Antioral Cancer Effects by the Nitrated [6,6,6]Tricycles Compound (SK1) In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. SK1 Preparation
2.2. Cell Culture and Reagents
2.3. Cell Cycle Assay
2.4. Annexin V/7AAD and Caspase 3/7 for Apoptosis Assays
2.5. Caspases 3/8/9 for Apoptosis Assays
2.6. ROS, Mitochondrial Superoxide (MitoSOX), and Mitochondrial Membrane Potential (MMP) for Oxidative Stress Assays
2.7. Cellular Antioxidant Glutathione (GSH) Assay
2.8. γH2AX and 8-Hydroxy-2-Deoxyguanosine (8-OHdG) for DNA Damage Assays
2.9. Statistical Analysis
3. Results
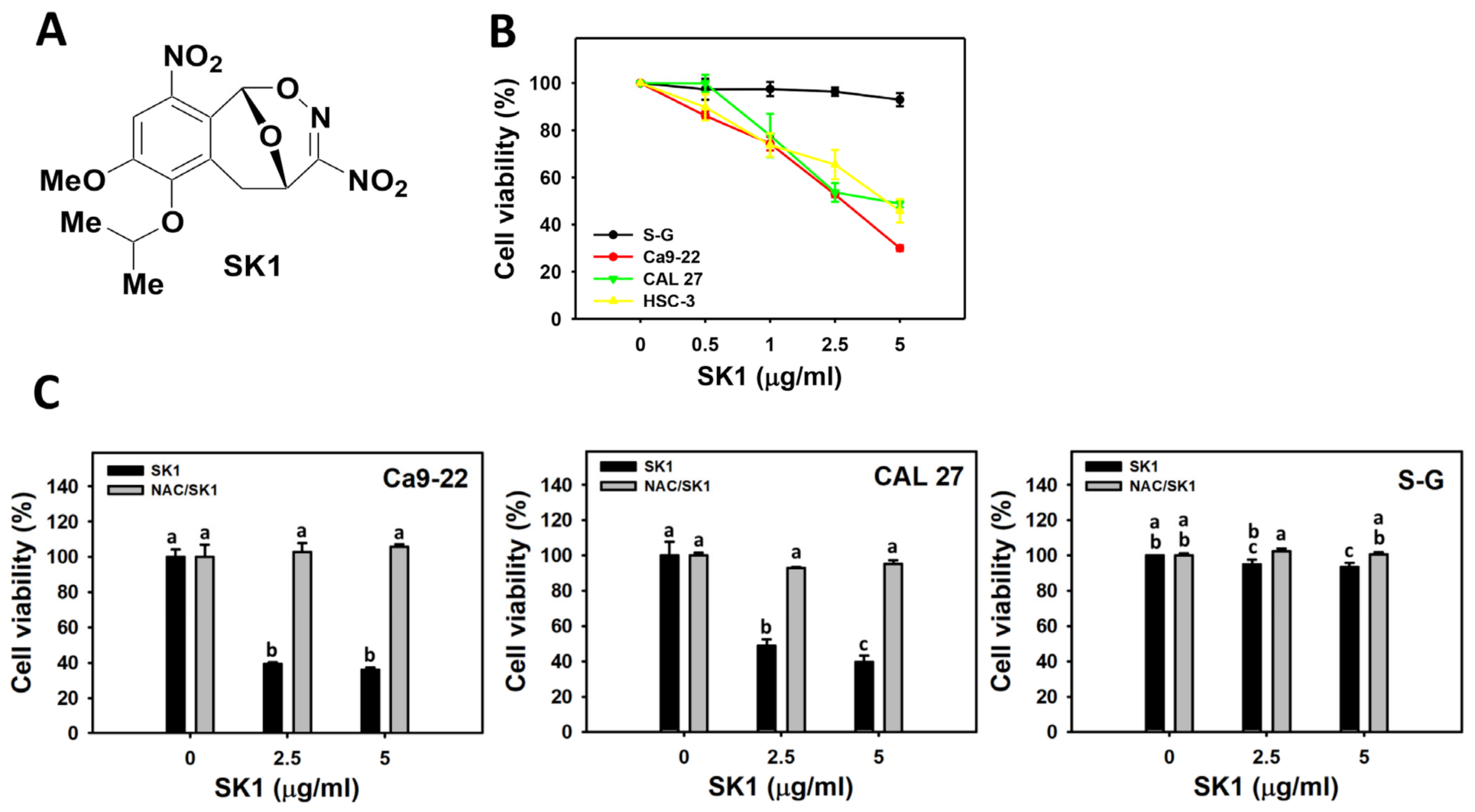
3.1. Cell Viability of SK1 (Oral Cancer vs. Non-Malignant Cells)
3.2. Cell Cycle of SK1 (Oral Cancer vs. Non-Malignant Cells)
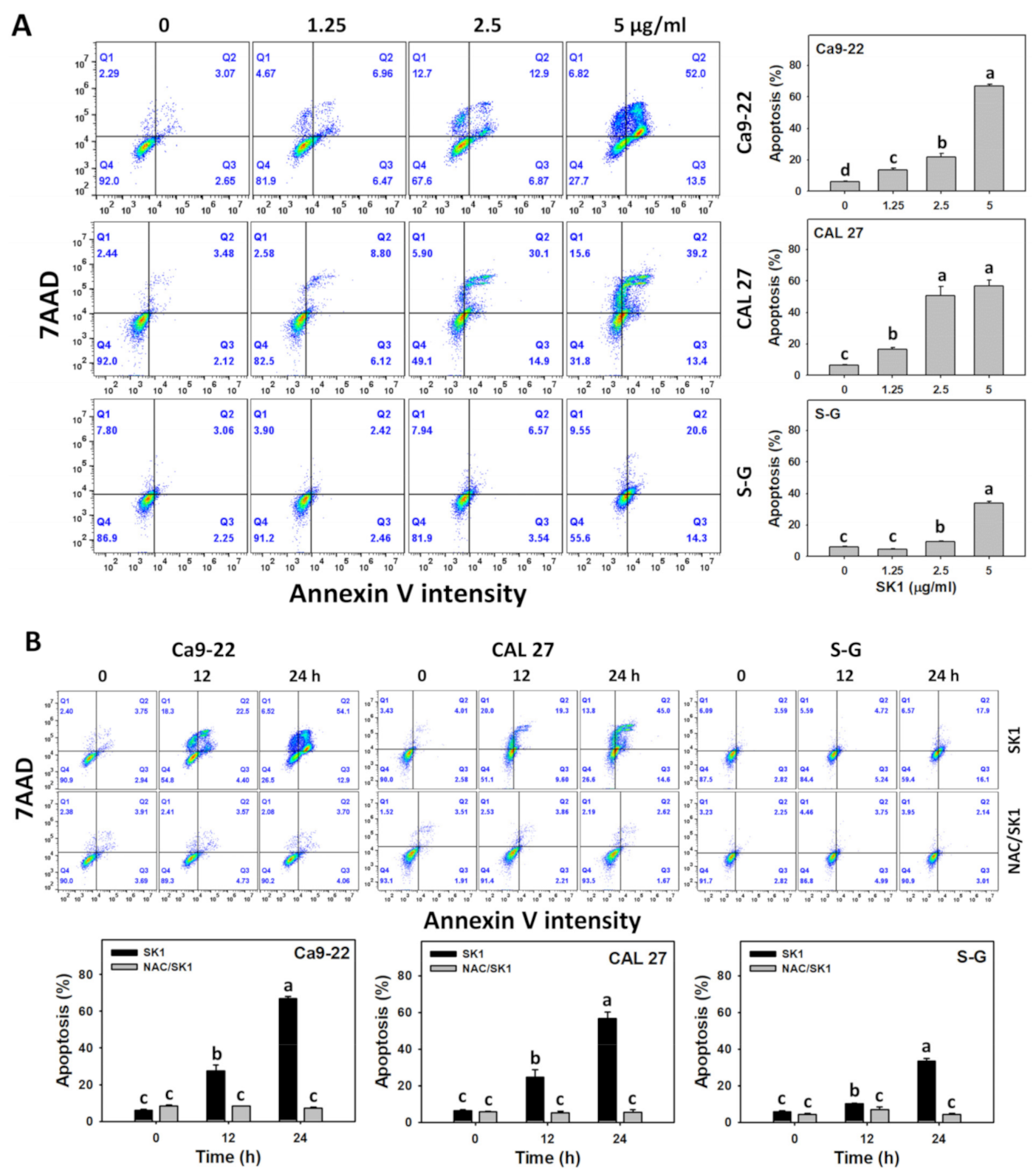
3.3. Apoptosis (Annexin V) of SK1 (Oral Cancer vs. Non-Malignant Cells)
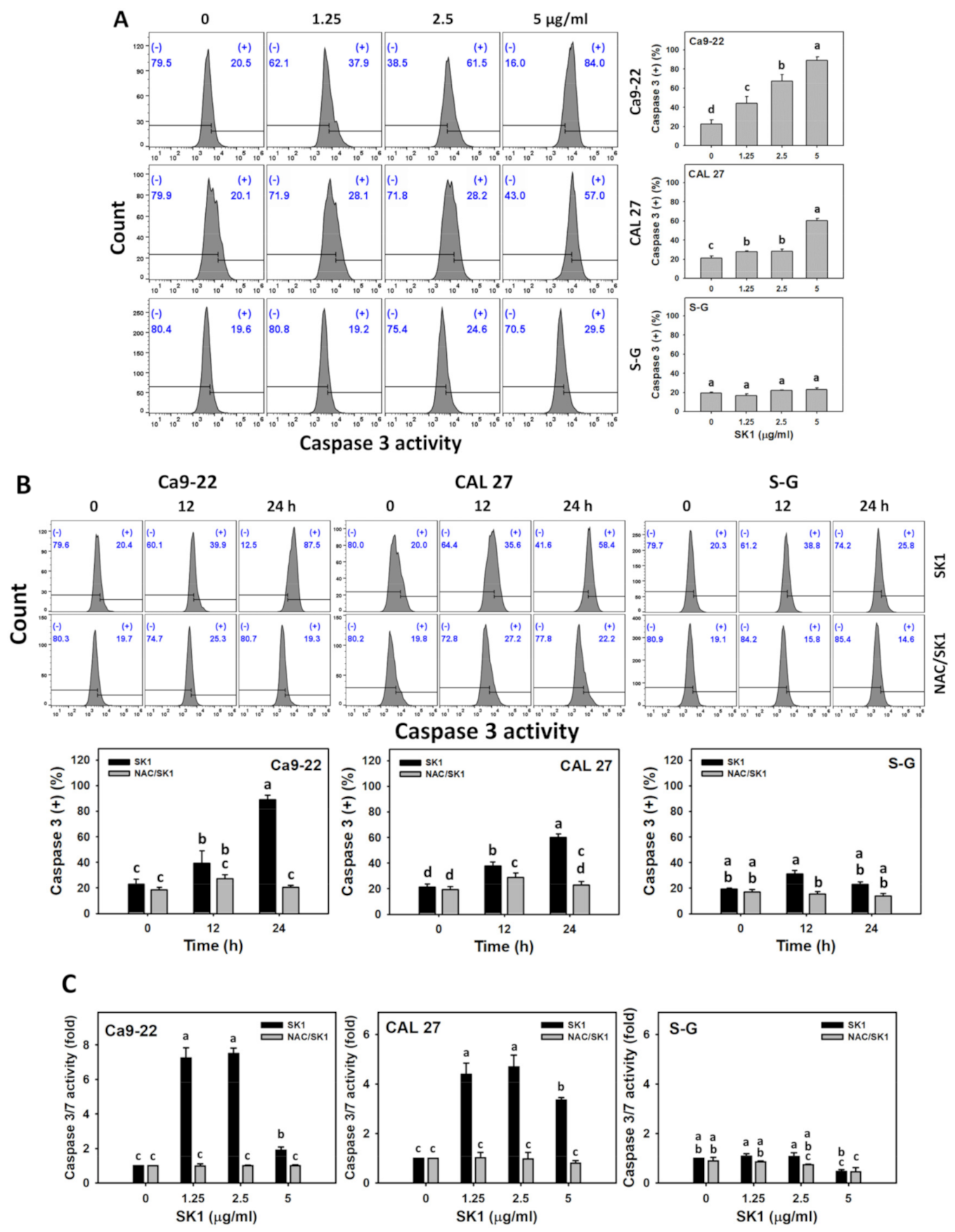
3.4. Apoptosis (Caspases 3 and 3/7) of SK1 (Oral Cancer vs. Non-Malignant Cells)
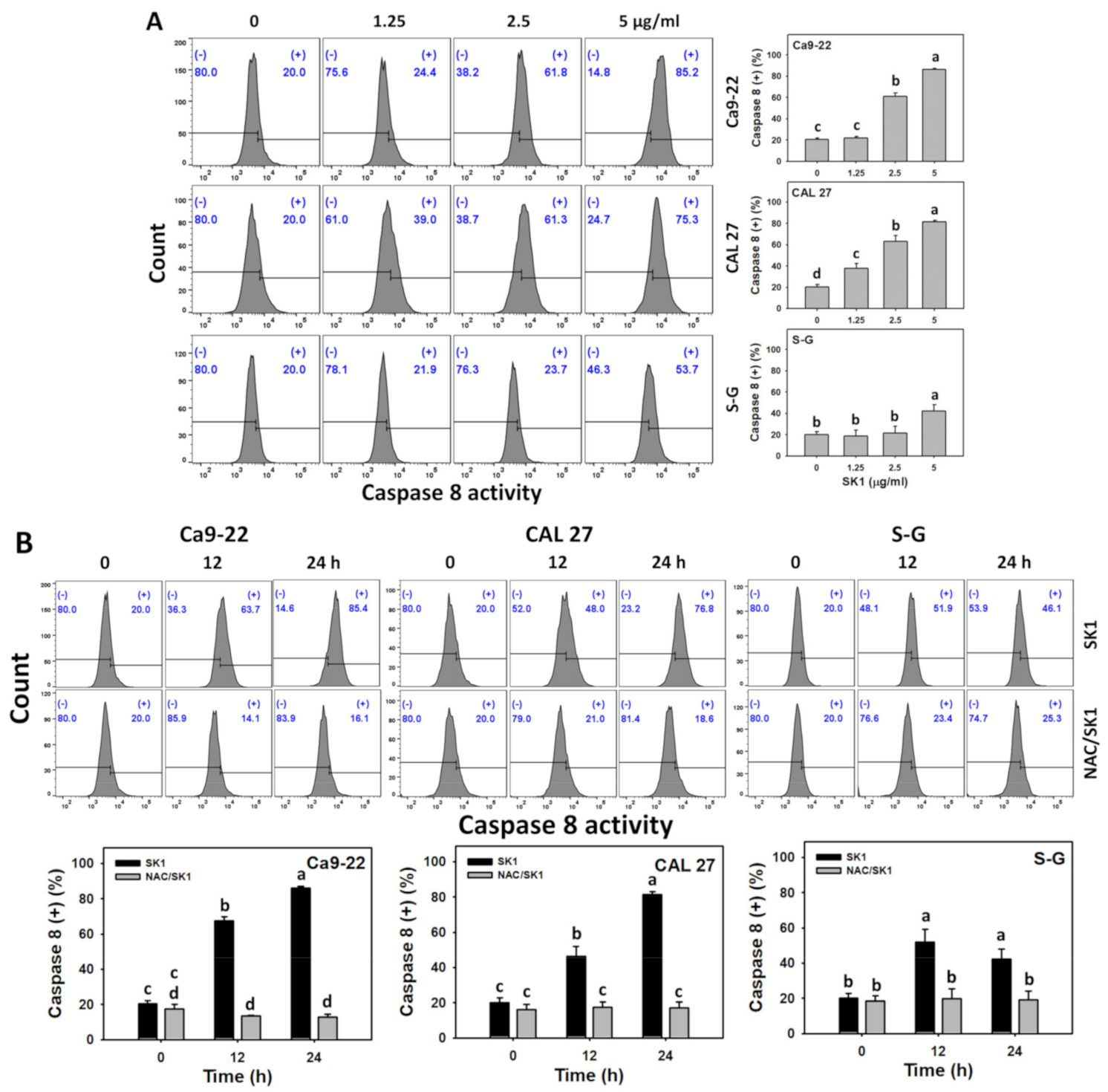
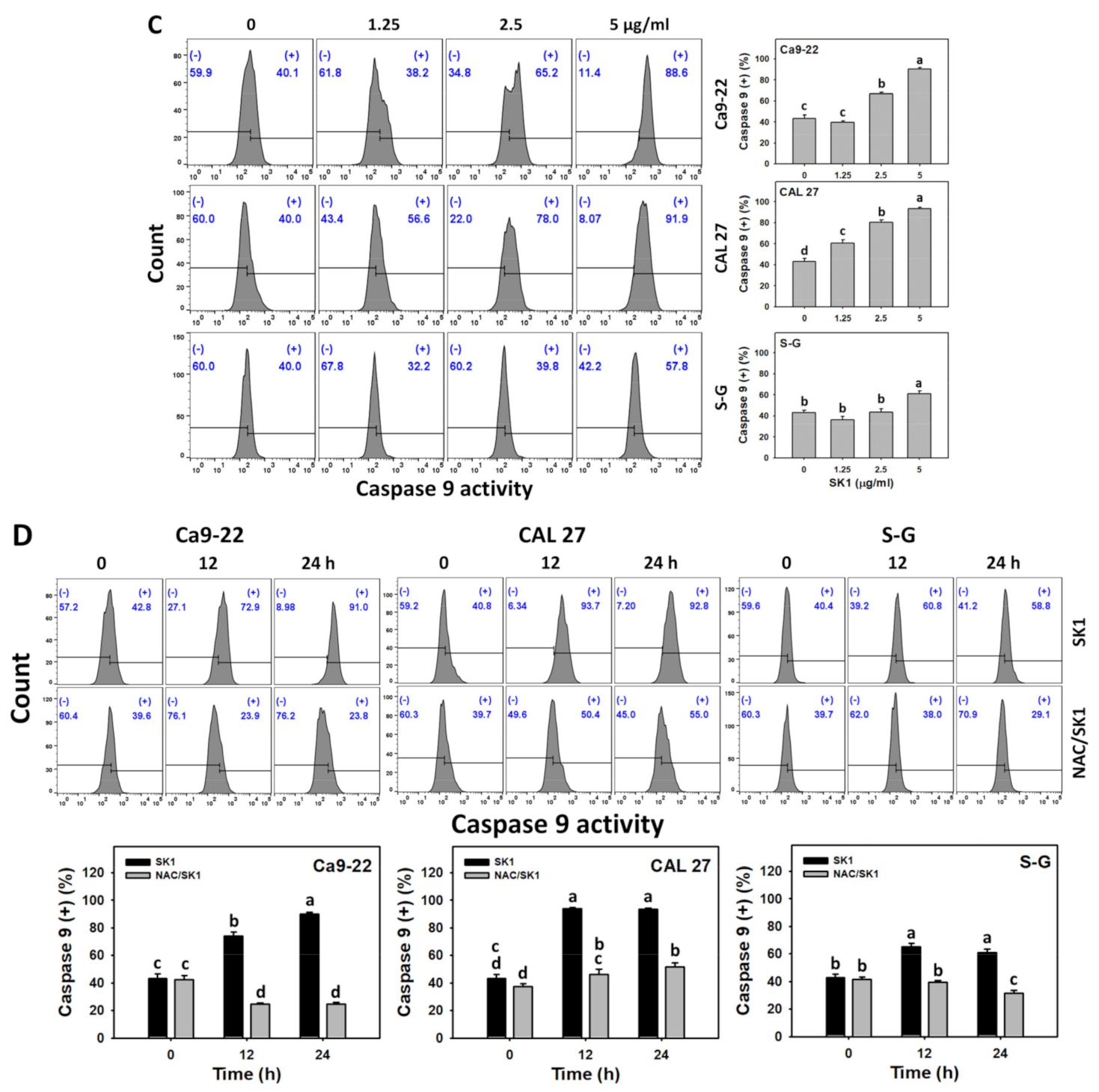
3.5. Apoptosis (Caspases 8 and 9) of SK1 (Oral Cancer vs. Non-Malignant Cells)
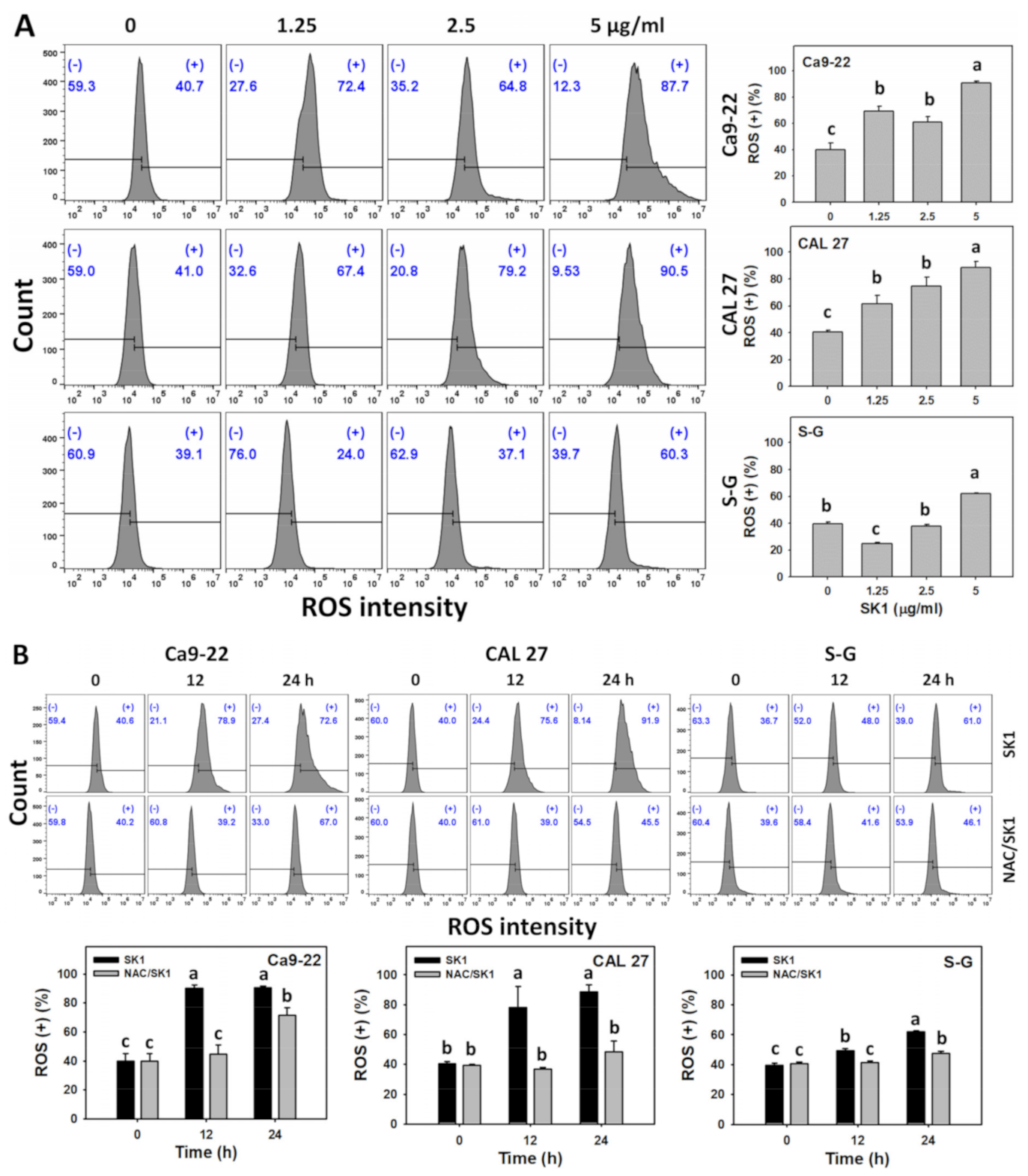
3.6. Oxidative Stress (ROS and MitoSOX) of SK1 (Oral Cancer vs. Non-Malignant Cells)
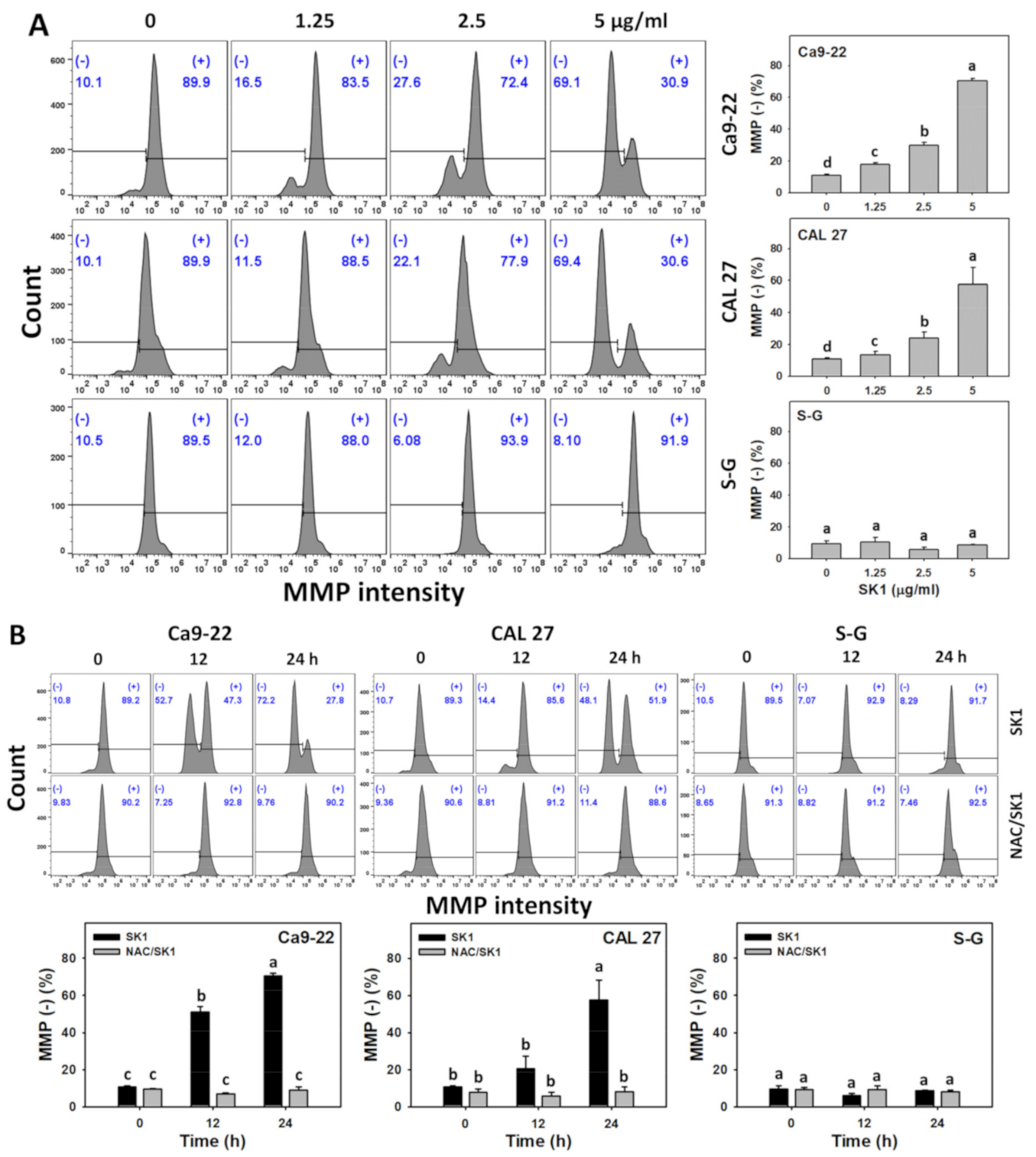
3.7. Oxidative Stress (MMP) of SK1 (Oral Cancer vs. Non-Malignant Cells)
3.8. Cellular Antioxidant (GSH) of SK1 (Oral Cancer vs. Non-Malignant Cells)
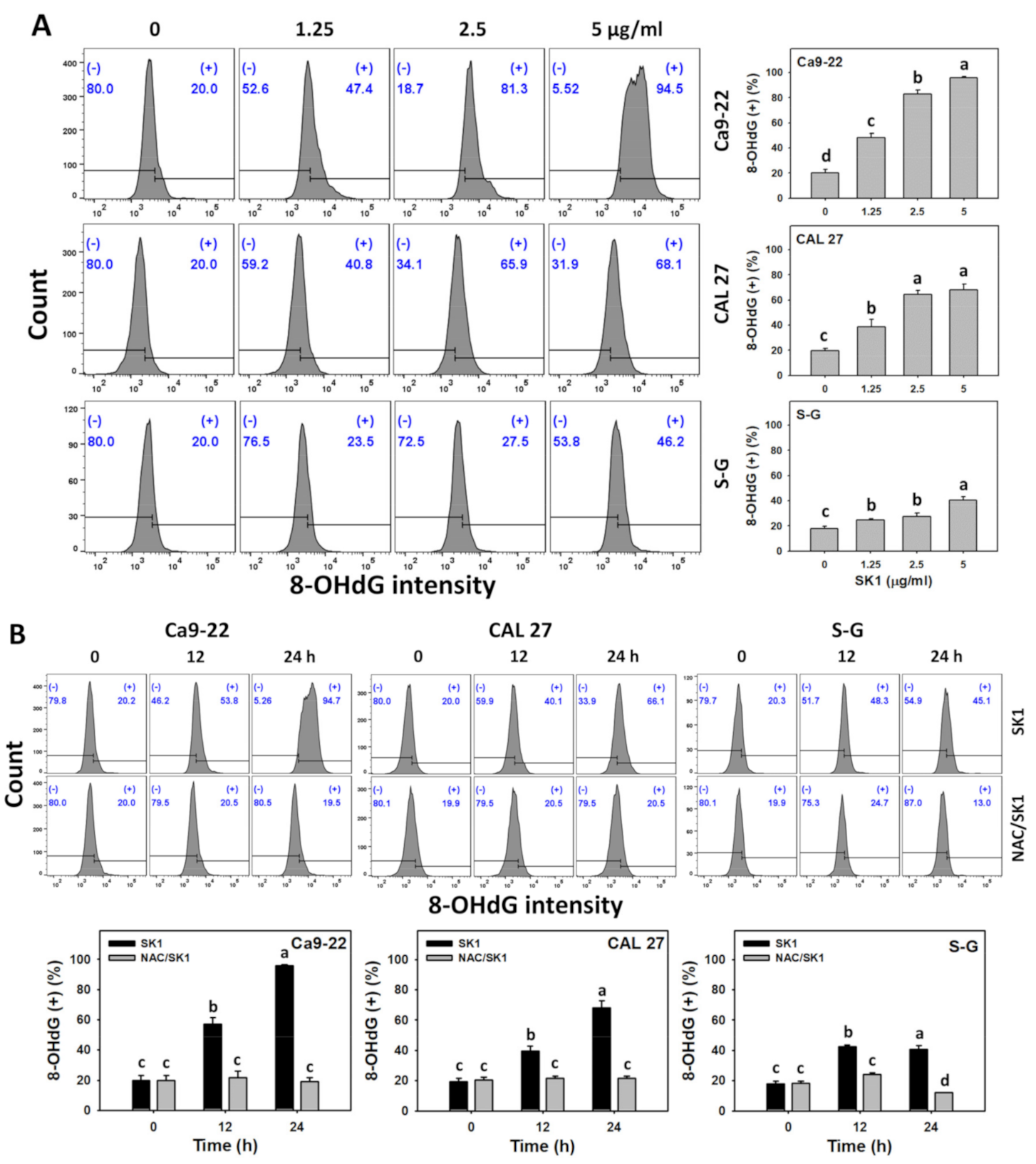
3.9. DNA Damages (γH2AX and 8-OHdG) of SK1 (Oral Cancer vs. Non-Malignant Cells)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Porter, S. ABC of oral health. Oral cancer. BMJ 2000, 321, 97–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ko, Y.C.; Huang, Y.L.; Lee, C.H.; Chen, M.J.; Lin, L.M.; Tsai, C.C. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral. Pathol. Med. 1995, 24, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar]
- Sarode, G.S.; Sarode, S.C.; Maniyar, N.; Anand, R.; Patil, S. Oral cancer databases: A comprehensive review. J. Oral. Pathol. Med. 2018, 47, 547–556. [Google Scholar] [CrossRef]
- Silverman, S., Jr. Oral cancer: Complications of therapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1999, 88, 122–126. [Google Scholar] [CrossRef]
- Liu, W.Z.; Ma, L.Y.; Liu, D.S.; Huang, Y.L.; Wang, C.H.; Shi, S.S.; Pan, X.H.; Song, X.D.; Zhu, R.X. Peniciketals A-C, new spiroketals from saline soil derived Penicillium raistrichii. Org. Lett. 2014, 16, 90–93. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Quamina, D.S.; Pelaez, F.; Teran, A.; Felock, P.; Hazuda, D.J. Integrastatins: Structure and HIV-1 integrase inhibitory activities of two novel racemic tetracyclic aromatic heterocycles produced by two fungal species. Tetrahedron Lett. 2002, 43, 2351–2354. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Dittrich, B.; Schüffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Saxena, S. Endophytic fungi: A source of potential antifungal compounds. J. Fungi 2018, 4, 77. [Google Scholar] [CrossRef]
- Chan, C.K.; Tsai, Y.L.; Chang, M.Y. Construction of nitrated benzo [3.3.1]bicyclic acetal/ketal core via nitration of o-carbonyl allylbenzenes. Org. Lett. 2017, 19, 1358–1361. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Koper, M.T.; Calle-Vallejo, F. Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis. J. Am. Chem. Soc. 2014, 136, 15694–15701. [Google Scholar] [CrossRef]
- Nurdin, L.; Spasyuk, D.M.; Fairburn, L.; Piers, W.E.; Maron, L. Oxygen-oxygen bond cleavage and formation in Co(II)-mediated stoichiometric O2 reduction via the potential intermediacy of a Co(IV) oxyl radical. J. Am. Chem. Soc. 2018, 140, 16094–16105. [Google Scholar] [CrossRef]
- Gamon, L.F.; Wille, U. Oxidative damage of biomolecules by the environmental pollutants NO2* and NO3*. Acc. Chem. Res. 2016, 49, 2136–2145. [Google Scholar] [CrossRef]
- Lubos, E.; Handy, D.E.; Loscalzo, J. Role of oxidative stress and nitric oxide in atherothrombosis. Front. Biosci. 2008, 13, 5323–5344. [Google Scholar] [CrossRef]
- Hsieh, H.J.; Liu, C.A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef]
- Hu, Y.; Yin, F.; Yu, Z.; Peng, Y.; Zhao, G.; Liu, Z.; Zhou, D.; Ma, X.; Shahidi, F.; Zhu, B. Trans, trans-2,4-decadienal impairs vascular endothelial function by inducing oxidative/nitrative stress and apoptosis. Redox. Biol. 2020, 34, 101577. [Google Scholar] [CrossRef]
- Iqbal, S.; Jabeen, F.; Peng, C.; Shah, M.A.; Ijaz, M.U.; Rasul, A.; Ali, S.; Rauf, A.; Batiha, G.E.; Klodzinska, E. Nickel nanoparticles induce hepatotoxicity via oxidative and nitrative stress-mediated apoptosis and inflammation. Toxicol. Ind. Health 2021, 37, 619–634. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Kasten, F.H.; Pineda, L.F.; Schneider, P.E.; Rawls, H.R.; Foster, T.A. Biocompatibility testing of an experimental fluoride releasing resin using human gingival epithelial cells in vitro. In Vitro Cell. Dev. Biol. 1989, 25, 57–62. [Google Scholar] [CrossRef]
- Kasten, F.H.; Soileau, K.; Meffert, R.M. Quantitative evaluation of human gingival epithelial cell attachment to implant surfaces in vitro. Int. J. Periodont. Restor. Dent. 1990, 10, 68–79. [Google Scholar]
- Wang, H.R.; Tang, J.Y.; Wang, Y.Y.; Farooqi, A.A.; Yen, C.Y.; Yuan, S.F.; Huang, H.W.; Chang, H.W. Manoalide preferentially provides antiproliferation of oral cancer cells by oxidative stress-mediated apoptosis and DNA damage. Cancers 2019, 11, 1303. [Google Scholar] [CrossRef]
- Shiau, J.P.; Chuang, Y.T.; Yang, K.H.; Chang, F.R.; Sheu, J.H.; Hou, M.F.; Jeng, J.H.; Tang, J.Y.; Chang, H.W. Brown algae-derived fucoidan exerts oxidative stress-dependent antiproliferation on oral cancer cells. Antioxidants 2022, 11, 841. [Google Scholar] [CrossRef]
- Huang, C.H.; Yeh, J.M.; Chan, W.H. Hazardous impacts of silver nanoparticles on mouse oocyte maturation and fertilization and fetal development through induction of apoptotic processes. Environ. Toxicol. 2018, 33, 1039–1049. [Google Scholar] [CrossRef]
- Hung, J.H.; Chen, C.Y.; Omar, H.A.; Huang, K.Y.; Tsao, C.C.; Chiu, C.C.; Chen, Y.L.; Chen, P.H.; Teng, Y.N. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environ. Toxicol. 2016, 31, 1888–1898. [Google Scholar] [CrossRef]
- Wang, T.S.; Lin, C.P.; Chen, Y.P.; Chao, M.R.; Li, C.C.; Liu, K.L. CYP450-mediated mitochondrial ROS production involved in arecoline N-oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018, 33, 1029–1038. [Google Scholar] [CrossRef]
- Vignon, C.; Debeissat, C.; Georget, M.T.; Bouscary, D.; Gyan, E.; Rosset, P.; Herault, O. Flow cytometric quantification of all phases of the cell cycle and apoptosis in a two-color fluorescence plot. PLoS ONE 2013, 8, e68425. [Google Scholar] [CrossRef]
- Fan, H.C.; Hsieh, Y.C.; Li, L.H.; Chang, C.C.; Janouskova, K.; Ramani, M.V.; Subbaraju, G.V.; Cheng, K.T.; Chang, C.C. Dehydroxyhispolon methyl ether, a hispolon derivative, inhibits WNT/beta-catenin signaling to elicit human colorectal carcinoma cell apoptosis. Int. J. Mol. Sci. 2020, 21, 8839. [Google Scholar] [CrossRef]
- Liu, W.; Lin, L.C.; Wang, P.J.; Chen, Y.N.; Wang, S.C.; Chuang, Y.T.; Tsai, I.H.; Yu, S.Y.; Chang, F.R.; Cheng, Y.B.; et al. Nepenthes ethyl acetate extract provides oxidative stress-dependent anti-leukemia effects. Antioxidants 2021, 10, 1410. [Google Scholar] [CrossRef]
- Lee, C.H.; Shih, Y.L.; Lee, M.H.; Au, M.K.; Chen, Y.L.; Lu, H.F.; Chung, J.G. Bufalin induces apoptosis of human osteosarcoma U-2 OS cells through endoplasmic reticulum stress, caspase- and mitochondria-dependent signaling pathways. Molecules 2017, 22, 437. [Google Scholar] [CrossRef]
- Shih, S.P.; Lu, M.C.; El-Shazly, M.; Lin, Y.H.; Chen, C.L.; Yu, S.S.F.; Liu, Y.C. The antileukemic and anti-prostatic effect of aeroplysinin-1 is mediated through ROS-induced apoptosis via NOX activation and inhibition of HIF-1a activity. Life 2022, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, X.; Shang, Y.; Li, Y.; Chen, S.Z. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells in vitro and in vivo. Cancer Commun. 2018, 38, 43. [Google Scholar] [CrossRef]
- Saulnier Sholler, G.L.; Brard, L.; Straub, J.A.; Dorf, L.; Illeyne, S.; Koto, K.; Kalkunte, S.; Bosenberg, M.; Ashikaga, T.; Nishi, R. Nifurtimox induces apoptosis of neuroblastoma cells in vitro and in vivo. J. Pediatr. Hematol. Oncol. 2009, 31, 187–193. [Google Scholar] [CrossRef]
- Deng, Y.; Zou, Y.; Yang, C.H.; Houk, K.N.; Smith, A.B., 3rd. Total syntheses of (+)-peniciketals A-B and (-)-diocollettines A exploiting a photoisomerization/cyclization union protocol. J. Org. Chem. 2021, 86, 13583–13597. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, C.H.; Smith, A.B., III. Enantioselective total synthesis of (+)-peniciketals A and B: Two architecturally complex spiroketals. J. Am. Chem. Soc. 2021, 143, 1740–1744. [Google Scholar] [CrossRef]
- Freitas, R.D.; Dias, R.B.; Vidal, M.T.A.; Valverde, L.F.; Gomes Alves Costa, R.; Damasceno, A.K.A.; Sales, C.B.S.; Siquara da Rocha, L.O.; Dos Reis, M.G.; Soares, M.B.P.; et al. Inhibition of CAL27 oral squamous carcinoma cell by targeting hedgehog pathway with vismodegib or itraconazole. Front. Oncol. 2020, 10, 563838. [Google Scholar] [CrossRef]
- Sirichoat, A.; Suwannakot, K.; Chaisawang, P.; Pannangrong, W.; Aranarochana, A.; Wigmore, P.; Welbat, J.U. Melatonin attenuates 5-fluorouracil-induced spatial memory and hippocampal neurogenesis impairment in adult rats. Life Sci. 2020, 248, 117468. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Willems, P.H.; Rossignol, R.; Dieteren, C.E.; Murphy, M.P.; Koopman, W.J. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects-involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef]
- Magda, D.; Miller, R.A. Motexafin Gadolinium: A Novel Redox Active Drug for Cancer Therapy; Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 466–476. [Google Scholar]
- Bey, E.A.; Bentle, M.S.; Reinicke, K.E.; Dong, Y.; Yang, C.R.; Girard, L.; Minna, J.D.; Bornmann, W.G.; Gao, J.; Boothman, D.A. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc. Natl. Acad. Sci. USA 2007, 104, 11832–11837. [Google Scholar] [CrossRef]
- Agnihotri, N.; Mishra, P. Mutagenic product formation due to reaction of guanine radical cation with nitrogen dioxide. J. Phys. Chem. B 2009, 113, 3129–3138. [Google Scholar] [CrossRef]
- Agnihotri, N.; Mishra, P. Formation of 8-nitroguanine due to reaction between guanyl radical and nitrogen dioxide: Catalytic role of hydration. J. Phys. Chem. B 2010, 114, 7391–7404. [Google Scholar] [CrossRef]
- Agnihotri, N.; Mishra, P. Reactivities of radicals of adenine and guanine towards reactive oxygen species and reactive nitrogen oxide species: OH and NO2. Chem. Phys. Lett. 2011, 503, 305–309. [Google Scholar] [CrossRef]
- Ceron-Carrasco, J.P.; Requena, A.; Zuniga, J.; Jacquemin, D. Mutagenic effects induced by the attack of NO2 radical to the guanine-cytosine base pair. Front. Chem. 2015, 3, 13. [Google Scholar] [CrossRef]
- Misiaszek, R.; Crean, C.; Geacintov, N.E.; Shafirovich, V. Combination of nitrogen dioxide radicals with 8-oxo-7,8-dihydroguanine and guanine radicals in DNA: Oxidation and nitration end-products. J. Am. Chem. Soc. 2005, 127, 2191–2200. [Google Scholar] [CrossRef]
- Sekiguchi, M. Molecular devices for high fidelity of DNA replication and gene expression. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 278–296. [Google Scholar] [CrossRef][Green Version]
- Huang, C.H.; Huang, Z.W.; Ho, F.M.; Chan, W.H. Berberine impairs embryonic development in vitro and in vivo through oxidative stress-mediated apoptotic processes. Environ. Toxicol. 2018, 33, 280–294. [Google Scholar] [CrossRef]
- Chang, H.W.; Li, R.N.; Wang, H.R.; Liu, J.R.; Tang, J.Y.; Huang, H.W.; Chan, Y.H.; Yen, C.Y. Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front. Physiol. 2017, 8, 634. [Google Scholar] [CrossRef]
- Shih, H.C.; El-Shazly, M.; Juan, Y.S.; Chang, C.Y.; Su, J.H.; Chen, Y.C.; Shih, S.P.; Chen, H.M.; Wu, Y.C.; Lu, M.C. Cracking the cytotoxicity code: Apoptotic induction of 10-acetylirciformonin B is mediated through ROS generation and mitochondrial dysfunction. Mar. Drugs 2014, 12, 3072–3090. [Google Scholar] [CrossRef]
- Wu, C.F.; Lee, M.G.; El-Shazly, M.; Lai, K.H.; Ke, S.C.; Su, C.W.; Shih, S.P.; Sung, P.J.; Hong, M.C.; Wen, Z.H.; et al. Isoaaptamine induces T-47D cells apoptosis and autophagy via oxidative stress. Mar. Drugs 2018, 16, 18. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-N.; Chan, C.-K.; Yen, C.-Y.; Shiau, J.-P.; Chang, M.-Y.; Wang, C.-C.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Antioral Cancer Effects by the Nitrated [6,6,6]Tricycles Compound (SK1) In Vitro. Antioxidants 2022, 11, 2072. https://doi.org/10.3390/antiox11102072
Chen Y-N, Chan C-K, Yen C-Y, Shiau J-P, Chang M-Y, Wang C-C, Jeng J-H, Tang J-Y, Chang H-W. Antioral Cancer Effects by the Nitrated [6,6,6]Tricycles Compound (SK1) In Vitro. Antioxidants. 2022; 11(10):2072. https://doi.org/10.3390/antiox11102072
Chicago/Turabian StyleChen, Yan-Ning, Chieh-Kai Chan, Ching-Yu Yen, Jun-Ping Shiau, Meng-Yang Chang, Cheng-Chung Wang, Jiiang-Huei Jeng, Jen-Yang Tang, and Hsueh-Wei Chang. 2022. "Antioral Cancer Effects by the Nitrated [6,6,6]Tricycles Compound (SK1) In Vitro" Antioxidants 11, no. 10: 2072. https://doi.org/10.3390/antiox11102072
APA StyleChen, Y.-N., Chan, C.-K., Yen, C.-Y., Shiau, J.-P., Chang, M.-Y., Wang, C.-C., Jeng, J.-H., Tang, J.-Y., & Chang, H.-W. (2022). Antioral Cancer Effects by the Nitrated [6,6,6]Tricycles Compound (SK1) In Vitro. Antioxidants, 11(10), 2072. https://doi.org/10.3390/antiox11102072







