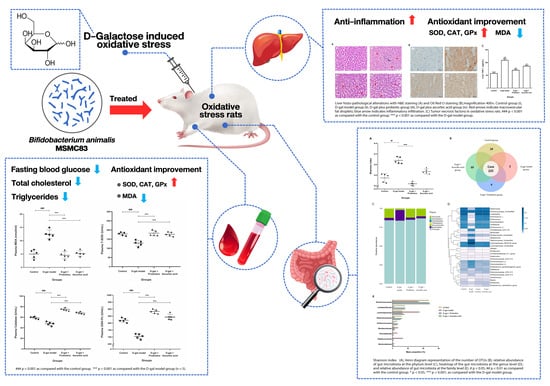
Bifidobacterium animalis MSMC83 Improves Oxidative Stress and Gut Microbiota in D-Galactose-Induced Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
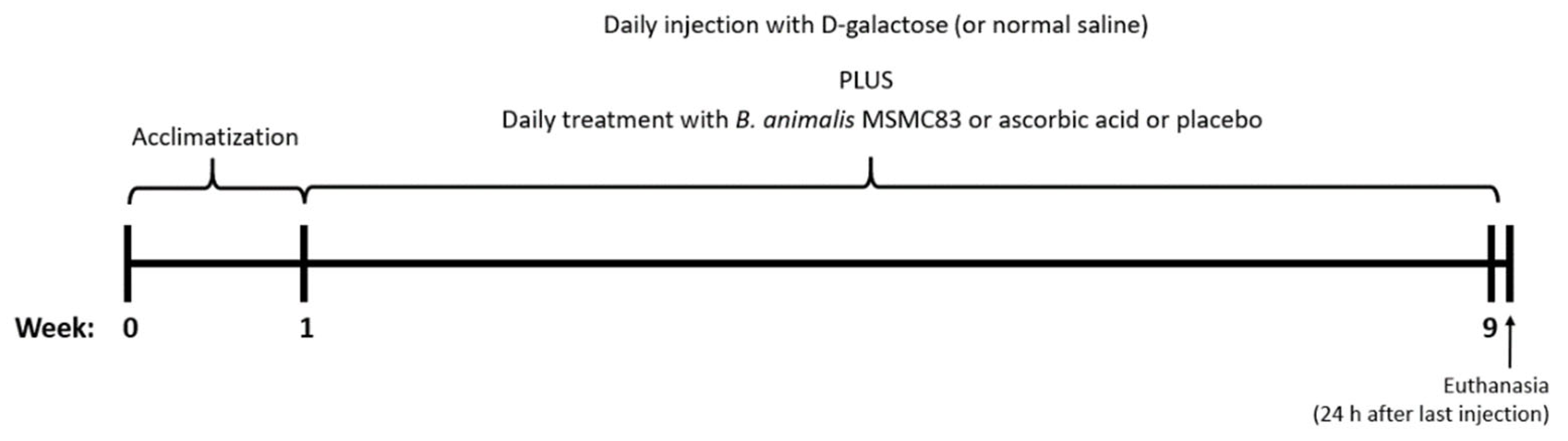
2.2. Experimental Design
2.3. Sample and Organ Collections
2.4. Serum Biochemical Estimation
2.5. Histological Analysis
2.6. Determination of TNF-α Level in Liver
2.7. Measurement of Oxidative Stress Markers in Plasma and Liver
2.8. Analysis of Gut Microbiota by Next-Generation Sequencing
2.9. Statistical Analysis
3. Results
3.1. Changes in Body and Organ Weight
3.2. Biochemical Changes in Plasma
3.3. Effect of B. animalis MSMC83 on Liver Inflammation
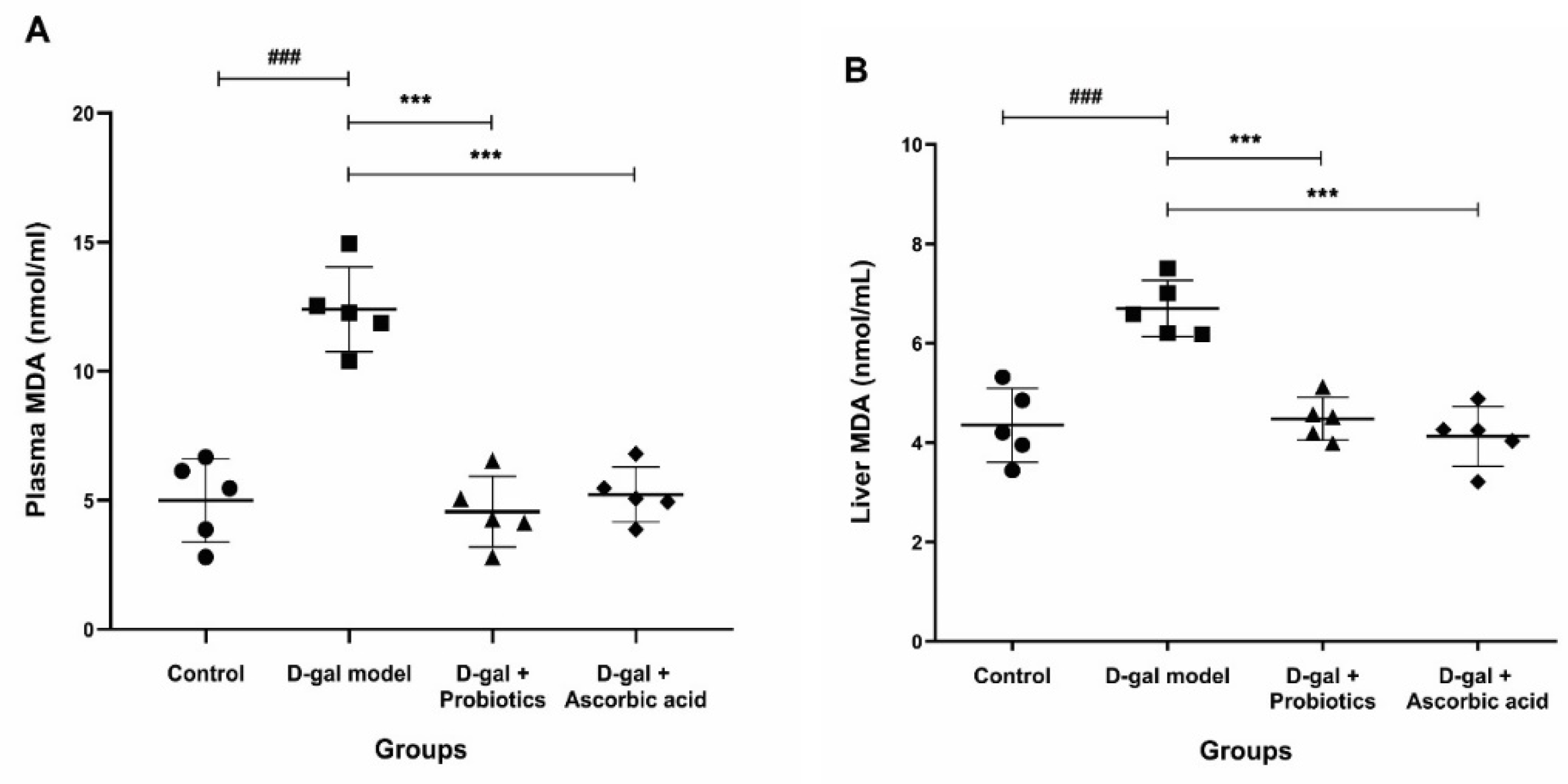
3.4. Effect of B. animalis MSMC83 on Oxidative Stress Markers in Plasma and Liver
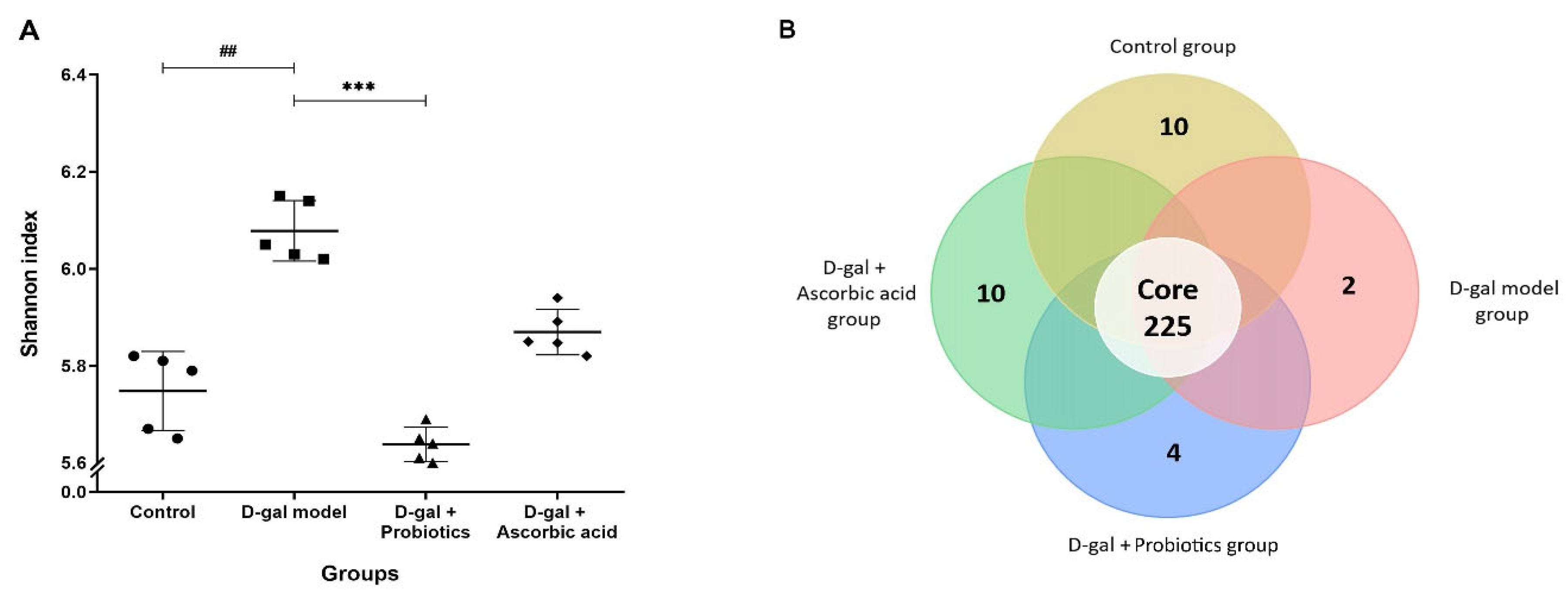
3.5. The Dysbiosis Induced by D-gal Was Prevented by B. animalis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preiser, J.C. Oxidative stress. J. Parenter. Enteral. Nutr. 2012, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hwang, K.T.; Chung, M.Y.; Cho, D.H.; Park, C.S. Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): Role for a metal ion chelating effect. J. Food. Sci. 2005, 70, m388–m391. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelombitko, M.A. Role of reactive oxygen species in inflammation: A minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.C.; Feng, M.Q.; Sun, J.; Xu, X.L.; Zhou, G.H. Screening of lactic acid bacteria with high protease activity from fermented sausages and antioxidant activity assessment of its fermented sausages. CyTA J. Food 2019, 17, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, B. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis: A role for bifidobacteria and lactobacilli? Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 205. [Google Scholar] [CrossRef]
- Anandharaj, M.; Sivasankari, B.; Parveen Rani, R. Effects of probiotics, prebiotics, and synbiotics on hypercholesterolemia: A review. Chin. J. Biol. 2014, 2014, 572754. [Google Scholar] [CrossRef] [Green Version]
- Ayeni, F.A.; Sánchez, B.; Adeniyi, B.A.; de los Reyes-Gavilán, C.G.; Margolles, A.; Ruas-Madiedo, P. Evaluation of the functional potential of Weissella and Lactobacillus isolates obtained from Nigerian traditional fermented foods and cow’s intestine. Int. J. Food Microbiol. 2011, 147, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azman, K.F.; Safdar, A.; Zakaria, R. D-galactose-induced liver aging model: Its underlying mechanisms and potential therapeutic interventions. Exp. Gerontol. 2021, 150, 111372. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Cao, X.; Cui, L.; Liu, H.; Wang, S.; Chen, T. Anti-aging effect of the combination of Bifidobacterium longum and B. animalis in a d-galactose-treated mice. J. Funct. Foods 2020, 69, 103938. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, F.; Yan, S.; Zhai, Q.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food. Funct. 2018, 9, 917–924. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, R.X.; Du, Y.T.; Xu, X.J.; Xue, Y.; Gao, D.; Gao, T.; Sheng, Z.; Zhang, L.Y.; Tuo, H.Z. Features of gut microbiota in patients with idiopathic Parkinson’s disease. Zhonghua Yi Xue Za Zhi 2020, 100, 1017–1022. [Google Scholar]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Ma, F.; Sun, M.; Song, Y.; Xu, D.; Mu, G.; Tuo, Y. Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food. Funct. 2021, 12, 373–386. [Google Scholar] [CrossRef]
- Vitheejongjaroen, P.; Kanthawang, P.; Loison, F.; Jaisin, Y.; Pachekrepapol, U.; Taweechotipatr, M. Antioxidant activity of Bifidobacterium animalis MSMC83 and its application in set-style probiotic yoghurt. Food. Biosci. 2021, 43, 101259. [Google Scholar] [CrossRef]
- Jantararussamee, C.; Rodniem, S.; Taweechotipatr, M.; Showpittapornchai, U.; Pradidarcheep, W. Hepatoprotective effect of probiotic lactic acid bacteria on thioacetamide-induced liver fibrosis in rats. Probiotics. Antimicrob. Proteins. 2021, 13, 40–50. [Google Scholar] [CrossRef]
- Guo, D.; Xie, M.; Xiao, H.; Xu, L.; Zhang, S.; Chen, X.; Wu, Z. Bacillus subtilis supplementation in a high-fat diet modulates the gut microbiota and ameliorates hepatic lipid accumulation in grass carp (Ctenopharyngodon idella). Fishes 2022, 7, 94. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food. Chem. 2020, 316, 126309. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Zhang, J.; Yang, H.; Huo, L.; Gao, J.; Chen, H.; Gao, W. Protective effect of tetrahydropalmatine against d-galactose induced memory impairment in rat. Physiol. Behav. 2016, 154, 114–125. [Google Scholar] [CrossRef]
- James, E.L.; Michalek, R.D.; Pitiyage, G.N.; de Castro, A.M.; Vignola, K.S.; Jones, J.; Mohney, R.P.; Karoly, E.D.; Prime, S.S.; Parkinson, E.K. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J. Proteome. Res. 2015, 14, 1854–1871. [Google Scholar] [CrossRef]
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B12 and omega-3 fatty acids on brain function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Dong, J.; Wang, Y.; Gond, B.K. Thermal oxidation ageing effects on silicone rubber sealing performance. Polym. Degrad. Stabil. 2017, 135, 43–53. [Google Scholar] [CrossRef]
- Politis-Barber, V.; Brunetta, H.S.; Paglialunga, S.; Petrick, H.L.; Holloway, G.P. Long-term, high-fat feeding exacerbates short-term increases in adipose mitochondrial reactive oxygen species, without impairing mitochondrial respiration. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E376–E387. [Google Scholar] [CrossRef]
- Li, B.; Evivie, S.E.; Lu, J.; Jiao, Y.; Wang, C.; Li, Z.; Liu, F.; Huo, G. Lactobacillus helveticus KLDS1.8701 alleviates d-galactose-induced aging by regulating Nrf-2 and gut microbiota in mice. Food. Funct. 2018, 9, 6586–6598. [Google Scholar] [CrossRef]
- Kenawy, S.; Hegazy, R.; Hassan, A.; El-Shenawy, S.; Gomaa, N.; Zaki, H.; Attia, A. Involvement of insulin resistance in D-galactose-induced age-related dementia in rats: Protective role of metformin and saxagliptin. PLoS ONE 2017, 12, e0183565. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, K.N.; Klotz, L.O.; Korsten, P.; Bornstein, S.R.; Barthel, A. Linking Alzheimer’s disease to insulin resistance: The FoxO response to oxidative stress. Mol. Psychiatry 2010, 15, 1046–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Huang, Q.; Fu, X.; Yue, X.J.; Liu, R.H.; You, L.J. Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, S.; Yang, D.; Qiu, L.; Wu, Y.; Wang, D.; Shah, N.P.; Xu, F.; Wei, H. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in d-galactose-induced aging mice. J. Dairy Sci. 2016, 99, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Li, W.; GW, L. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Navarrete, S.; Alarcon, M.; Palomo, I. Aqueous extract of tomato (Solanum lycopersicum L.) and ferulic acid reduce the expression of TNF-alpha and IL-1beta in LPS-activated macrophages. Molecules 2015, 20, 15319–15329. [Google Scholar] [CrossRef] [Green Version]
- Hritcu, L.; Bagci, E.; Aydin, E.; Mihasan, M. Antiamnesic and antioxidants effects of Ferulago angulata essential oil against scopolamine-induced memory impairment in laboratory rats. Neurochem. Res. 2015, 40, 1799–1809. [Google Scholar] [CrossRef]
- Xiao, M.H.; Xia, J.Y.; Wang, Z.L.; Hu, W.X.; Fan, Y.L.; Jia, D.Y.; Li, J.; Jing, P.W.; Wang, L.; Wang, Y.P. Ginsenoside Rg1 attenuates liver injury induced by D-galactose in mice. Exp. Ther. Med. 2018, 16, 4100–4106. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus plantarum AR501 alleviates the oxidative stress of d-galactose-induced aging mice liver by upregulation of nrf2-mediated antioxidant enzyme expression. J. Food. Sci. 2018, 83, 1990–1998. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Chen, K.C.; Guan, W.W.; Peng, C.C.; Peng, R.Y. Cylophosphamide elicited intracranial hemorrhage via mitochondrial ROS-hif-1α-ATP depleting pathway—Preventive trials with folic acid, resveratrol and vitamin E. RSC Adv. 2015, 5, 30342–30353. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Z.; Zhang, J.; Huo, L.; Gao, J.; Gao, W. Ferulic acid ameliorates memory impairment in d-galactose-induced aging mouse model. Int. J. Food. Sci. Nutr. 2016, 67, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and d-galactose-induced aging mice. Food. Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef]
- Yuan, L.; Kaplowitz, N. Glutathione in liver diseases and hepatotoxicity. Mol. Asp. Med. 2009, 30, 29–41. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut. Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Kullisaar, T.; Zilmer, M.; Mikelsaar, M.; Vihalemm, T.; Annuk, H.; Kairane, C.; Kilk, A. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food. Microbiol. 2002, 72, 215–224. [Google Scholar] [CrossRef]
- Aluwong, T.; Kawu, M.; Raji, M.; Dzenda, T.; Govwang, F.; Sinkalu, V.; Ayo, J. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants 2013, 2, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Food. 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Xu, R.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 2011, 17, 226–231. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lin, J.H.; Kuo, Y.W.; Chiang, P.R.; Ho, H.H. Probiotics and their metabolites reduce oxidative stress in middle-aged mice. Curr. Microbiol. 2022, 79, 104. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Yadav, U.C.; Reddy, A.B.; Saxena, A.; Tammali, R.; Shoeb, M.; Ansari, N.H.; Bhatnagar, A.; Petrash, M.J.; Srivastava, S.; et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011, 191, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Diao, Y.; Xin, Y.; Zhou, Y.; Li, N.; Pan, X.; Qi, S.; Qi, Z.; Xu, Y.; Luo, L.; Wan, H.; et al. Extracellular polysaccharide from Bacillus sp. strain LBP32 prevents LPS-induced inflammation in RAW 264.7 macrophages by inhibiting NF-κB and MAPKs activation and ROS production. Int. Immunopharmacol. 2014, 18, 12–19. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Z.; Zhu, G. Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct. 2013, 4, 982–989. [Google Scholar] [CrossRef]
- Tan, C.W.; Maziar, S.; Teo, E.H.T.; Tay, B.K. Microstructure and through-film electrical characteristics of vertically aligned amorphous carbon films. Diam. Relat. Mater. 2011, 20, 290–293. [Google Scholar] [CrossRef]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, J.W.; Jhun, J.; Kwon, J.Y.; Lee, B.I.; Yang, C.W.; Park, S.H.; Cho, M.L. Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of th17 and treg cell balance and fibrosis development. J. Med. Food 2018, 21, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [Green Version]
- Pino, A.; Benkaddour, B.; Inturri, R.; Amico, P.; Vaccaro, S.C.; Russo, N.; Vaccalluzzo, A.; Agolino, G.; Caggia, C.; Miloud, H.; et al. Characterization of Bifidobacterium asteroides isolates. Microorganisms 2022, 10, 655. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Borrelli, A.; Bonelli, P.; Tuccillo, F.M.; Goldfine, I.D.; Evans, J.L.; Buonaguro, F.M.; Mancini, A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox. Biol. 2018, 15, 467–479. [Google Scholar] [CrossRef]
- Soccol, C.R.; de Souza Vandenberghe, L.P.; Spier, M.R.; Pedroni Medeiros, A.B.; Yamaguishi, C.T.; de Dea Lindner, J.; Pandey, A.; Thomaz-Soccol, V. The potential of probiotics: A review. Food. Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Jones, R.M.; Neish, A.S. Redox signaling mediated by the gut microbiota. Free. Radic. Biol. Med. 2017, 105, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Lau, A.S.; Ong, J.S.; Kato, T.; Nakanishi, Y.; Azzam, G.; Azlan, A.; Ohno, H.; et al. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol. Res. 2019, 146, 104312. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Nardone, G.; Compare, D.; Liguori, E.; Di Mauro, V.; Rocco, A.; Barone, M.; Napoli, A.; Lapi, D.; Iovene, M.R.; Colantuoni, A. Rotective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 669–676. [Google Scholar] [CrossRef]



| Index | Control | D-gal Model | D-gal + Probiotics | D-gal + Ascorbic Acid |
|---|---|---|---|---|
| Body weight | 600.60 ± 8.79 | 635.67 ± 10.97 | 573.65 ± 16.44 | 576.69 ± 31.60 |
| Liver | 14.90 ± 0.35 | 17.05 ± 1.37 # | 14.03 ± 0.34 ** | 15.08 ± 0.49 * |
| Spleen | 0.82 ± 0.05 | 0.93 ± 0.03 | 0.77 ± 0.05 | 0.83 ± 0.13 |
| Stomach | 2.75 ± 0.17 | 2.91 ± 0.07 | 3.13 ± 0.27 | 2.72 ± 0.60 |
| Adipose tissue | 38.11 ± 0.64 | 42.81 ± 0.57 ## | 34.03 ± 1.23 *** | 34.56 ± 1.73 *** |
| Biochemical | Control | D-gal Model | D-gal+ Probiotics | D-gal + Ascorbic Acid |
|---|---|---|---|---|
| Glucose (mg/dL) | 203.67 ± 1.53 | 221.33 ± 5.13 ## | 105.00 ± 3.46 *** | 172.67 ± 6.43 *** |
| Total cholesterol (mg/dL) | 49.33 ± 3.22 | 60.67 ± 0.58 ## | 50.33 ± 1.53 *** | 50.67 ± 2.08 *** |
| Triglyceride (mg/dL) | 84.33 ± 3.79 | 229.67 ± 7.57 ## | 128.00 ± 5.57 *** | 102.00 ± 5.20 *** |
| HDL-C (mg/dL) | 31.33 ± 5.03 | 32.33 ± 1.53 | 35.67 ± 0.58 | 30.33 ± 1.53 |
| Direct LDL-C (mg/dL) | 5.00 ± 1.00 | 5.33 ± 0.58 | 5.67 ± 0.58 | 6.67 ± 0.58 |
| AST (U/L) | 74.00 ± 4.58 | 90.67 ± 5.77 ## | 68.07 ± 3.90 ** | 70.37 ± 9.02 * |
| ALT (U/L) | 26.67 ± 0.58 | 28.33 ± 4.04 | 25.67 ± 3.06 | 27.77 ± 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitheejongjaroen, P.; Kasorn, A.; Puttarat, N.; Loison, F.; Taweechotipatr, M. Bifidobacterium animalis MSMC83 Improves Oxidative Stress and Gut Microbiota in D-Galactose-Induced Rats. Antioxidants 2022, 11, 2146. https://doi.org/10.3390/antiox11112146
Vitheejongjaroen P, Kasorn A, Puttarat N, Loison F, Taweechotipatr M. Bifidobacterium animalis MSMC83 Improves Oxidative Stress and Gut Microbiota in D-Galactose-Induced Rats. Antioxidants. 2022; 11(11):2146. https://doi.org/10.3390/antiox11112146
Chicago/Turabian StyleVitheejongjaroen, Porntipha, Anongnard Kasorn, Narathip Puttarat, Fabien Loison, and Malai Taweechotipatr. 2022. "Bifidobacterium animalis MSMC83 Improves Oxidative Stress and Gut Microbiota in D-Galactose-Induced Rats" Antioxidants 11, no. 11: 2146. https://doi.org/10.3390/antiox11112146