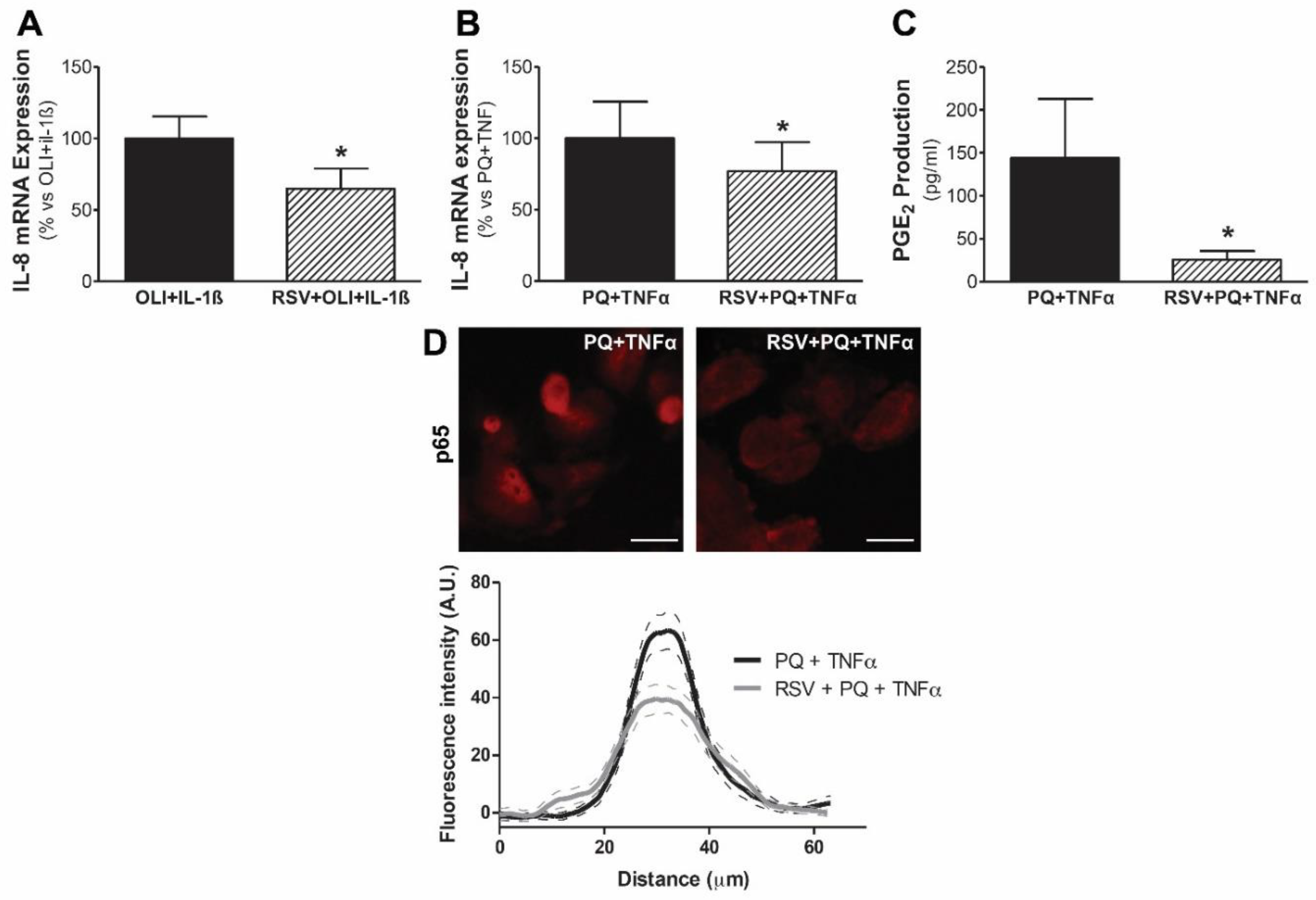
3.4. Resveratrol Protects Mesothelial Cells from Mitochondrial Dysfunction and Inflammatory Response
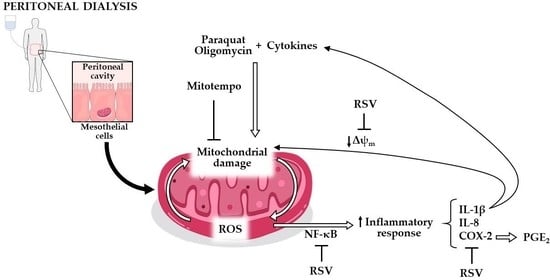
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.K.-T.; Chow, K.M.; Van De Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2016, 13, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Margetts, P.J.; Topley, N. The Pathophysiology of the Peritoneal Membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.-H. Loosening of the mesothelial barrier as an early therapeutic target to preserve peritoneal function in peritoneal dialysis. Kidney Res. Clin. Pract. 2020, 39, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015, 22, 204–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missiroli, S.; Genovese, I.; Perrone, M.; Vezzani, B.; Vitto, V.A.M.; Giorgi, C. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. J. Clin. Med. 2020, 9, 740. [Google Scholar] [CrossRef] [Green Version]
- Rego-Pérez, I.; Durán-Sotuela, A.; Ramos-Louro, P.; Blanco, F.J. Mitochondrial Genetics and Epigenetics in Osteoarthritis. Front. Genet. 2020, 10, 1335. [Google Scholar] [CrossRef] [Green Version]
- Tuppen, H.A.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta 2010, 1797, 113–128. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Xie, X.-H.; Chen, C.-H.; Peng, X.; Zhang, P.; Yang, C.; Wang, Y.-T. Molecular Regulation Mechanisms and Interactions Between Reactive Oxygen Species and Mitophagy. DNA Cell Biol. 2019, 38, 10–22. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the in-flammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef]
- Maneiro, E.; Martín, M.A.; de Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Care Res. 2003, 48, 700–708. [Google Scholar] [CrossRef]
- Ramil-Gómez, O.; Rodríguez-Carmona, A.; Fernández-Rodríguez, J.; Pérez-Fontán, M.; Ferreiro-Hermida, T.; López-Pardo, M.; Pérez-López, T.; López-Armada, M. Mitochondrial Dysfunction Plays a Relevant Role in Pathophysiology of Peritoneal Membrane Damage Induced by Peritoneal Dialysis. Antioxidants 2021, 10, 447. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; Riveiro-Naveira, R.R.; Valcárcel-Ares, M.N.; Hermida-Carballo, L.; Blanco, F.J.; López-Armada, M.J. Mito-chondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012, 64, 2927–2936. [Google Scholar] [CrossRef]
- Valcarcel-Ares, M.N.; Riveiro-Naveira, R.R.; Garcia, F.J.B.; Loureiro, J.; Hermida-Carballo, L.; Blanco, F.J.; López-Armada, M.J. Mitochondrial dysfunction promotes and aggravates the inflammatory response in normal human synoviocytes. Rheumatology 2014, 53, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Wang, J.; Xiang, S.; Chen, Z.; Zhang, X.; Chen, J. Dialysate cell-free mitochondrial DNA fragments as a marker of intraperitoneal inflammation and peritoneal solute transport rate in peritoneal dialysis. BMC Nephrol. 2019, 20, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.-Y.; Liu, S.-Y.; Yang, T.-C.; Liao, T.-L.; Kao, S.-H. High-Dialysate-Glucose-Induced Oxidative Stress and Mitochondrial-Mediated Apoptosis in Human Peritoneal Mesothelial Cells. Oxid. Med. Cell. Longev. 2014, 2014, 642793. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Sugimoto, T.; Ichikawa, Y.; Akatsuka, A.; Miyata, T.; Nangaku, M.; Tagawa, H.; Kurokawa, K. Glucose Dialysate Induces Mitochondrial DNA Damage in Peritoneal Mesothelial Cells. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2002, 22, 11–21. [Google Scholar] [CrossRef]
- Helmke, A.; Hüsing, A.M.; Gaedcke, S.; Brauns, N.; Balzer, M.S.; Reinhardt, M.; Hiss, M.; Shushakova, N.; de Luca, D.; Prinz, I.; et al. Peritoneal dialysate-range hypertonic glucose promotes T-cell IL-17 production that induces mesothelial inflammation. Eur. J. Immunol. 2020, 51, 354–367. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Induction of the Epithelial-to-Mesenchymal Transition of Human Colorectal Cancer by Human TNF-β (Lymphotoxin) and its Reversal by Resveratrol. Nutrients 2019, 11, 704. [Google Scholar] [CrossRef]
- Carozzi, S.; Nasini, M.G.; Ravera, M.; Sanna, A.; Tirotta, A.; Lamperi, S. Peritoneal dialysis effluent, cytokine levels, and peritoneal mesothelial cell viability in CAPD: A possible relationship. Adv. Perit. Dial. Conf. Perit. Dial. 1997, 13, 7–12. [Google Scholar]
- Topley, N.; Brown, Z.; Jörres, A.; Westwick, J.; Davies, M.; Coles, G.A.; Williams, J.D. Human peritoneal mesothelial cells synthesize interleukin-8. Synergistic induction by in-terleukin-1 beta and tumor necrosis factor-alpha. Am. J. Pathol. 1993, 142, 1876–1886. [Google Scholar]
- López-Armada, M.; Caramés, B.; Martín, M.; Cillero-Pastor, B.; Lires-Dean, M.; Fuentes-Boquete, I.; Arenas, J.; Blanco, F.J. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr. Cartil. 2006, 14, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef]
- Aroeira, L.S.; Lara-Pezzi, E.; Loureiro, J.; Aguilera, A.; Ramírez-Huesca, M.; González-Mateo, G.; Pérez-Lozano, M.L.; Albar-Vizcaíno, P.; Bajo, M.-A.; del Peso, G.; et al. Cyclooxygenase-2 mediates dialysate-induced alterations of the peritoneal mem-brane. J. Am. Soc. Nephrol. 2009, 20, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef]
- Terri, M.; Trionfetti, F.; Montaldo, C.; Cordani, M.; Tripodi, M.; Lopez-Cabrera, M.; Strippoli, R. Mechanisms of Peritoneal Fibrosis: Focus on Immune Cells-Peritoneal Stroma Interactions. Front. Immunol. 2021, 12, 607204. [Google Scholar]
- Zhou, Q.; Bajo, M.-A.; del Peso, G.; Yu, X.; Selgas, R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016, 90, 515–524. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, K.B.; Lam, C.W.; Chan, T.M.; Li, F.K.; Leung, J.C. Changes of cytokine profiles during peritonitis in patients on continu-ous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 2000, 35, 644–652. [Google Scholar]
- Brauner, A.; Hylander, B.; Wretlind, B. Tumor necrosis factor-α, interleukin-1β, and interleukin-1 receptor antagonist in dialysate and serum from patients on continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 1996, 27, 402–408. [Google Scholar] [CrossRef]
- Prasad, N.; Singh, K.; Gupta, A.; Prasad, K.N. Isolation of bacterial DNA followed by sequencing and differing cytokine response in peritoneal dialysis effluent help in identifying bacteria in culture negative peritonitis. Nephrology 2016, 23, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Wang, Q.; Li, Y.; Lin, H.; Luo, D.; Zhao, W.; Dou, X.; Liu, J.; Zhang, H.; Huang, Y.; et al. Inhibition of hyperglycolysis in mesothelial cells prevents peritoneal fibrosis. Sci. Transl. Med. 2019, 11, eaav5341. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Huang, Q.; Ma, Y.L.; Mao, K.; Yang, G.; Luo, P.; Ma, G.; Mei, P.; Jin, Y. Potential roles of IL-1 subfamily members in glycolysis in disease. Cytokine Growth Factor Rev. 2018, 44, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxidative Med. Cell. Longev. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Zorov, S.D.; Babenko, V.A.; Zorov, D.B. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, B.; Ucero, A.C.; Benito-Martin, A.; Vicent, M.J.; Orzaez, M.; Celdrán, A.; Selgas, R.; Ortega, M.R.; Ortiz, A. Biocompatibility Reduces Inflammation-Induced Apoptosis in Mesothelial Cells Exposed to Peritoneal Dialysis Fluid. Blood Purif. 2015, 39, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, J.; Shen, M.; Xu, H.; Yu, S.; Cheng, Q.; Ding, F. Pyrroloquinoline Quinone Inhibits Rotenone-Induced Microglia Inflammation by Enhancing Autophagy. Molecules 2020, 25, 4359. [Google Scholar] [CrossRef]
- Bajo, M.A.; Pérez-Lozano, M.L.; Albar-Vizcaino, P.; del Peso, G.; Castro, M.-J.; Gonzalez-Mateo, G.; Fernández-Perpén, A.; Aguilera, A.; Sánchez-Villanueva, R.; Sánchez-Tomero, J.A.; et al. Low-GDP peritoneal dialysis fluid (‘balance’) has less impact in vitro and ex vivo on epithelial-to-mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrol. Dial. Transplant. 2010, 26, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Farhat, K.; van Ittersum, F.J.; Ter Wee, P.M.; Paauw, N.J.; Beelen, R.H.J.; Douma, C.E. Initiation of peritoneal dialysis in the first weeks after catheter insertion: A comparison of a neutral-pH, low-GDP PD fluid and a conventional PD fluid. Clin. Nephrol. 2018, 89, 75–82. [Google Scholar] [CrossRef]
- Riesenhuber, A.; Kratochwill, K.; Bender, T.O.; Vargha, R.; Kasper, D.C.; Herzog, R.; Salzer, E.; Aufricht, C. Peritoneal Dialysis Fluid Induces P38-Dependent Inflammation in Human Mesothelial Cells. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2011, 31, 332–339. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Shan, Y.; Yu, M.; Shi, J.; Tang, L.; Cao, H.; Sheng, M. Tetramethylpyrazine Ameliorates Peritoneal Angiogenesis by Regulating VEGF/Hippo/YAP Signaling. Front. Pharmacol. 2021, 12, 649581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, Q.; Chau, M.K.; Yip, M.S.; Lui, S.L.; Liu, S.; Chu, K.M.; Ngan, H.Y.; Chan, T.M.; Yung, S. Anti-fibrotic effect of decorin in peritoneal dialysis and PD-associated peritonitis. eBioMedicine 2020, 52, 102661. [Google Scholar] [CrossRef] [Green Version]
- Aroeira, L.S.; Aguilera, A.; Selgas, R.; Ramírez-Huesca, M.; Pérez-Lozano, M.L.; Cirugeda, A.; Bajo, M.A.; del Peso, G.; Sánchez-Tomero, J.A.; Jiménez-Heffernan, J.A.; et al. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high so-lute transport rate in peritoneal dialysis: Role of vascular endothelial growth factor. Am. J. Kidney Dis. 2005, 46, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Fung, W.W.; Poon, P.Y.; Ng, J.K.; Kwong, V.W.-K.; Pang, W.-F.; Kwan, B.C.-H.; Cheng, P.M.-S.; Li, P.K.-T.; Szeto, C.-C. Longitudinal Changes of NF-κB Downstream Mediators and Peritoneal Transport Charac-teristics in Incident Peritoneal Dialysis Patients. Sci. Rep. 2020, 10, 6440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullian, M.E.; Luttrell, L.M.; Lee, M.-H.; Morinelli, T.A. Stimulation of Cyclooxygenase 2 Expression in Rat Peritoneal Mesothelial Cells. Nephron Exp. Nephrol. 2014, 128, 89–97. [Google Scholar] [CrossRef]
- Sitter, T.; Haslinger, B.; Mandl, S.; Fricke, H.; Held, E.; Sellmayer, A. High glucose increases prostaglandin E2 synthesis in human peritoneal mesothelial cells: Role of hyperosmolarity. J. Am. Soc. Nephrol. 1998, 9, 2005–2012. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, Q.; Zheng, Q.; Gong, L.; Su, L.; Ren, B.; Ju, Y.; Jia, Z.; Dou, X. Enhanced mPGES-1 Contributes to PD-Related Peritoneal Fibrosis via Activation of the NLRP3 Inflammasome. Front. Med. 2021, 8, 675363. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; López-Armada, M.J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 2019, 165, 181–195. [Google Scholar] [CrossRef]
- Fabbrini, P.; Schilte, M.N.; Zareie, M.; ter Wee, P.M.; Keuning, E.D.; Beelen, R.H.J.; Born, J.V.D. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol. Dial. Transplant. 2009, 24, 3669–3676. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.; Kang, H.-J.; Kim, D.-A.; Ryu, E.-S.; Yu, M.; Lee, H.; Lee, H.K.; Ryu, H.-M.; Park, S.-H.; Kim, Y.-L.; et al. Paricalcitol attenuates TGF-β1–induced phenotype transition of human peritoneal mesothelial cells (HPMCs) via modulation of oxidative stress and NLRP3 inflammasome. FASEB J. 2018, 33, 3035–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.-S.; Ko, J.; Kim, D.-A.; Ryu, E.-S.; Ryu, H.-M.; Park, S.-H.; Kim, Y.-L.; Oh, E.-S.; Kang, D.-H. Metformin ameliorates the Phenotype Transition of Peritoneal Mesothelial Cells and Peritoneal Fibrosis via a modulation of Oxidative Stress. Sci. Rep. 2017, 7, 5690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, E.; Wang, Z.; Shen, T.; Shen, C.; Liu, D.; Gao, Q.; Li, X.; Wei, G. TMIGD1 Inhibited Abdominal Adhesion Formation by Alleviating Oxidative Stress in the Mito-chondria of Peritoneal Mesothelial Cells. Oxid. Med. Cell. Longev. 2021, 2021, 9993704. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Yang, L.; Zhang, B.; Li, W.; Wang, S.; Jiang, S.; Chen, X.; Li, W.; Zeng, L. LincRNA Cox-2 Regulates Lipopolysaccharide-Induced Inflammatory Response of Human Peritoneal Mesothelial Cells via Modulating miR-21/NF-κ B Axis. Mediat. Inflamm. 2019, 2019, 8626703. [Google Scholar] [CrossRef]
- Nevado, J.; Vallejo, S.; El-Assar, M.; Peiró, C.; Sánchez-Ferrer, C.F.; Rodríguez-Mañas, L. Changes in the human peritoneal mesothe-lial cells during aging. Kidney Int. 2006, 69, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Arab-Nozari, M.; Ahangar, N.; Mohammadi, E.; Lorigooini, Z.; Shokrzadeh, M.; Amiri, F.T.; Shaki, F. Ginkgo biloba attenuated hepatotoxicity induced by combined exposure to cadmium and fluoride via modulating the redox imbalance, Bax/Bcl-2 and NF-kB signaling pathways in male rats. Mol. Biol. Rep. 2020, 47, 6961–6972. [Google Scholar] [CrossRef]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the auto-crine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Feng, Y.; Zheng, X.; Sun, L.; Wasan, H.S.; Ruan, S.; Shen, M. Resveratrol and Its Analogs: Potent Agents to Reverse Epithelial-to-Mesenchymal Transition in Tumors. Front. Oncol. 2021, 11, 644134. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.A.; Almonte-Becerril, M.; Ramil-Gómez, O.; Hermida-Carballo, L.; Viñas-Diz, S.; Vela-Anero, Á.; Concha, Á.; Camacho-Encina, M.; Blanco, F.J.; López-Armada, M.J. Autophagy Activation by Resveratrol Reduces Severity of Experimental Rheumatoid Arthritis. Mol. Nutr. Food Res. 2020, 65, e2000377. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Sun, X.Y.; Lin, A.X. Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis pa-tients: A prospective, randomized, double-blind study. Ren. Fail. 2016, 38, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riveiro-Naveira, R.R.; Valcarcel-Ares, M.N.; Almonte-Becerril, M.; Vaamonde-García, C.; Loureiro, J.; Hermida-Carballo, L.; López-Peláez, E.; Blanco, F.J.; López-Armada, M.J. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology 2016, 55, 1889–1900. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, C.; Peiró, C.; Rodríguez-Mañas, L.; Nevado, J. Polyphenols Attenuate Highly-Glycosylated Haemoglobin-Induced Damage in Human Peritoneal Mesothelial Cells. Antioxidants 2020, 9, 572. [Google Scholar] [CrossRef]
- Lin, K.; Ng, S.; Paul, C.R.; Chen, H.; Zeng, R.; Liu, J.; Padma, V.V.; Huang, C.; Kuo, W. MicroRNA-210 repression facilitates advanced glycation end-product (AGE)-induced cardiac mitochondrial dysfunction and apoptosis via JNK activation. J. Cell. Biochem. 2021, 122, 1873–1885. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Rago, C.; Lombardi, T.; Di Fulvio, G.; Di Liberato, L.; Arduini, A.; Divino-Filho, J.C.; Bonomini, M. A New Peritoneal Dialysis Solution Containing L-Carnitine and Xylitol for Patients on Continuous Ambulatory Peritoneal Dialysis: First Clinical Experience. Toxins 2021, 13, 174. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef] [Green Version]
- Roca-Agujetas, V.; De Dios, C.; Lestón, L.; Marí, M.; Morales, A.; Colell, A. Recent Insights into the Mitochondrial Role in Autophagy and Its Regulation by Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, X.; Zhu, G.; Zhang, Y.; He, M.; Zhang, J. The role of Resveratrol-induced mitophagy/autophagy in peritoneal mesothelial cells inflammatory injury via NLRP3 inflammasome activation triggered by mitochondrial ROS. Exp. Cell Res. 2016, 341, 42–53. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramil-Gómez, O.; López-Pardo, M.; Fernández-Rodríguez, J.A.; Rodríguez-Carmona, A.; Pérez-López, T.; Vaamonde-García, C.; Pérez-Fontán, M.; López-Armada, M.J. Involvement of Mitochondrial Dysfunction in the Inflammatory Response in Human Mesothelial Cells from Peritoneal Dialysis Effluent. Antioxidants 2022, 11, 2184. https://doi.org/10.3390/antiox11112184
Ramil-Gómez O, López-Pardo M, Fernández-Rodríguez JA, Rodríguez-Carmona A, Pérez-López T, Vaamonde-García C, Pérez-Fontán M, López-Armada MJ. Involvement of Mitochondrial Dysfunction in the Inflammatory Response in Human Mesothelial Cells from Peritoneal Dialysis Effluent. Antioxidants. 2022; 11(11):2184. https://doi.org/10.3390/antiox11112184
Chicago/Turabian StyleRamil-Gómez, Olalla, Mirian López-Pardo, Jennifer Adriana Fernández-Rodríguez, Ana Rodríguez-Carmona, Teresa Pérez-López, Carlos Vaamonde-García, Miguel Pérez-Fontán, and María José López-Armada. 2022. "Involvement of Mitochondrial Dysfunction in the Inflammatory Response in Human Mesothelial Cells from Peritoneal Dialysis Effluent" Antioxidants 11, no. 11: 2184. https://doi.org/10.3390/antiox11112184
APA StyleRamil-Gómez, O., López-Pardo, M., Fernández-Rodríguez, J. A., Rodríguez-Carmona, A., Pérez-López, T., Vaamonde-García, C., Pérez-Fontán, M., & López-Armada, M. J. (2022). Involvement of Mitochondrial Dysfunction in the Inflammatory Response in Human Mesothelial Cells from Peritoneal Dialysis Effluent. Antioxidants, 11(11), 2184. https://doi.org/10.3390/antiox11112184