Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach
Abstract
:1. Introduction
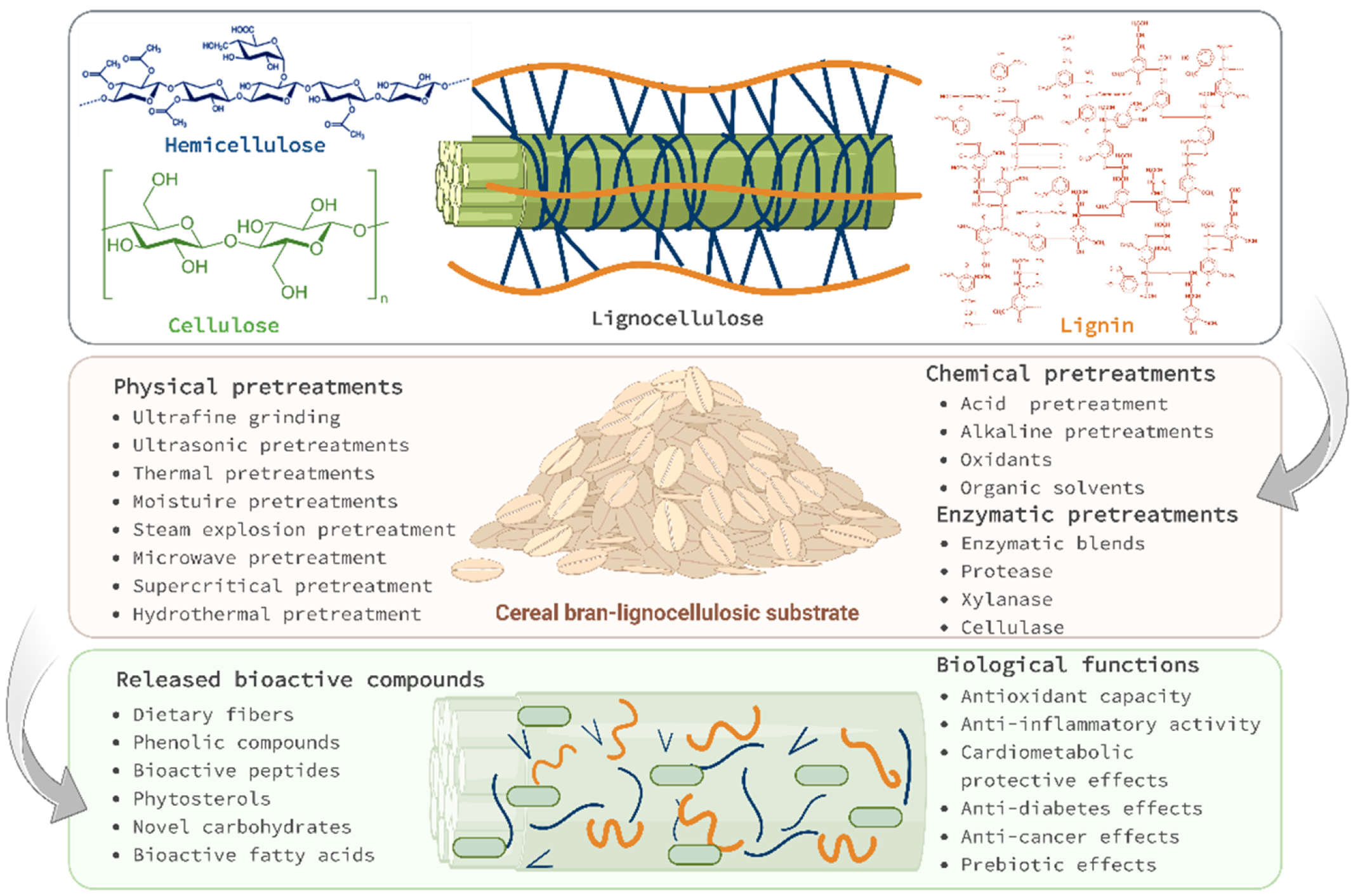
2. Intracellular Bioactive Phytochemicals of Cereal Bran
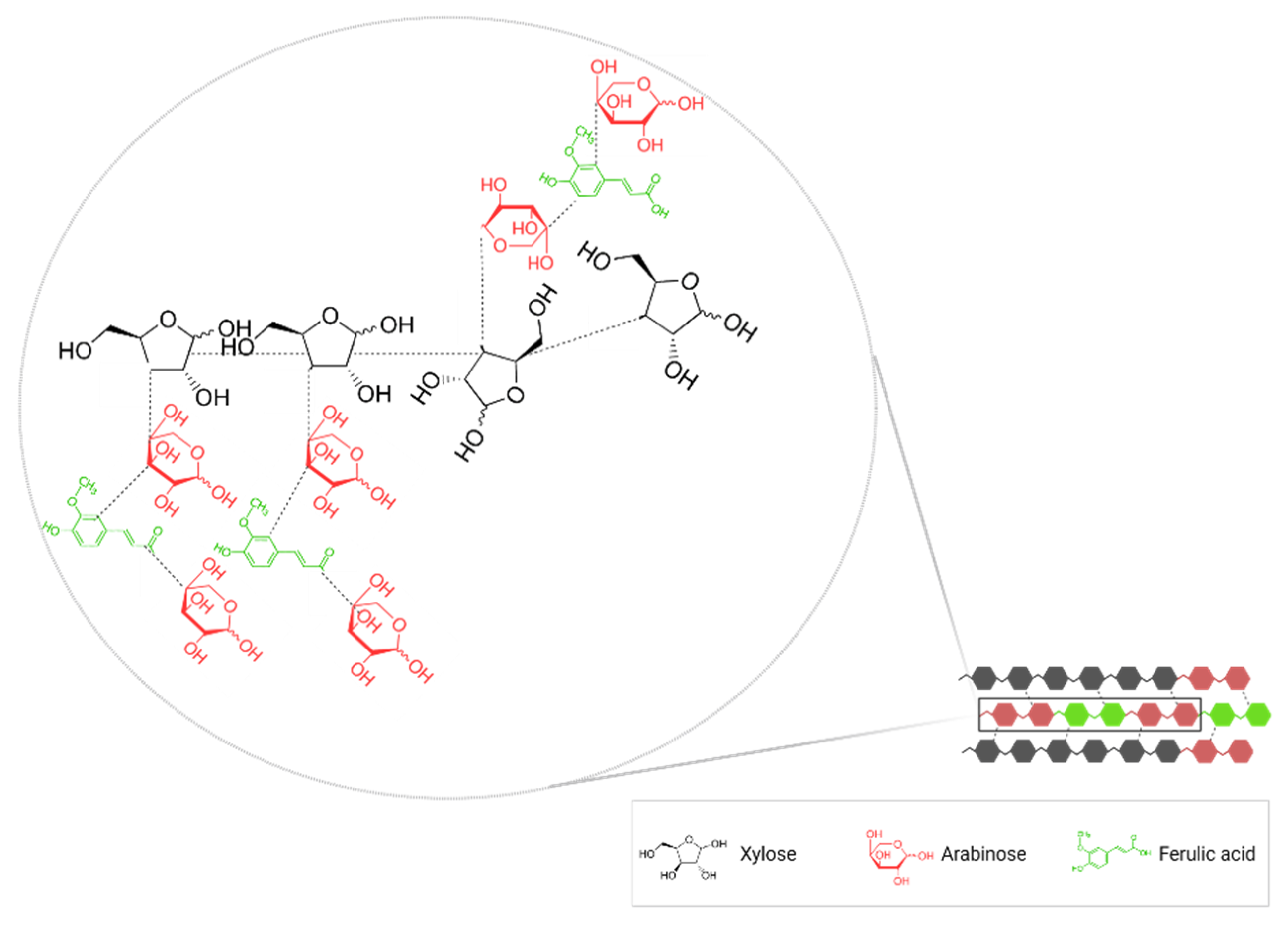
2.1. Dietary Fibers
2.2. Phenolic Compounds
2.3. Bioactive Peptides
2.4. Phytosterols
3. Biological Activities in the Human Body
3.1. Antioxidant Capacity
3.2. Anti-Inflammatory Activity
3.3. Cardiometabolic Protective Activity
3.4. Anti-Diabetes Activity
3.5. Anti-Cancer Activity
3.6. Prebiotic Effects
4. Pretreatments’ Effect on Cereal Bran Phytochemicals
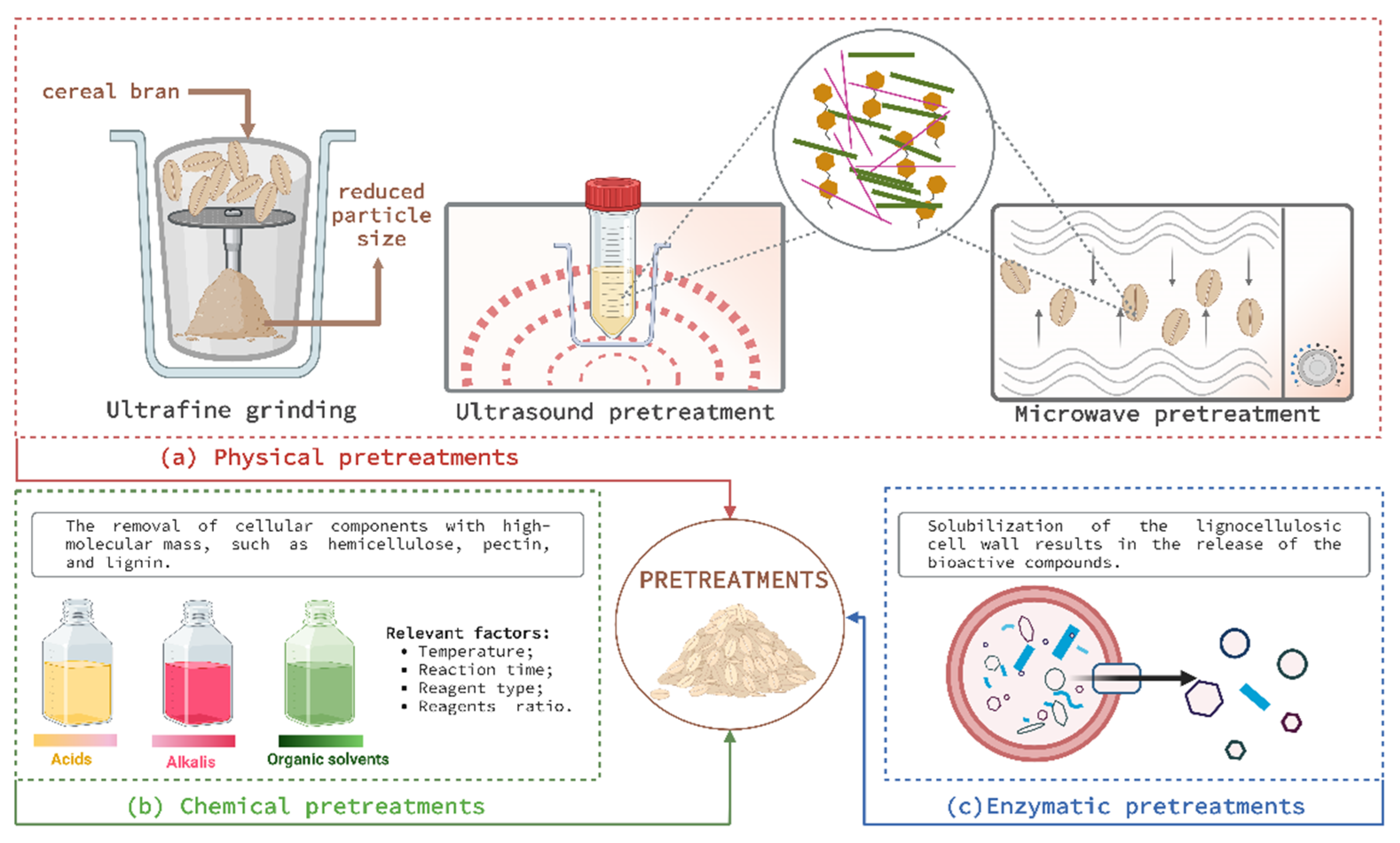
4.1. Physical Pretreatments
4.1.1. Ultrafine Grinding
4.1.2. Ultrasonic Pretreatments
4.1.3. Thermal and Moisture Pretreatments
4.1.4. Steam Explosion Pretreatment
4.1.5. Microwave Pretreatment
4.1.6. Supercritical Pretreatment
4.1.7. Hydrothermal Pretreatment
4.2. Chemical Pretreatments
4.3. Enzymatic Pretreatment
5. Cereal Bran Fermentation
| Fermentation Method | Inoculum | Substrate | Fermentation Condition | Results | References |
|---|---|---|---|---|---|
| SSF | Enterococcus faecalis M2 | WB | Inoculation rate: 10%; Moisture content: 60%; Time: 36 h; Temperature: 37 °C. | Soluble dietary fiber ↑ Phenols ↑ Flavonoids ↑ Alkylresorcinols ↑ Free amino acid ↑ Protein ↓ Phytic acid ↓ Antioxidant capacity ↑ | [103] |
| Bacillus sp. TMF–2 | Inoculation: 0.5 mL of bacterial suspension; Solid-to-liquid ratio: 1:1; Time: 11 days; Temperature: 30 °C. | Soluble phenolic content ↑ Antioxidant capacity ↑ Free radical scavenging rate ↑ Activity of hydrolytic enzymes (amylase, cellulase, pectinase, mannanase, protease, and phytase) ↑ Inorganic phosphorus ↑ Phytic acid ↓ | [104] | ||
| Aspergillus strains: A. brasiliensis, A. awamori, and A. sojae. | RB | Moisture: 1:1 (w/w); Fungal spores: 1% (v/w); Time: 8 days for A. brasiliensis and 14 days for A. awamori and A. sojae; Temperature: 25 °C. | Radical scavenging activity ↑ Tyrosinase inhibitory activity ↑ Lactase inhibitory activity ↑ Kojic acid ↑ Free phenolic acids ↑ Total flavonoid content ↑ | [100] | |
| Rhizopus oryzae | Moisture: 50%; Spore concentration: 4 × 106 spores/g bran; Time: 96 h; Temperature: 30 °C. | Total phenolic ↑ Total flavonoid ↑ Total carotenoid ↑ Total anthocyanin ↑ Antioxidant capacity ↑ Ferric reducing power ↑ Anti-inflammatory properties ↑ Anti-diabetic properties ↓ Radical scavenging ability ↓ | [102] | ||
| Aspergillus awamori and Aspergillus oryzae | Moisture: 30%; Inoculation: 1 mL of the spore suspension; Time: 5 days; Temperature: 30 °C. | Total phenolic content ↑ Protocatechuic acid ↑ Ferulic acid ↑ Radical scavenging activity ↑ Tyrosinase inhibitory activity ↑ | [31] | ||
| Saccharomyces cerevisiae | OB | Moisture: 45% (w/w); Inoculation: 5 mL of yeast suspensions (107 CFU/mL) per 100 g of dry weight; Time: 6 days; Temperature: 30 °C; Static conditions. | DPPH radical activity ↑ Total phenolic content ↑ Avenanthramides ↑ Ferulic acid ↑ Protocatechuic acid ↑ Caffeic acid ↑ Vanillic acid ↑ | [10] | |
| Monascus anka | Moisture: 60% (w/w); Inoculation: 0.1 mL of spore suspension per 1 g dry oats; Time: 14 days; Temperature: 30 °C | Ferulic acid ↑ Vanillic acid ↑ α-amylase activity ↑ Xylanase activity ↓ Total cellulase activity ↓ β-glucosidase activity ↓ | [101] | ||
| SmF | 3-member consortium of Bacillus subtilis, Bacillus coagulans, Bacillus cereus | WB | Wheat bran 2% (w/v); Time: 7 days; Temperature: 30 ± 2 °C; Agitation speed: 140 rpm. | Cellulases activities ↑ Digestibility of solid substrates ↑ Cellulose bioconversion ↑ Lignocellulose degradation ↑ β-glucosidase ↓ | [107] |
| Aspergillus phoenicis (Aspergillus saitoi) | Wheat bran: 1% (w/v); Temperature: 40 °C; pH: 6; Inoculation: 105 spores/mL. | Production of thermo-toleran mycelial β-D-fructofuranosidase and raffinose; | [108] | ||
| Pediococcus acidilactici | RB | Inoculation: 3% (v/v); Time: 24 h; Temperature 37 °C; pH: 5.6 | Bioconversion of ferulic acid into phenolic derivatives such as 4-ethylphenol, vanillin, vanillic acid, and vanillyl alcohol. | [105] | |
| Saccharomyces cerevisiae | Inoculation: 25 mL (1 × 108 cells/mL); Constant aeration; Agitation speed: 150 rpm; Time: 24 h; Temperature: 35–45 °C; pH: 3.5–4.5. | Antioxidant properties ↑ Cyanidin-3-glucoside and peonidin-3-glucoside were bioconverted to cyanidin and peonidin Bioactivity ↑ | [109] | ||
| Bacillus mojavensis | OB | Inoculation: 0.5%, 1%, and 2% (w/v); pH: 8.0 Time: 120 h; Temperature: 37 °C; Agitation speed: 180 rpm. | Xylanase yield of about 249.308 IU/mL | [110] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food Outlook—Biannual Report on Global Food Markets; FAO: Rome, Italy, 2019. [Google Scholar]
- Martín-Diana, A.B.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Peñas, E.; Rico, D. Wheat and Oat Brans as Sources of Polyphenol Compounds for Development of Antioxidant Nutraceutical Ingredients. Foods 2021, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Cui, L.; Qi, J.; Ojo, O.; Du, X.; Liu, Y.; Wang, X. The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Wang, Y.-F.; Lin, P.-Y.; Peng, S.-H.; Chou, M.-J. Seed peptide lunasin ameliorates obesity-induced inflammation and regulates immune responses in C57BL/6J mice fed high-fat diet. Food Chem. Toxicol. 2021, 147, 111908. [Google Scholar] [CrossRef]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet. 2020, 120, 1859–1883.e31. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, B.; Luo, S.; Wu, G.; Hui, X. Inducing apoptosis in human hepatocellular carcinoma cell lines via Nrf2/HO-1 signalling pathway of blueberry and blackcurrant powder manipulated oat bran paste extracts. J. Funct. Foods 2022, 89, 104967. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Cai, W.D.; Xiao, G.S.; Duan, Y.; Zhang, H. Subcritical water extraction-based methods affect the physicochemical and functional properties of soluble dietary fibers from wheat bran. Food Chem. 2019, 298, 124987. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Cătoi, A.-F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Martău, G.-A.; Unger, P.; Schneider, R.; Venus, J.; Vodnar, D.C.; López-Gómez, J.P. Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. J. Fungi 2021, 7, 766. [Google Scholar] [CrossRef]
- Spaggiari, M.; Ricci, A.; Calani, L.; Bresciani, L.; Neviani, E.; Dall’Asta, C.; Lazzi, C.; Galaverna, G. Solid state lactic acid fermentation: A strategy to improve wheat bran functionality. LWT 2020, 118, 108668. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef] [PubMed]
- Ndolo, V.U.; Beta, T. Comparative Studies on Composition and Distribution of Phenolic Acids in Cereal Grain Botanical Fractions. Cereal Chem. 2014, 91, 522–530. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Wheat Bran, Crude. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169722/nutrients (accessed on 26 October 2022).
- U.S. Department of Agriculture. Rice Bran, Crude. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169713/nutrients (accessed on 26 October 2022).
- U.S. Department of Agriculture. Oat Bran, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168872/nutrients (accessed on 26 October 2022).
- Cheng, W.; Sun, Y.; Fan, M.; Li, Y.; Wang, L.; Qian, H. Wheat bran, as the resource of dietary fiber: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 7269–7281. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Saeed, F.; Khan, M.A.; Niaz, B.; Khan, S.G.; Anjum, F.M.; Tufail, T.; Hussain, S. Comparative study of chemical treatments in combination with extrusion for the partial conversion of wheat and sorghum insoluble fiber into soluble. Food Sci. Nutr. 2019, 7, 2059–2067. [Google Scholar] [CrossRef] [Green Version]
- Escobar, E.L.N.; da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Teleky, B.-E.; Martău, G.A.; Vodnar, D.C. Physicochemical Effects of Lactobacillus plantarum and Lactobacillus casei Cocultures on Soy–Wheat Flour Dough Fermentation. Foods 2020, 9, 1894. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Cheng, W.; Yang, K.; Cai, L.; He, L.; Du, L.; Liu, Y.; Liu, A.; Zeng, Z.; et al. Impact of arabinoxylan on characteristics, stability and lipid oxidation of oil-in-water emulsions: Arabinoxylan from wheat bran, corn bran, rice bran, and rye bran. Food Chem. 2021, 358, 129813. [Google Scholar] [CrossRef]
- Revanappa, S.B.; Nandini, C.D.; Salimath, P.V. Structural variations of arabinoxylans extracted from different wheat (Triticum aestivum) cultivars in relation to chapati-quality. Food Hydrocoll. 2015, 43, 736–742. [Google Scholar] [CrossRef]
- Ballance, S.; Lu, Y.; Zobel, H.; Rieder, A.; Knutsen, S.H.; Dinu, V.T.; Christensen, B.E.; Ulset, A.-S.; Schmid, M.; Maina, N.; et al. Inter-laboratory analysis of cereal beta-glucan extracts of nutritional importance: An evaluation of different methods for determining weight-average molecular weight and molecular weight distribution. Food Hydrocoll. 2022, 127, 107510. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Bannikova, A.; Zyainitdinov, D.; Evteev, A.; Drevko, Y.; Evdokimov, I. Microencapsulation of polyphenols and xylooligosaccharides from oat bran in whey protein-maltodextrin complex coacervates: In-vitro evaluation and controlled release. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100236. [Google Scholar] [CrossRef]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia Amezquita, L.E.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Roasa, J.; De Villa, R.; Mine, Y.; Tsao, R. Phenolics of cereal, pulse and oilseed processing by-products and potential effects of solid-state fermentation on their bioaccessibility, bioavailability and health benefits: A review. Trends Food Sci. Technol. 2021, 116, 954–974. [Google Scholar] [CrossRef]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Wang, L.; Li, Z. Effect of particle size on the release behavior and functional properties of wheat bran phenolic compounds during in vitro gastrointestinal digestion. Food Chem. 2022, 367, 130751. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Cuevas-Rodríguez, E.O.; Mondor, M.; Ribéreau, S.; Arcand, Y.; Mackie, A.; Hernández-Álvarez, A.J. Impact of in vitro gastrointestinal digestion on peptide profile and bioactivity of cooked and non-cooked oat protein concentrates. Curr. Res. Food Sci. 2021, 4, 93–104. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Zhuang, Y.; Li, Y.; Shi, P.; Tian, H.; Li, X.; Chen, X. Isolation of novel ACE-inhibitory peptide from naked oat globulin hydrolysates in silico approach: Molecular docking, in vivo antihypertension and effects on renin and intracellular endothelin-1. J. Food Sci. 2020, 85, 1328–1337. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Yu, T.; He, J.; Cui, J.; Wang, J.; Cheng, X.; Fan, J. Oat globulin peptides regulate antidiabetic drug targets and glucose transporters in Caco-2 cells. J. Funct. Foods 2018, 42, 12–20. [Google Scholar] [CrossRef]
- Jahandideh, F.; Bourque, S.L.; Wu, J. A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides. Food Chem. X 2022, 13, 100222. [Google Scholar] [CrossRef] [PubMed]
- Shobako, N.; Ogawa, Y.; Ishikado, A.; Harada, K.; Kobayashi, E.; Suido, H.; Kusakari, T.; Maeda, M.; Suwa, M.; Matsumoto, M.; et al. A Novel Antihypertensive Peptide Identified in Thermolysin-Digested Rice Bran. Mol. Nutr. Food Res. 2018, 62, 1700732. [Google Scholar] [CrossRef]
- Martín-del-Campo, S.T.; Martínez-Basilio, P.C.; Sepúlveda-Álvarez, J.C.; Gutiérrez-Melchor, S.E.; Galindo-Peña, K.D.; Lara-Domínguez, A.K.; Cardador-Martínez, A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants 2019, 8, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbarbary, H.A.; Ejima, A.; Sato, K. Generation of antibacterial peptides from crude cheese whey using pepsin and rennet enzymes at various pH conditions. J. Sci. Food Agric. 2019, 99, 555–563. [Google Scholar] [CrossRef]
- Wang, J.-b.; Liu, X.-r.; Liu, S.-q.; Mao, R.-x.; Hou, C.; Zhu, N.; Liu, R.; Ma, H.-j.; Li, Y. Hypoglycemic Effects of Oat Oligopeptides in High-Calorie Diet/STZ-Induced Diabetic Rats. Molecules 2019, 24, 558. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Jiang, Y.; Zhou, X.; Bi, H.; Yang, B. Structure identification of soybean peptides and their immunomodulatory activity. Food Chem. 2021, 359, 129970. [Google Scholar] [CrossRef]
- Wang, X.M.; Chen, H.X.; Fu, X.G.; Li, S.Q.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Hatanaka, T.; Uraji, M.; Fujita, A.; Kawakami, K. Anti-oxidation Activities of Rice-Derived Peptides and Their Inhibitory Effects on Dipeptidylpeptidase-IV. Int. J. Pept. Res. Ther. 2015, 21, 479–485. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Lu, B.; Chen, Y.; Sun, P. Simultaneous Analysis of Free Phytosterols and Phytosterol Glycosides in Rice Bran by SPE/GC–MS. Food Chem. 2022, 387, 132S742. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, T.; Lampi, A.-M.; Nyström, L.; Hemery, Y.; Rouau, X.; Piironen, V. Distribution and composition of phytosterols and steryl ferulates in wheat grain and bran fractions. J. Cereal Sci. 2012, 56, 379–388. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Tao, G.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Characterization and determination of free phytosterols and phytosterol conjugates: The potential phytochemicals to classify different rice bran oil and rice bran. Food Chem. 2021, 344, 128624. [Google Scholar] [CrossRef]
- Deroover, L.; Tie, Y.; Verspreet, J.; Courtin, C.M.; Verbeke, K. Modifying wheat bran to improve its health benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 1104–1122. [Google Scholar] [CrossRef] [PubMed]
- Si, D.; Shang, T.; Liu, X.; Zheng, Z.; Hu, Q.; Hu, C.; Zhang, R. Production and characterization of functional wheat bran hydrolysate rich in reducing sugars, xylooligosaccharides and phenolic acids. Biotechnol. Rep. 2020, 27, e00511. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ma, Y.; Dong, L.; Jia, X.; Liu, L.; Huang, F.; Chi, J.; Xiao, J.; Zhang, M.; Zhang, R. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT 2019, 115, 108461. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Effect of extraction conditions on phenolic content, anthocyanin content and antioxidant activity of bran extracts from Thai rice cultivars. J. Cereal Sci. 2019, 86, 86–91. [Google Scholar] [CrossRef]
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R. Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo. J. Nutr. 2011, 141, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Mineo, S.; Takahashi, N.; Yamada-Hara, M.; Tsuzuno, T.; Aoki-Nonaka, Y.; Tabeta, K. Rice bran-derived protein fractions enhance sulforaphane-induced anti-oxidative activity in gingival epithelial cells. Arch. Oral Biol. 2021, 129, 105215. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtys, E.; Eisel, U.L.M.; Hageman, R.J.J.; Verkuyl, J.M.; Broersen, L.M.; Dierckx, R.; de Vries, E.F.J. Anti-inflammatory effects of rice bran components. Nutr. Rev. 2018, 76, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, F.; Salehi, M.; Ahmadi, A.; Fararoei, M.; Rusta, F.; Mahmoodianfard, S. Effects of supplementation with rice husk powder and rice bran on inflammatory factors in overweight and obese adults following an energy-restricted diet: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 833–843. [Google Scholar] [CrossRef]
- Mostafa, A.O.; Abdel-Kader, R.M.; Heikal, O.A. Enhancement of cognitive functions by rice bran extract in a neuroinflammatory mouse model via regulation of PPARγ. J. Funct. Foods 2018, 48, 314–321. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Alvarez de Sotomayor, M.; Herrera, M.D. Contribution of ferulic acid, γ-oryzanol and tocotrienols to the cardiometabolic protective effects of rice bran. J. Funct. Foods 2017, 32, 58–71. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Bermudez Pulgarin, B.; Alvarez de Sotomayor, M.; Herrera, M.D. Atherosclerosis-related inflammation and oxidative stress are improved by rice bran enzymatic extract. J. Funct. Foods 2016, 26, 610–621. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Herrera, M.D.; Laufs, U.; Alvarez de Sotomayor, M.; Werner, C. Food supplementation with rice bran enzymatic extract prevents vascular apoptosis and atherogenesis in ApoE−/− mice. Eur. J. Nutr. 2017, 56, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Xu, M.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466. [Google Scholar] [CrossRef]
- Ijarotimi, O.S.; Fakayejo, D.A.; Oluwajuyitan, T.D. Nutritional Characteristics, Glycaemic Index and Blood Glucose Lowering Property of Gluten-Free Composite Flour from Wheat (Triticum aestivum), Soybean (Glycine max), Oat-Bran (Avena sativa) and Rice-Bran (Oryza sativa). Appl. Food Res. 2021, 1, 100022. [Google Scholar] [CrossRef]
- Oluwajuyitan, T.D.; Ijarotimi, O.S.; Fagbemi, T.N.; Oboh, G. Blood glucose lowering, glycaemic index, carbohydrate-hydrolysing enzyme inhibitory activities of potential functional food from plantain, soy-cake, rice-bran and oat-bran flour blends. J. Food Meas. Charact. 2021, 15, 3761–3769. [Google Scholar] [CrossRef]
- Turrini, E.; Maffei, F.; Milelli, A.; Calcabrini, C.; Fimognari, C. Overview of the Anticancer Profile of Avenanthramides from Oat. Int. J. Mol. Sci. 2019, 20, 4536. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, H.; Otake, M.; Matsumoto, T.; Izutsu, R.; Jehung, J.P.; Goto, K.; Osaki, M.; Mayama, M.; Shikanai, M.; Kobayashi, H.; et al. Prevention of tumor progression in inflammation-related carcinogenesis by anti-inflammatory and anti-mutagenic effects brought about by ingesting fermented brown rice and rice bran with Aspergillus oryzae (FBRA). J. Funct. Foods 2022, 88, 104907. [Google Scholar] [CrossRef]
- Kannan, A.; Hettiarachchy, N.; Narayan, S. Colon and breast anti-cancer effects of peptide hydrolysates derived from rice bran. Open Bioact. Compd. J. 2009, 2, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61, 1500905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three—The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 91–119. [Google Scholar]
- Maki, K.C.; Gibson, G.R.; Dickmann, R.S.; Kendall, C.W.C.; Chen, C.Y.O.; Costabile, A.; Comelli, E.M.; McKay, D.L.; Almeida, N.G.; Jenkins, D.; et al. Digestive and physiologic effects of a wheat bran extract, arabino-xylan-oligosaccharide, in breakfast cereal. Nutrition 2012, 28, 1115–1121. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Wang, X.; Ma, S.; Sun, B.; Wang, F. Mechanochemical effects on the structural properties of wheat starch during vibration ball milling of wheat endosperm. Int. J. Biol. Macromol. 2022, 206, 306–312. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Zhang, X.; Kazem, A.E.; Iacomini, M.; Hamaker, B.R.; Cordeiro, L.M.C. Microwave treatment enhances human gut microbiota fermentability of isolated insoluble dietary fibers. Food Res. Int. 2021, 143, 110293. [Google Scholar] [CrossRef] [PubMed]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.K.; Fabiano-Tixier, A.S.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Song, L.-w.; Qi, J.-r.; Liao, J.-s.; Yang, X.-q. Enzymatic and enzyme-physical modification of citrus fiber by xylanase and planetary ball milling treatment. Food Hydrocoll. 2021, 121, 107015. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Wang, W.; Wang, L.; Jiang, L.; Yu, D.; Xie, F. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrason. Sonochem. 2021, 79, 105758. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Ultrasound-assisted extraction from defatted oat (Avena sativa L.) bran to simultaneously enhance phenolic compounds and β-glucan contents: Compositional and kinetic studies. J. Food Eng. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- Milić, P.S.; Rajković, K.M.; Stamenković, O.S.; Veljković, V.B. Kinetic modeling and optimization of maceration and ultrasound-extraction of resinoid from the aerial parts of white lady’s bedstraw (Galium mollugo L.). Ultrason. Sonochem. 2013, 20, 525–534. [Google Scholar] [CrossRef]
- Suresh, S.; Sivaramakrishnan, R.; Radha, K.V.; Incharoensakdi, A.; Pugazhendhi, A. Ultrasound pretreated rice bran for Rhizopus sp. phytase production as a feed. Food Biosci. 2021, 43, 101281. [Google Scholar] [CrossRef]
- Herrera-Ponce, A.L.; Salmeron-Ochoa, I.; Rodriguez-Figueroa, J.C.; Santellano-Estrada, E.; Garcia-Galicia, I.A.; Alarcon-Rojo, A.D. High-intensity ultrasound as pre-treatment in the development of fermented whey and oat beverages: Effect on the fermentation, antioxidant activity and consumer acceptance. J. Food Sci. Technol. 2022, 59, 796–804. [Google Scholar] [CrossRef]
- Vaitkeviciene, R.; Zadeike, D.; Gaizauskaite, Z.; Valentaviciute, K.; Marksa, M.; Mazdzieriene, R.; Bartkiene, E.; Lele, V.; Juodeikiene, G.; Jakstas, V. Functionalisation of rice bran assisted by ultrasonication and fermentation for the production of rice bran–lingonberry pulp-based probiotic nutraceutical. Int. J. Food Sci. Technol. 2022, 57, 1462–1472. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Wang, L.; Gao, H.; Chen, H. Process of steam explosion assisted superfine grinding on particle size, chemical composition and physico-chemical properties of wheat bran powder. Powder Technol. 2020, 371, 154–160. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, M.; Liu, X.; Zhong, K.; Tong, L.; Zhou, X.; Zhou, S. Effect of steam explosion-assisted extraction on phenolic acid profiles and antioxidant properties of wheat bran. J. Sci. Food Agric. 2016, 96, 3484–3491. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Long, X.; Huang, Y.; Yang, Y.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Tu, Z. Effects of microbial fermentation and microwave treatment on the composition, structural characteristics, and functional properties of modified okara dietary fiber. LWT 2020, 123, 109059. [Google Scholar] [CrossRef]
- Alonso, E. The role of supercritical fluids in the fractionation pretreatments of a wheat bran-based biorefinery. J. Supercrit. Fluids 2018, 133, 603–614. [Google Scholar] [CrossRef]
- Scapini, T.; dos Santos, M.S.N.; Bonatto, C.; Wancura, J.H.C.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery. Bioresour. Technol. 2021, 342, 126033. [Google Scholar] [CrossRef]
- Cantero, D.A.; Martínez, C.; Bermejo, M.; Cocero, M. Simultaneous and selective recovery of cellulose and hemicellulose fractions from wheat bran by supercritical water hydrolysis. Green Chem. 2015, 17, 610–618. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Chen, Y.; Chen, J. Effects of acid treatment on the physicochemical and functional properties of wheat bran insoluble dietary fiber. Cereal Chem. 2021, 99, 343–354. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Masamba, K.G.; Shoemaker, C.F.; Zhong, F.; Majeed, H.; Ma, J. The effect of chemical treatment on the In vitro hypoglycemic properties of rice bran insoluble dietary fiber. Food Hydrocoll. 2016, 52, 699–706. [Google Scholar] [CrossRef]
- Rahman, A.; Fehrenbach, J.; Ulven, C.; Simsek, S.; Hossain, K. Utilization of wheat-bran cellulosic fibers as reinforcement in bio-based polypropylene composite. Ind. Crops Prod. 2021, 172, 114028. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, X.; Zhang, Z.; Zhou, T.; Gao, R.; Li, Y.; Ding, X. Effect of β-endoxylanase and α-arabinofuranosidase enzymatic hydrolysis on nutritional and technological properties of wheat brans. Food Chem. 2020, 302, 125332. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Ritthibut, N.; Oh, S.-J.; Lim, S.-T. Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT 2021, 135, 110273. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascus anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Janarny, G.; Gunathilake, K.D.P.P. Changes in rice bran bioactives, their bioactivity, bioaccessibility and bioavailability with solid-state fermentation by Rhizopus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101510. [Google Scholar] [CrossRef]
- Mao, M.; Wang, P.; Shi, K.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lv, F. Effect of solid state fermentation by Enterococcus faecalis M2 on antioxidant and nutritional properties of wheat bran. J. Cereal Sci. 2020, 94, 102997. [Google Scholar] [CrossRef]
- Tanasković, S.J.; Šekuljica, N.; Jovanović, J.; Gazikalović, I.; Grbavčić, S.; Đorđević, N.; Sekulić, M.V.; Hao, J.; Luković, N.; Knežević-Jugović, Z. Upgrading of valuable food component contents and anti-nutritional factors depletion by solid-state fermentation: A way to valorize wheat bran for nutrition. J. Cereal Sci. 2021, 99, 103159. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D.; Kaur, G.; Kaur, G. Biotransformation of Rice Bran to Ferulic Acid by Pediococcal Isolates. Appl. Biochem. Biotechnol. 2013, 170, 854–867. [Google Scholar] [CrossRef]
- Martău, G.A.; Călinoiu, L.-F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- Vu, V.; Farkas, C.; Riyad, O.; Bujna, E.; Kilin, A.; Sipiczki, G.; Sharma, M.; Usmani, Z.; Gupta, V.K.; Nguyen, Q.D. Enhancement of the enzymatic hydrolysis efficiency of wheat bran using the Bacillus strains and their consortium. Bioresour. Technol. 2022, 343, 126092. [Google Scholar] [CrossRef] [PubMed]
- Rustiguel, C.B.; Jorge, J.A.; Guimarães, L.H.S. Characterization of a thermo-tolerant mycelial β-fructofuranosidase from Aspergillus phoenicis under submerged fermentation using wheat bran as carbon source. Biocatal. Agric. Biotechnol. 2015, 4, 362–369. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Pengkumsri, N.; Sirilun, S.; Peerajan, S.; Khongtan, S.; Sivamaruthi, B.S. Assessment of changes in the content of anthocyanins, phenolic acids, and antioxidant property of Saccharomyces cerevisiae mediated fermented black rice bran. AMB Express 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Akhavan Sepahy, A.; Ghazi, S.; Akhavan Sepahy, M. Cost-Effective Production and Optimization of Alkaline Xylanase by Indigenous Bacillus mojavensis AG13 Fermented on Agricultural Waste. Enzym. Res. 2011, 2011, 593624. [Google Scholar] [CrossRef]

| Nutrients | Wheat Bran [16] | Rice Bran [17] | Oat Bran [18] |
|---|---|---|---|
| Amount (g/100 g) | Amount (g/100 g) | Amount (g/100 g) | |
| Water | 9.89 | 6.13 | 6.55 |
| Energy | 216 kcal | 316 kcal | 246 |
| Protein | 15.6 | 13.4 | 17.3 |
| Total lipids | 4.25 | 20.8 | 7.03 |
| Polyunsaturated fatty acids | 2.21 | 7.46 | 2.77 |
| Monounsaturated fatty acids | 0.63 | 7.55 | 2.38 |
| Ash | 5.79 | 9.98 | 2.89 |
| Carbohydrates | 64.5 | 49.7 | 66.2 |
| Fibers | 42.8 | 21 | 15.4 |
| Total sugars | 0.41 | 0.9 | 1.45 |
| Source | Study | Pretreatments | Biological Activity | Key Findings | References |
|---|---|---|---|---|---|
| Wheat bran | in vitro | Ultrafine grinding: Coarse wheat bran Medium wheat bran Fine wheat bran Superfine wheat bran | Antioxidant activity; Digestive enzymes inhibitory activities | p-coumaric acid content in superfine WB was five times higher; Phenolic compounds bioaccessibility was increased by 65.51%; Starch digestibility was reduced. | [32] |
| in vivo | Enzyme pretreatment (xylanase, cellulose, β-glucanase, and feruloyl-esterase) Yeast fermentation (Baker’s Yeast) | Antioxidant capacity; Anti-inflammatory properties | Phenylpropionic acid and 3-hydroxyphenylpropionic acid were the main colonic metabolites identified; The pro-:anti-inflammatory cytokines ratio was significantly lower after the consumption of 300 g of bioprocessed bran-based bread for 3 days. | [54] | |
| in vitro | Grinding | Antioxidant activity | The extracted xylose, mannose, glucose, and galactose exhibited remarkable antioxidant activities (DPPH, ABTS, hydroxyl, and superoxide radical scavenging tests) | [56] | |
| in vivo | - | Prebiotic effect | Arabino-xylan-oligosaccharide provided prebiotic properties increasing fecal bifidobacteria and postprandial ferulic acid concentrations. | [74] | |
| Rice bran | in vitro | Extrusion | Antioxidant activity; | Extractability of the bound phenolics was increased; Increased free bound and total phenolic content by 23.0%, 50.7%, and 36.3%; No effect was observed on CAA antioxidant activity; Bioaccessibile phenolics increased by 40.5% | [52] |
| in vitro | Fungal fermentation | Antioxidant activity; | Extractability of the bound phenolics was increased; Increased free bound and total phenolic content by 99.4%, 40%, and 71.6%; ORAC antioxidant activity increased 1.8-fold; CAA antioxidant activity increased 4.1-fold; Bioaccessibile phenolics increased by 64.5% | [52] | |
| in vitro | Enzymatic pretreatment (protease from Aspergillus oryzae) | Anti-diabetic activity; Antioxidant activity; | Dipeptides Ile-Pro, Met-Pro, Val-Pro, and Leu-Pro had shown inhibitory activity against DPP-IV (dipeptidylpeptidase-IV); No inhibitory activity against human maltase–glucoamylase was observed. | [43] | |
| in vivo (animal study-mouse model) | Maceration in ethanol | Neuroinflammatory responses (memory and cognitive performance) | Spatial working, reference memory, and non-spatial recognition memory were improved | [60] | |
| in vivo (animal study-mouse model) | Enzymatic hydrolysis | Antioxidant capacity; Anti-inflammatory properties | Oxidative stress was reduced; Ferulic acid and γ-oryzanol reduced pro-inflammatory monocyte phenotype; Atherosclerosis-related oxidative stress and inflammation were reduced. | [62] | |
| in vivo (animal study-mouse model) | Fermentation with Aspergillus oryzae | Anti-inflammatory activity; Anti-mutagenic effects | Fermented brown rice and rice bran provided chemopreventive effectiveness against inflammation-related carcinogenesis | [68] | |
| Oat bran | in vivo (animal study-mouse model) | Grinding | Antidiabetic activity; Carbohydrate hydrolyzing inhibitory activity; Antihyperglycaemia activity. | The samples with 70% wheat, 20% soy cake, 5% rice bran, and 5% oat bran provided low glycaemic index, high carbohydrate hydrolyzing enzyme inhibitory potential, and blood glucose lowering potential. | [65] |
| in vivo | Maceration | Antidiabetic activity | The samples with plantain 60%, soy cake 30%, rice bran 5%, and oat bran 5% had the highest blood glucose-reducing activity. | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, S.A.; Călinoiu, L.F.; Dulf, F.V.; Fărcas, A.C.; Vodnar, D.C. Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach. Antioxidants 2022, 11, 2159. https://doi.org/10.3390/antiox11112159
Nemes SA, Călinoiu LF, Dulf FV, Fărcas AC, Vodnar DC. Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach. Antioxidants. 2022; 11(11):2159. https://doi.org/10.3390/antiox11112159
Chicago/Turabian StyleNemes, Silvia Amalia, Lavinia Florina Călinoiu, Francisc Vasile Dulf, Anca Corina Fărcas, and Dan Cristian Vodnar. 2022. "Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach" Antioxidants 11, no. 11: 2159. https://doi.org/10.3390/antiox11112159
APA StyleNemes, S. A., Călinoiu, L. F., Dulf, F. V., Fărcas, A. C., & Vodnar, D. C. (2022). Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach. Antioxidants, 11(11), 2159. https://doi.org/10.3390/antiox11112159










